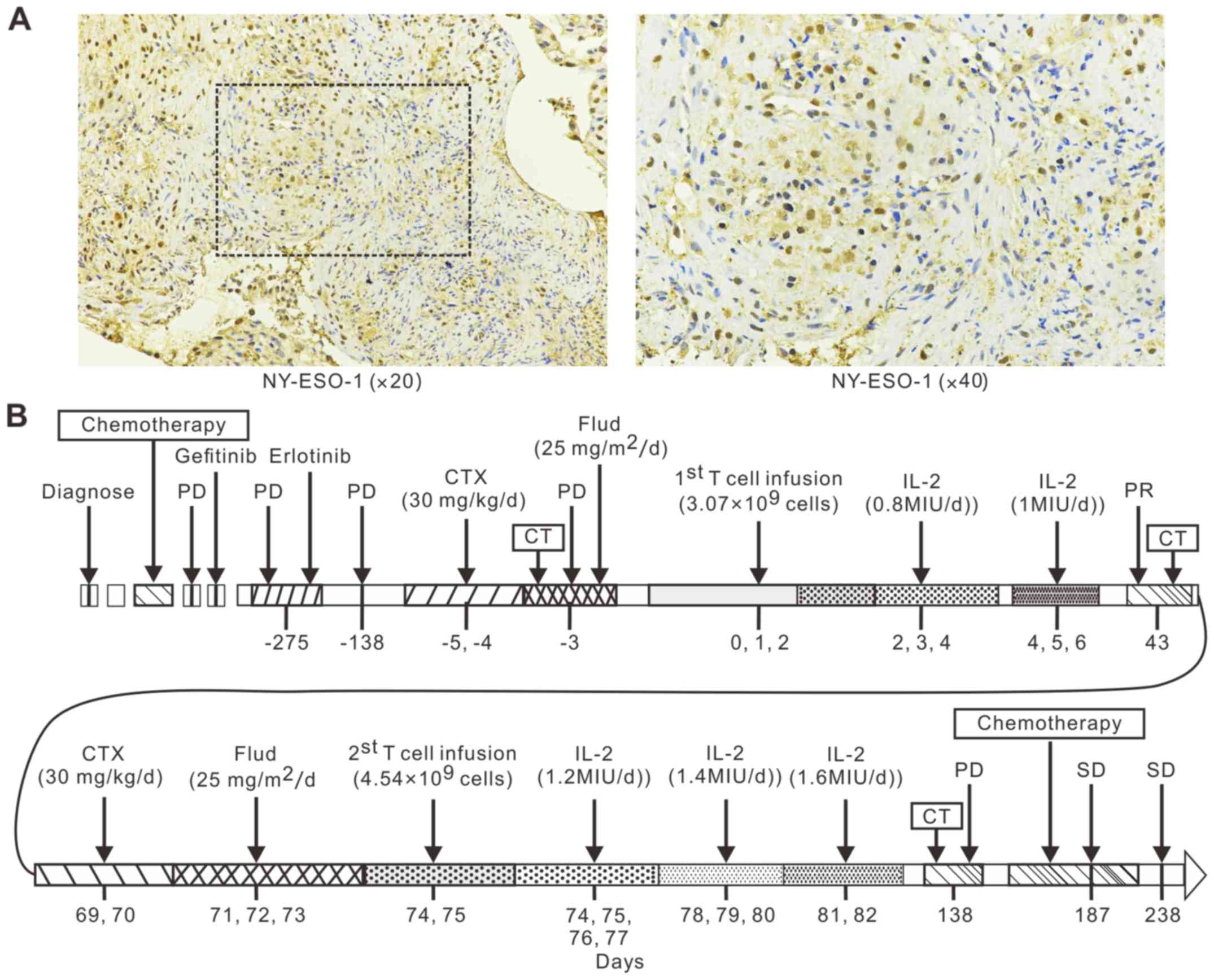
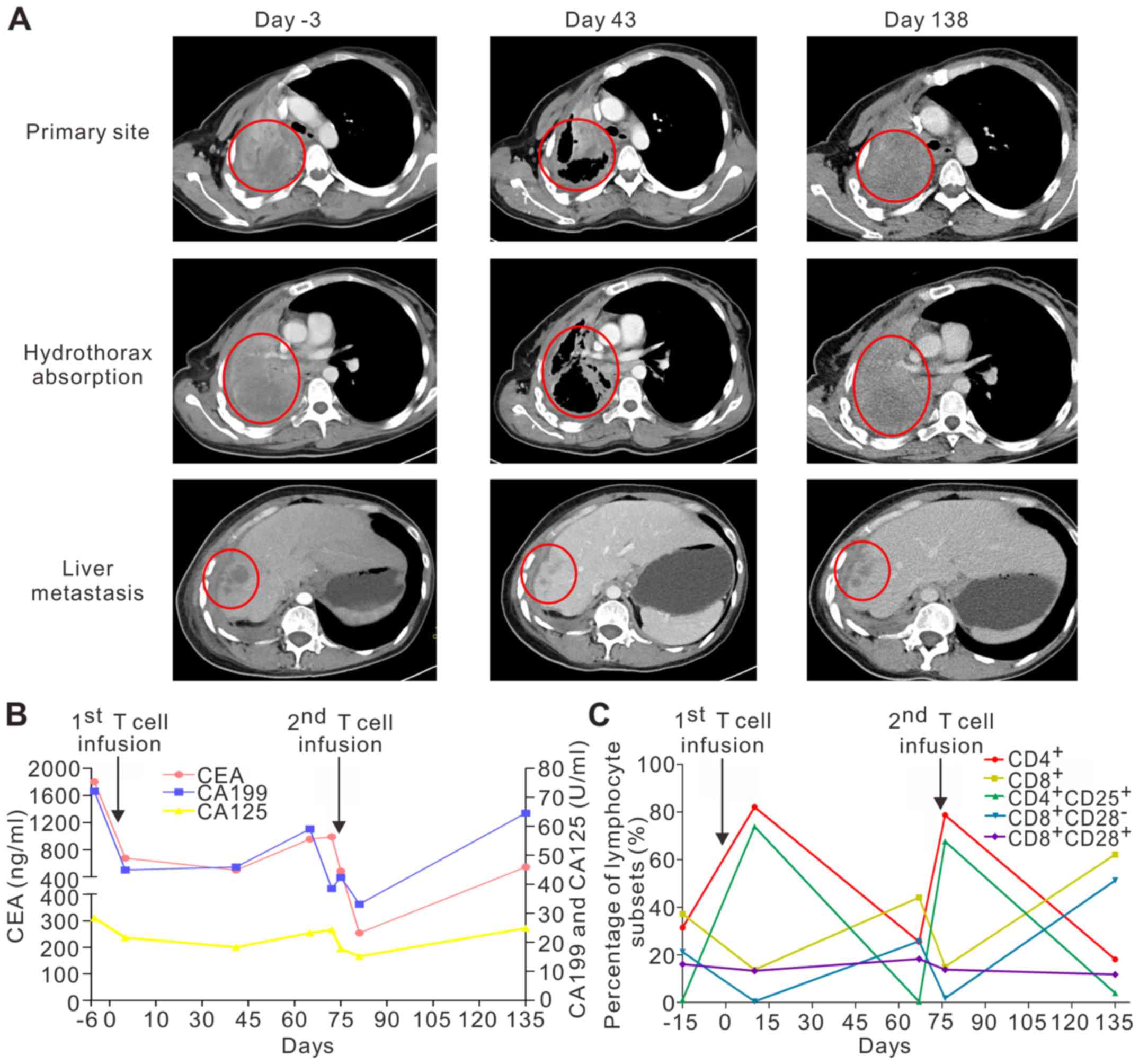
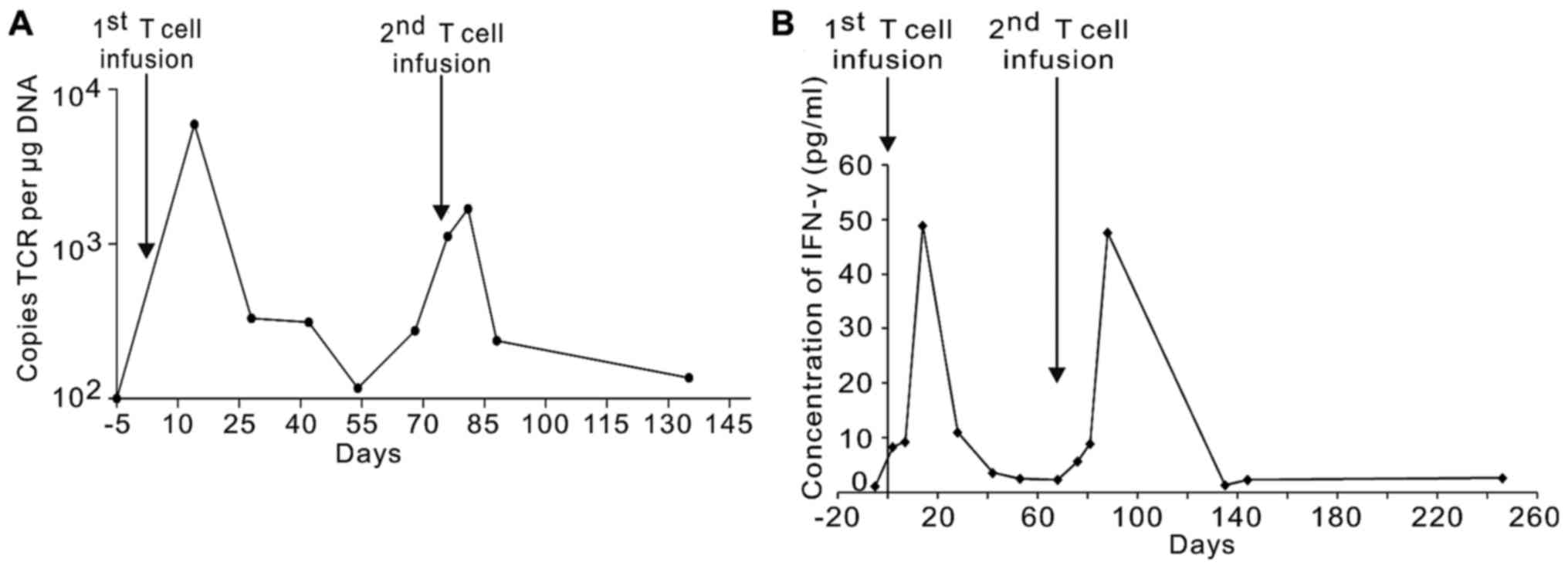
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zarogoulidis K, Zarogoulidis P, Darwiche
K, Boutsikou E, Machairiotis N, Tsakiridis K, Katsikogiannis N,
Kougioumtzi I, Karapantzos I, Huang H and Spyratos D: Treatment of
non-small cell lung cancer (NSCLC). J Thorac Dis. 5 (Suppl
4):S389–S396. 2013.PubMed/NCBI
|
|
3
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito M, Suzuki H, Kono K, Takenoshita S
and Kohno T: Treatment of lung adenocarcinoma by molecular-targeted
therapy and immunotherapy. Surg Today. 48:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined Nivolumab and Ipilimumab or
Monotherapy in Untreated Melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaufman HL, Russell J, Hamid O, Bhatia S,
Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M,
et al: Avelumab in patients with chemotherapy-refractory metastatic
Merkel cell carcinoma: A multicentre, single-group, open-label,
phase 2 trial. Lancet Oncol. 17:1374–1385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer JR: Immune checkpoint blockade:
The hope for immunotherapy as a treatment of lung cancer? Semin
Oncol. 41:126–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stevanovic S, Draper LM, Langhan MM,
Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry
RM, Kammula US, et al: Complete regression of metastatic cervical
cancer after treatment with human papillomavirus-targeted
tumor-infiltrating T cells. J Clin Oncol. 33:1543–1550. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robbins PF, Lu YC, El-Gamil M, Li YF,
Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al:
Mining exomic sequencing data to identify mutated antigens
recognized by adoptively transferred tumor-reactive T cells. Nat
Med. 19:747–752. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
San Miguel JF, Paiva B and Lasarte JJ:
Engineering anti-myeloma responses using affinity-enhanced
TCR-engineered T cells. Cancer Cell. 28:281–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robbins PF, Morgan RA, Feldman SA, Yang
JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ,
Mackall CL, et al: Tumor regression in patients with metastatic
synovial cell sarcoma and melanoma using genetically engineered
lymphocytes reactive with NY-ESO-1. J Clin Oncol. 29:917–924. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh H, Huls H, Kebriaei P and Cooper LJ:
A new approach to gene therapy using Sleeping Beauty to genetically
modify clinical-grade T cells to target CD19. Immunol Rev.
257:181–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. N Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kochenderfer JN, Dudley ME, Kassim SH,
Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al: Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively
treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol. 33:540–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu YC, Parker LL, Lu T, Zheng Z, Toomey
MA, White DE, Yao X, Li YF, Robbins PF, Feldman SA, et al:
Treatment of patients with metastatic cancer using a major
histocompatibility complex class II-restricted T-cell receptor
targeting the cancer germline antigen MAGE-A3. J Clin Oncol.
35:3322–3329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boon T and Old LJ: Cancer tumor antigens.
Curr Opin Immunol. 9:681–683. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scanlan MJ, Gure AO, Jungbluth AA, Old LJ
and Chen YT: Cancer/testis antigens: An expanding family of targets
for cancer immunotherapy. Immunol Rev. 188:22–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salmaninejad A, Zamani MR, Pourvahedi M,
Golchehre Z, Hosseini Bereshneh A and Rezaei N: Cancer/testis
antigens: Expression, regulation, tumor invasion, and use in
immunotherapy of cancers. Immunol Invest. 45:619–640. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stockert E, Jäger E, Chen YT, Scanlan MJ,
Gout I, Karbach J, Arand M, Knuth A and Old LJ: A survey of the
humoral immune response of cancer patients to a panel of human
tumor antigens. J Exp Med. 187:1349–1354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maio M, Coral S, Sigalotti L, Elisei R,
Romei C, Rossi G, Cortini E, Colizzi F, Fenzi G, Altomonte M, et
al: Analysis of cancer/testis antigens in sporadic medullary
thyroid carcinoma: Expression and humoral response to NY-ESO-1. J
Clin Endocrinol Metab. 88:748–754. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scanlan MJ, Altorki NK, Gure AO,
Williamson B, Jungbluth A, Chen YT and Old LJ: Expression of
cancer-testis antigens in lung cancer: Definition of bromodomain
testis-specific gene (BRDT) as a new CT gene, CT9. Cancer Lett.
150:155–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scanlan MJ, Gout I, Gordon CM, Williamson
B, Stockert E, Gure AO, Jäger D, Chen YT, Mackay A, O'Hare MJ and
Old LJ: Humoral immunity to human breast cancer: antigen definition
and quantitative analysis of mRNA expression. Cancer Immun.
1:42001.PubMed/NCBI
|
|
27
|
Chen YT: The journey from autologous
typing to SEREX, NY-ESO-1 and cancer/testis antigens. Cancer Immun.
12:82012.PubMed/NCBI
|
|
28
|
Jager E, Chen YT, Drijfhout JW, Karbach J,
Ringhoffer M, Jäger D, Arand M, Wada H, Noguchi Y, Stockert E, et
al: Simultaneous humoral and cellular immune response against
cancer-testis antigen NY-ESO-1: Definition of human
histocompatibility leukocyte antigen (HLA)-A2-binding peptide
epitopes. J Exp Med. 187:265–270. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hunder NN, Wallen H, Cao J, Hendricks DW,
Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA and Yee
C: Treatment of metastatic melanoma with autologous CD4+ T cells
against NY-ESO-1. N Engl J Med. 358:2698–2703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robbins PF, Kassim SH, Tran TL, Crystal
JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR,
Sherry RM, et al: A pilot trial using lymphocytes genetically
engineered with an NY-ESO-1-reactive T-cell receptor: Long-term
follow-up and correlates with response. Clin Cancer Res.
21:1019–1027. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rapoport AP, Stadtmauer EA, Binder-Scholl
GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B,
Finklestein J, et al: NY-ESO-1-specific TCR-engineered T cells
mediate sustained antigen-specific antitumor effects in myeloma.
Nat Med. 21:914–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SH, Lee S, Lee CH, Lee MK, Kim YD,
Shin DH, Choi KU, Kim JY, Park DY and Sol MY: Expression of
cancer-testis antigens MAGE-A3/6 and NY-ESO-1 in non-small-cell
lung carcinomas and their relationship with immune cell
infiltration. Lung. 187:401–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gjerstorff MF, Pøhl M, Olsen KE and Ditzel
HJ: Analysis of GAGE NY-ESO-1 and SP17 cancer/testis antigen
expression in early stage non-small cell lung carcinoma. BMC
Cancer. 13:4662013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oshima Y, Shimada H, Yajima S, Nanami T,
Matsushita K, Nomura F, Kainuma O, Takiguchi N, Soda H, Ueda T, et
al: NY-ESO-1 autoantibody as a tumor-specific biomarker for
esophageal cancer: Screening in 1969 patients with various cancers.
J Gastroenterol. 51:30–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tureci O, Mack U, Luxemburger U, Heinen H,
Krummenauer F, Sester M, Sester U, Sybrecht GW and Sahin U: Humoral
immune responses of lung cancer patients against tumor antigen
NY-ESO-1. Cancer Lett. 236:64–71. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laport GG, Levine BL, Stadtmauer EA,
Schuster SJ, Luger SM, Grupp S, Bunin N, Strobl FJ, Cotte J, Zheng
Z, et al: Adoptive transfer of costimulated T cells induces
lymphocytosis in patients with relapsed/refractory non-Hodgkin
lymphoma following CD34+-selected hematopoietic cell
transplantation. Blood. 102:2004–2013. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rapoport AP, Stadtmauer EA, Aqui N, Badros
A, Cotte J, Chrisley L, Veloso E, Zheng Z, Westphal S, Mair R, et
al: Restoration of immunity in lymphopenic individuals with cancer
by vaccination and adoptive T-cell transfer. Nat Med. 11:1230–1237.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klebanoff CA, Khong HT, Antony PA, Palmer
DC and Restifo NP: Sinks, suppressors and antigen presenters: How
lymphodepletion enhances T cell-mediated tumor immunotherapy.
Trends Immunol. 26:111–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Robson NC, McAlpine T, Knights AJ, Schnurr
M, Shin A, Chen W, Maraskovsky E and Cebon J: Processing and
cross-presentation of individual HLA-A, -B, or -C epitopes from
NY-ESO-1 or an HLA-A epitope for Melan-A differ according to the
mode of antigen delivery. Blood. 116:218–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robbins PF, Li YF, El-Gamil M, Zhao Y,
Wargo JA, Zheng Z, Xu H, Morgan RA, Feldman SA, Johnson LA, et al:
Single and dual amino acid substitutions in TCR CDRs can enhance
antigen-specific T cell functions. J Immunol. 180:6116–6131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Johnson LA, Morgan RA, Dudley ME, Cassard
L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich
JR, et al: Gene therapy with human and mouse T-cell receptors
mediates cancer regression and targets normal tissues expressing
cognate antigen. Blood. 114:535–546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hughes MS, Yu YY, Dudley ME, Zheng Z,
Robbins PF, Li Y, Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA
and Morgan RA: Transfer of a TCR gene derived from a patient with a
marked antitumor response conveys highly active T-cell effector
functions. Hum Gene Ther. 16:457–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Topalian SL, Solomon D and Rosenberg SA:
Tumor-specific cytolysis by lymphocytes infiltrating human
melanomas. J Immunol. 142:3714–3725. 1989.PubMed/NCBI
|
|
44
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kalos M, Levine BL, Porter DL, Katz S,
Grupp SA, Bagg A and June CH: T cells with chimeric antigen
receptors have potent antitumor effects and can establish memory in
patients with advanced leukemia. Sci Transl Med. 3:95ra732011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Linette GP, Stadtmauer EA, Maus MV,
Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM,
Cimino PJ, et al: Cardiovascular toxicity and titin
cross-reactivity of affinity-enhanced T cells in myeloma and
melanoma. Blood. 122:863–871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morgan RA, Chinnasamy N, Abate-Daga D,
Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry
RM, et al: Cancer regression and neurological toxicity following
anti-MAGE-A3 TCR gene therapy. J Immunother. 36:133–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
LeWinter MM and Granzier H: Cardiac titin:
A multifunctional giant. Circulation. 121:2137–2145. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Herman DS, Lam L, Taylor MR, Wang L,
Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B,
Sparks E, et al: Truncations of titin causing dilated
cardiomyopathy. N Engl J Med. 366:619–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene Ciloleucel CAR T-Cell Therapy in
Refractory Large B-Cell Lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Muranski P, Boni A, Antony PA, Cassard L,
Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K,
et al: Tumor-specific Th17-polarized cells eradicate large
established melanoma. Blood. 112:362–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Quezada SA, Simpson TR, Peggs KS, Merghoub
T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et
al: Tumor-reactive CD4(+) T cells develop cytotoxic activity and
eradicate large established melanoma after transfer into
lymphopenic hosts. J Exp Med. 207:637–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wei G, Ding L, Wang J, Hu Y and Huang H:
Advances of CD19-directed chimeric antigen receptor-modified T
cells in refractory/relapsed acute lymphoblastic leukemia. Exp
Hematol Oncol. 6:102017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tasian SK and Gardner RA: CD19-redirected
chimeric antigen receptor-modified T cells: A promising
immunotherapy for children and adults with B-cell acute
lymphoblastic leukemia (ALL). Ther Adv Hematol. 6:228–241. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Greaves M: Evolutionary determinants of
cancer. Cancer Discov. 5:806–820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yee C, Thompson JA, Byrd D, Riddell SR,
Roche P, Celis E and Greenberg PD: Adoptive T cell therapy using
antigen-specific CD8+ T cell clones for the treatment of patients
with metastatic melanoma: In vivo persistence, migration and
antitumor effect of transferred T cells. Proc Natl Acad Sci USA.
99:16168–16173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mackensen A, Meidenbauer N, Vogl S, Laumer
M, Berger J and Andreesen R: Phase I study of adoptive T-cell
therapy using antigen-specific CD8+ T cells for the treatment of
patients with metastatic melanoma. J Clin Oncol. 24:5060–5069.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hay KA and Turtle CJ: Chimeric antigen
receptor (CAR) T cells: Lessons learned from targeting of CD19 in
B-cell malignancies. Drugs. 77:237–245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Irving M, Vuillefroy de Silly R, Scholten
K, Dilek N and Coukos G: Engineering chimeric antigen receptor
T-cells for racing in solid tumors: Don't forget the fuel. Front
Immunol. 8:2672017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Frigault MJ and Maus MV: Chimeric antigen
receptor-modified T cells strike back. Int Immunol. 28:355–363.
2016. View Article : Google Scholar : PubMed/NCBI
|