In recent years, the dysregulation of Ezrin and
matrix metalloprotease-9 (MMP-9) has been determined to be an
important metastasis-associated mechanism in tumor cells of
different tissue origins (7–12). Ezrin, a 69 kDa cytoplasmic peripheral
membrane protein belonging to the Ezrin/radixin/moesin (ERM)
protein family, is an essential factor for gynecologic cancer
(13), osteosarcoma (14), rectal cancer (15,16) and
melanoma (17) metastasis. Together
with other ERM family proteins, Ezrin serves as an intermediate
between cell membrane proteins and the actin cytoskeleton (18), regulating a series of cellular
activities including cell survival, adhesion, migration and
invasion (18,19). It has been confirmed in multiple
different tumor models that Ezrin overexpression may contribute to
tumor metastasis and invasion (20–24),
potentially by inducing and promoting the EMT process (22,25–27). MMP-9
is a 92 kD matrixin (a type of enzyme), belonging to the
zinc-metalloproteinase family, which functions to degrade the
extracellular matrix (28). Mostly
secreted as inactive pro-proteins and activated following cleavage
by other extracellular proteinases, MMPs serve a role in
extracellular matrix breakdown in certain physiological processes,
including embryonic development, angiogenesis, wound healing and
cell migration, as well as in some pathological progressions,
including cancer cell invasion and metastasis (29). Emerging evidence has indicated that
the overexpression or hyper-activation of MMP-9 contributes to
tumor metastasis by facilitating tumor cell migration and invasion
in different models (10,30,31).
Recently, abnormal changes in microRNA (miRNA or
miR) levels have been proposed to be a primary mechanism for the
dysregulation of effector proteins associated with tumor
development (32–36). MiRNAs are small non-coding RNAs that
serve a function in gene silencing via sequence-specific base
pairing with the 3′ or 5′ untranslated regions of target mRNAs
(37). This results in mRNA
destabilization and degradation, thus serving as an important
post-transcriptional regulatory mechanism (37). The dysregulation or loss of function
of certain key regulatory miRNAs may therefore contribute to tumor
development via the subsequential dysregulation of tumor suppressor
proteins or oncoproteins (38).
Several studies have indicated that a change in human miR-183 level
is closely associated with tumor development (39–44).
However, the influence of miR-183 expression change on melanoma is
yet to be fully elucidated.
Previous studies have demonstrated that Ezrin and
MMP-9 are direct targets of miR-183 regulation, such that a
decrease of miR-183 causes the upregulation of Ezrin and MMP-9,
which leads to the facilitated migration and invasion of tumor
cells with different tissue origins (45–51). To
assess the impact of miR-183 dysregulation in melanoma, the present
study constructed an A375 human melanoma cell line with stable
miR-183 overexpression or knockdown via lentiviral transfection
with an miR-183 mimic or inhibitor, respectively. Changes in
miR-183 expression in the miR-183 mimic, inhibitor and control
group was verified using a reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay following total RNA
isolation. Furthermore, the impact of miR-183 on the migration and
invasion of A375 cells was evaluated using a scratch and Transwell
assay, respectively. Ezrin and MMP-9 levels were analyzed using
western blotting in each group. The results indicated that miR-183
functions as a tumor suppressor in melanoma by inhibiting tumor
cell migration and invasion via the downregulation of Ezrin and
MMP-9 protein and the deregulation of miR-183. Therefore, miR-183
may be an important factor for melanoma metastasis and development.
These results may provide novel insights into the molecular
mechanism of melanoma progression and may also elucidate a target
for the development of novel gene therapy against melanoma and
other metastatic tumors.
A375 cells (Chinese Academy of Sciences Cell Bank,
Beijing, China) were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Biological Industries, Kibbutz Beit Haemek,
Israel) and maintained at 37°C in a humidified incubator with 5%
CO2, unless otherwise indicated.
The sequence of miR-183 is
5′-GUGAAUUACCGAAGGGCCAUAA-3′. MiR-183 overexpression and knockdown
in A375 human melanoma cells was achieved using an miR-183 mimic,
an miRNA mimic negative control (NC) [an insignificant short
nucleotide sequence], an miR-183 inhibitor, an miRNA inhibitor NC
and blank plasmids were all obtained from Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China) following the manufacturer's protocol.
Following transfection miR-183 (250 µg/ml) for 12 h by
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol, total RNA from each cell group was
isolated via TRIzol-chloroform-isopropanol (TaKaRa, Bio, Inc.,
Otsu, Japan) extraction and miR-183 mRNA expression was assessed
using RT-qPCR analysis with an All-in-One™ miRNA RT-qPCR detection
kit (GeneCopoeia, Inc., Rockville, MD, USA) following the
manufacturer's protocol. The sequences of miR-183 primers, forward:
5′-CGCGGTATGGCACTGGTAGA-3′, and reverse:
5′-AGTGCAGGGTCCGAGGTATTC-3′. MiR-183 expression levels were
normalized to levels of U6 snRNA (5′-AAATTCGTGAAGCGTTCC-3′) using
the 2−ΔΔCq method (52).
Detection was achieved by SYBR green qPCR with the following
conditions: 95°C for 10 min followed by 40 cycles of 95°C for 10
sec, 60°C for 20 sec and 72°C for 20 sec.
A375 cells transfected with the miR-183 mimic, mimic
NC, miR-183 inhibitor or inhibitor NC plasmid were cultured (37°C)
overnight into confluent monolayers. Scratches were generated
gently in each cell culture using a sterile 200 µl pipette tip.
Cell migration towards the mid-line of the scratch was monitored at
0 and 24 h using a light microscope at ×100 magnification.
Following pre-culture in serum-free RPMI-1640 medium
for 24 h at 37°C, A375 cells with the aforementioned transfections
were trypsinized, re-suspended in serum-free RPMI-1640 medium and
diluted to a density of ~10,600 cells/ml. Cells (100 µl of each
group) were then seeded in the inner chamber membrane of the
Transwell apparatus. For the assessment of invasion activity, the
inner chamber was coated with fibronectin under serum-free
conditions and the uncoated inner chamber was used for the
evaluation of migration activity. RPMI-1640 medium supplemented
with 50% fetal bovine serum was added in the lower chamber of the
Transwell apparatus in each group and cells were cultured for 24 h
at 37°C in a humidified incubator with 5% CO2. A549
cells on the lower surface of the inner chamber were then gently
rinsed with PBS and stained with crystal violet staining solution
at room temperature for 10 min (Beyotime Institute of
Biotechnology, Haimen, China) prior to being counted using light
microscopy at ×200 magnification. Five randomly selected fields of
view were selected.
A375 cells that underwent the aforementioned
transfections were cultured in 6-well plates under normal culture
conditions (37°C for 24 h). A375 cells were homogenized in lysis
buffer [10 mM Tris base, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA,
1% Triton X-100, 0.5% NP-40, and a protease and phosphatase
inhibitor cocktail (Thermo Fisher Scientific, Inc.)]. Protein
concentrations were determined using a BCA assay kit (Thermo Fisher
Scientific, Inc.) with bovine serum albumin (2 mg/ml) as a
standard. The total protein of each cell group (20 µl) was
separated using polyacrylamide gel (15%) electrophoresis under
reducing conditions, followed by transferral onto nitrocellulose
membranes. Following blocking at 4°C overnight with non-fat milk
and preparation with PBS, membranes were probed with antibodies
(incubated 16 h at 4°C) targeting human Ezrin (cat. no. ab40839;
Abcam, Cambridge, UK; dilution of 1:2,000) and MMP-9 (cat. no.
ab76003; Abcam; dilution of 1:1,000), with β-actin (cat. no.
ab8227; Abcam; dilution of 1:2,000) as an internal reference. The
membranes were then incubated with horseradish peroxidase
conjugated secondary antibodies (dilution of 1:5,000, incubated 1 h
at room temperature) (Goat Anti-Rabbit IgG H&L; cat. no.
ab6702; Abcam) and colorized using an ECL substrate (Thermo Fisher
Scientific, Inc.). The image of each sample was captured using
chemo-fluorescence-sensitive film and the gray value of each band
was analyzed. The quantity of target protein was evaluated by the
ratio of its gray value to the internal reference using ImageJ
software, version 1.48 (National Institutes of Health, Bethesda,
MD, USA).
All experiments were independently repeated at least
three times. Statistical analysis was performed using SPSS
statistical software, version 17.0 (SPSS, Inc., Chicago, IL, USA).
All statistical data were presented as the mean ± standard
deviation. A Student's t-test was performed to assess significance
and P<0.05 was considered to indicate a statistically
significant difference.
To determine the influence of increased and
decreased miR-183 expression in human melanoma, A375 cells were
transfected with miR-183 mimic and miR-183 inhibitor sequences,
which served as the experimental groups. MiRNA mimic and inhibitor
NC sequences were utilized as internal reference groups, using a
lentiviral plasmid transfection system. A375 cells that were
non-transfected and transfected with blank plasmids were used as
external references. MiR-183 expression in each group 12 h
following transfection was analyzed using RT-qPCR following total
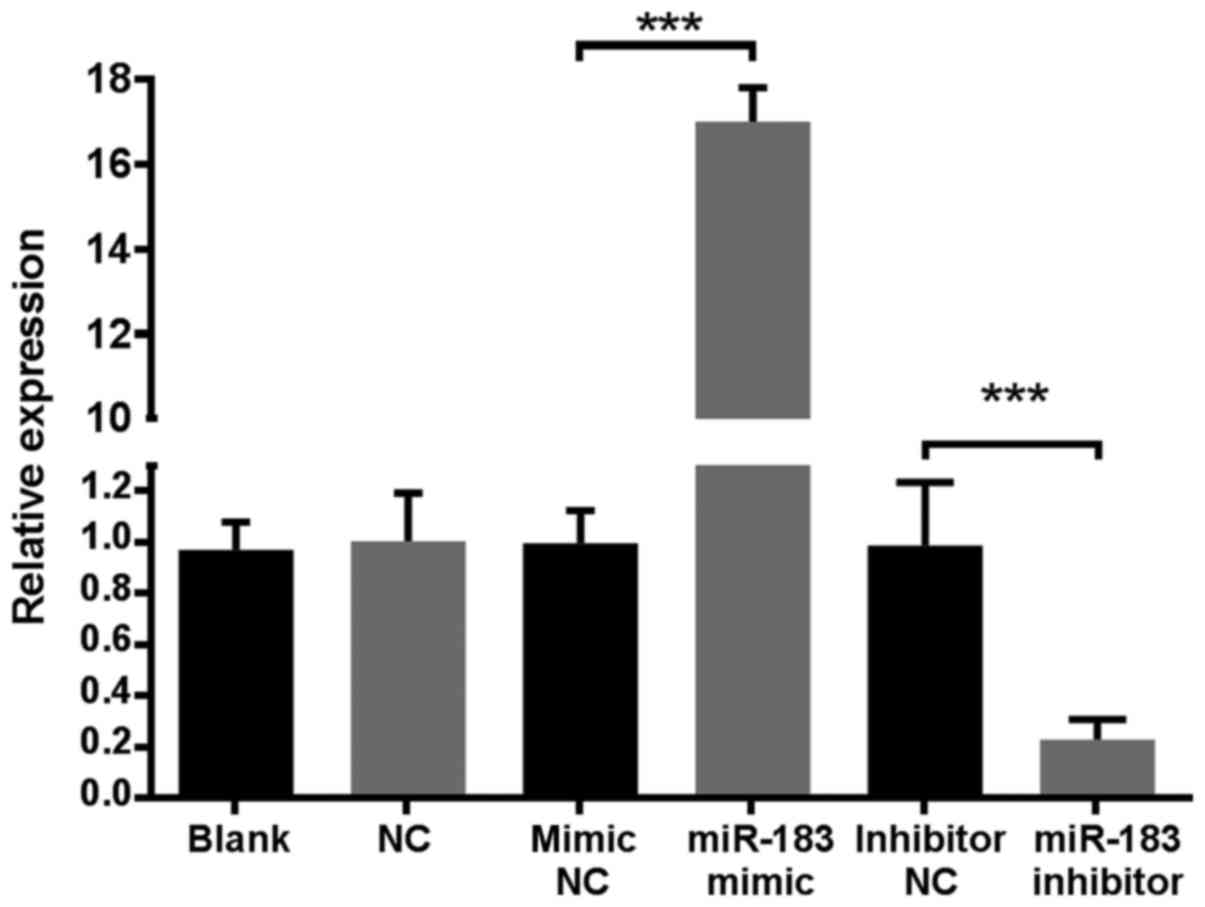
RNA isolation. The results demonstrated that the expression of
miR-183 in the miR-183 mimic group was >16-fold higher compared
with all control groups, while the miR-183 inhibitor group was
5-fold lower compared with control groups (Fig. 1). These results indicated that the
established cell line was stable. MiR-183 levels in the mimic and
inhibitor NC groups were similar to those of the blank and NC
groups, indicating that transfection alone did not affect miR-183
expression. Additionally, no notable morphological changes were
observed among A375 cells in the blank, NC, mimic NC and inhibitor
NC groups (data not shown). Therefore, A375 cells in mimic NC and
inhibitor NC groups alone were utilized as NCs in future
experiments.
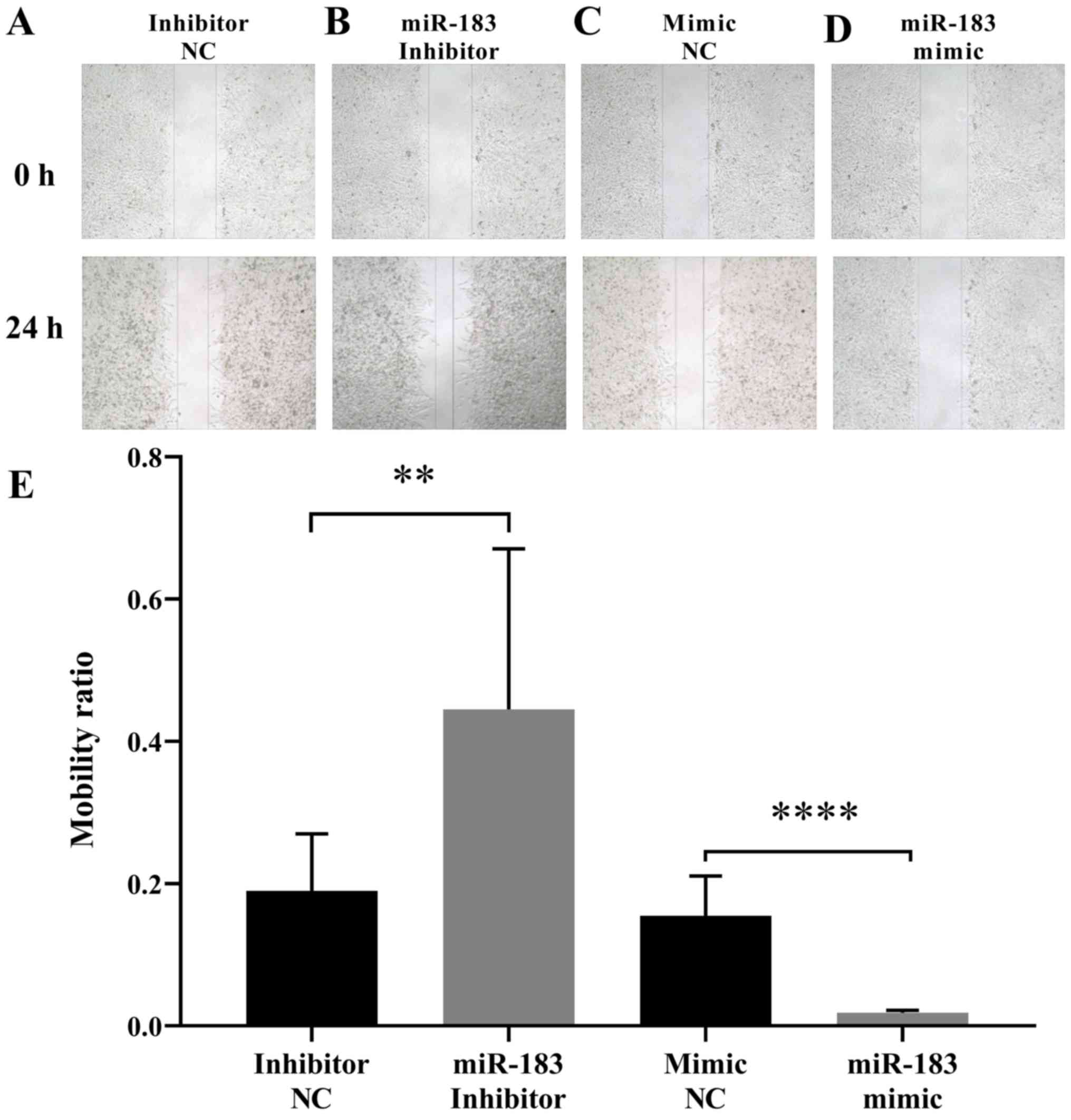
To assess the influence of miR-183 expression
changes on A375 cell migration, a scratch assay was performed as
aforementioned. The results demonstrated that the artificial
upregulation of miR-183 expression significantly suppressed A375
cell migration, whereas miR-183 downregulation promoted A375 cell
migration when compared with their respective controls (Fig. 2). This indicated that miR-183 inhibits
the migration of A375 cells in vitro. To further evaluate
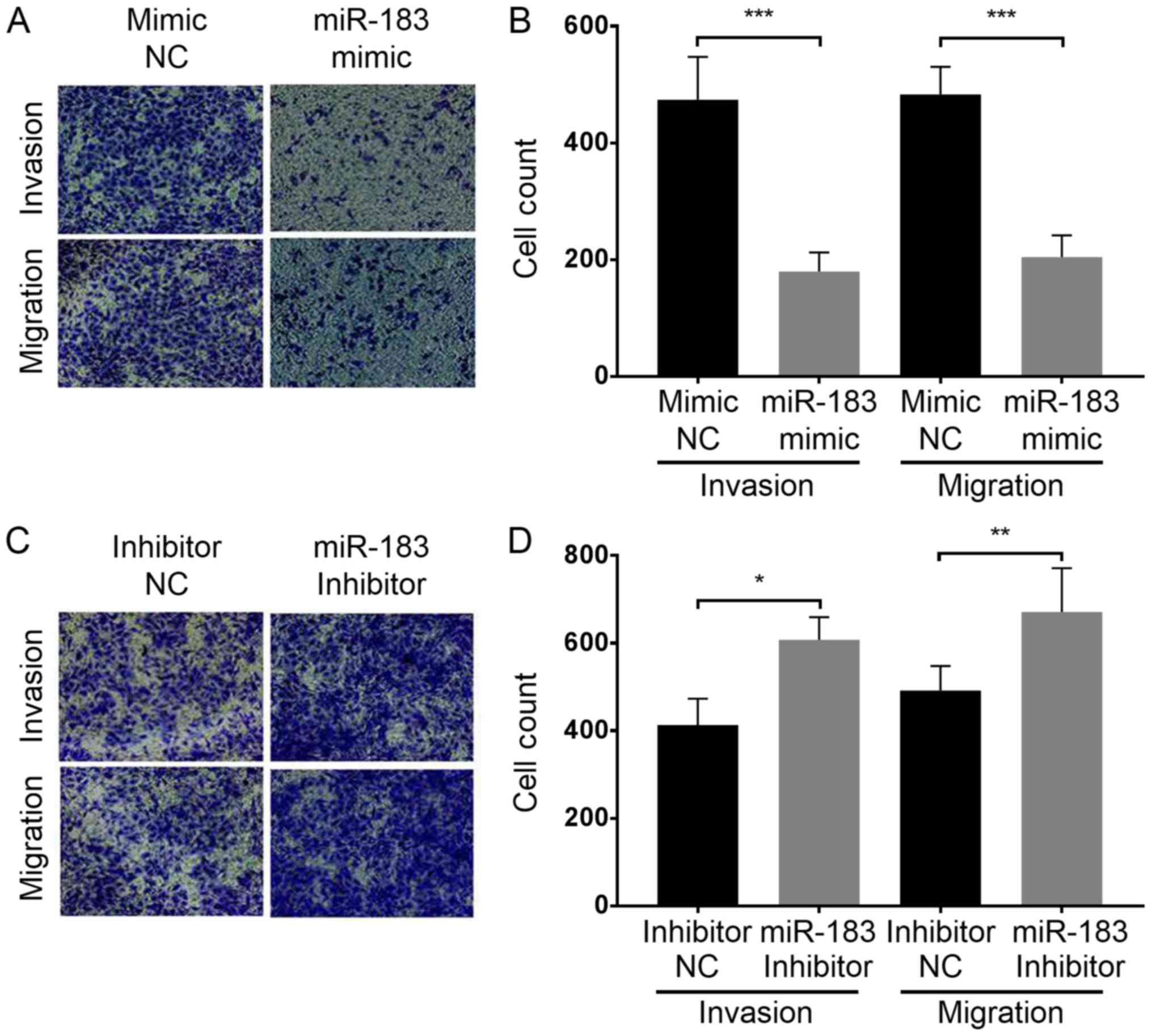
the influence of miR-183 expression change on A375 cell migration
and invasion, a Transwell assay was performed with each group of
cells as aforementioned. The results demonstrated that miR-183
overexpression in the miR-183 mimic group significantly inhibited
the migration and invasion of A375 cells in vitro compared
with the mimic NC group (Fig. 3A and
B). Furthermore, miR-183 knockdown in the inhibitor group
promoted A375 cell migration and invasion compared with the
inhibitor NC group (Fig. 3C and D).
Taken together, this indicates that miR-183 suppresses A375 cell
migration and invasion in vitro, and migration and invasion
are increased by the downregulation of miR-183.
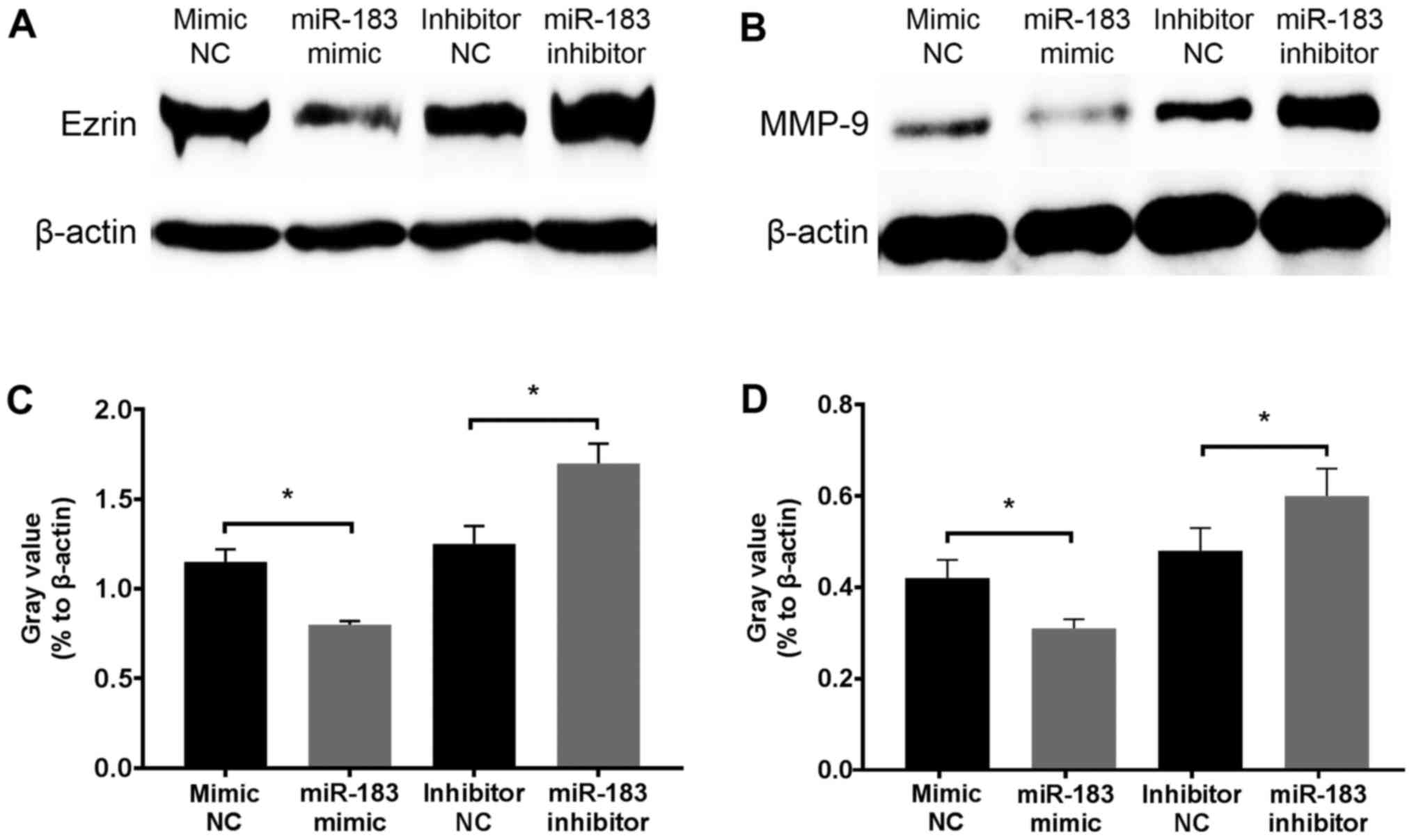
To determine the inhibitory mechanism of miR-183,
changes in Ezrin and MMP-9 protein expression in A375 cells were
assessed at 12 h following transfection with an miR-183 mimic or
inhibitor. Western blotting revealed that Ezrin and MMP-9 were
downregulated and upregulated following miR-183 overexpression and
knockdown in vitro, respectively (Fig. 4). Considering the well-established
association of Ezrin and MMP-9 with the migratory and invasive
activity of melanoma, and that these proteins act as direct targets
of miR-183 (45,46,49,53), the
results demonstrated that miR-183 inhibits A375 cell migration and
invasion potentially via the downregulation of Ezrin and MMP-9
expression.
The results of the present study support the theory
that miR-183 may serve as a tumor suppressor in melanoma. The
migratory activity and invasiveness of A375 human melanoma cells
was demonstrated to be negatively associated with miR-183
expression. Furthermore, Ezrin and MMP-9, which have been
previously regarded as important promoters of melanoma metastasis
(54–59), were revealed to be negatively
regulated by miR-183, indicating that miR-183 inhibits A375 human
melanoma cell migration and invasion, possibly through the
downregulation of Ezrin and MMP-9 expression. Thus, the abnormal
decrease of miR-183 in melanoma may cause Ezrin and MMP-9
overexpression, resulting in an increased metastatic activity.
Several studies have revealed that miR-183 may exhibit either pro-
or anti-metastatic functions in different tumor cells (60–65). More
specifically, miR-183 has been considered to promote metastasis in
most tumor models, including breast cancer (66), medulloblastoma (67), hepatocellular carcinoma (68), follicular thyroid carcinoma (43), esophageal cancer (69), gastric cancer (70), pancreatic cancer (71) and synovial sarcoma (72), while inhibiting metastasis in lung
(73), colon (74) and ovarian cancer (75). This is possibly due to the
heterogeneity of cancer and the differences in ontogenesis of
different types of cancer.
MiR-183 is located and transcribed in cluster with
miR-96 and miR-182, which exerts various functions (40). It regulates multiple mRNA targets;
however, not all targets have been fully elucidated (38). Further studies are thus required to
assess the transcriptional regulation of the miR-183-96-182 cluster
under tumor pathological conditions. Considering the complexity of
cytokinetic regulation, proteomic methods that assess the function
and regulatory network of miR-183 target proteins, and particularly
their interaction with cytoskeleton regulators and integrins, which
interact with the extracellular matrix, are being employed
(42). However, further studies are
required to obtain a better understanding of the regulatory
mechanism of miR-183. This may provide novel insights into the
development of anti-metastasis gene therapy.
Not applicable.
No funding was received.
All data generated or analyzed during this study are
included in this published article.
YSZ and GQW completed all the experiments and the
statistical analysis. YSZ wrote and revised the manuscript. All
authors read and approved the final the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Jemal A, Ward EM, Johnson CJ, Cronin KA,
Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, et al:
Annual report to the nation on the status of cancer, 1975-2014,
featuring survival. J Natl Cancer Inst. 109:2017. View Article : Google Scholar
|
|
2
|
Nikolaou V and Stratigos AJ: Emerging
trends in the epidemiology of melanoma. Br J Dermatol. 170:11–19.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Damsky WE, Theodosakis N and Bosenberg M:
Melanoma metastasis: New concepts and evolving paradigms. Oncogene.
33:2413–2422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zbytek B, Carlson JA, Granese J, Ross J,
Mihm MC Jr and Slominski A: Current concepts of metastasis in
melanoma. Expert Rev Dermatol. 3:569–585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bravo-Cordero JJ, Hodgson L and Condeelis
J: Directed cell invasion and migration during metastasis. Curr
Opin Cell Biol. 24:277–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Long ZY and Wang TH: Advances of the role
of Ezrin in migration and invasion of breast cancer cells. Sheng Li
Ke Xue Jin Zhan. 47:21–26. 2016.(In Chinese). PubMed/NCBI
|
|
8
|
Liu HY, Gu WJ, Wang CZ, Ji XJ and Mu YM:
Matrix metalloproteinase-9 and −2 and tissue inhibitor of matrix
metalloproteinase-2 in invasive pituitary adenomas: A systematic
review and meta-analysis of case-control trials. Medicine.
95:e39042016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Candido S, Abrams SL, Steelman LS,
Lertpiriyapong K, Fitzgerald TL, Martelli AM, Cocco L, Montalto G,
Cervello M, Polesel J, et al: Roles of NGAL and MMP-9 in the tumor
microenvironment and sensitivity to targeted therapy. Biochim
Biophys Acta. 1863:438–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pârvănescu V, Georgescu M, Georgescu I,
Șurlin V, Pătraşcu Ș, Picleanu AM and Georgescu E: The role of
matrix metalloproteinase-9 (MMP-9) as a prognostic factor in
epithelial and lymphatic neoplasia. Chirurgia. 110:506–510.
2015.PubMed/NCBI
|
|
11
|
Li J, Wei K, Yu H, Jin D, Wang G and Yu B:
Prognostic value of Ezrin in various cancers: A systematic review
and updated meta-analysis. Sci Rep. 5:179032015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao DH, Zhu J, Wang WB, Dong F, Zhang Q,
Fan HW, Zhang JZ and Wang YM: Correlations of ezrin expression with
pathological characteristics and prognosis of osteosarcoma: A
meta-analysis. ScientificWorldJournal. 2014:8375432014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi SD, Fadiel A and Naftolin F: Erratum
to: Ezrin is an essential marker for metastasis of gynecologic
cancer. J Menopausal Med. 22:1882016. View Article : Google Scholar
|
|
14
|
Khanna C, Wan X, Bose S, Cassaday R, Olomu
O, Mendoza A, Yeung C, Gorlick R, Hewitt SM and Helman LJ: The
membrane-cytoskeleton linker ezrin is necessary for osteosarcoma
metastasis. Nat Med. 10:182–186. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Korkeila EA, Syrjänen K, Bendardaf R,
Laulajainen M, Carpén O, Pyrhönen S and Sundström J: Preoperative
radiotherapy modulates ezrin expression and its value as a
predictive marker in patients with rectal cancer. Hum Pathol.
42:384–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patara M, Santos EM, de Almeida Coudry R,
Soares FA, Ferreira FO and Rossi BM: Ezrin expression as a
prognostic marker in colorectal adenocarcinoma. Pathol Oncol Res.
17:827–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ilmonen S, Vaheri A, Asko-Seljavaara S and
Carpen O: Ezrin in primary cutaneous melanoma. Mod Pathol.
18:503–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bretscher A, Edwards K and Fehon RG: ERM
proteins and merlin: Integrators at the cell cortex. Nat Rev Mol
Cell Biol. 3:586–599. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
McClatchey AI: Merlin and ERM proteins:
Unappreciated roles in cancer development? Nat Rev Cancer.
3:877–883. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Feng YM and Fang SQ: Overexpression
of ezrin and galectin-3 as predictors of poor prognosis of cervical
cancer. Braz J Med Biol Res. 50:e53562017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horwitz V, Davidson B, Stern D, Trope CG,
Tavor Re'em T and Reich R: Ezrin is associated with disease
progression in ovarian carcinoma. PLoS One. 11:e01625022016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong J, Di C, Piao J, Sun J, Han L, Chen
L, Yan G and Lin Z: Ezrin contributes to cervical cancer
progression through induction of epithelial-mesenchymal transition.
Oncotarget. 7:19631–19642. 2016.PubMed/NCBI
|
|
23
|
McRobert EA and Bach LA: Ezrin contributes
to impaired podocyte migration and adhesion caused by advanced
glycation end products. Nephrology. 21:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piao J and Liu S, Xu Y, Wang C, Lin Z, Qin
Y and Liu S: Ezrin protein overexpression predicts the poor
prognosis of pancreatic ductal adenocarcinomas. Exp Mol Pathol.
98:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Lin Z, Chen B, Chen S, Jiang Z, Zhou
T, Hou Z and Wang Y: Ezrin/NF-κB activation regulates
epithelial-mesenchymal transition induced by EGF and promotes
metastasis of colorectal cancer. Biomed Pharmacother. 92:140–148.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He J, Ma G, Qian J, Zhu Y, Liang M, Yao N,
Ding Q, Chen L, Liu X, Xia T, et al: Interaction between ezrin and
cortactin in promoting epithelial to mesenchymal transition in
breast cancer cells. Med Sci Monit. 23:1583–1596. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lan M, Kojima T, Murata M, Osanai M,
Takano K, Chiba H and Sawada N: Phosphorylation of ezrin enhances
microvillus length via a p38 MAP-kinase pathway in an immortalized
mouse hepatic cell line. Exp Cell Res. 312:111–120. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dang B, Duan X, Wang Z, He W and Chen G: A
therapeutic target of cerebral hemorrhagic stroke: Matrix
metalloproteinase-9. Curr Drug Targets. 18:1358–1366. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boziki M and Grigoriadis N: An update on
the role of matrix metalloproteinases in the pathogenesis of
multiple sclerosis. Med Chem. 14:155–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Banday MZ, Sameer AS, Mir AH, Mokhdomi TA,
Chowdri NA and Haq E: Matrix metalloproteinase (MMP) −2, −7 and −9
promoter polymorphisms in colorectal cancer in ethnic Kashmiri
population - A case-control study and a mini review. Gene.
589:81–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gong L, Wu D, Zou J, Chen J, Chen L, Chen
Y, Ni C and Yuan H: Prognostic impact of serum and tissue MMP-9 in
non-small cell lung cancer: A systematic review and meta-analysis.
Oncotarget. 7:18458–18468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yonemori K, Kurahara H, Maemura K and
Natsugoe S: MicroRNA in pancreatic cancer. J Hum Genet. 62:33–40.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xue J, Yang J, Luo M, Cho WC and Liu X:
MicroRNA-targeted therapeutics for lung cancer treatment. Expert
Opin Drug Discov. 12:141–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manasa VG and Kannan S: Impact of microRNA
dynamics on cancer hallmarks: An oral cancer scenario. Tumour Biol.
39:10104283176959202017.doi: 10.1177/1010428317695920. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Latchana N, Ganju A, Howard JH and Carson
WE III: MicroRNA dysregulation in melanoma. Surg Oncol. 25:184–189.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kanekura K, Nishi H, Isaka K and Kuroda M:
MicroRNA and gynecologic cancers. J Obstet Gynaecol Res.
42:612–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
D'Angelo B, Benedetti E, Cimini A and
Giordano A: MicroRNAs: A puzzling tool in cancer diagnostics and
therapy. Anticancer Res. 36:5571–5575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lima CR, Gomes CC and Santos MF: Role of
microRNAs in endocrine cancer metastasis. Mol Cell Endocrinol.
456:62–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moridikia A, Mirzaei H, Sahebkar A and
Salimian J: MicroRNAs: Potential candidates for diagnosis and
treatment of colorectal cancer. J Cell Physiol. 233:901–913. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma Y, Liang AJ, Fan YP, Huang YR, Zhao XM,
Sun Y and Chen XF: Dysregulation and functional roles of
miR-183-96-182 cluster in cancer cell proliferation, invasion and
metastasis. Oncotarget. 7:42805–42825. 2016.PubMed/NCBI
|
|
41
|
Shimono Y, Mukohyama J, Nakamura S and
Minami H: MicroRNA regulation of human breast cancer stem cells. J
Clin Med. 5(pii): E22015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dambal S, Shah M, Mihelich B and Nonn L:
The microRNA-183 cluster: The family that plays together stays
together. Nucleic Acids Res. 43:7173–7188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wojtas B, Ferraz C, Stokowy T, Hauptmann
S, Lange D, Dralle H, Musholt T, Jarzab B, Paschke R and Eszlinger
M: Differential miRNA expression defines migration and reduced
apoptosis in follicular thyroid carcinomas. Mol Cell Endocrinol.
388:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang QH, Sun HM, Zheng RZ, Li YC, Zhang
Q, Cheng P, Tang ZH and Huang F: Meta-analysis of microRNA-183
family expression in human cancer studies comparing cancer tissues
with noncancerous tissues. Gene. 527:26–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ruan H, Liang X, Zhao W, Ma L and Zhao Y:
The effects of microRNA-183 promots cell proliferation and invasion
by targeting MMP-9 in endometrial cancer. Biomed Pharmacother.
89:812–818. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan D, Wang Y, Qi P, Chen Y, Xu P, Yang X,
Jin X and Tian X: MicroRNA-183 functions as the tumor suppressor
via inhibiting cellular invasion and metastasis by targeting MMP-9
in cervical cancer. Gynecol Oncol. 141:166–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Zuo J, Lei M, Wu S, Zang X and
Zhang C: Ezrin promotes invasion and migration of the MG63
osteosarcoma cell. Chin Med J. 127:1954–1959. 2014.PubMed/NCBI
|
|
48
|
Mu Y, Zhang H, Che L and Li K: Clinical
significance of microRNA-183/Ezrin axis in judging the prognosis of
patients with osteosarcoma. Med Oncol. 31:8212014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cao LL, Xie JW, Lin Y, Zheng CH, Li P,
Wang JB, Lin JX, Lu J, Chen QY and Huang CM: miR-183 inhibits
invasion of gastric cancer by targeting Ezrin. Int J Clin Exp
Pathol. 7:5582–5594. 2014.PubMed/NCBI
|
|
50
|
Zhu J, Feng Y, Ke Z, Yang Z, Zhou J, Huang
X and Wang L: Down-regulation of miR-183 promotes migration and
invasion of osteosarcoma by targeting Ezrin. Am J Pathol.
180:2440–2451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao H, Guo M, Zhao G, Ma Q, Ma B, Qiu X
and Fan Q: miR-183 inhibits the metastasis of osteosarcoma via
downregulation of the expression of Ezrin in F5M2 cells. Int J Mol
Med. 30:1013–1020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang G, Mao W and Zheng S: MicroRNA-183
regulates Ezrin expression in lung cancer cells. FEBS Lett.
582:3663–3668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hsu YY, Shi GY, Kuo CH, Liu SL, Wu CM, Ma
CY, Lin FY, Yang HY and Wu HL: Thrombomodulin is an
ezrin-interacting protein that controls epithelial morphology and
promotes collective cell migration. FASEB J. 26:3440–3452. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brambilla D and Fais S: The Janus-faced
role of ezrin in ‘linking’ cells to either normal or metastatic
phenotype. Int J Cancer. 125:2239–2245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Federici C, Brambilla D, Lozupone F,
Matarrese P, de Milito A, Lugini L, Iessi E, Cecchetti S, Marino M,
Perdicchio M, et al: Pleiotropic function of ezrin in human
metastatic melanomas. Int J Cancer. 124:2804–2812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim A, Im M, Yim NH and Ma JY: Reduction
of metastatic and angiogenic potency of malignant cancer by
Eupatorium fortunei via suppression of MMP-9 activity and VEGF
production. Sci Rep. 4:69942014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee KR, Lee JS, Kim YR, Song IG and Hong
EK: Polysaccharide from Inonotus obliquus inhibits migration and
invasion in B16-F10 cells by suppressing MMP-2 and MMP-9 via
downregulation of NF-κB signaling pathway. Oncol Rep. 31:2447–2453.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tang ZY, Liu Y, Liu LX, Ding XY, Zhang H
and Fang LQ: RNAi-mediated MMP-9 silencing inhibits mouse melanoma
cell invasion and migration in vitro and in vivo. Cell Biol Int.
37:849–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cheung CC, Lun SW, Chung GT, Chow C, Lo C,
Choy KW and Lo KW: MicroRNA-183 suppresses cancer stem-like cell
properties in EBV-associated nasopharyngeal carcinoma. BMC Cancer.
16:4952016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Song C, Zhang L, Wang J, Huang Z, Li X, Wu
M, Li S, Tang H and Xie X: High expression of microRNA-183/182/96
cluster as a prognostic biomarker for breast cancer. Sci Rep.
6:245022016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhu C, Deng X, Wu J, Zhang J, Yang H, Fu
S, Zhang Y, Han Y, Zou Y, Chen Z, et al: MicroRNA-183 promotes
migration and invasion of CD133+/CD326+ lung adenocarcinoma
initiating cells via PTPN4 inhibition. Tumour Biol. 37:11289–11297.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Miao F, Zhu J, Chen Y, Tang N, Wang X and
Li X: MicroRNA-183-5p promotes the proliferation, invasion and
metastasis of human pancreatic adenocarcinoma cells. Oncol Lett.
11:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xu F, Zhang H, Su Y, Kong J, Yu H and Qian
B: Up-regulation of microRNA-183-3p is a potent prognostic marker
for lung adenocarcinoma of female non-smokers. Clin Transl Oncol.
16:980–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhou T, Zhang GJ, Zhou H, Xiao HX and Li
Y: Overexpression of microRNA-183 in human colorectal cancer and
its clinical significance. Eur J Gastroenterol Hepatol. 26:229–233.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li P, Sheng C, Huang L, Zhang H, Huang L,
Cheng Z and Zhu Q: MiR-183/-96/-182 cluster is up-regulated in most
breast cancers and increases cell proliferation and migration.
Breast Cancer Res. 16:4732014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Weeraratne SD, Amani V, Teider N,
Pierre-Francois J, Winter D, Kye MJ, Sengupta S, Archer T, Remke M,
Bai AH, et al: Pleiotropic effects of miR-183~96~182 converge to
regulate cell survival, proliferation and migration in
medulloblastoma. Acta Neuropathol. 123:539–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li ZB, Li ZZ, Li L, Chu HT and Jia M:
MiR-21 and miR-183 can simultaneously target SOCS6 and modulate
growth and invasion of hepatocellular carcinoma (HCC) cells. Eur
Rev Med Pharmacol Sci. 19:3208–3217. 2015.PubMed/NCBI
|
|
69
|
Yang M, Liu R, Li X, Liao J, Pu Y, Pan E,
Yin L and Wang Y: miRNA-183 suppresses apoptosis and promotes
proliferation in esophageal cancer by targeting PDCD4. Mol Cells.
37:873–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li C, Deng L, Zhi Q, Meng Q, Qian A, Sang
H, Li X and Xia J: MicroRNA-183 functions as an oncogene by
regulating PDCD4 in gastric cancer. Anticancer Agents Med Chem.
16:447–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang YY and Feng HM: MEG3 suppresses
human pancreatic neuroendocrine tumor cells growth and metastasis
by down-regulation of Mir-183. Cell Physiol Biochem. 44:345–356.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sarver AL, Li L and Subramanian S:
MicroRNA miR-183 functions as an oncogene by targeting the
transcription factor EGR1 and promoting tumor cell migration.
Cancer Res. 70:9570–9580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yang CL, Zheng XL, Ye K, Ge H, Sun YN, Lu
YF and Fan QX: MicroRNA-183 acts as a tumor suppressor in human
non-small cell lung cancer by down-regulating MTA1. Cell Physiol
Biochem. 46:93–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gao P, He M, Zhang C and Geng C:
Integrated analysis of gene expression signatures associated with
colon cancer from three datasets. Gene. 654–695. 2018.
|
|
75
|
Li J, Liang S, Jin H, Xu C, Ma D and Lu X:
Tiam1, negatively regulated by miR-22, miR-183 and miR-31, is
involved in migration, invasion and viability of ovarian cancer
cells. Oncol Rep. 27:18352012.PubMed/NCBI
|