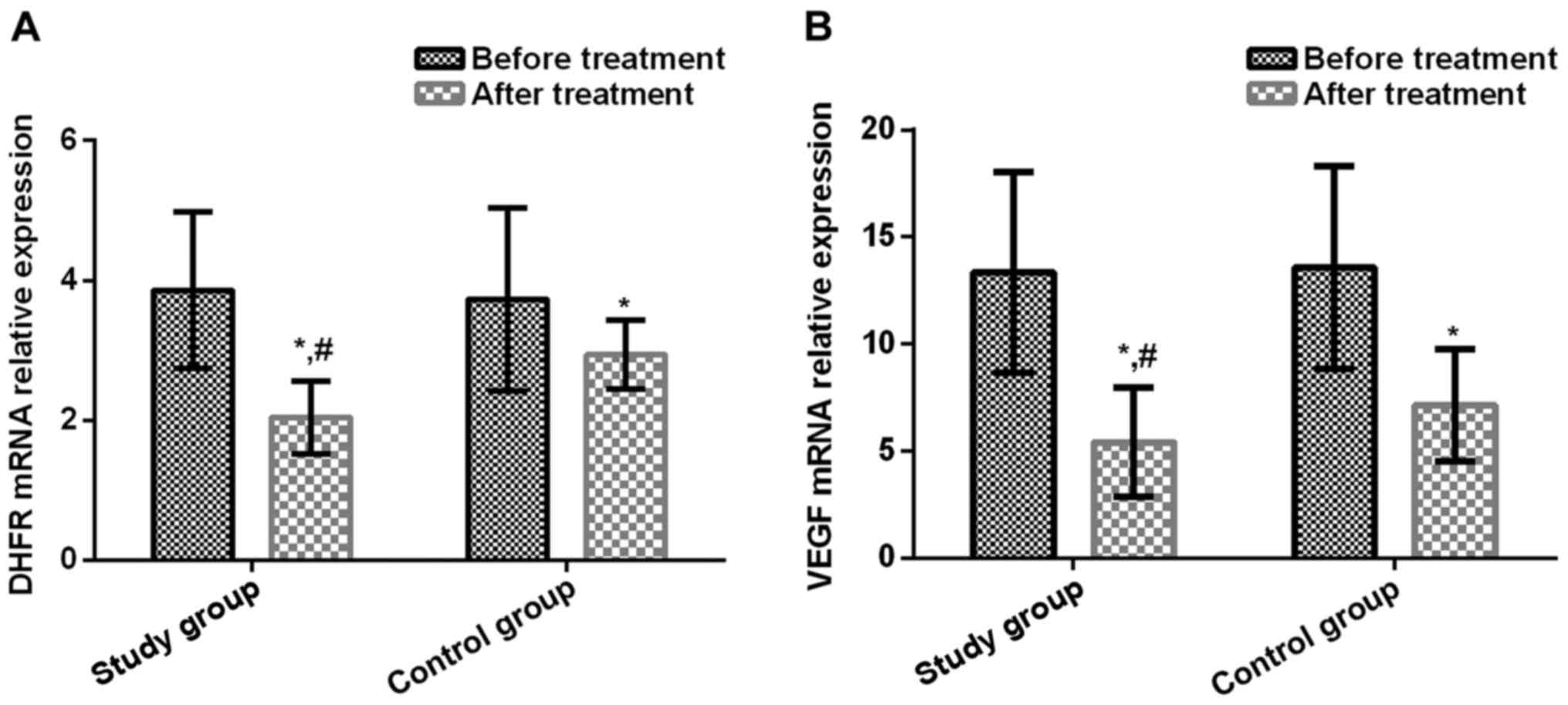
|
1
|
Alfred Witjes J, Lebret T, Compérat EM,
Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J,
Rouanne M, et al: Updated 2016 EAU guidelines on muscle-invasive
and metastatic bladder cancer. Eur Urol. 71:462–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: a
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arcangeli G, Strigari L and Arcangeli S:
Radical cystectomy versus organ-sparing trimodality treatment in
muscle-invasive bladder cancer: A systematic review of clinical
trials. Crit Rev Oncol Hematol. 95:387–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tekin A, Aki FT and Ozen H: Radical
cystectomy versus alternative treatments for muscle-confined
bladder cancer. Int Urol Nephrol. 33:357–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HS, Jeong CW, Kwak C, Kim HH and Ku
JH: Adjuvant chemotherapy for muscle-invasive bladder cancer: A
systematic review and network meta-analysis of randomized clinical
trials. Oncotarget. 8:81204–81214. 2017.PubMed/NCBI
|
|
6
|
Tanino R, Tsubata Y, Harashima N, Harada M
and Isobe T: Novel drug-resistance mechanisms of pemetrexed-treated
non-small cell lung cancer. Oncotarget. 9:16807–16821. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhushan B, Ahuja D, Verma S, Saluja S,
Siddiqui S and Kapur S: Relation of cell viability and apoptosis
with clinical remission following induction chemotherapy in ALL and
AML. J Exp Clin Cancer Res. 26:313–321. 2007.PubMed/NCBI
|
|
8
|
Depau L, Brunetti J, Falciani C, Scali S,
Riolo G, Mandarini E, Pini A and Bracci L: Coupling to a
cancer-selective heparan-sulfate-targeted branched peptide can
by-pass breast cancer cell resistance to methotrexate. Oncotarget.
8:76141–76152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchi E, Mangone M, Zullo K and O'Connor
OA: Pralatrexate pharmacology and clinical development. Clin Cancer
Res. 19:6657–6661. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nemoto A, Saida S, Kato I, Kikuchi J,
Furukawa Y, Maeda Y, Akahane K, Honna-Oshiro H, Goi K, Kagami K, et
al: Specific antileukemic activity of PD0332991, a CDK4/6
inhibitor, against Philadelphia chromosome-positive lymphoid
leukemia. Mol Cancer Ther. 15:94–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fawal MA, Jungas T, Kischel A, Audouard C,
Iacovoni JS and Davy A: Cross talk between one-carbon metabolism,
Eph signaling, and histone methylation promotes neural stem cell
differentiation. Cell Rep. 23:2864–2873.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raghunathachar Sahana K, Akila P, Prashant
V, Sharath Chandra B and Nataraj Suma M: Quantitation of vascular
endothelial growth factor and interleukin-6 in different stages of
breast cancer. Rep Biochem Mol Biol. 6:33–39. 2017.PubMed/NCBI
|
|
13
|
Chang SS, Boorjian SA, Chou R, Clark PE,
Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD,
et al: Diagnosis and treatment of non-muscle invasive bladder
cancer: AUA/SUO guideline. J Urol. 196:1021–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazza P, Moran GW, Li G, Robins DJ,
Matulay JT, Herr HW, Decastro GJ, McKiernan JM and Anderson CB:
Conservative management following clinical complete response to
neoadjuvant chemotherapy of muscle invasive bladder cancer:
Contemporary outcomes of a multi-institutional cohort study. J
Urol. May 19–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bell GC, Caudle KE, Whirl-Carrillo M,
Gordon RJ, Hikino K, Prows CA, Gaedigk A, Agundez J, Sadhasivam S,
Klein TE, et al: Clinical Pharmacogenetics Implementation
Consortium (CPIC) guideline for CYP2D6 genotype and use of
ondansetron and tropisetron. Clin Pharmacol Ther. 102:213–218.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al; OAK Study Group, . Atezolizumab versus docetaxel in
patients with previously treated non-small-cell lung cancer (OAK):
A phase 3, open-label, multicentre randomised controlled trial.
Lancet. 389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dueck AC, Mendoza TR, Mitchell SA, Reeve
BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM,
O'Mara AM, et al: Validity and reliability of the US National
Cancer Institute's patient-reported outcomes version of the common
terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol.
1:1051–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perkins JR, Dawes JM, McMahon SB, Bennett
DL, Orengo C and and Kohl M: ReadqPCR and NormqPCR: R packages for
the reading, quality checking and normalisation of RT-qPCR
quantification cycle (Cq) data. BMC Genomics. 13:2962012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mak RH, Hunt D, Shipley WU, Efstathiou JA,
Tester WJ, Hagan MP, Kaufman DS, Heney NM and Zietman AL: Long-term
outcomes in patients with muscle-invasive bladder cancer after
selective bladder-preserving combined-modality therapy: A pooled
analysis of Radiation Therapy Oncology Group protocols 8802, 8903,
9506, 9706, 9906, and 0233. J Clin Oncol. 32:3801–3809. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soave A, Riethdorf S, Dahlem R, von
Amsberg G, Minner S, Weisbach L, Engel O, Fisch M, Pantel K and
Rink M: A nonrandomized, prospective, clinical study on the impact
of circulating tumor cells on outcomes of urothelial carcinoma of
the bladder patients treated with radical cystectomy with or
without adjuvant chemotherapy. Int J Cancer. 140:381–389. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi X, Wong BL, Lau SH, Ng KT, Kwok SY,
Kin-Wai Sun C, Tzang FC, Shao Y, Li CX, Geng W, et al: A
hemoglobin-based oxygen carrier sensitized Cisplatin based
chemotherapy in hepatocellular carcinoma. Oncotarget.
8:85311–85325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Narayan V, Mamtani R, Keefe S, Guzzo T,
Malkowicz SB and Vaughn DJ: Cisplatin, gemcitabine, and lapatinib
as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer
Res Treat. 48:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cognetti F, Ruggeri EM, Felici A, Gallucci
M, Muto G, Pollera CF, Massidda B, Rubagotti A, Giannarelli D and
Boccardo F; Study Group, . Adjuvant chemotherapy with cisplatin and
gemcitabine versus chemotherapy at relapse in patients with
muscle-invasive bladder cancer submitted to radical cystectomy: An
Italian, multicenter, randomized phase III trial. Ann Oncol.
23:695–700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cipolleschi MG, Marzi I, Rovida E,
Olivotto M and Dello Sbarba P: Low-dose methotrexate enhances
cycling of highly anaplastic cancer cells. Cell Cycle. 16:280–285.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta SC, Sung B, Prasad S, Webb LJ and
Aggarwal BB: Cancer drug discovery by repurposing: Teaching new
tricks to old dogs. Trends Pharmacol Sci. 34:508–517. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Connor OA, Hamlin PA, Portlock C,
Moskowitz CH, Noy A, Straus DJ, Macgregor-Cortelli B, Neylon E,
Sarasohn D, Dumetrescu O, et al: Pralatrexate, a novel class of
antifol with high affinity for the reduced folate carrier-type 1,
produces marked complete and durable remissions in a diversity of
chemotherapy refractory cases of T-cell lymphoma. Br J Haematol.
139:425–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hydbring P, Malumbres M and Sicinski P:
Non-canonical functions of cell cycle cyclins and cyclin-dependent
kinases. Nat Rev Mol Cell Biol. 17:280–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toogood PL, Harvey PJ, Repine JT, Sheehan
DJ, VanderWel SN, Zhou H, Keller PR, McNamara DJ, Sherry D, Zhu T,
et al: Discovery of a potent and selective inhibitor of
cyclin-dependent kinase 4/6. J Med Chem. 48:2388–2406. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Knowles MA and Hurst CD: Molecular biology
of bladder cancer: New insights into pathogenesis and clinical
diversity. Nat Rev Cancer. 15:25–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen MJ, Shimada T, Moulton AD, Cline A,
Humphries RK, Maizel J and Nienhuis AW: The functional human
dihydrofolate reductase gene. J Biol Chem. 259:3933–3943.
1984.PubMed/NCBI
|
|
31
|
Banerjee D, Mayer-Kuckuk P, Capiaux G,
Budak-Alpdogan T, Gorlick R and Bertino JR: Novel aspects of
resistance to drugs targeted to dihydrofolate reductase and
thymidylate synthase. Biochim Biophys Acta. 1587:164–173. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–1338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Poyet C, Thomas L, Benoit TM, Delmo DA,
Luberto L, Banzola I, Günthart MS, Sais G, Eberli D, Sulser T, et
al: Implication of vascular endothelial growth factor A and C in
revealing diagnostic lymphangiogenic markers in node-positive
bladder cancer. Oncotarget. 8:21871–21883. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirata S, Matsubara T, Saura R, Tateishi H
and Hirohata K: Inhibition of in vitro vascular endothelial cell
proliferation and in vivo neovascularization by low-dose
methotrexate. Arthritis Rheum. 32:1065–1073. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jocham D, Witjes F, Wagner S, Zeylemaker
B, van Moorselaar J, Grimm MO, Muschter R, Popken G, König F and
Knüchel R: Improved detection and treatment of bladder cancer using
hexaminolevulinate imaging: A prospective, phase III multicenter
study. J Urol. 174:862–866. 2005. View Article : Google Scholar : PubMed/NCBI
|