Introduction
Breast cancer (BC) is one of the most common cancer
types in women. Approximately 1.68 million women are diagnosed with
BC worldwide each year and it ranks as the leading cause of
cancer-associated cases of mortality in women (1). Although China is a country with a lower
incidence of BC, incidence and mortality still ranks the highest
for BC in female malignant cancer types (2–4). BC is a
heterogeneous disease that exhibits high sensitivity to
chemotherapeutic treatment; approximately 50% of patients with
advanced breast cancer benefit from combined chemotherapy, whereas
the other half experience only the adverse effects from
chemotherapy drugs (5). Therefore, it
is important to further refine BC molecular classification to more
precisely guide treatment decisions. There are four BC molecular
subtypes, including luminal A, luminal B, human epidermal growth
factor receptor 2 (HER2) overexpression and triple-negative (TN)
(6). Significant differences have
been identified in the prognosis and treatment efficacy among
patients with different molecular subtypes of BC (7). Although the existing molecular subtyping
provides an improved basis for the treatment and prognosis of
patients, certain patients with heterogeneous BC remain insensitive
to clinical treatment (8).
Sperm-associated antigen 5 (SPAG5), first identified
in 2001, is also known as mitotic spindle-associated protein 5
(9–11). SPAG5 is involved in the formation of
spindles during the cell cycle and may be closely associated with
the function and movement of spindles during mitosis (12,13).
Certain studies have also reported that SPAG5 may inhibit cell
apoptosis through the inhibition of mammalian target of rapamycin
complex 1 (14). In 2016, Abdel-Fatah
et al (15) revealed that
SPAG5 is an amplified gene in BC and may have clinical utility as a
biomarker in estrogen receptor (ER)-negative BC, however this study
did not examine the association between SPAG5 and the molecular
classification of BC.
The current study analyzed SPAG5 expression in
Chinese patients with primary BC and compared it with the
expression level in matched adjacent nontumor tissues. The
association between SPAG5 expression, clinical characteristics,
ER/progesterone receptor (PR)/HER2/Ki-67 expression and overall
survival (OS) in patients with BC was also investigated.
Materials and methods
Human tissue specimens and patient
clinical information
A total of 379 formalin-fixed, paraffin-embedded
(FFPE) breast tissue samples were collected from 159 female
patients with an age range of 24–87 years. This included 159 cancer
tissue samples, 159 matched adjacent nontumor tissue samples and 61
benign tumor tissue samples. All tissue blocks were obtained from
the Department of Pathology at the Affiliated Hospital of Nantong
University (Jiangsu, China) between July 2003 and March 2010.
Medical records for tissue donor patients included information on
age, histological grade, ER expression, PR expression, HER2
expression, Ki-67 expression, tumor size, lymph node metastasis and
tumor-node-metastasis (TNM) stage. No patients received treatment,
including radiation therapy, chemotherapy or immunotherapy, prior
to surgical resection. OS was defined as the period from initial
diagnosis of BC via biopsy until mortality. Information on patients
who were alive at the last follow-up date was removed from the
analysis. Disease-free survival was defined as the period from
surgical treatment until disease recurrence. In addition, a total
of 35 freshly frozen BC tumor tissues and matching adjacent
nontumor tissues were obtained from a subset of the 159 patients at
the Affiliated Hospital of Nantong University. Written informed
consent was obtained from all patients involved in the current
study. The Human Research Ethics Committee of the Affiliated
Hospital of Nantong University approved the study protocol.
Tissue microarrays (TMAs) and
immunohistochemistry (IHC)
TMAs were generated at the Department of Pathology,
Affiliated Hospital of Nantong University, using a Quick Ray manual
tissue microarrayer system (cat. no. UT06; Unitma Co., Ltd., Seoul,
Korea). Specifically, core tissue biopsies (2 mm in diameter) were
obtained from 159 individuals. FFPE blocks were made and then
arranged in new recipient paraffin blocks (cat. no. 62595-08;
Quick-Ray™ Recipient Block; Head Biotechnology Co., Ltd., Beijing,
China), according to the manufacturer's protocol. A total of five
breast TMAs were made. Sections (4 µm) were cut and placed on super
frost-charged glass microscope slides to generate TMA slides.
Tissue sections were deparaffinized and rehydrated through a graded
series of alcohols. Endogenous peroxidase activity was blocked by
incubation in 3% H2O2. Tissues were placed in
0.01 M citrate buffer at pH 6.0 and heated to boiling in a
microwave for antigen retrieval. SPAG5 was detected using a
polyclonal rabbit anti-human SPAG5 antibody for 2 h at room
temperature (1:1,000; cat. no. HPA022008; Atlas Antibodies, Bromma,
Sweeden). Reactions were detected using an Envision™ peroxidase kit
(Dako; Agilent Technologies, Inc., Santa Clara, CA, USA). Tissues
were then incubated in 0.05% 3,3′-diaminobenzidine (Dako, Agilent
Technologies, Inc.) for 30 sec at room temperature, counterstained
with 0.45% hematoxylin for 3 min, dehydrated through a graded
series of alcohols, cleared in xylene and coverslipped with
permanent mounting media at room temperature.
Staining was quantified in all tissues without
knowledge of clinical characteristics. SPAG5 expression was scored
using the semi-quantitative H-score method, which takes into
account both the staining intensity and the percentage of cells at
that intensity (10,11). The following staining intensity scores
were used: 0 indicated no staining; 1+ indicated weak staining; 2+
indicated moderate staining; and 3+ indicated intense staining. The
total number of cells at each intensity level was multiplied by the
corresponding intensity score to yield an intensity percentage
score. Final staining scores were calculated by summing the four
intensity percentage scores. The minimum possible final staining
score was 0 (no staining) and the maximum possible score was 300
(100% of cells with 3+ staining intensity).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to determine the SPAG5 mRNA
expression level in 35 pairs of human BC tissue and matched
adjacent nontumor tissue. Tissue samples were snap-frozen in liquid
nitrogen and stored at −80°C prior to RNA extraction. Total RNA was
extracted from frozen samples using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and reverse
transcribed into cDNA using a PrimeScript™ RT reagent kit (Clontech
Laboratories, Inc., Mountainview, CA, USA), according to the
manufacturer's protocol. Human β-actin served as the internal
control for determining SPAG5 mRNA levels. The following primers
were used for qPCR: human β-actin forward,
5′-TGGAGAAAATCTGGCACCAC-3′ and reverse, 5′-GATGATGCCTCGTTCTAC-3′;
and SPAG5 forward, 5′-CATCTCACAGTGGGATAACTAATAAAC-3′ and reverse,
5′-CAGGGATAGGTGAAGCAAGGATA-3′ (GenScript, Nanjing, Jiangsu China).
qPCR was performed using an ABI PRISM 7500HT Sequence Detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) in
96-well plates. The final volume for each reaction was 20 µl, which
included 2 µl of cDNA template (corresponding to ~40 ng of
retro-transcribed total RNA), the primers (20 nmol/l each) and 2X
SYBR-Green PCR Master mixture (10 µl). Thermocycling conditions
were as follows: Following an initial 2 min at 50°C to allow
AmpErase-UNG activity and 10 min at 95°C, samples were cycled 40
times at 95°C for 15 sec and 58°C for 1 min. All experiments were
performed in triplicate. mRNA levels were quantified using the
2−ΔΔCq method (16) and
normalized to respective internal controls.
Statistical analysis
All statistical analyses were performed using the
SPSS 18.0 statistical software package (SPSS Inc., Chicago, IL,
USA). For statistical analysis, the continuous SPAG5 expression
data from IHC were first converted into dichotic data (low vs.
high) using specific cutoff values, which were selected based on
significant differences in OS using the X-tile software program
(www.tissuearray.org/rimmlab)
(14). Statistical analysis of the
RT-qPCR data was performed using an unpaired Student's t-test when
two groups were compared. χ2 test was used to identify
statistical differences between groups. Cumulative patient survival
was estimated using the Kaplan-Meier method and a log-rank test was
used to compare the survival curves. A Cox proportional hazards
model was used to calculate univariate and multivariate hazard
ratios (HRs) for the variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
SPAG5 mRNA is highly expressed in BC
tissues
The expression levels of SPAG5 mRNA in BC tissues
and matched adjacent nontumor tissues were examined using RT-qPCR.
The SPAG5 mRNA expression level was 3.41±0.41-fold higher in BC
tissues compared with matched adjacent nontumor tissues
(P<0.001; Fig. 1A). In addition,
it was identified that the SPAG5 mRNA expression level was
1.81±0.31-fold higher in TNBC tissues compared with other BC
molecular subtypes (P<0.001; Fig.
1B).
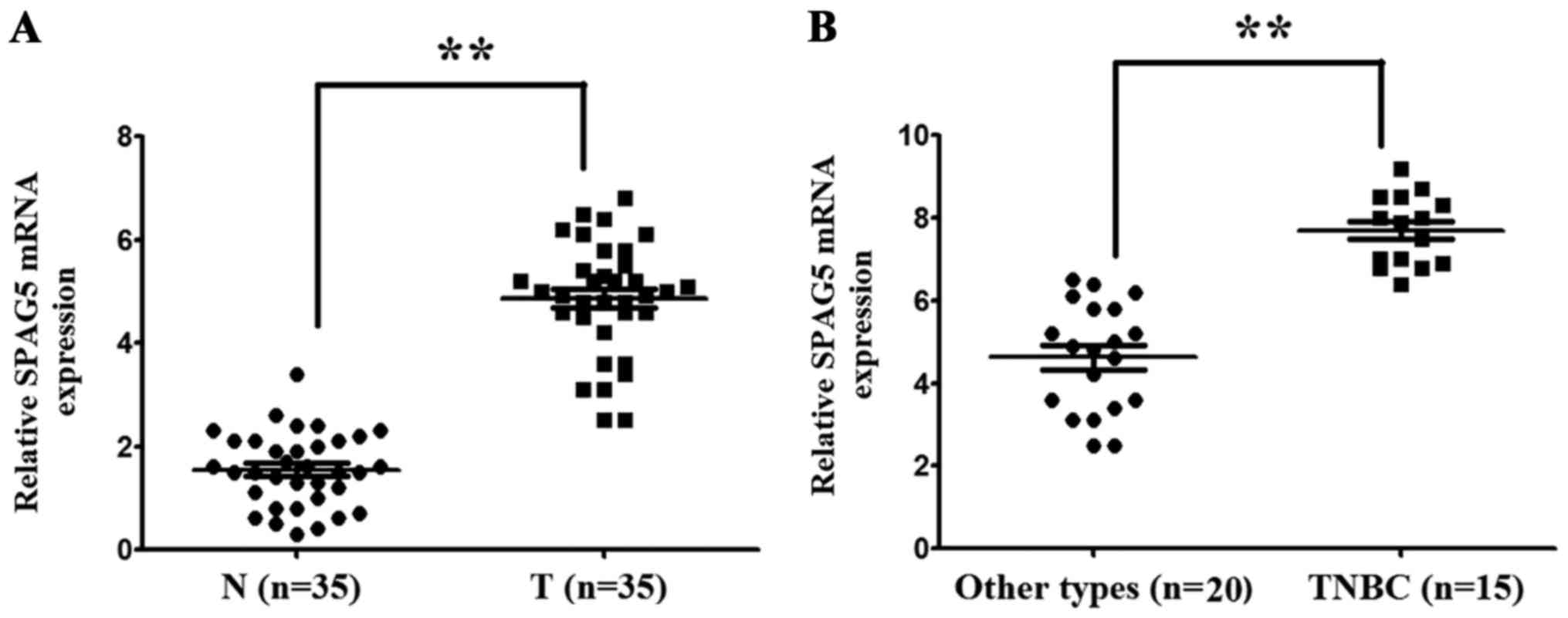 | Figure 1.SPAG5 mRNA expression in 35 pairs of
BC tissues and matched adjacent nontumor tissues. SPAG5 mRNA
expression was detected using reverse transcription-quantitative
polymerase chain reaction and normalized to β-actin. The expression
level of SPAG5 mRNA in BC tissues, normal breast tissues, TNBC
tissues and non-TNBC tissues was 4.41±0.53, 1.73±0.28, 7.83±0.13
and 4.37±0.19, respectively. (A) SPAG5 mRNA level was significantly
higher in BC tissues compared with matched adjacent nontumor
tissues. (B) SPAG5 mRNA level was significantly higher in TNBC
tissues compared with other molecular subtypes of BC. Data are
presented as mean ± standard deviation. **P<0.001. SPAG5,
sperm-associated antigen 5; BC, breast cancer; TNBC,
triple-negative breast cancer; N, matched adjacent nontumor
tissues; T, BC tissues. |
SPAG5 protein is highly expressed in
BC tissues
SPAG5 protein expression was demonstrated to be
localized to the cytoplasm using IHC (Fig. 2). Furthermore, IHC revealed that SPAG5
protein was highly expressed in BC tissues. Using X-tile software
to analyze TMA data, SPAG5 expression was classified as high or low
according to OS for patients with BC. The cutoff value was selected
as 130; a score of 0–130 was considered low or no expression and a
score of 131–300 was considered high expression. High SPAG5
expression was detected more frequently in BC tissues (116/159,
72.96%) compared with benign tumor tissues (2/61, 3.28%) or matched
adjacent nontumor tissues (0/159, 0.00%; Table I).
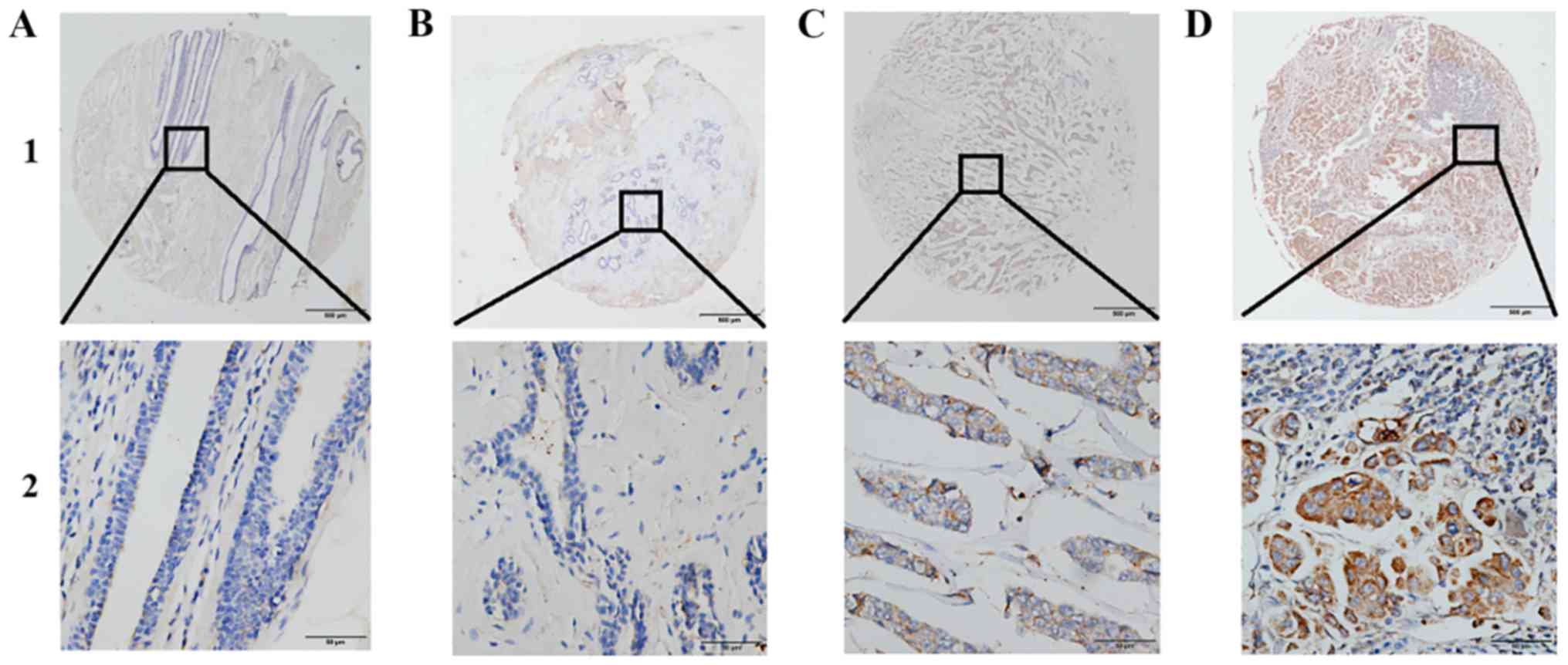 | Figure 2.Representative patterns of SPAG5
protein expression in BC tissues on tissue microarray sections. (A)
Low expression of SPAG5 in matched adjacent nontumor tissue (IHC
score=60). (B) Low expression of SPAG5 in benign tumor tissue (IHC
score=50). (C) Weakly positive expression of SPAG5 in BC tissue
(IHC score=150). (D) High expression of SPAG5 in BC tissue (IHC
score=300). Row 1, SPAG5 staining with magnification, ×4, (scale
bar, 500 µm); row 2, SPAG5 staining with magnification, ×40 (scale
bar, 50 µm). SPAG5, sperm-associated antigen 5; BC, breast cancer;
IHC, immunohistochemistry. |
 | Table I.SPAG5 protein expression in breast
tissues. |
Table I.
SPAG5 protein expression in breast
tissues.
|
|
| SPAG5 expression, n
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Tissue type | n | Low or no | High | χ2 | P-value |
|---|
| Breast cancer
tissues | 159 | 43 (27.04) | 116 (72.96) |
|
|
| Matched adjacent
tissues | 159 | 159 (100.00) | 0 (0.00) | 60.700 | <0.001 |
| Benign tumor
tissues | 61 | 59 (96.72) | 2 (3.28) |
|
|
High SPAG5 expression is associated
with clinicopathological characteristics in BC
The association between SPAG5 protein expression and
clinicopathologic characteristics in BC tissues was investigated.
Results indicated that high expression of SPAG5 in BC was
associated with histological grade (χ2=9.965, P=0.007),
ER expression (χ2=10.940, P<0.001), Ki-67 expression
(χ2=21.799, P<0.001), tumor size
(χ2=15.913, P=0.001), lymph node status
(χ2=18.519, P<0.001), TNM stage
(χ2=11.511, P=0.003) and TNBC subtype
(χ2=10.429 P=0.015). However, no significant association
was identified between SPAG5 expression and age, PR expression or
HER2 expression (Table II).
 | Table II.Association of SPAG5 protein
expression levels with clinicopathological characteristics in
patients with breast cancer. |
Table II.
Association of SPAG5 protein
expression levels with clinicopathological characteristics in
patients with breast cancer.
|
|
| SPAG5 expression, n
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | n | Low or none | High | χ2 | P-value |
|---|
| Total | 159 |
|
|
|
|
| Age, years |
|
|
| 1.078 | 0.299 |
| ≤60 | 81 | 62 (76.54) | 19 (23.46) |
|
|
|
>60 | 78 | 54 (69.23) | 24 (30.77) |
|
|
| Histological
grade |
|
|
| 9.965 | 0.007 |
| I | 57 | 33 (57.89) | 24 (42.11) |
|
|
| II | 63 | 51 (80.95) | 12 (19.05) |
|
|
| III | 39 | 32 (82.05) | 7 (17.95) |
|
|
| ER expression |
|
|
| 10.940 | <0.001 |
|
Positive | 110 | 89 (80.91) | 21 (19.09) |
|
|
|
Negative | 49 | 27 (55.10) | 22 (44.90) |
|
|
| PR expression |
|
|
| 3.614 | 0.057 |
|
Positive | 97 | 76 (78.35) | 21 (21.65) |
|
|
|
Negative | 62 | 40 (64.52) | 22 (35.48) |
|
|
| HER2
expression |
|
|
| 2.856 | 0.091 |
|
Positive | 57 | 37 (64.91) | 20 (35.09) |
|
|
|
Negative | 102 | 79 (77.45) | 23 (22.55) |
|
|
| Ki-67
expression |
|
|
| 21.799 | <0.001 |
|
Positive | 63 | 58 (92.06) | 5 (7.94) |
|
|
|
Negative | 96 | 58 (60.42) | 38 (39.58) |
|
|
| Tumor size |
|
|
| 15.913 | <0.001 |
| T1 | 75 | 45 (60.00) | 30 (40.00) |
|
|
| T2 | 54 | 43 (79.63) | 11 (20.37) |
|
|
| T3 and
4 | 30 | 28 (93.33) | 2 (6.67) |
|
|
| Lymph node
metastasis |
|
|
| 18.519 | <0.001 |
| N0 | 67 | 37 (55.22) | 30 (44.78) |
|
|
|
N1-3 | 92 | 79 (85.87) | 13 (14.13) |
|
|
| TNM stage |
|
|
| 11.511 | 0.003 |
| I | 51 | 30 (56.82) | 21 (41.18) |
|
|
| II | 69 | 51 (73.91) | 18 (26.09) |
|
|
|
III | 39 | 35 (89.74) | 4 (10.26) |
|
|
| TNBC |
|
|
| 10.429 | 0.015 |
|
Positive | 140 | 87 (62.14) | 53 (37.86) |
|
|
|
Negative | 19 | 15 (78.96) | 4 (21.05) |
|
|
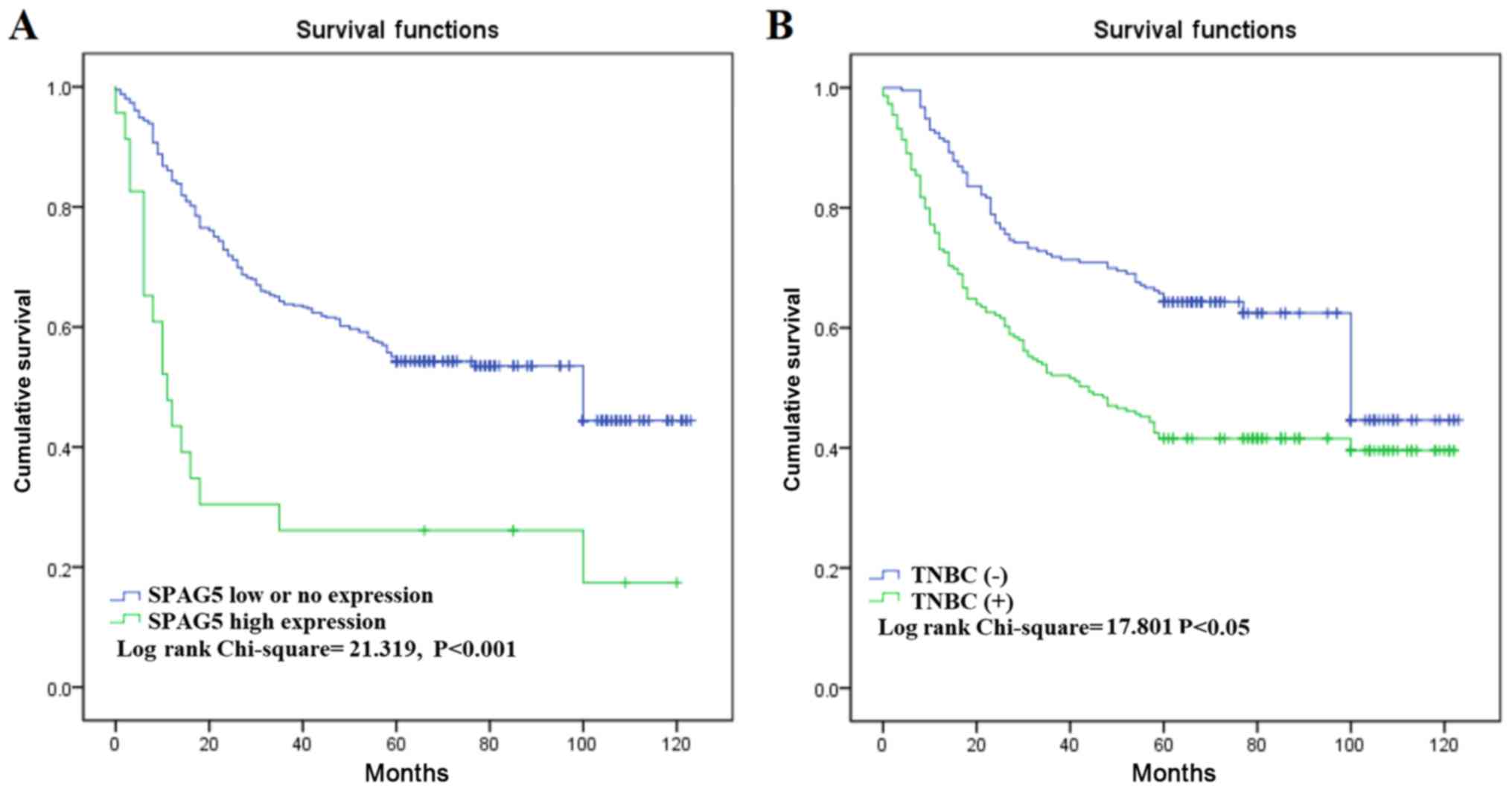
High expression of SPAG5 protein is
associated with a poor prognosis in BC
Lastly, the current study investigated the
association between SPAG5 expression and prognostic factors in BC
through univariate and multivariate analyses. High SPAG5 expression
level was associated with poor OS in univariate analysis [HR=1.956;
95% confidence interval (CI)=1.040–2.009; P<0.001). OS was also
identified to be associated with other prognostic markers,
including TNM stage (HR=10.92; 95% CI=4.109–18.723; P<0.001),
lymph node status (HR=2.192; 95% CI=1.528–2.428; P<0.001) and
TNBC (HR=3.892; 95% CI=2.829–5.628; P<0.001). Multivariate
analysis revealed that only high SPAG5 expression, molecular
classification and TNM stage were associated with poor OS (Table III). Similar results were identified
using Kaplan-Meier survival analysis; this indicated that SPAG5
high expression and the TNBC-positive subtype were significantly
associated with poor OS (Fig. 3).
 | Table III.Univariate and multivariate analysis
of prognostic markers for overall survival in breast cancer. |
Table III.
Univariate and multivariate analysis
of prognostic markers for overall survival in breast cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| SPAG5 | 1.956 | <0.001 | 1.041–2.009 | 1.523 | 0.043 | 0.323–1.934 |
| High
vs. low or none |
|
|
|
|
|
|
| Age, years |
|
|
|
|
|
|
| ≤60 vs.
>60 | 1.014 | 0.778 | 0.652–1.627 |
|
|
|
| Histological
grade |
|
|
|
|
|
|
| I vs.
II vs. III | 1.018 | 0.819 | 0.602–1.713 |
|
|
|
| ER |
|
|
|
|
|
|
|
Positive vs. negative | 1.012 | 0.969 | 0.621–1.589 |
|
|
|
| PR |
|
|
|
|
|
|
|
Positive vs. negative | 0.892 | 0.391 | 0.521–1.052 |
|
|
|
| HER2 |
|
|
|
|
|
|
|
Positive vs. negative | 0.832 | 0.437 | 0.519–1.192 |
|
|
|
| Ki-67 |
|
|
|
|
|
|
|
Positive vs. negative | 0.732 | 0.398 | 0.492–1.382 |
|
|
|
| Tumor size, cm |
|
|
|
|
|
|
| ≤2 vs.
>2 | 0.819 | 0.308 | 0.528–1.103 |
|
|
|
| Lymph node
status |
|
|
|
|
|
|
| N0 vs.
N1-3 | 10.92 | 0.000 | 4.109–18.723 | 3.829 | <0.001 | 1.292–7.303 |
| TNM stage |
|
|
|
|
|
|
| I vs.
II vs. III | 2.192 | <0.001 | 1.528–2.428 | 1.368 | 0.061 | 0.928–1.927 |
| TNBC |
|
|
|
|
|
|
|
Positive vs. negative | 3.892 | <0.001 | 2.829–5.628 | 3.923 | <0.001 | 1.802–6.193 |
Discussion
BC is regarded as a heterogeneous disease that can
be classified into several molecular subtypes with prognostic
significance (15). According to ER,
PR, HER2 and Ki-67 expression status, BC can be categorized into
four molecular subtypes, which can be used to predict outcome and
contribute to the management of BC (7). TNBC typically predicts a poor prognosis
and is characterized by no ER and PR expression, and no
overexpression of HER2 (17). The
majority of patients with TNBC have an aggressive tumor phenotype
and exhibit a partial response to chemotherapy (17). Due to the aforementioned reasons, new
targets are required for TNBC therapy.
SPAG5 serves an important role in cell meiosis and
spermatid morphogenesis (10,18). Certain studies have indicated that
knockdown of SPAG5 expression in cervical cancer cells can inhibit
cell proliferation and induce cell apoptosis, thereby decreasing
the ability of cancer cells to invade (19,20).
According to the aforementioned studies, SPAG5 is currently
considered to serve a role in promoting tumor cell growth. Cornen
et al (21) reported that
SPAG5 expression may be associated with the molecular
classification of BC and is a potential oncogene in luminal BC.
Using RT-qPCR, the current study identified SPAG5
mRNA overexpression in fresh BC tumor tissues compared with matched
adjacent nontumor tissues, particularly in TNBC tissues. A
monoclonal antibody that recognizes an epitope in the SPAG5 protein
was then used to demonstrate that SPAG5 was highly expressed in BC
tissues and was associated with histological grade, ER expression,
Ki-67 expression, lymph node status, TNM stage and TNBC subtype.
Previously, studies have demonstrated that SPAG5 is highly
expressed in BC tissues and is associated with tumor
classification, p53 mutation and the amplification of HER2
(6). The current study also revealed
that overexpression of SPAG5 predicted poor OS in patients with BC.
This is similar to findings made by Abdel-Fatah et al
(15), who reported that higher
expression of SPAG5 was associated with a poor prognosis in
patients with BC. Abdel-Fatah et al (15) reported that the role of SPAG5 could
replace Ki67 as another proliferation factor. The current study
also identified that SPAG5 protein expression was associated with
Ki67 expression. In contrast to the study by Abdel-Fatah et
al (15), the current study
analyzed the association between SPAG5 protein expression and TNBC
type, which may provide a new standard for the diagnosis of TNBC
type in the future.
In conclusion, the current study indicated that high
expression of SPAG5 may be associated with a poor prognosis in
patients with BC, which may lead to the development of useful and
effective targeted drugs for the treatment of BC.
Acknowledgments
Not applicable.
Funding
The current study was supported by the Science and
Technology Development Fund of Nanjing Medical University (grant
no. 2017NJMUZD107).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and QT designed the study; LJ, YS acquired the
data and drafted the article; LJ, XW, LX and XZ analyzed and
interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study has been approved by the Human Research
Ethics Committee of the Affiliated Hospital of Nantong University
(Nantong, China). Written informed consent was obtained from all
patients involved in the current study.
Patient consent for publication
Written informed consent was obtained from all
patients involved in the current study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beiki O, Hall P, Ekbom A and Moradi T:
Breast cancer incidence and case fatality among 4.7 million women
in relation to social and ethnic background: A population-based
cohort study. Breast Cancer Res. 14:R52012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang YL, Qian XL and Fu L: Comparison of
HER2 status of breast cancer between Chinese women in China and
Chinese-American women in the US. Breast J. 23:764–765. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Li Q, Xu B, Zhang T, Chen S and
Luo Y: Efficacy and safety of duloxetine in Chinese breast cancer
patients with paclitaxel-induced peripheral neuropathy. Chin J
Cancer Res. 29:411–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng R, Luo C, Guo Q, Cao J, Yang Q, Dong
K, Wang S, Wang K and Song C: Association analyses of genetic
variants in long non-coding RNA MALAT1 with breast cancer
susceptibility and mRNA expression of MALAT1 in Chinese Han
population. Gene. 642:241–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abrahams HJ, Gielissen MF, Schmits IC,
Verhagen CA, Rovers MM and Knoop H: Risk factors, prevalence, and
course of severe fatigue after breast cancer treatment: A
meta-analysis involving 12 327 breast cancer survivors. Ann Oncol.
27:965–974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rossing M, Østrup O, Majewski WW, Kinalis
S, Jensen MB, Knoop A, Kroman N, Talman ML, Hansen TVO, Ejlertsen B
and Nielsen FC: Molecular subtyping of breast cancer improves
identification of both high and low risk patients. Acta Oncol.
57:58–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stathopoulos GP, Malamos NA, Markopoulos
C, Polychronis A, Armakolas A, Rigatos S, Yannopoulou A, Kaparelou
M and Antoniou P: The role of Ki-67 in the proliferation and
prognosis of breast cancer molecular classification subtypes.
Anticancer Drugs. 25:950–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue J, Tarnasky HA, Rancourt DE and van
Der Hoorn FA: Targeted disruption of the testicular SPAG5/deepest
protein does not affect spermatogenesis or fertility. Mol Cell
Biol. 22:1993–1997. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao X, Xue J and van der Hoorn FA:
Testicular protein Spag5 has similarity to mitotic spindle protein
Deepest and binds outer dense fiber protein Odf1. Mol Reprod Dev.
59:410–416. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fitzgerald CJ, Oko RJ and van der Hoorn
FA: Rat Spag5 associates in somatic cells with endoplasmic
reticulum and microtubules but in spermatozoa with outer dense
fibers. Mol Reprod Dev. 73:92–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong W, Zhou Y, Li J, Mysore R, Luo W, Li
S, Chang MS, Olkkonen VM and Yan D: OSBP-related protein 8 (ORP8)
interacts with Homo sapiens sperm associated antigen 5 (SPAG5) and
mediates oxysterol interference of HepG2 cell cycle. Exp Cell Res.
322:227–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kersten FF, van Wijk E, Hetterschijt L,
Bauβ K, Peters TA, Aslanyan MG, van der Zwaag B, Wolfrum U, Keunen
JE, Roepman R and Kremer H: The mitotic spindle protein
SPAG5/Astrin connects to the Usher protein network postmitotically.
Cilia. 1:22012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan LJ, Li JD, Zhang L, Wang JH, Wan T,
Zhou Y, Tu H, Yun JP, Luo RZ, Jia WH and Zheng M: SPAG5
upregulation predicts poor prognosis in cervical cancer patients
and alters sensitivity to taxol treatment via the mTOR signaling
pathway. Cell Death Dis. 6:e17842015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdel-Fatah TMA, Agarwal D, Liu DX,
Russell R, Rueda OM, Liu K, Xu B, Moseley PM, Green AR, Pockley AG,
et al: SPAG5 as a prognostic biomarker and chemotherapy sensitivity
predictor in breast cancer: A retrospective, integrated genomic,
transcriptomic, and protein analysis. Lancet Oncol. 17:1004–1018.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stovgaard ES, Nielsen D, Hogdall E and
Balslev E: Triple negative breast cancer-prognostic role of
immune-related factors: A systematic review. Acta Oncol. 57:74–82.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kierszenbaum AL: Spermatid manchette:
Plugging proteins to zero into the sperm tail. Mol Reprod Dev.
59:347–349. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Podo F, Buydens LM, Degani H, Hilhorst R,
Klipp E, Gribbestad IS, Van Huffel S, van Laarhoven HW, Luts J,
Monleon D, et al: Triple-negative breast cancer: Present challenges
and new perspectives. Mol Oncol. 4:209–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Li S, Yang X, Qiao B, Zhang Z and
Xu Y: miR-539 inhibits prostate cancer progression by directly
targeting SPAG5. J Exp Clin Cancer Res. 35:602016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cornen S, Guille A, Adélaïde J,
Addou-Klouche L, Finetti P, Saade MR, Manai M, Carbuccia N,
Bekhouche I, Letessier A, et al: Candidate luminal B breast cancer
genes identified by genome, gene expression and DNA methylation
profiling. PLoS One. 9:e818432014. View Article : Google Scholar : PubMed/NCBI
|