Introduction
Rectal cancer is a common malignant tumor of the
digestive tract (1). The recurrence
and mortality rates of rectal cancer are high due to the unique
anatomy of the rectum. The standard treatment for locally advanced
rectal cancer is preoperative concurrent chemoradiotherapy or
short-range radiotherapy+total mesorectal excision+postoperative
adjuvant chemotherapy. Neoadjuvant therapy may improve the anus
preservation rate and reduce the risk of tumor recurrence. However,
the efficacy of neoadjuvant therapy is variable and little is known
about the factors associated with therapeutic efficacy. The
disadvantages of therapy failure include delayed surgery and
immunosuppression. The tumor microenvironment comprises
immunological cells with local infiltration of cancer stromal cells
together with their secreted active mediators and tumor cells. In
the 1880s, Paget (2) established the
concept of ‘seed and soil’. As a ‘soil,’ the tumor microenvironment
provides the basis for tumor occurrence, development, invasion and
metastasis (3). Tumor-infiltrating
lymphocytes (TILs) are an important component of this
microenvironment and serve a vital role in tumor progression and
treatment outcome. However, alterations in the expression levels of
TILs in the tumor microenvironment, pre- and post-neoadjuvant
therapy, are not fully understood.
Cluster of differentiation (CD)4+T and
CD8+T serve a crucial role in tumor recognition and
removal. CD4+T cells kill tumor cells through
interferon-γ (IFN-γ) and activate CD8+T cells in various
ways. Activated CD8+T cells are recruited to the tumor
site and induce apoptosis (4).
Regulatory T cells (Tregs) are a subgroup of T cells that inhibit
the immune response to autologous tumor cells, and this inhibition
is considered the main cause of the failure of immunotherapy
(5–8).
The transcription factor forkhead box P3 (Foxp3) is considered to
be the most specific Treg marker. Tregs may inhibit T cells by
expressing cytotoxic T lymphocyte-associated antigen-4 (CTLA-4),
which binds to B7 molecules on the surface of activated T cells. It
also reduces the activation of T cells and degrades activated T
cells by combining with CD80 and CD86 on the surface of antigen
presenting cells, which converts transduction signals, producing
indolamine 2,3-dioxygenase and degrading tryptophan (9). Previous studies have reported that Foxp3
may be associated with poor prognosis; however, its role in the
prognosis of rectal cancer is controversial. In addition, the
activation of T cells requires the concomitant release of secondary
signals by costimulators (10,11).
CTLA-4 inhibits the activation of T cells by interacting with B7
(CD80/CD86) (12), and previous
studies have reported that CTLA-4 is associated with poor prognosis
(13,14). Another inhibitor located on the
surface of T lymphocytes, B7-H1 (10), also known as programmed death ligand-1
(PD-L1), is expressed in T cells, B cells, macrophages and
dendritic cells, and its expression is upregulated following the
activation of antigen-presenting cells. PD-L1 may inhibit the
proliferation of T cells and the production of cytokines in T cells
by combining with PD-1, and thus serves a critical role in immune
tolerance and escape (15,16). It has been reported that PD-L1 is
upregulated in numerous malignant tumors, including melanoma, lung
cancer, renal cell carcinoma, ovarian cancer, colorectal cancer
(17), breast cancer (18) and osteosarcoma (19), and may serve an important role in
tumor-immune system interactions (20,21).
In the present study, the clinical treatment and
prognosis of rectal cancer was evaluated by selecting immune
markers associated with tumor progression in TILs, including CD4,
CD8, CTLA-4, Foxp3 and PD-L1. Alterations in the tumor
microenvironment were assessed pre- and post-neoadjuvant therapy by
immunohistochemistry (IHC), and the tumor microenvironment,
curative effect and prognosis of rectal cancer were compared
between neoadjuvant chemotherapy (NAC) and neoadjuvant
chemoradiotherapy (NACR).
Materials and methods
Patients
The present study investigated 109 patients who
underwent neoadjuvant therapy in the Shanxi Provincial Cancer
Hospital (Shanxi, China) between January 2012 and December 2015, of
whom 50 patients were treated using the FOLFOX4 regimen every 2–3
weeks (3 days of chemotherapy + 2 weeks of rest) for two to four
cycles of preoperative chemotherapy, and 59 patients were treated
with chemoradiotherapy. In the latter group, the total dose of
radiotherapy was 25–50 Gy, and two to four cycles of the FOLFOX4
regimen were provided during the same period. The Research Ethics
Committee of the Shanxi Cancer Hospital approved the study and
patient consent was obtained.
Tissue microarray
Tissue microarrays consisted of paraffin blocks in
which 48 separate tissue cores were assembled in an array. The
paraffin blocks were from 109 patients with rectal cancer who had
undergone neoadjuvant treatment. A hollow needle was used to remove
tissue cores as small as 1.8 mm in diameter from regions of
interest in paraffin-embedded tissues. These tissue cores were
inserted in a recipient paraffin block in a precisely spaced array.
The tissue microarray block was placed upside down on the slide,
incubated in an oven at 55°C for 10 min, and cooled to room
temperature. The tissue cores and the recipient paraffin fusion
blocks were repeatedly produced. Sections from these blocks were
cut using a microtome, mounted on a microscope slide, and analyzed
using a microscope.
IHC
All biopsy specimens collected prior to treatment
and resected specimens collected following treatment were analyzed
using IHC. The paraffin sections (3 µm) were dewaxed in xylene and
hydrated in gradient ethanol solutions, and antigen retrieval was
performed in a microwave for 2 min. The tissue slides were
incubated in 3% hydrogen peroxide for 10 min, and non-specific
binding was blocked using normal goat serum (SP900, working
solution; OriGene Technologies, Inc., Beijing, China) for 5–10 min
at 25°C. The slides were washed in PBS and incubated with the
primary antibody at 4°C overnight. The slides were again washed in
PBS, incubated with the secondary antibody (GK600705A, goat
anti-mouse/rabbit IgG, multimer, working solution; GeneTech
Biotechnology Co., Ltd.) for 30 min at 25°C, and visualized for 5
min with a diaminobenzidine color reaction kit (GK347005; GeneTech
Biotechnology Co., Ltd.) at 25°C. The slides were counterstained
with hematoxylin for 50 s at 25°C, dehydrated and mounted following
transparency. The primary antibodies were as follows: CD4 (cat. no.
EP204; GeneTech Biotechnology Co., Ltd., Shanghai, China); CD8
(cat. no. C8/114B; GeneTech Biotechnology Co., Ltd.); CTLA-4 (cat.
no. sc-376016; Santa Cruz Biotechnology, Inc., Dallas, TX, USA);
FOXP3 (cat. no. 236A/E7; Abcam, USA); PD-L1 (cat. no. sp142;
GeneTech Biotechnology Co., Ltd.). The positive controls were human
tonsils for CTLA4 and FOXP3, and human placenta for PD-L1. An
isotype control was used as a negative control for each case
stained for CTLA-4, FOXP3 and PD-L1, to control for potential false
positive staining. The microscope we use is OLYMPUS, BX46 (Olympus
Corporation, Tokyo, Japan) at ×40 magnification.
Histological analysis
The double-blind method was used for the
interpretation of histological sections by two pathologists. The
percentage and average number of positive TILs were calculated in
five fields at ×40 magnification. Tumor response was evaluated
using the tumor regression grade (TRG) system proposed by Dworak
et al (22) as follows: i)
Grade 0, no regression; ii) grade 1, minor tumor regression,
dominant tumor mass with evident fibrosis in £25% of the tumor
mass; iii) grade 2, moderate tumor regression, with fibrosis in
26–50% of the tumor mass; iv) grade 3, high tumor regression
(>50%), fibrosis in the majority of the tumor mass; and v) grade
4, total tumor regression, absence of viable tumor cells, only
fibrotic mass remaining. In the present study, TRGs 3 and 4
indicated a good response to therapy, whereas TRGs 0–2 indicated a
poor response to therapy. The mean value was set as the cut-off
value for the density of each type of TIL and patients were
classified into high- and low-TIL groups based on this cut-off
value.
Follow-up
From the initial diagnosis, all patients were
followed-up until August 15th 2017, and the median follow-up period
was 42 months. All patients were monitored by outpatient
appointment or telephone follow-up. Overall survival (OS) was
defined as the period from pathological diagnosis to mortality.
Statistical analysis
SPSS version 23.0 (IBM Corp., Armonk, NY, USA) was
used for data analysis. The χ2 test was used to compare
categorical data. Data are expressed as the mean ± standard
deviation. A t-test was used for group comparisons. Pearson
correlation was used for correlation analysis. The Kaplan-Meier
test was used for single-factor analysis of patient survival. The
Cox proportional hazard regression model was used for multi-factor
analysis of prognosis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Among the 109 patients, 62 were male and 47 were
female. The age of the study population was 32–78 years (mean,
54.78±10.71 years). The pathological types included high
differentiation (eight cases), moderate differentiation (76 cases),
and low differentiation (25 cases). With respect to the depth of
infiltration: 33 cases were in stage T3 and 76 cases were in stage
T4. A total of 35 patients did not present lymph node metastasis,
whereas 74 patients did present lymph node metastasis. Tumor
distance from the anal margin was 0–14 cm (mean 5.02±2.55 cm).
Overall, 34 patients had a good response, whereas 75 patients
presented a poor response (Table
I).
 | Table I.Clinicopathological characteristics
of the 109 patients. |
Table I.
Clinicopathological characteristics
of the 109 patients.
| Clinicopathological
parameters | No. patients
(n=109) |
|---|
| Sex |
|
|
Male | 62 (57%) |
|
Female | 47 (43%) |
| Age, mean (SD) | 54.78 (10.71) |
|
≤55 | 57 (52%) |
|
>55 | 52 (48%) |
| Histology |
|
|
Low | 8 (7%) |
|
Middle | 76 (70%) |
|
High | 25 (23%) |
| T stage |
|
| T3 | 33 (30%) |
| T4 | 76 (70%) |
| Lymphatic
invasion |
|
|
Negative | 35 (32%) |
|
Positive | 74 (68%) |
| DFTAV, cm [mean
(SD)] | 5.02 (2.55) |
| ≤5 | 69 (63%) |
|
>5 | 40 (37%) |
| TRG |
|
| Poor
response | 75 (69%) |
| Good
response | 34 (31%) |
| Survival rate |
|
|
Survival | 89 (82%) |
|
Mortality | 20 (18%) |
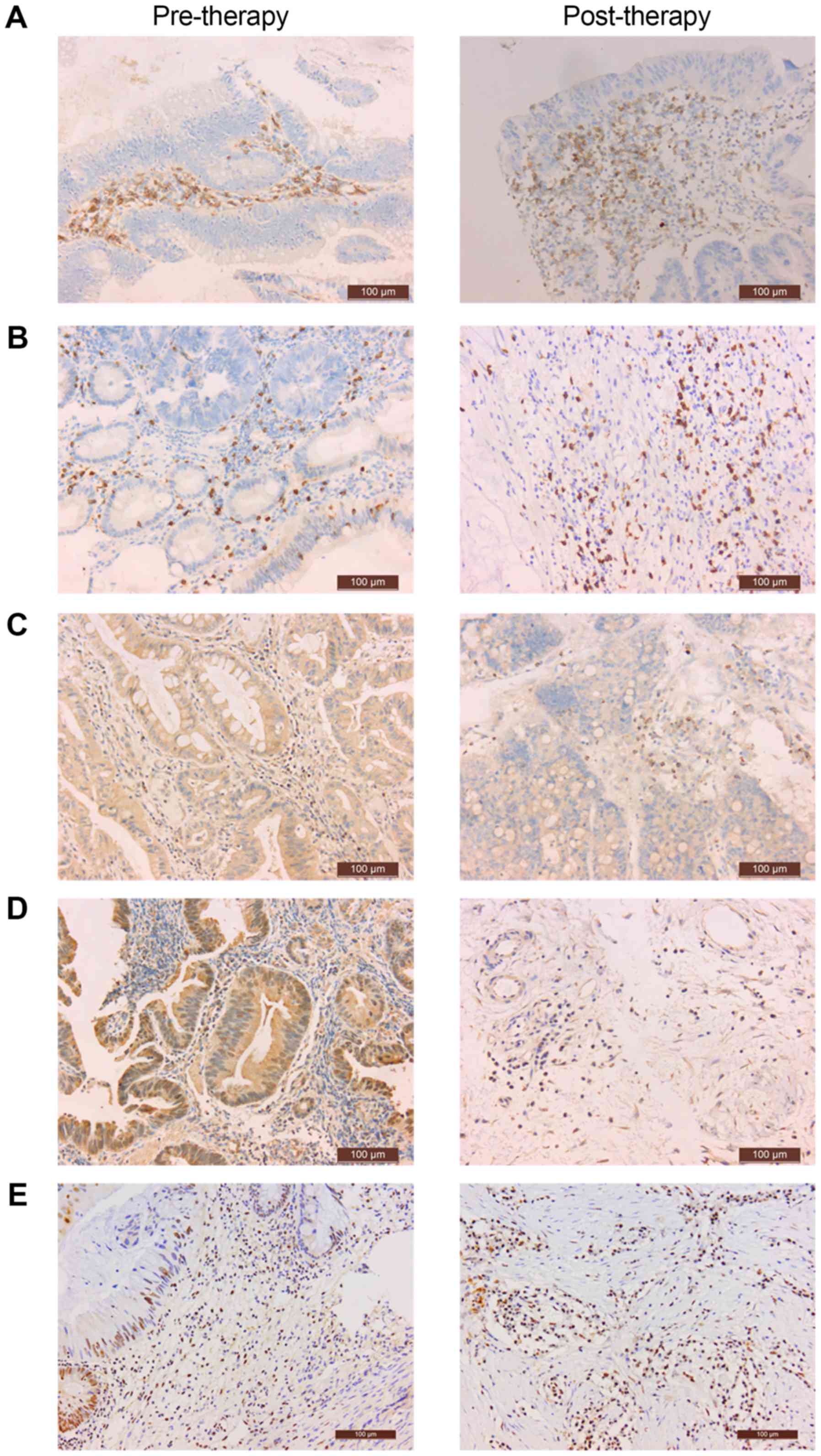
Evaluation of immune markers
CD4 was expressed on the cell membrane of
interstitial TILs (Fig. 1A). CD8 was
expressed in the cytoplasm of interstitial TILs (Fig. 1B). CTLA4 and FOXP3 were expressed in
the cytoplasm of TILs and tumor cells; positive cells were
identified by staining (Fig. 1C and
D). PD-L1 was expressed in the nucleus of TILs and tumor cells;
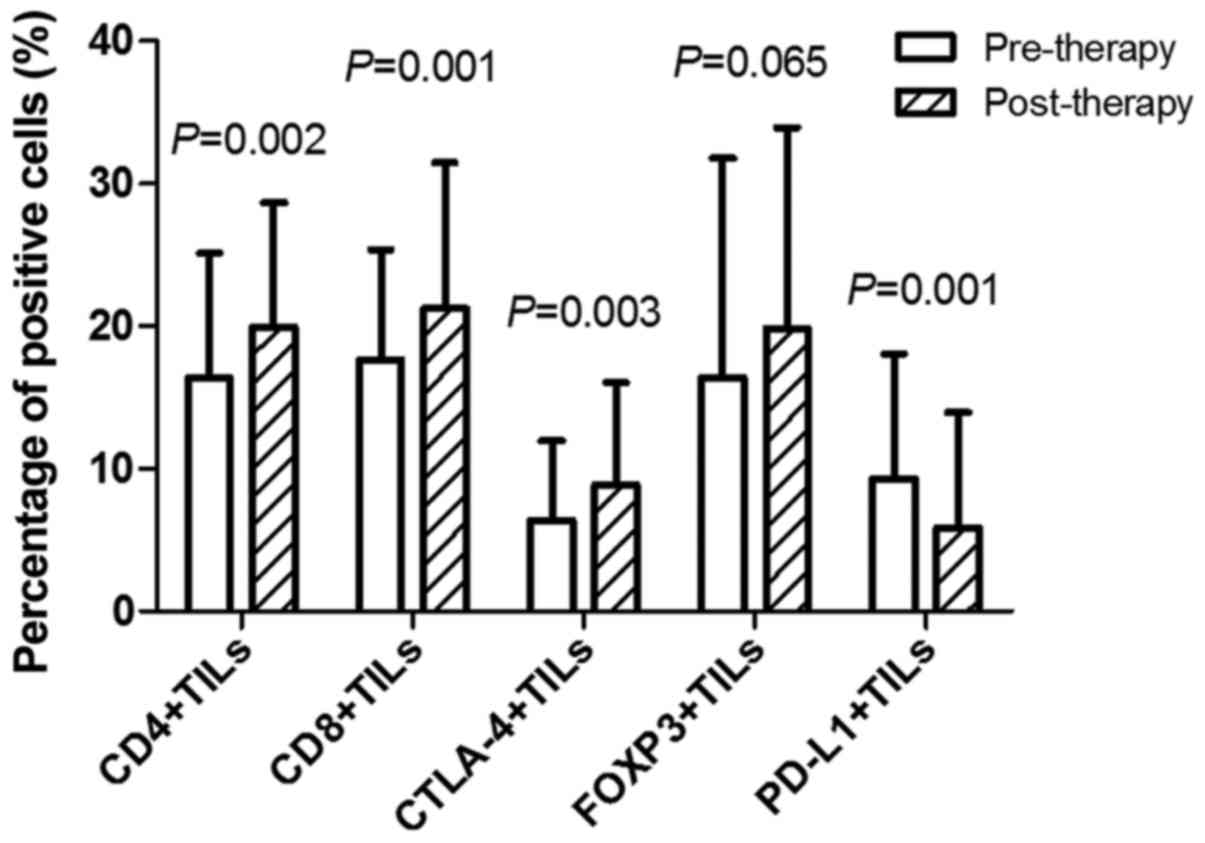
positive cells were identified by staining (Fig. 1E). The percentage of positive cells in
CD4+TILs pre- and post-neoadjuvant therapy was
16.35±8.76 and 19.95±8.73%, respectively (P=0.002). The percentage
of positive cells in CD8+TILs pre- and post-neoadjuvant
therapy was 17.64±7.74 and 21.27±10.21%, respectively (P=0.001).
The percentage of positive cells in CTLA-4+TILs pre- and
post-neoadjuvant therapy was 6.34±5.66 and 8.88±7.17%, respectively
(P=0.003). There was no significant difference between the
percentage of positive cells in FOXP3+TILs pre- and
post-neoadjuvant therapy (P=0.065). The percentage of positive
cells in PD-L1+TILs pre- and post-neoadjuvant therapy
was 9.28±8.77 and 5.83±8.12%, respectively (P=0.001; Fig. 2).
Association between immune markers,
clinicopathological features and curative effect pre- and
post-neoadjuvant therapy
Patients with poorly differentiated adenocarcinoma
were more likely to have a good response to neoadjuvant therapy
(P=0.007; Table II). Prior to
neoadjuvant therapy, patients with low FOXP3+TILs, and
high PD-L1+TILs were more likely to have a favorable
therapeutic response (P<0.001 and P=0.001, respectively).
Following neoadjuvant therapy, patients with high
CD4+TILs, high CD8+TILs, low
FOXP3+TILs and high PD-L1+TILs were more
likely to have a good response to therapy (P=0.026, 0.007, 0.007
and <0.001, respectively). The other clinicopathological
features and tumor immune markers were not significantly associated
with the curative effect.
 | Table II.Association between immune markers,
clinicopathological features, and curative effect pre- and
post-neoadjuvant therapy. |
Table II.
Association between immune markers,
clinicopathological features, and curative effect pre- and
post-neoadjuvant therapy.
| Clinicopathological
features | Poor response | Good response | P-value |
|---|
| Sex |
|
Male | 43 | 19 | 0.887 |
|
Female | 32 | 15 |
|
| Age, years | 55.87±10.94 | 52.38±9.89 | 0.116 |
| Histology |
|
Low | 11 | 14 | 0.007a |
|
Middle | 57 | 19 |
|
|
High | 7 | 1 |
|
| T stage |
| T3 | 25 | 8 | 0.302 |
| T4 | 50 | 26 |
|
| Lymphatic
invasion |
|
Negative | 21 | 14 | 0.172 |
|
Positive | 54 | 20 |
|
| Distance from the
anal verge, cm |
| ≤5 | 44 | 25 | 0.136 |
|
>5 | 31 | 9 |
|
| Pre-therapy
CD4+TILs, (%) |
|
Low | 45 | 14 | 0.068 |
|
High | 30 | 20 |
|
| Post-therapy
CD4+TILs, (%) |
|
Low | 48 | 14 | 0.026a |
|
High | 27 | 20 |
|
| Pre-therapy
CD8+TILs (%) |
|
Low | 47 | 16 | 0.126 |
|
High | 28 | 18 |
|
| Post-therapy
CD8+TILs (%) |
|
Low | 45 | 11 | 0.007a |
|
High | 30 | 23 |
|
| Pre-therapy
CTLA-4+TILs (%) |
|
Low | 48 | 21 | 0.823 |
|
High | 27 | 13 |
|
| Post-therapy
CTLA-4+TILs (%) |
|
Low | 44 | 26 | 0.072 |
|
High | 31 | 8 |
|
| Pre-therapy
FOXP3+TILs (%) |
|
Low | 36 | 30 |
<0.001a |
|
High | 39 | 4 |
|
| Post-therapy
FOXP3+TILs (%) |
|
Low | 39 | 27 | 0.007a |
|
High | 36 | 7 |
|
| Pre-therapy
PD-L1+TILs (%) |
|
Low | 52 | 12 | 0.001a |
|
High | 23 | 22 |
|
| Post-therapy
PD-L1+TILs (%) |
|
Low | 62 | 14 |
<0.001a |
|
High | 13 | 20 |
|
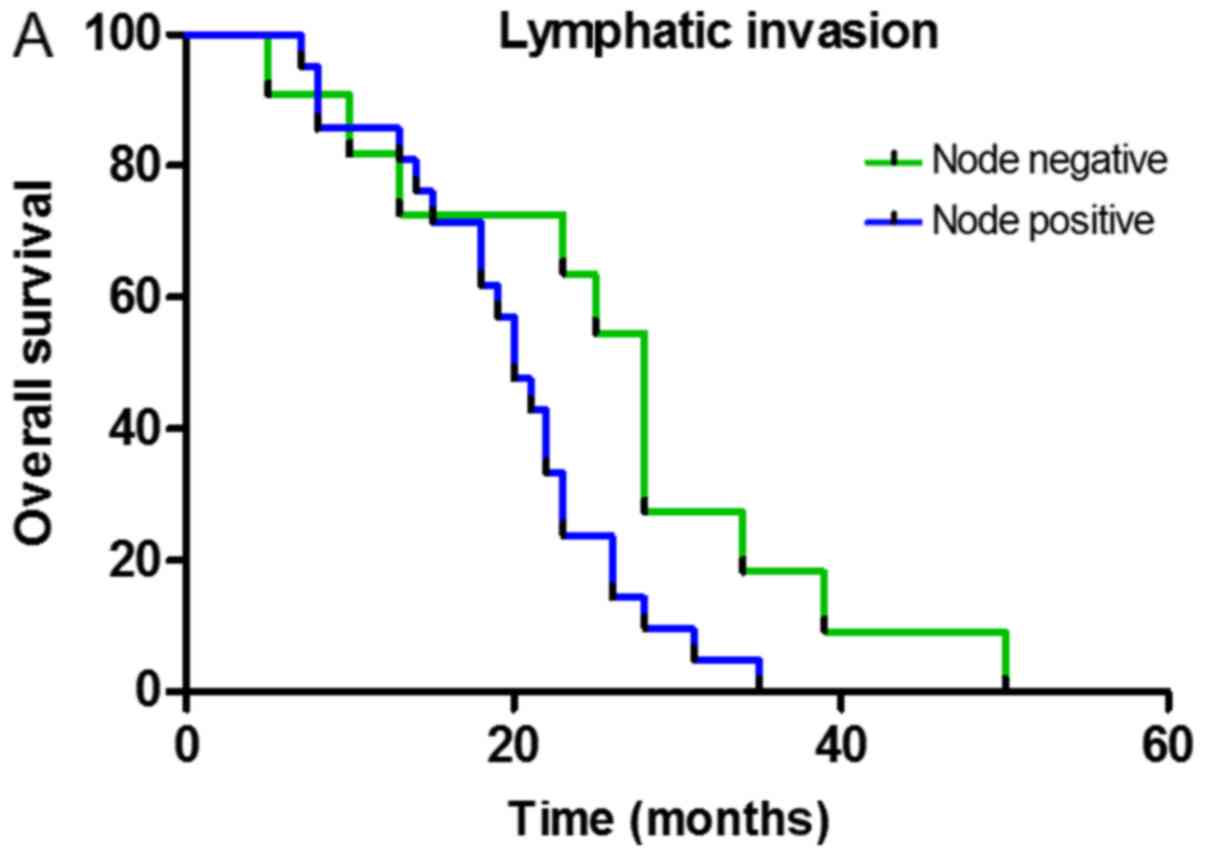
Survival analysis
Single-factor analysis of the total survival time
was conducted using the Kaplan-Meier test and indicated the
presence of an association between the total survival period,
lymphatic invasion, CD8+TILs and FOXP3+TILs
following neoadjuvant therapy. The survival function curve
demonstrated that the total survival time of patients without lymph
node metastasis was long and the mean survival time was 25.73±3.93
months (P=0.038; Fig. 3A). Total
survival time in patients with high levels of CD8+TILs
was long, and the average survival time was 26.25±2.15 months
(P=0.032; Fig. 3B). The total
survival time in patients with low FOXP3+TIL levels was
long and the mean survival time was 25.06±2.53 months (P=0.016;
Fig. 3C). The median survival time of
patients whose CD4+TILs did not increase following
neoadjuvant therapy was 19 months. The median survival time of
patients whose CD8+TILs did not increase following
neoadjuvant therapy was 21.5 months. Multiple factor analysis with
the Cox proportional risk regression model was used to analyze the
single factors that displayed statistical significance. Multiple
stepwise regression analysis indicated that CD8+TILs and
FOXP3+TILs were independent prognostic factors and may
affect the total survival of patients subjected to neoadjuvant
therapy for rectal cancer (P=0.037 and 0.013, respectively;
Table III).
 | Table III.Correlation between
clinicopathological features, immune markers and total survival
time. |
Table III.
Correlation between
clinicopathological features, immune markers and total survival
time.
|
| Single factor
analysis | Multifactor
analysis |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Log-rank | P-value | B | SD | P-value |
|---|
| Gender | 0.764 | 0.382 |
|
|
|
| Age | 0.360 | 0.548 |
|
|
|
| T staging | 0.016 | 0.898 |
|
|
|
| Lymphatic
invasion | 4.313 | 0.038a | 0.455 | 0.424 | 0.063 |
| DFTAV, cm | 0.486 | 0.486 |
|
|
|
| Pre-therapy
CD4+TILs, % | 0.759 | 0.383 |
|
|
|
| Post-therapy
CD4+TILs, % | 0.144 | 0.704 |
|
|
|
| Pre-therapy
CD8+TILs, % | 0.399 | 0.528 |
|
|
|
| Post-therapy
CD8+TILs, % | 4.680 | 0.032a | 2.191 | 0.377 | 0.037a |
| Pre-therapy
CTLA-4+TILs, % | 0.090 | 0.764 |
|
|
|
| Post-therapy
CTLA-4+TILs, % | 2.831 | 0.092 |
|
|
|
| Pre-therapy
FOXP3+TILs, % | 0.630 | 0.427 |
|
|
|
| Post-therapy
FOXP3+TILs, % | 5.826 | 0.016a | 0.357 | 0.417 | 0.013a |
| Pre-therapy
PD-L1+TILs, % | 0.007 | 0.935 |
|
|
|
| Post-therapy
PD-L1+TILs, % | 2.267 | 0.132 |
|
|
|
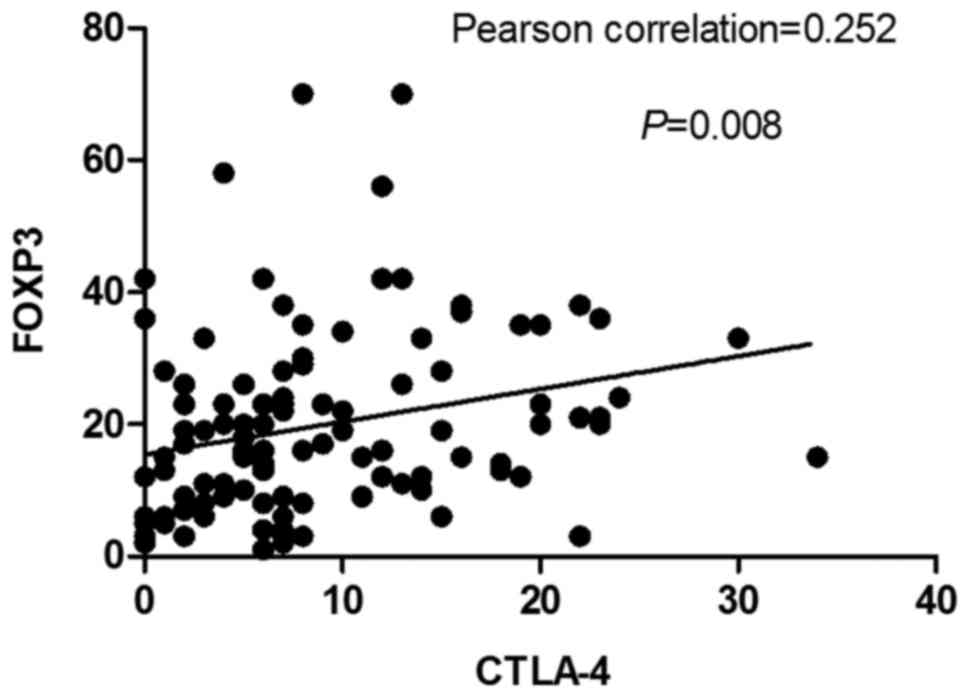
Correlation between immune
markers
There was no significant correlation between
CD4+TIL levels with CTLA-4+TIL and
FOXP3+TIL levels following neoadjuvant therapy (P-values
of 0.398 and 0.186, respectively). There was no correlation between
CD8+TIL levels with CTLA-4+TIL and
FOXP3+TIL levels following neoadjuvant therapy (P=0.845
and 0.655, respectively) (Data not shown). However, there was a
strong correlation between CTLA-4+TIL levels and
FOXP3+TIL levels (Pearson correlation coefficient=0.252,
P=0.008; Fig. 4).
Comparison between NAC and NACR
The comparison of immune markers, survival time,
curative effect and survival rate between NAC and NACR indicated
that the percentage of CTLA-4+TILs-positive cells was
significantly higher in NACR compared with NAC (P<0.001). The
differences in other indexes were not statistically significant
(Table IV).
 | Table IV.Comparison between NAC and NACR. |
Table IV.
Comparison between NAC and NACR.
| Clinicopathological
features | NAC | NACR | P-value |
|---|
| Post-therapy
CD4+TILs (%) |
|
Low | 29 | 33 | 0.828 |
|
High | 21 | 26 |
|
| Post-therapy
CD8+TILs (%) |
|
Low | 26 | 30 | 0.905 |
|
High | 24 | 29 |
|
| Post-therapy
CTLA-4+TILs (%) |
|
Low | 41 | 29 |
<0.001a |
|
High | 9 | 30 |
|
| Post-therapy
FOXP3+TILs (%) |
|
Low | 28 | 38 | 0.371 |
|
High | 22 | 21 |
|
| Post-therapy
PD-L1+TILs (%) |
|
Low | 38 | 38 | 0.189 |
|
High | 12 | 21 |
|
| Survival time,
months | 19.31±5.99 | 22.92±10.98 | 0.294 |
| TRG |
| Poor
response | 37 | 38 | 0.281 |
| Good
response | 13 | 21 |
|
| Survival rate | 80 | 85 | 0.931 |
|
Survival | 41 | 48 |
|
|
Mortality | 9 | 11 |
|
Discussion
The results of the present study indicated that the
expression of CD4+TILs and CD8+TILs was
significantly higher post-neoadjuvant therapy compared with
pre-neoadjuvant therapy, and patients with high levels of
CD4+TILs and CD8+TILs following neoadjuvant
therapy were more responsive to neoadjuvant therapy. This result
demonstrated that these patients may have had immunodeficiencies
prior to neoadjuvant therapy, and radiotherapy and chemotherapy may
increase the expression levels of CD4+TILs and
CD8+TILs. A previous study has reported that
chemotherapy may induce the expression of death receptors, increase
the levels of CD4+TILs, CD8+TILs and other
immune cells, and activate the immune microenvironment of the tumor
(23), which may improve the response
to neoadjuvant therapy. However, there was no significant
difference in the effect of CD4+TIL and
CD8+TIL levels prior to neoadjuvant therapy on its
curative effect, indicating that the effect of chemotherapy and/or
radiotherapy on the tumor immune microenvironment may be more
important in neoadjuvant therapy compared with the pre-existing
local immune response. Following neoadjuvant therapy, prognosis in
patients with high CD8+TIL levels was significantly
better compared with patients with low CD8+TIL levels,
suggesting that the levels of CD8+TILs in the tumor
microenvironment may limit the occurrence and development of tumors
and improve prognosis in patients with high CD8+TILs.
This result is consistent with those of other studies (24,25).
The CTLA-4 costimulatory pathway is a negative
signal activated by T cells. In the present study, the expression
level of CTLA-4+TILs post-neoadjuvant therapy was
significantly higher compared with pre-neoadjuvant therapy. This
result may be due to the killing effect of chemotherapy or
chemoradiotherapy in the tumor, leading to the release of vascular
endothelial growth factor, interleukin-10 (IL-10), transforming
growth factor-β and other inhibitory factors by tumor cells
(26). Moreover, the expression of
major histocompatibility complex molecules or costimulatory
molecules CD80/86 on the surface of dendritic cells, and CTLA-4
molecules as CD80/86 receptors alters accordingly (26). However, there was no significant
correlation between CTLA-4+TILs levels and the effect of
neoadjuvant therapy pre- and post-neoadjuvant therapy.
Compared with levels prior to neoadjuvant therapy,
the number of FOXP3+TILs was not significantly increased following
neoadjuvant therapy. However, low numbers of FOXP3+TILs were more
effective pre- and post-neoadjuvant therapy. Tregs undergo rapid
turnover compared with other T cell subsets and are selectively
depleted by a number of chemotherapeutic drugs, including
5-fluorouracil (27–29). In numerous patients, selective
depletion by chemotherapy may enhance the ‘window of opportunity’
for anti-tumor immunity and promote tumor regression. However, the
possibility that the number of adverse reactions is increased due
to Tregs in the neoplasm following neoadjuvant therapy cannot be
excluded. The results of the present study indicate that the
prognosis of patients with low expression levels of FOXP3+TILs
following neoadjuvant therapy is improved. However, the association
between FOXP3+TILs levels and the occurrence and
development of neoplasms remains unclear. Frey et al
(30) demonstrated that the high
expression level of FOXP3+ TILs is associated with good prognosis,
whereas Lin et al (31)
reported that levels of FOXP3+ TILs are associated with
tumor progression. Therefore, the correlation between FOXP3+TILs
and tumor progression requires further investigation.
Anti PD-1 and anti PD-L1 antibodies have been
demonstrated to have a notable and lasting effect in patients with
melanoma, renal cell carcinoma and non-small cell lung cancer
(31). However, clinical trials have
demonstrated that the blocking effect of anti PD-1 or PD-L1
antibodies in rectal cancer is poor (32). In the present study, the percentage of
positive PD-L1+TILs post-neoadjuvant therapy was lower
compared with pre-neoadjuvant therapy. However, the effect of
chemotherapy on the expression levels of PD-L1 is unknown. Saigusa
et al (33) used quantitative
polymerase chain reaction analysis to measure alterations in the
expression levels of the PD-L1 gene in four types of colon
cancer cell lines, prior to irradiation and 1, 3, and 5 days
following irradiation. It was observed that the expression levels
of PD-L1 were reduced following irradiation. However, the
mechanism underlying this process requires further investigation.
Patients with high expression levels of PD-L1+TILs
pre-and post-neoadjuvant therapy may achieve a good therapeutic
response, which contradicts the results of a previous study
(33). The PD-1/PD-Ll signaling
pathway may serve a negative role in regulating T cells through
multiple mechanisms, although PD-1 is not the only receptor
mediating PD-L1 activity. PD-L1 also causes apoptosis of
PD-1-negative T lymphocytes, suggesting that there may be
additional receptors expressed on T lymphocytes that bind to PD-L1
and induce immunosuppression (34).
Moreover, in contrast with its immunosuppressive activity, PD-L1
may also stimulate the immune response. PD-L1 protein in
combination with low levels of anti-CD3 antibody in resting T cells
may enhance the proliferation of T cells as well as the secretion
of IL-10 and IFN γ-1b (35). These
contradictory results suggest that PD-L1 may have multiple
receptors, which exert different immunomodulatory effects through
binding with different receptors (36).
A previous study (37)
reported that CTLA-4 and FOXP3 may inhibit the activation of T
cells, however the results indicated no statistically significant
association between the expression levels of CD4+TILs or
CD8+TILs and CTLA-4+TILs or
FOXP3+TILs following neoadjuvant therapy. This may be
due to the small sample size. Nonetheless, there was a positive
association between CTLA-4+TILs and
FOXP3+TILs following neoadjuvant therapy. Tregs may
inhibit the activity of effector cells by transducing a reverse
signal of the crosslink between CTLA-4 and antigen-presenting cell
or B7 (CD80 and CD86) on the surface of activated T cells (38). In addition, it was reported that the
expression level of CTLA-4 on the surface of
CD4+D25+Treg was decreased following knockdown of FOXP3
expression in mice, yet was increased in mice transfected with high
levels of FOXP3 (39). The comparison
between NAC and NACR indicated that the percentage of
CTLA-4+TILs-positive cells in NACR was significantly
higher compared with NAC. It is possible that the body produces an
immunosuppressive response following radiation exposure. The
expression of CTLA-4 may be beneficial for avoiding or reducing the
occurrence of autoimmune reactions caused by radiation exposure.
Radiation has been reported to activate anti-tumor immune responses
by killing cancer cells and inducing distant effects (40). In addition, studies have demonstrated
that radiation may increase the proportion of Treg cells in humans
(41,42), and Tregs may induce the expression of
CTLA-4 through various pathways. Moreover, the present study
observed a positive correlation between CTLA-4+TIL
levels and FOXP3+TIL levels, which also supports this
hypothesis. In addition, it was reported that NACR was more
effective and increased the OS compared with NAC; however, the
difference was not statistically significant, which may be due to
the small sample size.
Compared with previous studies, the number of
patients in the current study was notably larger; the majority of
studies currently in the literature were conducted with <100
patients (33,43,44). In
the analysis, the differences between the two methods of NAC and
NACR were compared, and it was reported that the effects of these
two treatments on numerous immune factors differed. However, the
association between these indicators and the curative effect or
prognosis with different treatment methods was not analyzed. There
may be a number of differences in the results due to differences in
the criteria for evaluating the pathological response and the
standard of interpretation of IHC.
In conclusion, CD4+TILs and
CD8+TILs may inhibit the growth of tumor cells, and
FOXP3+TILs are associated with a poor response to
therapy. CD8+TILs and FOXP3+TILs following
neoadjuvant therapy are independent prognostic factors and affect
total survival in patients undergoing neoadjuvant therapy for
rectal cancer. The role of PD-L1+TILs remains to be
investigated. The effects of NAC and NACR on the tumor
microenvironment may be different. The tumor microenvironment is
complex, thus in-depth studies on immunoregulatory mechanisms in
the inflammatory microenvironment of rectal cancer cells, and a
possible association between the tumor microenvironment and signal
transduction and metabolic pathways of rectal cancer may elucidate
the pathological mechanisms and potential immunotherapy of rectal
cancer.
Acknowledgements
The authors would like to thank Ms. Wenxia Yan for
her assistance with IHC; Mr. Liwu Xie for his assistance with data
collection; and Dr Xiaojuan Wang and Dr Jing Li (Department of
Pathology of Shanxi Provincial Cancer Hospital, Taiyuan, China) for
their assistance with the image analysis and pathological
assessment.
Funding
This study was funded by the Major Research and
Development Project of Shanxi Science and Technology Department of
Shanxi (grant no. 201603 D321049) and the Scientific Research Tsak
of the Shanxi Health and Family Planning Commission of Shanxi
(grant no. 2014052).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SYZ, WQB and YFX were responsible for the study
design, original article drafting and editing, data acquisition and
data analysis. XNT was responsible for original article drafting,
data analysis and article revision. PB was responsible for the
immunohistochemistry. JX and YFX performed the immunohistochemical
evaluations and critically revised the manuscript.
Ethics approval and consent to
participate
Ethics for the use of human tissues was approved by
the Shanxi Provincial Cancer Hospital Ethics Committee (no. 201722)
and patient consent was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
3
|
Albini A, Magnani E and Noonan DM: The
tumor microenvironment: Biology of a complex cellular and tissue
society. Q J Nucl Med Mol Imaging. 54:244–248. 2010.PubMed/NCBI
|
|
4
|
Kumamoto Y, Mattei LM, Sellers S, Payne GW
and Iwasaki A: CD4+ T cells support cytotoxic T lymphocyte priming
by controlling lymph node input. Proc Natl Acad Sci USA.
108:8749–8754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grossman WJ, Verbsky JW, Barchet W,
Colonna M, Atkinson JP and Ley TJ: Human T regulatory cells can use
the perforin pathway to cause autologous target cell death.
Immunity. 21:589–601. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paust S, Lu L, McCarty N and Cantor H:
Engagement of B7 on effector T cells by regulatory T cells prevents
autoimmune disease. Proc Natl Acad Sci USA. 101:10398–10403. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Boehmer H: Mechanisms of suppression
by suppressor T cells. Nat Immunol. 6:338–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaudhary B, Khaled YS, Ammori BJ and
Elkord E: Neuropilin 1: Function and therapeutic potential in
cancer. Cancer Immunol Immunother. 63:81–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bloch O, Crane CA, Kaur R, Safaee M,
Rutkowski MJ and Parsa AT: Gliomas promote immunosuppression
through induction of B7-H1 expression in tumor-associated
macrophages. Clin Cancer Res. 19:3165–3175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu B, Chen L, Liu L, Zhu Y, Wu C, Jiang J
and Zhang X: T-cell-mediated tumor immune surveillance and
expression of B7 co-inhibitory molecules in cancers of the upper
gastrointestinal tract. Immunol Res. 50:269–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brunner MC, Chambers CA, Chan FK, Hanke J,
Winoto A and Allison JP: CTLA-4-Mediated inhibition of early events
of T cell proliferation. J Immunol. 162:5813–5820. 1999.PubMed/NCBI
|
|
13
|
Salvi S, Fontana V, Boccardo S, Merlo DF,
Margallo E, Laurent S, Morabito A, Rijavec E, Dal Bello MG, Mora M,
et al: Evaluation of CTLA-4 expression and relevance as a novel
prognostic factor in patients with non-small cell lung cancer.
Cancer Immunol Immunother. 61:1463–1472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karabon L, Markiewicz M, Kosmaczewska A,
Partyka A, Pawlak-Adamska E, Tomkiewicz A, Ciszak L, Jagoda K,
Dzierzak-Mietla M, Kyrcz-Krzemien S and Frydecka I: Pretransplant
donor and recipient CTLA-4 mRNA and protein levels as a prognostic
marker for aGvHD in allogeneic hematopoietic stem cell
transplantation. Immunol Lett. 165:52–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sabatier R, Finetti P, Mamessier E,
Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D and
Bertucci F: Prognostic and predictive value of PDL1 expression in
breast cancer. Oncotarget. 6:5449–5464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen JK, Cote GM, Choy E, Yang P, Harmon
D, Schwab J, Nielsen GP, Chebib I, Ferrone S, Wang X, et al:
Programmed cell death ligand 1 expression in osteosarcoma. Cancer
Immunol Res. 2:690–698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng
P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et
al: Blockade of B7-H1 improves myeloid dendritic cell-mediated
antitumor immunity. Nat Med. 9:562–567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Gajewski TF and Kline J:
PD-1/PD-L1 interactions inhibit antitumor immune responses in a
murine acute myeloid leukemia model. Blood. 114:1545–1552. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dworak O, Keilholz L and Hoffmann A:
Pathological features of rectal cancer after preoperative
radiochemotherapy. Int J Colorectal Dis. 12:19–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalbasi A, June CH, Haas N and Vapiwala N:
Radiation and immunotherapy: A synergistic combination. J Clin
Invest. 123:2756–2763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yasuda K, Nirei T, Sunami E, Nagawa H and
Kitayama J: Density of CD4(+) and CD8(+) T lymphocytes in biopsy
samples can be a predictor of pathological response to
chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 6:492011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garcia-Martinez E, Gil GL, Benito AC,
González-Billalabeitia E, Conesa MA, García García T, García-Garre
E, Vicente V and de la Peña Ayala F: Tumor-infiltrating immune cell
profiles and their change after neoadjuvant chemotherapy predict
response and prognosis of breast cancer. Breast Cancer Res.
16:4882014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J and Guo Z: Immune escape of tumor.
Chin J Cancer Biother. 4:315–317. 2006.(In Chinese).
|
|
27
|
Rettig L, Seidenberg S, Parvanova I,
Samaras P, Curioni A, Knuth A and Pascolo S: Gemcitabine depletes
regulatory T-cells in human and mice and enhances triggering of
vaccine-specific cytotoxic T-cells. Int J Cancer. 129:832–838.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maeda K, Hazama S, Tokuno K, Kan S, Maeda
Y, Watanabe Y, Kamei R, Shindo Y, Maeda N, Yoshimura K, et al:
Impact of chemotherapy for colorectal cancer on regulatory T-cells
and tumor immunity. Anticancer Res. 31:4569–4574. 2011.PubMed/NCBI
|
|
29
|
Van der Most RG, Currie AJ, Mahendran S,
Prosser A, Darabi A, Robinson BW, Nowak AK and Lake RA: Tumor
eradication after cyclophosphamide depends on concurrent depletion
of regulatory T cells: A role for cycling TNFR2-expressing
effector-suppressor T cells in limiting effective chemotherapy.
Cancer Immunol Immunother. 58:1219–1228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frey DM, Droeser RA, Viehl CT, Zlobec I,
Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L and
Tornillo L: High frequency of tumor-infiltrating FOXP3(+)
regulatory T cells predicts improved survival in mismatch
repair-proficient colorectal cancer patients. Int J Cancer.
126:2635–2643. 2010.PubMed/NCBI
|
|
31
|
Lin YC, Mahalingam J, Chiang JM, Su PJ,
Chu YY, Lai HY, Fang JH, Huang CT, Chiu CT and Lin CY: Activated
but not resting regulatory T cells accumulated in tumor
microenvironment and correlated with tumor progression in patients
with colorectal cancer. Int J Cancer. 132:1341–1350. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saigusa S, Toiyama Y, Tanaka K, Inoue Y,
Mori K, Ide S, Imaoka H, Kawamura M, Mohri Y and Kusunoki M:
Implication of programmed cell death ligand 1 expression in tumor
recurrence and prognosis in rectal cancer with neoadjuvant
chemoradiotherapy. Int J Clin Oncol. 21:946–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Butte MJ, Keir ME, Phamduy TB, Sharpe AH
and Freeman GJ: Programmed death-1 ligand 1 interacts specifically
with the B7-1 costimulatory molecule to inhibit T cell responses.
Immunity. 27:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tamura H, Dong H, Zhu G, Sica GL, Flies
DB, Tamada K and Chen L: B7-H1 costimulation preferentially
enhances CD28-independent T-helper cell function. Blood.
97:1809–1816. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Parsa AT, Waldron JS, Panner A, Crane CA,
Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et
al: Loss of tumor suppressor PTEN function increases B7-H1
expression and immunoresistance in glioma. Nat Med. 13:84–88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walker LS: Treg and CTLA-4: Two
intertwining pathways to immune tolerance. J Autoimmun. 45:49–57.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Birebent B, Lorho R, Lechartier H, de
Guibert S, Alizadeh M, Vu N, Beauplet A, Robillard N and Semana G:
Suppressive properties of human CD4+CD25+ regulatory T cells are
dependent on CTLA-4 expression. Eur J Immunol. 34:3485–3496. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu C, Li W and Yao Y: Regulating mechanism
of regulatory T cells in immunoregulatory responses. Int J Pathol
Clin Med. 28:199–204. 2008.
|
|
40
|
Postow MA, Callahan MK, Barker CA, Yamada
Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al:
Immunologic correlates of the abscopal effect in a patient with
melanoma. N Engl J Med. 366:925–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qu Y, Zhang B, Liu S, Zhang A, Wu T and
Zhao Y: 2-Gy whole-body irradiation significantly alters the
balance of CD4+ CD25- T effector cells and CD4+ CD25+ Foxp3+ T
regulatory cells in mice. Cell Mol Immunol. 7:419–427. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Billiard F, Buard V, Benderitter M and
Linard C: Abdominal γ-radiation induces an accumulation of
function-impaired regulatory T cells in the small intestine. Int J
Radiat Oncol Biol Phys. 80:869–876. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mizukami Y, Kono K, Kawaguchi Y, Akaike H,
Kamimura K, Sugai H and Fujii H: Localisation pattern of Foxp3+
regulatory T cells is associated with clinical behaviour in gastric
cancer. Br J Cancer. 98:148–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsutani S, Shibutani M, Maeda K,
Nagahara H, Fukuoka T, Nakao S, Hirakawa K and Ohira M:
Significance of tumor-infiltrating lymphocytes before and after
neoadjuvant therapy for rectal cancer. Cancer Sci. 109:966–979.
2018. View Article : Google Scholar : PubMed/NCBI
|