Introduction
Gastric cancer (GC) is the fourth most common cause
of cancer-associated mortality worldwide, as the majority of
patients initially present at an advanced stage of the disease; the
prognosis is poor with a 6-month survival rate of <15% (1). Since GC is a heterogeneous disease with
various genetic mutations, in addition to current histological
classification systems, including Lauren (2) and the World Health Organization
(3) systems, it has been of great
focus to determine a novel molecular classification system in the
age of high-throughput technology. As reviewed by Cisło et
al (4), The Cancer Genome Atlas
(TCGA) sought to identify the subtypes of GC using complex
statistical analyses of molecular data that had been obtained from
six molecular analysis platforms that included DNA sequencing, RNA
sequencing and protein arrays. A total of four major genomic
subtypes of GC were proposed, including Epstein Barr
Virus-positive, microsatellite instability, genomically stable and
chromosomal instability.
In the present study, never-in-mitosis A-related
kinase 8 (NEK8) gene expression data and relapse-free and overall
survival information were further analyzed from Gene Expression
Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo; Affymetrix
microarrays only; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and the European Genome-Phenome Archive (EGA; http://www.ebi.ac.uk/ega/home), in addition to
TCGA, using online tool (http://kmplot.com/analysis/) (5). It was revealed that higher expression
levels of NEK8 lead to poor survival outcomes associated with GC,
indicating that NEK8 may be associated with the development of
GC.
NEK8 is a member of the serine/threonine protein
kinase family associated with never in mitosis, gene A (NIMA) of
Aspergillus nidulans (6–8), and it
has been reported to serve a role in the progression of the cell
cycle from the G2 to M phase (9),
primary cilia disassembly (10,11) and
activation of the DNA damage response signaling pathway (9,12). Holland
et al (13) identified NEK8 as
a novel NIMA-associated kinase and also its candidate target BICD
cargo adaptor 2, which has been associated with microtubule
dynamics, independent of the cell cycle. In addition, it has been
reported in several studies that NEK8 regulates ciliary stability
via polycystin-1, polycystin-2 (11),
yes-associated protein (14),
galectin-1, sorcin and vimentin proteins (15). It has previously been reported that
NEK8 may serve a potential role in tumorigenesis and the DNA damage
response via MYC proto-oncogene, BHLH transcription factor
(16), RAD51 recombinase (17) and the serine/threonine kinase
signaling pathway (12). However, the
underlying mechanism of NEK8 regulation requires further
investigation (18). In another
previous study (19), it was revealed
that NEK8 may be a novel target gene for hypoxia-inducible factors
(HIFs) and that the von-Hippel-Lindau tumor suppressor protein
(pVHL) may downregulate NEK8 expression via HIFs.
It has been well reported that the functions of
pVHL, the product of the VHL tumor suppressor gene, may be
associated with its role as the substrate-recognition component of
an E3 ubiquitin ligase complex, which also contains elongin B,
elongin C, cullin 2 and ring-box 1 (20). The aforementioned complex targets
HIF-α for ubiquitination and degradation. Although less thoroughly
characterized, pVHL also exhibits HIF-independent functions,
including regulation of microtubule dynamics, extracellular matrix
deposition, responses to DNA damage and primary cilia maintenance
(20).
In the present study, it was demonstrated that pVHL
may interact with NEK8 and act as an E3 ubiquitin ligase that
promotes NEK8 degradation. The results of the present study may
improve current knowledge of NEK8 function and regulation.
Materials and methods
Cell culture and reagents
SGC-7901 and SNU-1 cells were obtained from the Type
Culture Collection of the Chinese Academy of Science (CASTCC;
Shanghai, China) and cultured in an appropriate medium, RPMI-1640
plus 10% fetal bovine serum (FBS), as suggested by CASTCC. Cells
were incubated at 37°C with 5% CO2. Cycloheximide (CHX)
and MG-132 were obtained from MedChemExpress (Shanghai, China).
Plasmid transfection
The plasmids (2 µg) pcDNA3.0-VHL, enhanced green
fluorescent protein (EGFP)-NEK8 and influenza hemagglutinin-tagged
ubiquitin (HA-Ub) were transfected using Lipofectamine
2000® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. At 48 h
post-transfection, the cells were treated or harvested and
subjected to further analyses.
NEK8-knockdown
Stable knockdown of NEK8 in SGC-7901 cells was
conducted with the pSUPER RNAi system using Lipofectamine
2000® (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. To establish
pSUPER-NEK8, the sequences selected from human NEK8 cDNA,
5′-TCGTCAAGATCGGTGATTT-3′ and 5′-CTGGAAGACAAAGCCCTTA-3′, were
synthesized by Sangon Biotech Co., Ltd (Shanghai, China).
NEK8-shRNA sequence was inserted in pSUPER vector and empty pSUPER
vector was used as the negative control.
Ubiquitination assays
SGC-7901 cells were co-transfected with EGFP-NEK8,
HA-Ub and VHL or vector plasmids, respectively. Cells were treated
with 10 µM MG-132 for 4 h prior to harvesting. GFP-NEK8 protein was
immunoprecipitated from cell lysates using anti-GFP antibody (2 µg;
cat. no. sc-101525), and IgG antibody (2 µg; cat. no. sc-2025; both
Santa Cruz Biotechnology Inc., Dallas, TX, USA) was used as the
control. Proteins from the precipitates were subjected to western
blotting with anti-HA antibody (1:1,000; cat. no. sc-7392; Santa
Cruz Biotechnology Inc.).
NEK8 half-life assays
The SGC-7901 cells at 48 h after transfection with
pcDNA3.0-VHL were exposed to 100 µg/ml cycloheximide for 10, 30 and
60 min to block protein synthesis. The cells were collected for
immunoblotting analyses. Changes in relative protein expression
levels over time were determined by measuring the optical density
of the NEK8 protein bands.
Immunoblotting and
immunoprecipitation
Immunoblotting and immunoprecipitation were
conducted as previously described (17), using antibodies against NEK8 (1:500;
cat. no. sc-50761), HA (1:1,000; cat. no. sc-7392), GFP (1:1,000;
cat. no. sc-101525), GAPDH (1:5,000; cat. no. sc-47724; all Santa
Cruz Biotechnology Inc.) and VHL (1:500; cat. no. 68547; Cell
Signaling Technology Inc., Danvers, MA, USA). In brief, 50 ng
protein samples was extracted using RIPA lysis and lysed in sodium
dodecyl sulfate (SDS) buffer, separated by 10% SDS-polyacrylamide
gel electrophoresis (PAGE), and transferred to PVDF membranes. The
membranes were incubated with primary antibodies at 4°C overnight,
followed by peroxidase-labeled secondary antibodies (1:2,000; cat.
nos. sc-2005 and sc-2030; Santa Cruz Biotechnology Inc.) at room
temperature for 1 h. Immunoblots were developed using the ECL Prime
Western Blotting Detection system (GE Healthcare Life Sciences,
Little Chalfont, UK). For immunoprecipitation, 1 µg primary
antibody against VHL was added to 200 µl lysates of
2×106 cells and incubated with rotation at 4°C for 2 h.
Rabbit immunoglobulin G (IgG) was used as a negative control.
Subsequent to precipitation with agarose A/G (Santa Cruz
Biotechnology Inc.), proteins were washed in 100 µl SDS loading
buffer (Beyotime Institute of Biotechnology, Haimen, China); 20 µl
of each sample was separated by 12% SDS-PAGE and analyzed by
immunoblotting using an antibody against NEK8.
Kaplan-Meier survival analysis
The Kaplan-Meier plotter is capable of assessing the
effect of 54,675 genes on survival using 10,461 cancer samples,
including 5,143 patients with breast cancer, 1,816 with ovarian
cancer, 2,437 with lung cancer and 1,065 with GC, with a mean
follow-up of 69, 40, 49 and 33 months, respectively. The primary
purpose of the tool was a meta-analysis-based biomarker
assessment.
Gene expression data and relapse-free and overall
survival information were downloaded from GEO (https://www.ncbi.nlm.nih.gov/geo; Affymetrix
microarrays only; Affymetrix; Thermo Fisher Scientific Inc.), EGA
(https://www.ebi.ac.uk/ega/home) and TCGA
(https://cancergenome.nih.gov). The
database was handled by a PostgreSQL server (https://www.postgresql.org), which integrates gene
expression and clinical data simultaneously. To analyze the
prognostic value of a particular gene, the patient samples were
split into two groups, according to varying quantile expression of
the proposed biomarker. A Kaplan-Meier survival plot was used to
compare the two patient cohorts, and the hazard ratio with 95%
confidence intervals, and the log-rank P-values were calculated.
Each database is updated biannually. For NEK8, the Affy IDs were
1557172_x_at and 1557170_at. All probe sets per gene were selected
in the probe set option.
MTT assay
Cells (5×103 cells/well) were seeded in
96-well plates in complete culture medium. After 24, 48, 72 and 96
h, the reaction was terminated by adding MTT to each well with a
final concentration of 0.5 mg/ml. The reaction was allowed to
proceed for 3–4 h at 37°C. The formazan crystals were then
dissolved by adding 0.1 ml dimethyl sulfoxide. The intensity of the
color developed, which is a reflection of the number of live cells,
was measured at 490 nm using a multiwell spectrophotometer. All
assays were performed with 3 replicates.
Transwell assay
SGC-7901 cell migration was evaluated using an 8-mm
pore size Transwell system (Corning Star; Corning Life Sciences,
Cambridge, MA, USA). Briefly, short hairpin RNA (shRNA)-transfected
cells were resuspended in serum-free RPMI-1640 medium at a density
of 2×105 cells/ml. The upper chamber of the Transwell
was loaded with 100 µl cell suspension and the lower chamber was
loaded with 0.6 ml RPMI-1640 containing 10% fetal bovine serum.
Following 8 h of incubation, the cells that had migrated to the
lower chamber were fixed with 100% methanol at 4°C for 15 min and
stained with 0.1% crystal violet at room temperature for 15 min.
The cell images were captured under a stereomicroscope (Olympus
Corporation, Tokyo, Japan), then the cells were washed with in 10%
acetic acid for the measurement of the optical density at a
wavelength of 595 nm.
Colony formation assay
A total of 1,000 cells transfected with negative
control plasmid associated with the NEK8-shRNA-transfected SGC-7901
cells were mixed with RPMI-1640 containing 0.3% agar and 10% FBS,
and plated on 0.6% basal agar in triplicate 6-well plates. Each
well was then further covered with RPMI-1640 containing 10% FBS and
incubated at 37°C with 5% CO2 for 2 weeks, prior to the
colony numbers being counted by eye.
Antitumor activity assay in vivo
BALB/C-nu/nu mice aged 4–5 weeks were obtained from
Zhejiang Academy of Medical Sciences (Hangzhou, China). A total of
24 male mice (mean weight, ~20 g) were housed in sterile cages
under laminar airflow hoods at 20°C in a specific pathogen-free
environment under a 12-h light/dark cycle and provided with
autoclaved chow and water ad libitum. The present study was
ethically approved by the Medical Ethics Committee of Taizhou
College of Medicine (approval, no. TZYXY2017-003; Taizhou, China).
A total of 1×106 SGC-7901 and SNU-1 gastric cancer cells
were transfected with 1 µg negative control shRNA or NEK8 shRNA,
separately. The shRNA-transfected cells were transplanted
subcutaneously into the left and right flanks of the mice,
respectively. Tumor volumes were measured with calipers twice per
week and were calculated as: Volume=(width)2 × length/2
(3). After 3 weeks, animals were
sacrificed by cervical dissociation and the solid tumors were
removed and weighed. The largest diameter of tumors developed in
all mice examined was 1.634 cm, with no multiple tumors.
Statistical analysis
The data were analyzed using SPSS 13.0 (SPSS Inc.,
Chicago, IL, USA). The results were compared using one-way analysis
of variance followed by Dunnett's post-hoc test for multiple
comparisons. All results are expressed as the mean ± standard
deviation from three replicates. P<0.05 was considered to
indicate a statistically significant difference.
Results
pVHL interacts with NEK8 in SGC-7901
cells
In a previous study, it was demonstrated that pVHL
may regulate NEK8 indirectly via the HIF-1 signaling pathway
(17). To verify whether pVHL
interacts with NEK8 in cells, immunoprecipitation was performed. As
indicated in Fig. 1A, the pull-down
effect of the anti-VHL antibody on NEK8 protein was marked,
indicating an association between the two proteins. Further
investigation was required to examine whether pVHL interacts with
NEK8 directly.
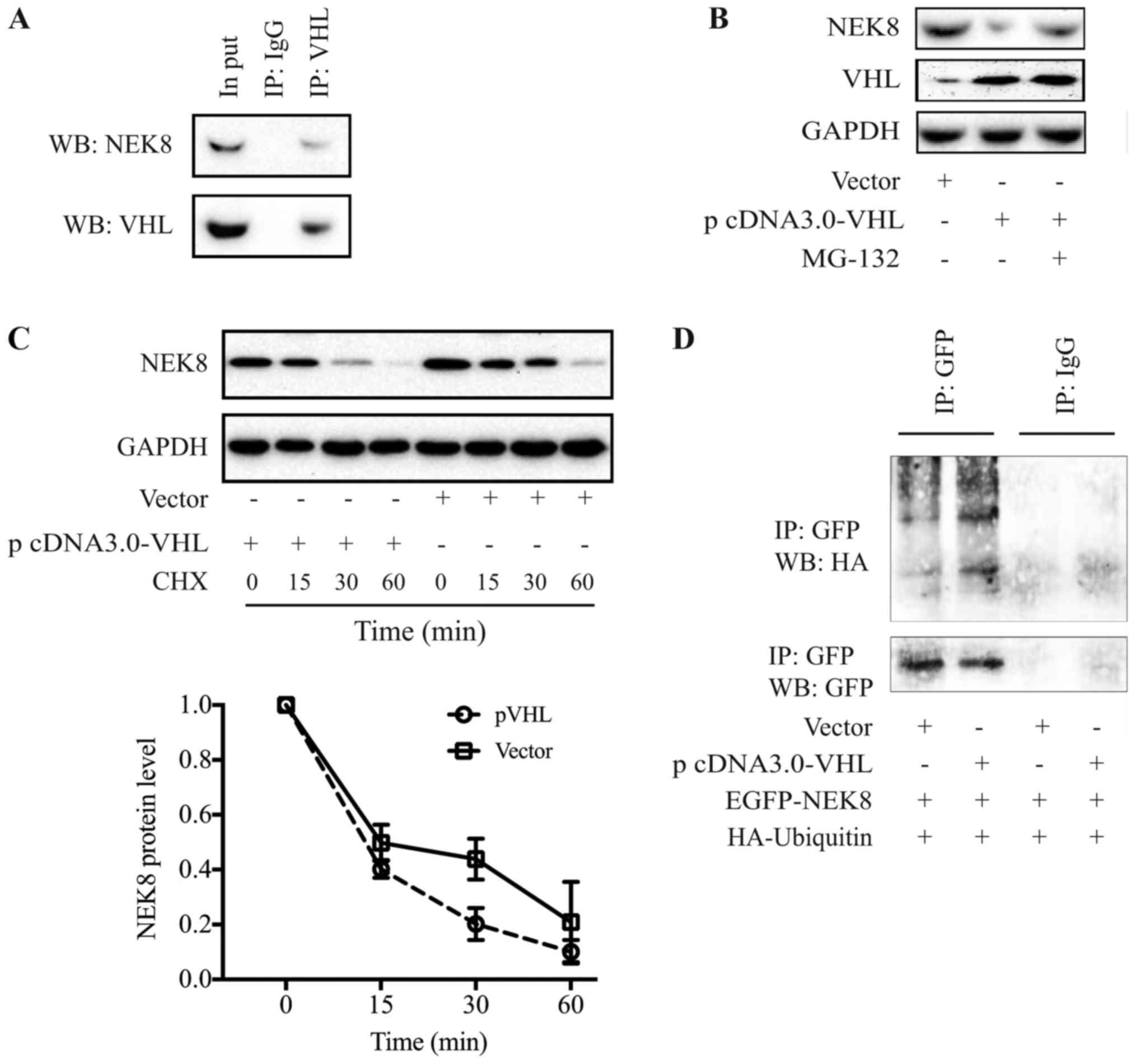 | Figure 1.pVHL interacts with NEK8 and promotes
NEK8 degradation through the proteasome-ubiquitination signaling
pathway. (A) Immunoprecipitation and immunoblot analysis revealing
the protein association between pVHL and NEK8. (B) Treatment with
the 26S proteasome inhibitor, MG-132 (10 µM), rescued the
downregulation of NEK-8 in the pVHL-overexpressing SGC-7901 cells.
(C) pVHL promotes NEK8 degradation. (D) pVHL promoted NEK8
ubiquitination. VHL, von-Hippel-Lindau tumor suppressor protein;
NEK8, never-in-mitosis A-related kinase 8; EGFP, enhanced green
fluorescent protein; HA, influenza hemagglutinin; Ub, ubiquitin;
IgG, rabbit immunoglobulin G; IP, immunoprecipitation; CHX,
cycloheximide; vector, vector of VHL; WB, western blotting. |
pVHL promotes NEK8 protein degradation
via the proteasome-ubiquitin signaling pathway
The present study proposed that pVHL, an E3 ligase,
may promote the degradation of NEK8 protein. Firstly, cells were
treated with the 26S proteasome inhibitor, MG-132 (10 µM). This
proteasome inhibitor rescued the reduction in NEK8 expression
levels induced by pVHL overexpression, indicating that pVHL may
mediate the decrease in NEK8 protein by proteasome-executed
degradation (Fig. 1B). Subsequently,
the half-life of NEK8 protein was determined in the presence or
absence of pVHL overexpression. SGC-7901 cells were transfected
with pVHL and NEK8 expression levels were subsequently evaluated by
immunoblot analysis at various time points following the inhibition
of de novo protein synthesis by CHX. The half-life of NEK8
was reduced from >30 to <15 min when pVHL was ectopically
expressed (Fig. 1C). The findings of
the present study demonstrated that pVHL may promote the rapid
degradation of NEK8.
To further confirm whether pVHL promotes NEK8
degradation via the ubiquitin-proteasomal pathway, an
ubiquitination experiment was performed. SGC-7901 cells were
co-transfected with GFP-NEK8, HA-Ub and pVHL plasmids.
Immunoprecipitation was performed with anti-GFP or IgG antibodies,
and the ubiquitination of NEK8 was evaluated by western blotting
with an anti-HA antibody. As indicated in Fig. 1D, pVHL promoted the ubiquitination
NEK8.
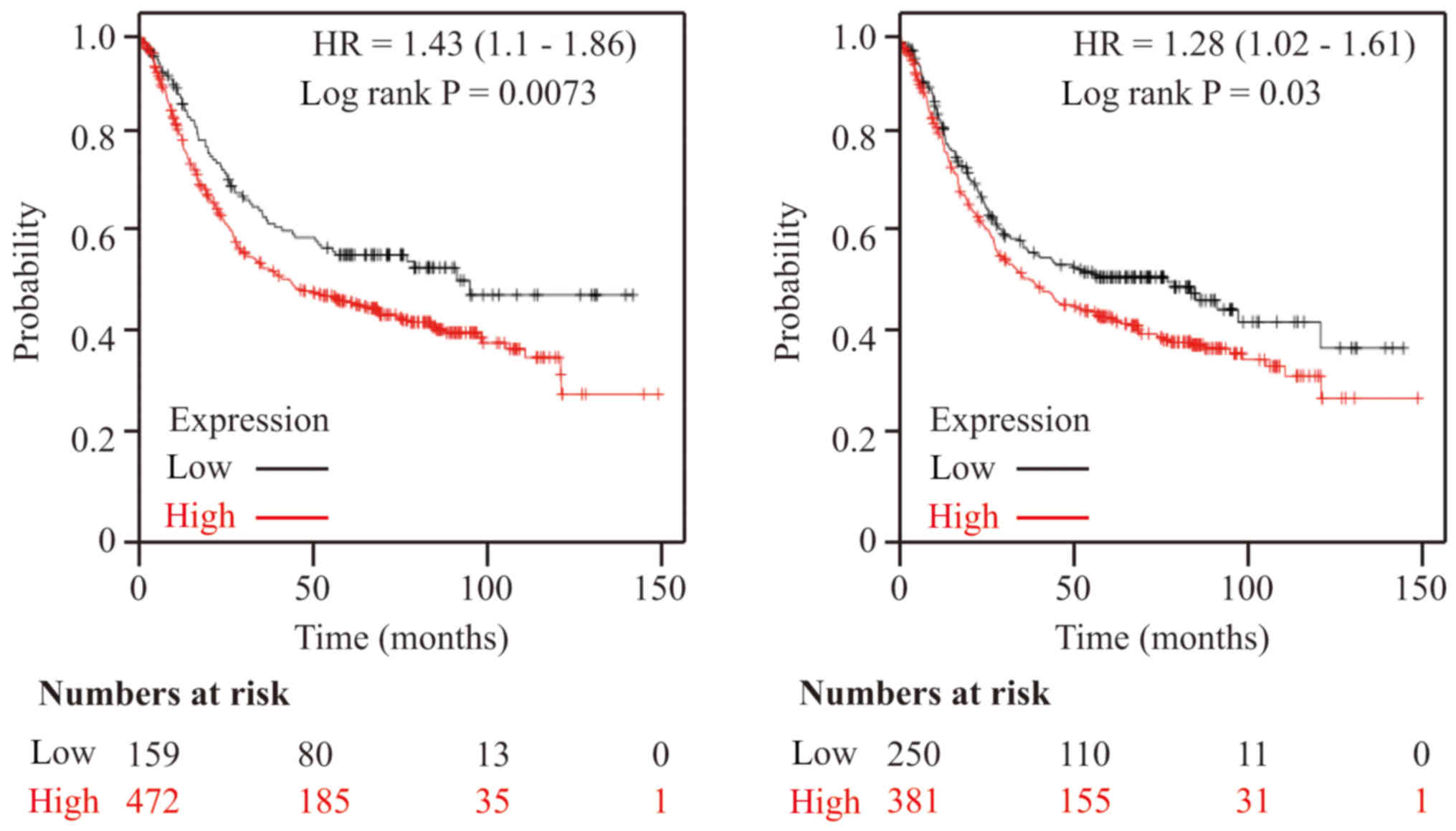
NEK8 promotes cancer progression
The Kaplan-Meier estimate was used to assess the
effects of 54,675 genes on survival using 1,065 cancer samples from
patients with GC, with a mean follow-up of 33 months (http://kmplot.com/analysis/). The primary purpose of
the tool is meta-analysis-based biomarker assessment. Online
analysis using Kaplan-Meier survival plots based on the
transcriptomic data of 1,065 patients demonstrated that higher
expression levels of NEK8 may lead to poor survival in patients
with GC (Fig. 2). All analyses were
conducted using standard procedures (19).
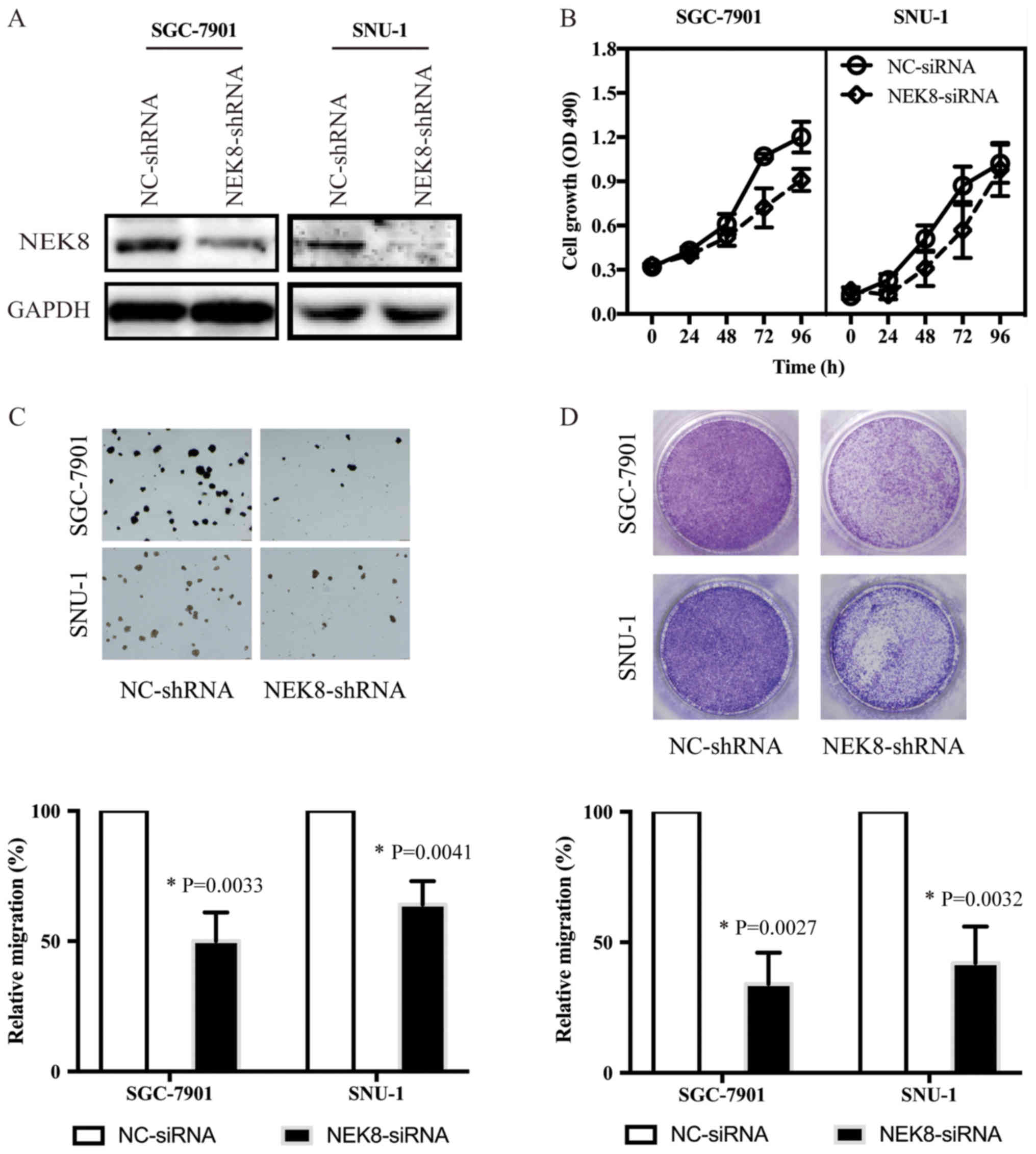
To confirm whether NEK8 may promote the progression
of cancer, NEK8 expression was downregulated by shRNA. As
demonstrated by western blotting (Fig.
3A), NEK8 was efficiently knocked down in SGC-7901 and SNU-1
cells. In addition, NEK8 downregulation inhibited the
proliferation, colony formation ability and migration of SGC-7901
and SNU-1 cells (Fig. 3B-D).
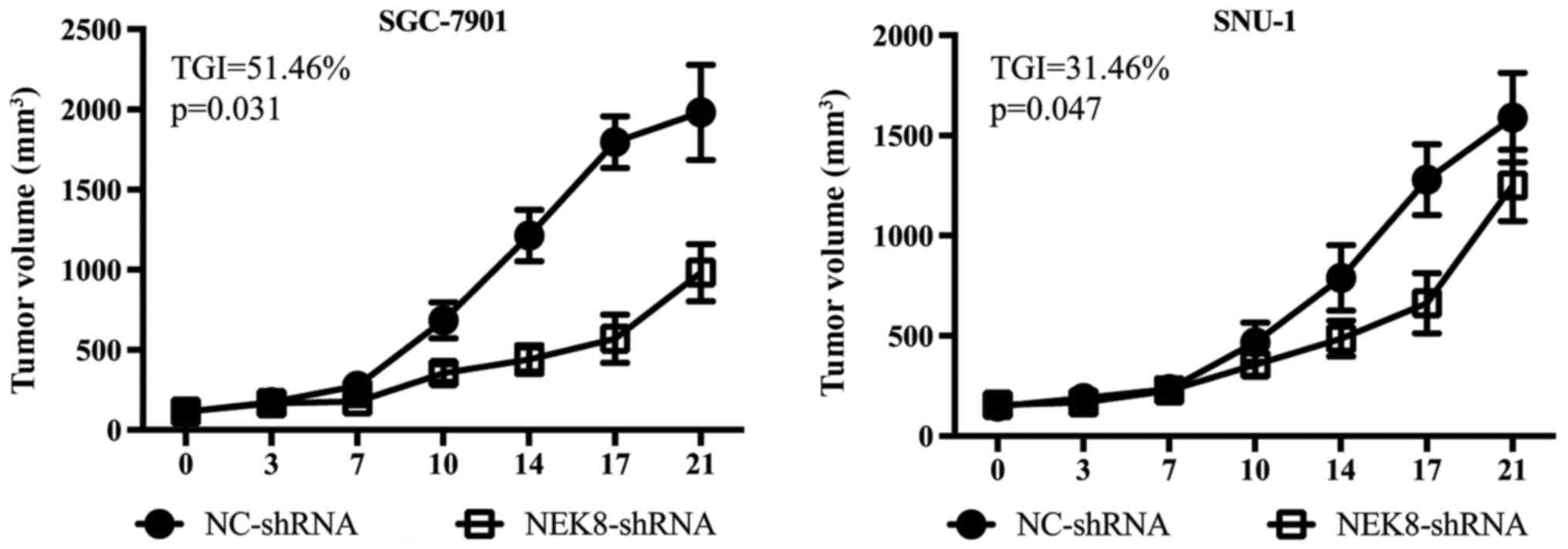
Based on the aforementioned in vitro
findings, the present study investigated whether NEK8 may serve a
role in gastric cancer cell proliferation in vivo. SGC-7901
and SNU-1 cells transfected with control or NEK8-shRNAs were
implanted subcutaneously into the flanks of female nude mice,
respectively. As presented in Fig. 4,
implanted control cells formed significantly larger tumors compared
with NEK8-shRNAs-transfected cells. Additional research is required
to investigate the potential underlying mechanisms.
Discussion
In the present study, it was demonstrated that pVHL
may interact with NEK8 in vitro, as determined by
immunoprecipitation, and that pVHL may promote NEK8 degradation via
the ubiquitin-proteasome signaling pathway. Additionally,
NEK8-knockdown mediated by shRNA inhibited of the proliferation,
colony formation and migration of the GC SGC-7901 cell line in
vitro, and proliferation in vivo.
Bowers and Boylan (21) reported that NEK8 may be a novel
tumor-associated gene as revealed by its variable expression in
healthy human breast tissue and breast tumors. It has been reported
that NEK8 may serve a critical role in the development of cancer
via the regulation of DNA damage/repair (17). The present study generated
Kaplan-Meier survival plots, which revealed that higher expression
levels of NEK8 were associated with a poor survival rate.
Furthermore, the present study revealed that RNAi-mediated
knockdown of NEK8 effectively suppressed GC cell proliferation,
soft-agar colony formation and migration in vitro, and
xenograft growth in vivo. The aforementioned data indicate a
possible role for NEK8 in gastric tumor progression.
Indirect regulation of NEK8 by pVHL via the HIF-1
signaling pathway has been reported (19). The association between pVHL and NEK8
in SGC-7901 cells was investigated in the present study. Numerous
HIF-independent roles of pVHL have been observed by biochemical
interactions (22). However, the most
documented function of pVHL is its role as the
substrate-recognition component of an E3 ubiquitin ligase complex
(20). This suggests that NEK8 may
serve as a novel target of pVHL. The results of the present study
revealed the promoting effects of pVHL on NEK8 protein degradation
and ubiquitination. In addition, the study suggested that MG-132
rescued the reduction in NEK8 expression levels induced by pVHL
overexpression, which supported the aforementioned hypothesis.
In summary, the findings of the present study may
provide novel insight into the signaling pathway underlying the
regulation of NEK8 in cancer. Additionally, the data of the present
study may provide further insight into the role of pVHL in NEK8
regulation, as well as the role of the NEK8 in the progression of
GC. This may improve the understanding of tumor progression and
contribute to the development of novel therapeutic strategies.
Acknowledgements
The authors would like to thank Mr. Jian-Xing Zhang
(Laboratory for Biological Medicine, School of Medicine, Taizhou
University) for providing technical assistance in the animal
procedures.
Funding
The present study was supported by the Zhejiang
Provincial Natural Science Foundation (grant nos. LY16H310006,
LY15H310002 and LY15H310001), The National Natural Science
Foundation of China (grant no. 81201530), the Public Technology
Research Projects of the Science Technology Department of Zhejiang
Province (grant nos. 2016C37111, 2015C37093, 2016C33235 and
2018C37109) and the Science Technology Department of Taizhou City
(grant no. 15yw08).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XFD, GC and YLW designed the study; XFD, JC, JZ and
GC performed the research; XFD, GC and YLW analyzed the data; and
GC and YLW wrote the manuscript.
Ethics approval and consent to
participate
The present study was ethically approved by the
Medical Ethics Committee of Taizhou College of Medicine (approval
no. TZYXY2017-003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim HJ and Oh SC: Novel systemic therapies
for advanced gastric cancer. J Gastric Cancer. 18:1–19. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An Attempt At A Histo-Clinical Classification. Acta
Pathol Microbio Scand. 64:31–49. 1965. View Article : Google Scholar
|
|
3
|
Hamilton SR and Aaltonen LA: World Health
Organization Classification of Tumours. Pathology and Genetics of
Tumours of the Digestive System. IARC Press; Lyon, France: 2000
|
|
4
|
Cisło M, Filip AA, Arnold Offerhaus GJ,
Ciseł B, Rawicz-Pruszyński K, Skierucha M and Polkowski WP:
Distinct molecular subtypes of gastric cancer: From Laurén to
molecular pathology. Oncotarget. 9:19427–19442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren D, Lin B, Zhang X, Peng Y, Ye Z, Ma Y,
Liang Y, Cao L, Li X, Li R, et al: Maintenance of cancer stemness
by miR-196b-5p contributes to chemoresistance of colorectal cancer
cells via activating STAT3 signaling pathway. Oncotarget.
8:49807–49823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fry AM, O'Regan L, Sabir SR and Bayliss R:
Cell cycle regulation by the NEK family of protein kinases. J Cell
Sci. 125:4423–4433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moniz L, Dutt P, Haider N and Stambolic V:
Nek family of kinases in cell cycle, checkpoint control and cancer.
Cell Div. 6:182011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prosser SL, O'Regan L and Fry AM: Novel
insights into the mechanisms of mitotic spindle assembly by NEK
kinases. Mol Cell Oncol. 3:e10629522015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jackson PK: Nek8 couples renal
ciliopathies to DNA damage and checkpoint control. Mol Cell.
51:407–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Otto EA, Trapp ML, Schultheiss UT, Helou
J, Quarmby LM and Hildebrandt F: NEK8 mutations affect ciliary and
centrosomal localization and may cause nephronophthisis. J Am Soc
Nephrol. 19:587–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sohara E, Luo Y, Zhang J, Manning DK,
Beier DR and Zhou J: Nek8 regulates the expression and localization
of polycystin-1 and polycystin-2. J Am Soc Nephrol. 19:469–476.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi HJ, Lin JR, Vannier JB, Slaats GG,
Kile AC, Paulsen RD, Manning DK, Beier DR, Giles RH, Boulton SJ and
Cimprich KA: NEK8 links the ATR-regulated replication stress
response and S phase CDK activity to renal ciliopathies. Mol Cell.
51:423–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holland PM, Milne A, Garka K, Johnson RS,
Willis C, Sims JE, Rauch CT, Bird TA and Virca GD: Purification,
cloning, and characterization of Nek8, a novel NIMA-related kinase,
and its candidate substrate Bicd2. J Biol Chem. 277:16229–16240.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grampa V, Delous M, Zaidan M, Odye G,
Thomas S, Elkhartoufi N, Filhol E, Niel O, Silbermann F, Lebreton
C, et al: Novel NEK8 mutations cause severe syndromic renal cystic
dysplasia through YAP dysregulation. PLoS Genet. 12:e10058942016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valkova N, Yunis R, Mak SK, Kang K and
Kültz D: Nek8 mutation causes overexpression of galectin-1, sorcin,
and vimentin and accumulation of the major urinary protein in renal
cysts of jck mice. Mol Cell Proteomics. 4:1009–1018. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frank V, Habbig S, Bartram MP, Eisenberger
T, Veenstra-Knol HE, Decker C, Boorsma RA, Göbel H, Nürnberg G,
Griessmann A, et al: Mutations in NEK8 link multiple organ
dysplasia with altered Hippo signalling and increased c-MYC
expression. Hum Mol Genet. 22:2177–2185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abeyta A, Castella M, Jacquemont C and
Taniguchi T: NEK8 regulates DNA damage-induced RAD51 foci formation
and replication fork protection. Cell Cycle. 16:335–347. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Czarnecki PG, Gabriel GC, Manning DK,
Sergeev M, Lemke K, Klena NT, Liu X, Chen Y, Li Y, San Agustin JT,
et al: ANKS6 is the critical activator of NEK8 kinase in embryonic
situs determination and organ patterning. Nat Commun. 6:60232015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding XF, Zhou J, Hu QY, Liu SC and Chen G:
The tumor suppressor pVHL down-regulates never-in-mitosis A-related
kinase 8 via hypoxia-inducible factors to maintain cilia in human
renal cancer cells. J Biol Chem. 290:1389–1394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gossage L, Eisen T and Maher ER: VHL, the
story of a tumour suppressor gene. Nat Rev Cancer. 15:55–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bowers AJ and Boylan JF: Nek8, a NIMA
family kinase member, is overexpressed in primary human breast
tumors. Gene. 328:135–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M and Kim WY: Two sides to every story:
The HIF-dependent and HIF-independent functions of pVHL. J Cell Mol
Med. 15:187–195. 2011. View Article : Google Scholar : PubMed/NCBI
|