Introduction
Genomic analysis is an important tool used in the
development of therapies against cancer. Current therapies for
cancer often fail, which eventually leads to a high mortality rate.
A study by Kim et al (2010) revealed that distinct subtypes
of breast cancer exhibit different sensitivities to systemic
chemotherapy (1). Causative gene
mutations may also affect the sensitivity of cancer cells towards
certain chemotherapeutic drugs (2),
and so developing genomic biomarkers specific to each cancer
subtype is essential for screening, diagnosis, predicting patient
prognosis and selecting effective cancer treatments (2,3).
S100A10, a member of the S100 proteins family, forms
a homodimer comprising two EF-hand motifs; an N-terminal
S100-specific EF hand and a C-terminal canonical EF hand, linked by
a hinge region known as the Ca2+-binding loop (4). The function of the EF hand remains
elusive due to limited knowledge regarding its structural effects
on downstream targets, despite thorough studies of the interaction
between the EF hand and Ca2+ (5). Genetic mutations, such as substitutions
and deletions, have been identified in the calcium-binding residues
of the EF hand, which render S100A10 unable to bind to calcium
(6). S100A10 has been reported to
interact with numerous ion channels, such as TRPV5 and TRPV6 for
Ca2+ and Mg2+ transport, as well as the
serotonin 5-HT1B receptor, which is involved in the regulation of
serotonin signaling (7).
Additionally, the expression of S100A10 in epithelial and stromal
cells of the endometrium might promote embryo implantation
(8). Furthermore, S100A10
upregulation is involved in the progression of angiogenesis of the
embryo (9). Based on these reports,
it appears that S100A10 is involved in a variety of normal
functions in several tissues via interactions with various
biomolecules.
Annexin 2 (ANXA2) is the most common ligand of
S100A10, which, along with other ligands, forms a heterotetrameric
complex known as AIIt (A2 heterotetramer). Several studies have
reported that the ANXA2-S100A10 complex prevents ubiquitinylation
of S100A10 (10,11). Allt is an essential regulator of
cellular plasmin generation. Plasminogen circulates in the blood it
its inactive form, and the conversion of S100A10-bound plasminogen
to plasmin is mediated by tissue plasminogen activator (tPA) and
urokinase-type plasminogen activator (uPA) (11–13).
Binding to Allt prevents inactivation of the plasmin, which may
eventually contribute to cancer progression (14–18).
Tumor-promoting activities of S100A10 in
cancer
It has been established that cancer cells undergo
modifications that make them functionally different to normal
cells. These modifications result in certain characteristics, known
as the ‘Hallmarks of Cancer’. In 2011, Hanahan and Weinberg
reported the hallmarks of cancer as well as the enabling
characteristics of cancer cells, which are: i) Sustained
proliferative signaling; ii) resistance to cell death; iii) evasion
of growth suppressors; iv) replicative immortality; v)
tumor-promoting inflammation; vi) avoidance of immune destruction,
vii) angiogenesis induction; viii) invasion and metastasis
activation; ix) deregulation of cellular energetics and x) genome
instability and mutations (19). In
order to evaluate the role of S100A10 in cancer, the effects of
S100A10 in the development of these hallmarks need to be
investigated.
Sustained proliferative signaling,
resistance to cell death, evasion of growth suppressors and
replicative immortality
Tumor cells are considered to be persistent due to
their ability to survive in unfavorable conditions, which is a
result of the abovementioned modifications. This persistence be
achieved by direct action via the release of their own growth
factors, thus preserving autocrine signaling, or indirect action
via interfering with the apoptotic pathway to inhibit apoptosis
(19). The intrinsic apoptotic
pathway, which involves the Bcl-2 family, regulates apoptosis
through the mitochondria. Possible mechanisms by which Bcl-2 family
proteins regulate apoptosis have been postulated (20), however these mechanisms have yet to be
definitely elucidated. It has been reported that Bad, a
pro-apoptotic member of the Bcl-2 family, induces cytochrome C
release from the mitochondria, activating caspase 9 which in turn
leads to the activation of caspase-3 and the initiation of
apoptosis (20). However, S100A10 has
been reported to interact with Bad and hinder its pro-apoptotic
activity, suggesting that S100A10 may have anti-apoptotic effects
in cancer cells (21,22). This may explain reports of increased
caspase-3 expression following S100A10 downregulation in
vitro (23) and in
S100A10-knockout mice (24).
Furthermore, S100A10 downregulation suppresses cell growth by
reducing the expression of Cyclin D1 (24), which is the critical downstream
effector protein of epidermal growth factor receptor (EGFR)
signaling (25). It is important to
note that S100A10 is overexpressed in patients with mutated EGFR in
comparison to patients with normal EGFR, suggesting a correlation
between the two (26). Reduced growth
of murine Lewis lung carcinoma or T241 fibrosarcoma has also been
reported in S100A10-deficient mice (12). In accordance with the in vivo
evidence, the regulation of tumor cell proliferation by S100A10 has
been observed in patients with a variety of cancers, including
squamous cell carcinoma (27) and
colorectal cancer (28), as well as
in COLO201, COLO205, COLO320, DLD-1, HCT-15, HCT-116, HT29, LOVO,
LS174T, SW480, SW620, SW1116 and WiDR colorectal cancer cell lines
(22). Furthermore, reduced S100A10
expression caused by ANXA2 knockdown resulted in decreased tumor
growth and proliferation in GL621 mouse glioma cells (29).
Tumor-promoting inflammation and
avoidance of immune destruction
Inflammation is one of the critical traits that
contributes to tumor progression and cancer development. Chronic
inflammation is known to increase the incidence of cancer,
primarily by causing DNA damage and inducing the inflammatory
response, which give rise to a pro-tumorigenic microenvironment
(30,31). When tumor growth reaches a certain
point, tumors begin to produce pro-inflammatory factors,
predominantly matrix metalloproteinases (MMPs), which induce
further inflammation at the tumor site. This results in further
recruitment of immune cells and cytokine production, which in turn
promotes tumor progression (32).
This recurrent positive loop of inflammation in tumorigenesis is
integral to the rapid progression of cancer.
Tumor cells regulate inflammation via various
mechanisms, and S100A10 has been identified to serve a
pro-inflammatory role. As discussed, AIIt converts plasminogen into
plasmin, which may lead to inflammation. The amino-terminal peptide
is a byproduct of plasmin cleavage (33). AIIt-derived cell surface plasmin
triggers the phosphorylation of PKC signaling molecules, which
leads to ANXA2 cleavage, resulting in the activation of toll-like
receptor 4 (TLR-4) and NFκB signaling (34). This pathway has been reported in
hepatocellular carcinoma, in which activation of the Akt/NFκB
signaling pathway promoted liver carcinogenesis. AIIt disassembly
occurs after ANXA2 phosphorylation, following which tPA binds to
the S100A10 subunit within the carboxyl-terminal lysine residue, to
activate the CD11b-dependent integrin-linked kinase (ILK) pathway
(13,35). Together with plasmin, ILK can induce
nuclear translocation of NFκB, which promotes the production of
pro-inflammatory factors, including IL-1, IL-6 and TNFα (13,33,36).
Although AIIt-dependent macrophage activation may occur via the
MAPK and NFκB pathways, TLR-4 knockdown inhibits AIIt-driven
cytokine production (16); this
suggests that TLR-4 serves an important role in AIIt-mediated
inflammation. TLR-4 activation induces tumor-associated IL-6
expression in bladder cancer through p38 and Erk signaling
(37), which is activated by
JAK1/TYK2 and STAT3 stimulation. Inhibiting JAK, p38, and NFκB
results in a significant reduction in IL-6 and TNF-α expression,
suggesting that this pathway is important for releasing
plasmin-dependent cytokines (38).
TLR-4 activation triggers AIIt to recruit and
activate macrophages (16) via
extravasation and migration within extravascular tumor tissues. The
effects of S100A10 in cellular migration and invasion may
significantly contribute to the recruitment of immune cells at the
tumor inflammation site by inducing fibrinolysis. It has been
hypothesized that S100A10 expression in macrophages induces the
production of plasmin by cell surface plasminogen receptors, which
allows for the migration of macrophages by facilitating the
proteolysis of basement membrane and extracellular matrices
(21). S100A10 has also been observed
to have a direct effect in macrophage infiltration in vivo;
S100A10−/− mice exhibited a significant reduction in
macrophage recruitment compared with wild type mice (39). In addition, S100A10 indirectly
stimulates the release of MCP-1 under hypoxic conditions (40), which might aid in the recruitment of
monocytes in the tumor microenvironment via chemotaxis (33).
Prolonged inflammation at the tumor site suppresses
the anti-tumoral activities of immune cells due to the secretion of
tumor-promoting cytokines, including IL-1, IL-6 and TNF-α (41). The release of these cytokines and
prostaglandin E2 (PGE2) stimulates the
infiltration of myeloid derived suppressor cells (MDSCs) into the
tumor microenvironment (42).
Infiltrating MDSCs elicit immunosuppressive effects via a number of
mechanisms, such as inducing anergy in NK cells via membrane-bound
TGF-β, STAT-5 activity and ARG-1. Furthermore, it can suppress the
cytotoxicity of NK cells by inhibiting interferon-γ (IFN-γ)
production (43) and downregulating
NKG2D, as is observed in glioma (44). TGF-β also been reported to induce the
activation of induced Treg (iTreg) cells by MDSC (45). Moreover, together with IL-6, TGF-β is
able to stimulate Th17 and enhance the pro-tumoral effects of MDSC
(41). The binding of TNF-α to its
receptor on CD11b+Gr1+ myeloid cells results
in TGF-β release, which in turn suppresses the anti-tumoral
activity of CD8+ T cells (46), intensifying immunosuppression in the
tumor microenvironment. S100A10's ability to release these
pro-inflammatory cytokines indirectly facilitates immune-escape
mechanisms by mitigating T cell cytotoxicity and evading
immunosurveillance (36).
Angiogenesis induction
Due to its altered metabolism, the tumor
microenvironment is hypoxic. This triggers the release of hypoxia
inducible factor-1α (HIF-1α), which stimulates oxygen delivery to
the hypoxic site by promoting angiogenesis by regulating
pro-angiogenic genes, including vascular endothelial growth factor
(VEGF), platelet-derived growth factor (PDGF) and monocyte
chemoattractant protein 1 (MCP-1) (40,47). This
results in increases in vascular permeability, endothelial cell
proliferation and sprouting, creating a vast tumor vasculature
(47). However, tumor vessels
typically function poorly due to their irregular and leaky
structure (48). Inadequate tumor
vessels leads to the stabilization of HIF-1α, which further
promotes angiogenesis to generate a positive feedback loop
(47). The feedback loop is
exacerbated by HIF-1α-induced ANXA2 transcription, which occurs via
binding to the hormone response element of ANXA2 gene (18,49). ANXA2
upregulation leads to the stabilization of S100A10 (10,18).
Together, the heterotetrameric complex of ANXA2 and S100A10
enhances the generation of plasmin, which is able to activate a
number of MMPs (50,51). Both plasmin and MMPs further promote
angiogenesis via the extracellular matrix (ECM) -associated
pro-angiogenic growth factors (36,52–54).
Studies have reported the significance of ANXA2 and
S100A10 in the initiation and progression of angiogenesis.
ANXA2-deficient mice exhibited decreased angiogenic activity
(55,56). This disturbance in angiogenic activity
may occur due to the impaired plasmin-MMP axis of angiogenesis.
Inhibition of heterotetramer formation via competitive binding
results in a substantial reduction in vascular branching (57). It can therefore be inferred that the
ANXA2-S100A10 complex plays a pivotal role in the initiation and
progression of angiogenesis.
Cell-cell interactions are mediated by interactions
between cancer cells and opposing endothelial cells via Annexin 2
and S100A10. This has been observed in breast cancer, where
interactions between Annexin 2 and S100A10 resulted in the
generation of activated plasmin, promoting ECM proteolysis and
initiating the release of ECM-sequestered VEGF via MMP-9 activation
(13,58,59).
Activation of invasion and metastasis. Cancer
malignancy is determined by its metastasis and invasion potential.
This hallmark of cancer relies on the ability of cancer cells to
modify ECM and induce epithelial to mesenchymal transition (EMT)
(19). In order to invade and
metastasize, cancer cells must cross the basement membrane. ECM is
promoted by proteases, such as plasmin and MMPs (60). Plasmin proteolytic activity allows for
the degradation of fibronectin and laminin within the basement
membrane, simultaneously initiating a proteolytic cascade via the
activation of proteases such as MMPs (61), which helps to remodel the ECM. An
essential process in MMP regulation is the conversion of zymogen
into active proteolytic enzyme, which is mediated by plasmin
(62). S100A10 directly effects MMP
regulation to influence plasmin generation. Following the binding
of plasminogen to AIIt, the uPA-mediated generation and activation
of plasmin upregulates MMP-1 via the Erk1/2, p38, cyclooxygenase-2
and PGE2 pathways (51).
Specifically, extracellular AIIt allows cancer cells to utilize
plasmin, cathepsin B and MMPs to degrade cellular adhesion factors
(21). Plasmin-dependent ECM
proteolysis activates uPA, which binds to the uPA receptor (uPAR)
to cleave and activate plasmin (21,63).
Activated plasmin subsequently activates pro-uPA, generating a
positive feedback loop once again (21).
The positive feedback loop is intensified by
pathways regulated by oncogenic Ras. It has been reported that
oncogenic HRas upregulates MMP-2 and MMP-9 via increasing the
expression of uPAR, suggesting that invasion and metastasis may be
Ras-dependent. Although oncogenic Ras is a key regulator of plasmin
generation, S100A10 knockdown results in a significant reduction in
Ras-dependent plasmin generation (64). This verifies the existence of a
positive feedback loop between S100A10, plasmin generation and
oncogenic Ras. S100A10 overexpression has been reported to induce
invasion and metastasis in lung adenocarcinoma and is correlated
with higher TNM stages (65), thyroid
neoplasms (66) and acute
promyelocytic leukemia (APL) (36).
S100A10 overexpression is observed in the breast cancer cell line
MDA-MB-435 (67) and colorectal
cancer (28). S100A10 downregulation,
on the other hand, reduces plasmin generation, which leads to a
loss of invasiveness of cancer cells (67).
S100A10 is an independent prognostic biomarker for
serous ovarian cancer. A previous study reported that high S100A10
mRNA levels and S100A10 cytoplasmic positivity was correlated with
decreased overall patient survival and a 2-fold increase in ovarian
cancer mortality (68). In APL
patients, S100A10 overexpression and activation promotes the
migration of cancerous leukemic cells as well as hyperfibrinolysis,
often causing excessive bleeding (13). One study reported that the AIlt
overexpression resulted in forced expression of leukemia/retinoic
acid receptor a (PML/RARα) fusion protein, which led to a 27.6%
increase in cell invasiveness, whereas antibodies inhibiting Allt
reduced invasion and migration (36).
It has been reported that the invasion of CCL-222 colorectal cells
via ECM degradation was significantly reduced by a loss of S100A10
(69). S100A10 knockdown in HT-1080
cells results in the depletion of metastatic lung foci, whereas
S100A10 upregulation increases the metastatic potential of these
cells (61).
DLC-1, a Rho GTPase-activating protein, is a ligand
that competitively binds wit S100A10 at the ANXA2 binding site. The
coupling of S100A10 and DCL-1 prevents ANXA2 from inhibiting the
ubiquitin-dependent degradation of S100A10, resulting in a decrease
in S100A10 and, consequently, reduced migration and invasion in
non-small-cell lung cancer lines (A549 and H1395) (70). This confirms that plasmin generation
and plasminogen-dependent cell invasion occurs due to the surface
protein loss of S100A10, not ANXA2. However, ANXA2 expression has
been reported to be an independent predictor of metastasis in
clear-cell renal cell carcinoma. It was demonstrated that the
5-year metastasis-free rate is significantly lower in
ANXA2-negative tumors compared with ANXA2-positive tumors (71). As ANXA2 expression is proportional to
S100A10 expression (10,18,29), and
S100A10 is highly expressed in renal cancer (72), it could be that renal cancer
metastasis is initiated by the interaction between S100A10 and
ANXA2. This has also been suggested in pancreatic (73) and gastric cancers (38,74,75).
S100A10 expression is increased in advanced pancreatic tumors
compared with benign pancreatic tumors (15) and, furthermore, is correlated with the
proportion of lymph node metastases and the depth of gastric cancer
(38). However, the exact mechanism
of S100A10 in cancer invasiveness requires further investigation.
ANXA2 has been found to have an invasion-promoting role in in
pancreatic ductal adenocarcinoma, which is achieved via the
initiation of hedgehog signaling, inducing the binding of tenascin
C to ANXA2 (76).
Deregulating cellular energetics
One of the factors that allow cancer cells to
survive in unfavorable conditions is their ability to alter
metabolic processes (19). Altered
metabolism in cancer cells occurs via upregulation of glucose
transporter 1 (GLUT1), which results in an elevated glucose intake
to support energy production (77–79). A
direct role of S100A10 in altering cellular metabolism has not yet
been identified; however, the aforementioned metabolism
dysregulation is highly associated with oncogenic Ras and HIF-1α
(77,78), suggesting an indirect effect of
S100A10. As discussed above, S100A10 influences oncogenic Ras
expression and HIF-1α stabilization, resulting in KRas mutations
that cause GLUT1 upregulation and consequently increased glucose
uptake (80). As well as
S100A10-mediated HIF-1α stabilization, Ras activation also induces
HIF-1α translation via the Ras/Raf/Mek/Erk kinase signaling cascade
(81). HIF-1α then binds to
hypoxia-response elements in the promoter region of the GLUT1 gene
to increase GLUT1 expression (80).
In ovarian cancer, S100A10 has eight potential
binding motifs for c-Myc transcriptional factor (82), which play an important role in the
regulation of glycolysis via targeting the lactate dehydrogenase A
(LDHA) (83). Overexpression and
stabilization of c-Myc by S100A10 amplifies glycolysis, resulting
in a persistent increase in the availability of nutrients necessary
for cancer cell proliferation. There is a clear correlation between
S100A10 expression and altered metabolism in tumor cells. However,
further studies are required in order to explore the potential
mechanisms by which S100A10 may directly affect tumor cellular
energetics.
Genome instability and mutation
The hallmarks and characteristics of cancer develop
via genetic or epigenetic modifications. Simply put, these
modifications allow tumor cells to gain abilities that are
beneficial for their growth. The underlying mechanism by which
tumor cells obtain these characteristics is via mutations in
caretaker genes. However, tumorigenesis may also be initiated by
epigenetic changes that result in a downregulation of tumor
suppressor genes (19). The location
of the S100A10 gene is susceptible to epigenetic changes that may
contribute to cancer development. These changes may affect the
regulation of S100A10 expression, which corresponds to tumor
malignancies (84).
No direct correlation between S100A10 and genomic
changes has been identified. Nonetheless, its interaction with
ANXA2 is associated with increased susceptibility to human
papilloma virus (HPV) infection (85–87), in
which integration of the viral genome into the host causes the
degradation of p53 and Rb (88,89).
Conclusion and future studies
S100A10 is a novel gene that may have potential as a
biomarker and treatment target due to its persistent overexpression
in a variety of tumor cells, as well as its contribution to several
key hallmarks of cancer. Recently, S100A10 expression has been
recognized as a potential malignancy biomarker in colorectal cancer
(28), renal cell carcinoma (72), non-small cell lung carcinoma (90) and gallbladder cancer (91).
It is thought that S100A10 might play role in
cellular differentiation and cell cycle progression, making it a
potent prognostic biomarker and a potential predictive marker of
sensitivity to chemotherapeutic drugs. Oxaliplatin-based
chemotherapy, which hinders the growth and proliferation of
advanced cancer by activating certain apoptotic pathways, has been
reported to be less effective in colorectal cancer with forced
expression of S100A10 (47). Forced
S100A10 expression significantly increases the 50% inhibitory
concentration (IC50) of oxaliplatin (22). This suggests that S100A10 expression
may be used to predict resistance to chemotherapeutic agents.
S100A10s is often expressed together with ANXA2,
whose role in cancer has been well studied, as a heterotetramer
complex localized in the intracellular cytoplasm and extracellular
membrane of various cancer cells (13,16,49).
Despite the observed correlation between S100A10 expression and
cancer development, little is known with regard to the underlying
biological mechanisms.
In summary, this review demonstrates that S100A10
interacts with a variety of proteins in different pathways to
promote cancer development (Fig. 1).
One of the persistent roles of S100A10 that contributes to the
hallmarks of cancer is plasmin generation, which significantly
remodels the ECM (13,61); this ECM modulation occurs in invasion,
metastasis, inflammation, evasion of immune destruction, and
angiogenesis. Furthermore, S100A10 appears to serve a greater role
in the activation of invasion and metastasis compared with the
other hallmarks of cancer (Table I).
These findings may provide a basis for the development of effective
treatment regimes for advanced cancer.
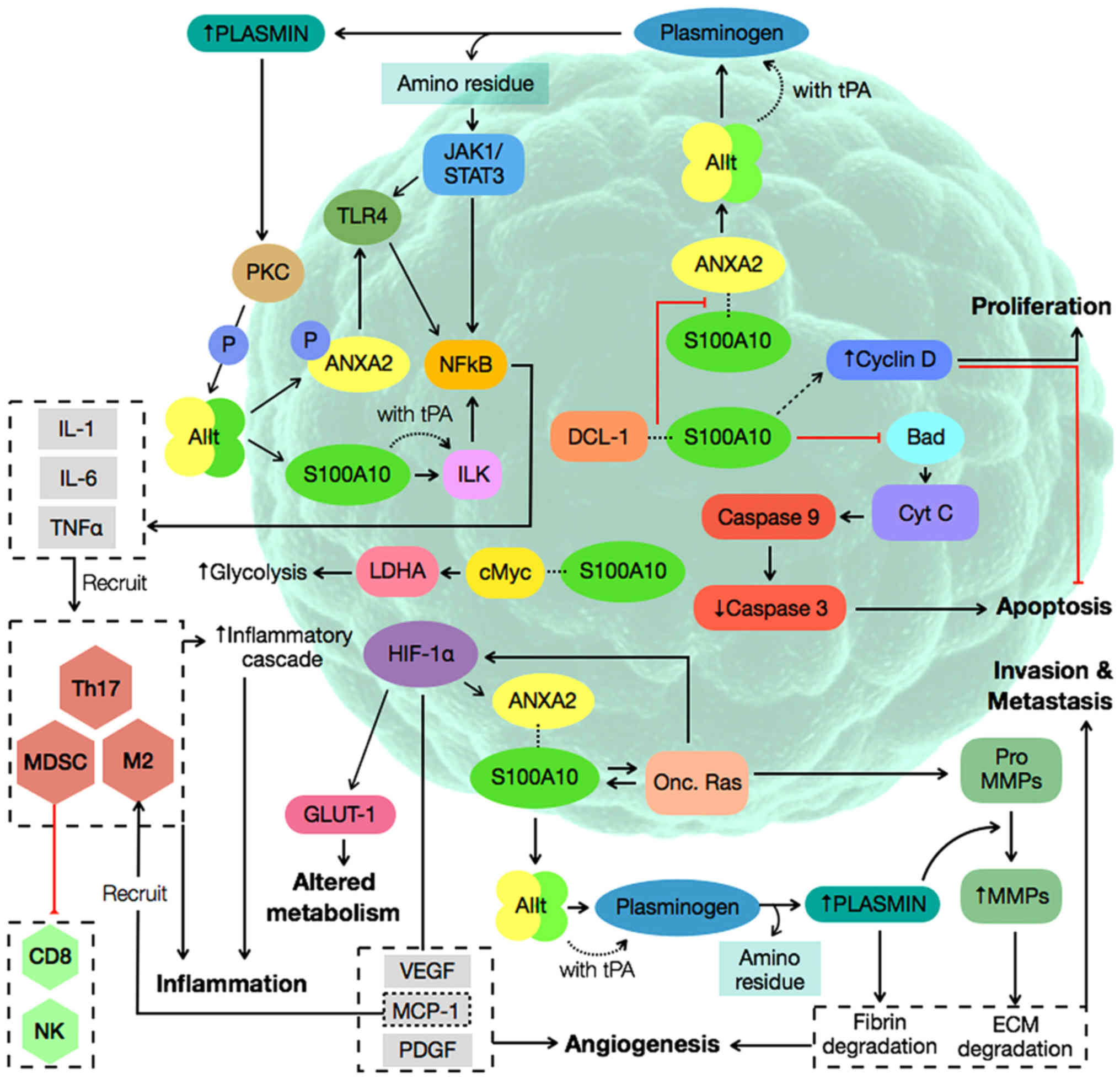 | Figure 1.Cancer progression pathways involving
S100A10. AIIt, Annexin A2 and S100A10 heterotetrameric complex;
ANXA2, Annexin A2; CD8, CD8+ T cells; Cyt C, Cytochrome
C; c-Myc; HIF-1α, Hypoxia inducible factor-1α; IL-1, interleukin 1;
IL-6, Interleukin 6; ILK, integrin-linked kinase; M2, type 2
macrophages; LDHA, lactate dehydrogenase A; MCP-1, monocyte
chemoattractant protein 1; MDSC, myeloid-derived suppressor cells;
MMPs, matrix metalloproteinases; NFκB, nuclear factor κB; NK,
natural killer cells; Onc. Ras, oncogenic Ras; P, phosphate; PDGF,
platelet derived growth factor; PKC, protein kinase C; Th17, T
helper 17 cells; TLR4, toll-like receptor 4; TNF-α, tumor necrosis
factor α; tPA, tissue plasminogen activator; VEGF, vascular
endothelial growth factor. |
 | Table I.Association between S100A10
expression and the hallmarks of cancer in different cancer
types. |
Table I.
Association between S100A10
expression and the hallmarks of cancer in different cancer
types.
|
| Hallmarks of
cancer |
|---|
|
|
|
|---|
| Types of
cancer | Apoptosis and
proliferation | Immune escape and
tumor-promoting inflammation | Angiogenesis | Invasion and
metastasis | Cellular energetics
deregulation | Genome instability
and mutationa |
|---|
| Breast |
|
| ✓ | ✓ |
|
|
| Colorectal | ✓ |
|
| ✓ |
|
|
| Gastric |
|
|
| ✓ |
|
|
| Glioma | ✓ | ✓ |
|
|
|
|
| Leukemia |
|
|
| ✓ |
|
|
| Liver |
| ✓ |
|
|
|
|
| Lung | ✓ |
|
| ✓ |
|
|
| Ovarian |
|
|
| ✓ | ✓ |
|
| Pancreas |
|
|
| ✓ |
|
|
| Renal |
|
|
| ✓ |
|
|
| SCC | ✓ |
|
|
|
|
|
| Thyroid |
|
|
| ✓ |
|
|
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
NAT and ASK devised the main conceptual ideas and
proof outline. ASK designed the figures. NAT, ASK, SZR, MRGS and AD
interpreted the results and drafted the manuscript. NAT took the
lead in writing the manuscript. AS supervised the project, took
part in the conceptualization of the whole manuscript and helped
with the interpretations of the results obtained during the
research process. In addition, AS also revised the manuscript
thoroughly prior to submission and gave the final approval for its
submission to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim SI, Sohn J, Koo JS, Park SH, Park HS
and Park BW: Molecular subtypes and tumor response to neoadjuvant
chemotherapy in patients with locally advanced breast cancer.
Oncology. 79:324–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Novelli G, Ciccacci C, Borgiani P,
Papaluca Amati M and Abadie E: Genetic tests and genomic
biomarkers: Regulation, qualification and validation. Clin Cases
Miner Bone Metab. 5:149–154. 2008.PubMed/NCBI
|
|
3
|
Jin H, Lee HC, Park SS, Jeong YS and Kim
SY: Serum cancer biomarker discovery through analysis of gene
expression data sets across multiple tumor and normal tissues. J
Biomed Inform. 44:1076–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donato R, Cannon BR, Sorci G, Riuzzi F,
Hsu K, Weber DJ and Geczy CL: Functions of S100 proteins. Curr Mol
Med. 13:24–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chazin WJ: Relating form and function of
EF-hand calcium binding proteins. Acc Chem Res. 44:171–179. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santamaria-Kisiel L and Shaw GS:
Identification of regions responsible for the open conformation of
S100A10 using chimaeric S100A11-S100A10 proteins. Biochem J.
434:37–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Svenningsson P and Greengard P: p11
(S100A10)-an inducible adaptor protein that modulates neuronal
functions. Curr Opin Pharmacol. 7:27–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bissonnette L, Drissennek L, Antoine Y,
Tiers L, Hirtz C, Lehmann S, Perrochia H, Bissonnette F, Kadoch IJ,
Haouzi D and Hamamah S: Human S100A10 plays a crucial role in the
acquisition of the endometrial receptivity phenotype. Cell Adhes
Migr. 10:282–298. 2016. View Article : Google Scholar
|
|
9
|
Domínguez F, Garrido-Gómez T, López JA,
Camafeita E, Quiñonero A, Pellicer A and Simón C: Proteomic
analysis of the human receptive versus non-receptive endometrium
using differential in-gel electrophoresis and MALDI-MS unveils
stathmin 1 and annexin A2 as differentially regulated. Hum Reprod.
24:2607–2617. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He KL, Deora AB, Xiong H, Ling Q, Weksler
BB, Niesvizky R and Hajjar KA: Endothelial cell annexin A2
regulates polyubiquitination and degradation of its binding partner
S100A10/p11. J Biol Chem. 283:19192–19200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Connell PA, Madureira PA, Berman JN,
Liwski RS and Waisman DM: Regulation of S100A10 by the PML-RAR-α
oncoprotein. Blood. 117:4095–4105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Phipps KD, Surette AP, O'Connell PA and
Waisman DM: Plasminogen receptor S100A10 is essential for the
migration of tumor-promoting macrophages into tumor sites. Cancer
Res. 71:6676–6683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bharadwaj A, Bydoun M, Holloway R and
Waisman D: Annexin A2 heterotetramer: Structure and function. Int J
Mol Sci. 14:6259–6305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carpenter SL and Mathew P:
Alpha2-antiplasmin and its deficiency: Fibrinolysis out of balance.
Haemophilia. 14:1250–1254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sitek B, Sipos B, Alkatout I, Poschmann G,
Stephan C, Schulenborg T, Marcus K, Lüttges J, Dittert DD, Baretton
G, et al: Analysis of the pancreatic tumor progression by a
quantitative proteomic approach and immunhistochemical validation.
J Proteome Res. 8:1647–1656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Swisher JF, Burton N, Bacot SM, Vogel SN
and Feldman GM: Annexin A2 tetramer activates human and murine
macrophages through TLR4. Blood. 115:549–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miles LA and Parmer RJ: S100A10: A complex
inflammatory role. Blood. 116:1022–1024. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang B, Deora AB, He KL, Chen K, Sui G,
Jacovina AT, Almeida D, Hong P, Burgman P and Hajjar KA:
Hypoxia-inducible factor-1 drives annexin A2 system-mediated
perivascular fibrin clearance in oxygen-induced retinopathy in
mice. Blood. 118:2918–2929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duronio RJ and Xiong Y: Signaling pathways
that control cell proliferation. Cold Spring Harb Perspect Biol.
5:a0089042013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Madureira PA, O'Connell PA, Surette AP,
Miller VA and Waisman DM: The biochemistry and regulation of
S100A10: A multifunctional plasminogen receptor involved in
oncogenesis. J Biomed Biotechnol. 2012:3536872012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki S and Tanigawara Y: Forced
expression of S100A10 reduces sensitivity to oxaliplatin in
colorectal cancer cells. Proteome Sci. 12:262014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan X, Miao Y, Fan R, Qian H, Chen P, Liu
H, Yan X, Li J and Zhou F: MiR-590-5P inhibits growth of HepG2
cells via decrease of S100A10 expression and inhibition of the Wnt
pathway. Int J Mol Sci. 14:8556–8569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Egeland M, Warner-Schmidt J, Greengard P
and Svenningsson P: Neurogenic effects of fluoxetine are attenuated
in p11 (S100A10) knockout mice. Biol Psychiatry. 67:1048–1056.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu W, Ren H, Ren J, Yin T, Hu B, Xie S,
Dai Y, Wu W, Xiao Z, Yang X and Xie D: The role of
EGFR/PI3K/Akt/cyclinD1 signaling pathway in acquired middle ear
cholesteatoma. Mediators Inflamm. 2013:6512072013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnson H, Del Rosario AM, Bryson BD,
Schroeder MA, Sarkaria JN and White FM: Molecular characterization
of EGFR and EGFRvIII signaling networks in human glioblastoma tumor
xenografts. Mol Cell Proteomics. 11:1724–1740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Riau AK, Setiawan M, Mehta JS, Ti
SE, Tong L, Tan DT and Beuerman RW: S100A expression in normal
corneal-limbal epithelial cells and ocular surface squamous cell
carcinoma tissue. Mol Vis. 17:2263–2271. 2011.PubMed/NCBI
|
|
28
|
Shang J, Zhang Z, Song W, Zhou B, Zhang Y,
Li G and Qiu S: S100A10 as a novel biomarker in colorectal cancer.
Tumour Biol. 34:3785–3790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang CY and Lin CF: Annexin A2: Its
molecular regulation and cellular expression in cancer development.
Dis Markers. 2014:3089762014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neurath MF and Finotto S: IL-6 signaling
in autoimmunity, chronic inflammation and inflammation-associated
cancer. Cytokine Growth Factor Rev. 22:83–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dufour A and Overall CM: Missing the
target: Matrix metalloproteinase antitargets in inflammation and
cancer. Trends Pharmacol Sci. 34:233–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Godier A and Hunt BJ: Plasminogen
receptors and their role in the pathogenesis of inflammatory,
autoimmune and malignant disease. J Thromb Haemost. 11:26–34. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hajjar KA: The Biology of Annexin A2: From
vascular fibrinolysis to innate immunity. Trans Am Clin Climatol
Assoc. 126:144–155. 2015.PubMed/NCBI
|
|
35
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang D, Yang Y, Sun J, Dong X, Wang J,
Liu H, Lu C, Chen X, Shao J and Yan J: Annexin A2-S100A10
heterotetramer is upregulated by PML/RARα fusion protein and
promotes plasminogen-dependent fibrinolysis and matrix invasion in
acute promyelocytic leukemia. Front Med. 11:410–422. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qian Y, Deng J, Xie H, Geng L, Zhou L,
Wang Y, Yin S, Feng X and Zheng S: Regulation of TLR4-induced IL-6
response in bladder cancer cells by opposing actions of MAPK and
PI3K signaling. J Cancer Res Clin Oncol. 135:379–386. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li C, Hou Y, Zhang J and Zhang L: The
expressions and roles of S100A6 and S100A10 in gastric cancer.
Biomed Res. 28:2131–2138. 2017.
|
|
39
|
O'Connell PA, Surette AP, Liwski RS,
Svenningsson P and Waisman DM: S100A10 regulates
plasminogen-dependent macrophage invasion. Blood. 116:1136–1146.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: The central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Y and Cao X: Immunosuppressive cells
in tumor immune escape and metastasis. J Mol Med (Berl).
94:509–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhatia A and Kumar Y: Cellular and
molecular mechanisms in cancer immune escape: A comprehensive
review. Expert Rev Clin Immunol. 10:41–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han J, Alvarez-Breckenridge CA, Wang QE
and Yu J: TGF-β signaling and its targeting for glioma treatment.
AM J Cancer Res. 5:945–955. 2015.PubMed/NCBI
|
|
44
|
Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier
LL and Parsa AT: TGF-beta downregulates the activating receptor
NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol.
12:7–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lindau D, Gielen P, Kroesen M, Wesseling P
and Adema GJ: The immunosuppressive tumour network: Myeloid-derived
suppressor cells, regulatory T cells and natural killer T cells.
Immunology. 138:105–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Terabe M and Berzofsky JA: The role of NKT
cells in tumor immunity. Adv Cancer Res. 101:277–348. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Krock BL, Skuli N and Simon MC:
Hypoxia-induced angiogenesis: Good and evil. Genes Cancer.
2:1117–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Siemann DW: The unique characteristics of
tumor vasculature and preclinical evidence for its selective
disruption by Tumor-vascular disrupting agents. Cancer Treat Rev.
37:63–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luo M and Hajjar KA: Annexin A2 system in
human biology: Cell surface and beyond. Semin Thromb Hemost.
39:338–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bydoun M and Waisman DM: On the
contribution of S100A10 and annexin A2 to plasminogen activation
and oncogenesis: An enduring ambiguity. Future Oncol. 10:2469–2479.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deryugina EI and Quigley JP: Cell surface
remodeling by plasmin: A new function for an old enzyme. J Biomed
Biotechnol. 2012:5642592012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
van Hinsbergh VW and Koolwijk P:
Endothelial sprouting and angiogenesis: Matrix metalloproteinases
in the lead. Cardiovasc Res. 78:203–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Montuori N and Ragno P: Role of uPA/uPAR
in the modulation of angiogenesis. Chem Immunol Allergy.
99:105–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kumari S and Malla R: New Insight on the
role of plasminogen receptor in cancer progression. Cancer Growth
Metastasis. 8:35–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Surette AP, Madureira PA, Phipps KD,
Miller VA, Svenningsson P and Waisman DM: Regulation of
fibrinolysis by S100A10 in vivo. Blood. 118:3172–3181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu W and Hajjar KA: The annexin A2 system
and angiogenesis. Biol Chem. 397:1005–1016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Valapala M, Thamake SI and Vishwanatha JK:
A competitive hexapeptide inhibitor of annexin A2 prevents
hypoxia-induced angiogenic events. J Cell Sci. 124:1453–1464. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vempati P, Mac Gabhann F and Popel AS:
Quantifying the proteolytic release of extracellular
matrix-sequestered VEGF with a computational model. PLoS One.
5:e118602010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Myrvang HK, Guo X, Li C and Dekker LV:
Protein interactions between surface annexin A2 and S100A10 mediate
adhesion of breast cancer cells to microvascular endothelial cells.
FEBS Lett. 587:3210–3215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
López-Soto A, Gonzalez S, Smyth MJ and
Galluzzi L: Control of Metastasis by NK Cells. Cancer Cell.
32:135–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Surette A and Waisman D: S100A10: A Key
Regulator of Fibrinolysis. Fibrinolysis Thrombolysis. 2014.
View Article : Google Scholar
|
|
62
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hitchcock JK, Katz AA and Schäfer G:
Dynamic reciprocity: The role of annexin A2 in tissue integrity. J
Cell Commun Signal. 8:125–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Madureira PA, Bharadwaj AG, Bydoun M,
Garant K, O'Connell P, Lee P and Waisman DM: Cell surface protease
activation during RAS transformation: Critical role of the
plasminogen receptor, S100A10. Oncotarget. 7:47720–47737. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Katono K, Sato Y, Jiang SX, Kobayashi M,
Saito K, Nagashio R, Ryuge S, Satoh Y, Saegusa M and Masuda N:
Clinicopathological significance of S100A10 expression in lung
adenocarcinomas. Asian Pac J Cancer Prev. 17:289–294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ito Y, Arai K, Nozawa R, Yoshida H,
Higashiyama T, Takamura Y, Miya A, Kobayashi K, Kuma K and Miyauchi
A: S100A10 expression in thyroid neoplasms originating from the
follicular epithelium: Contribution to the aggressive
characteristic of anaplastic carcinoma. Anticancer Res.
27:2679–2683. 2007.PubMed/NCBI
|
|
67
|
Zhang J, Guo B, Zhang Y, Cao J and Chen T:
Silencing of the annexin II gene down-regulates the levels of
S100A10, c-Myc and plasmin and inhibits breast cancer cell
proliferation and invasion. Saudi Med J. 31:374–381.
2010.PubMed/NCBI
|
|
68
|
Lokman NA, Pyragius CE, Ruszkiewicz A,
Oehler MK and Ricciardelli C: Annexin A2 and S100A10 are
independent predictors of serous ovarian cancer outcome. Transl
Res. 171:83–95.e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
O'Connell PA and Waisman DM: Regulation of
plasmin generation by the annexin A2 heterotetramer: A shift in
perspective. Future Oncol. 8:763–765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yang X, Popescu NC and Zimonjic DB: DLC1
interaction with S100A10 mediates inhibition of in vitro cell
invasion and tumorigenicity of lung cancer cells through a
RhoGAP-independent mechanism. Cancer Res. 71:2916–2925. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ohno Y, Izumi M, Kawamura T, Nishimura T,
Mukai K and Tachibana M: Annexin II represents metastatic potential
in clear-cell renal cell carcinoma. Br J Cancer. 101:287–294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Domoto T, Miyama Y, Suzuki H, Teratani T,
Arai K, Sugiyama T, Takayama T, Mugiya S, Ozono S and Nozawa R:
Evaluation of S100A10, annexin II and B-FABP expression as markers
for renal cell carcinoma. Cancer Sci. 98:77–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yamamoto N, Nakamura Y, Morinaga S, Numata
K, Sawazaki S, Watanabe T, Numata M, Tamagawa H, Godai T, Shiozawa
M, et al: The clinical significance of S100A10 in pancreatic
cancer. J Clin Oncol. 31:1942013. View Article : Google Scholar
|
|
74
|
Liu J, Li X, Dong GL, Zhang HW, Chen DL,
Du JJ, Zheng JY, Li JP and Wang WZ: In silico analysis and
verification of S100 gene expression in gastric cancer. BMC Cancer.
8:2612008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang Q, Zhu M, Cheng W, Xing R, Li W,
Zhao M, Xu L, Li E, Luo G and Lu Y: Downregulation of 425G>a
variant of calcium-binding protein S100A14 associated with poor
differentiation and prognosis in gastric cancer. J Cancer Res Clin
Oncol. 141:691–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Foley K, Muth S, Jaffee E and Zheng L:
Hedgehog signaling stimulates Tenascin C to promote invasion of
pancreatic ductal adenocarcinoma cells through Annexin A2. Cell
Adhes Migr. 11:514–523. 2017. View Article : Google Scholar
|
|
77
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jones RG and Thompson CB: Tumor
suppressors and cell metabolism: A recipe for cancer growth. Genes
Dev. 23:537–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Labak CM, Wang PY, Arora R, Guda MR,
Asuthkar S, Tsung AJ and Velpula KK: Glucose transport: Meeting the
metabolic demands of cancer and applications in glioblastoma
treatment. Am J Cancer Res. 6:1599–1608. 2016.PubMed/NCBI
|
|
80
|
Barron CC, Bilan PJ, Tsakiridis T and
Tsiani E: Facilitative glucose transporters: Implications for
cancer detection, prognosis and treatment. Metabolism. 65:124–139.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sertel S, Eichhorn T, Simon CH, Plinkert
PK, Johnson SW and Efferth T: Pharmacogenomic identification of
c-Myc/Max-regulated genes associated with cytotoxicity of
artesunate towards human colon, ovarian and lung cancer cell lines.
Molecules. 15:2886–2910. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Miller DM, Thomas SD, Islam A, Muench D
and Sedoris K: c-Myc and cancer metabolism. Clin Cancer Res.
18:5546–5553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Leśniak W: Epigenetic regulation of S100
protein expression. Clin Epigenetics. 2:77–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Woodham AW, Da Silva DM, Skeate JG, Raff
AB, Ambroso MR, Brand HE, Isas JM, Langen R and Kast WM: The
S100A10 subunit of the annexin A2 heterotetramer facilitates
L2-mediated human papillomavirus infection. PLoS One. 7:e435192012.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Dziduszko A and Ozbun MA: Annexin A2 and
S100A10 regulate human papillomavirus type 16 entry and
intracellular trafficking in human keratinocytes. J Virol.
87:7502–7515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Woodham AW, Taylor JR, Jimenez AI, Skeate
JG, Schmidt T, Brand HE, Da Silva DM and Kast WM: Small molecule
inhibitors of the annexin A2 heterotetramer prevent human
papillomavirus type 16 infection. J Antimicrob Chemother.
70:1686–1690. 2015.PubMed/NCBI
|
|
88
|
Litwin TR, Clarke MA, Dean M and
Wentzensen N: Somatic host cell alterations in HPV carcinogenesis.
Viruses. 9:2062017. View Article : Google Scholar
|
|
89
|
Stiasny A, Freier CP, Kuhn C, Schulze S,
Mayr D, Alexiou C, Janko C, Wiest I, Dannecker C, Jeschke U and
Kost BP: The involvement of E6, p53, p16, MDM2 and Gal-3 in the
clinical outcome of patients with cervical cancer. Oncol Lett.
14:4467–4476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Genova C, Rijavec E and Grossi F: Tumor
microenvironment as a potential source of clinical biomarkers in
non-small cell lung cancer: Can we use enemy territory at our
advantage? J Thorac Dis. 9:4300–4304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tan Y, Ma SY, Wang FQ, Meng HP, Mei C, Liu
A and Wu HR: Proteomic-based analysis for identification of
potential serum biomarkers in gallbladder cancer. Oncol Rep.
26:853–859. 2011.PubMed/NCBI
|















