Introduction
Breast cancer (BC) is the leading cause of
cancer-associated mortality among women worldwide (1). In the past decade, the mortality rate
has decreased in the majority of high-income countries; however,
the incidence and mortality rates have increased in China (1). This may be due to a number of factors,
including the one-child policy, lower cancer screening rates and
delays in cancer diagnosis (2). In
addition, the median age at diagnosis of BC is 48–50 years in China
and 62.9% of patients are premenopausal at that time (2).
BC in younger women has been recognized to be more
aggressive and exhibits a worse prognosis compared with BC in older
women (3,4). Previous studies have identified that,
compared with older patients, younger women with BC present with a
larger tumor size, a higher incidence of lymph node involvement
(4,5)
and an increased 5-year risk of developing metastasis (3,6). Compared
with older women, young women exhibit higher proportions of hormone
receptor (HR)+/human epidermal growth factor receptor 2
(HER-2)+, HR−/HER2+ and
triple-negative BC (5,7). Diverse molecular subtypes usually have
distinct disease-free survival (DFS) and overall survival (OS)
rates (6,8), and age has been identified to serve
different roles (9,10). Clinicians use certain risk scores in
clinical practice, including the commonly used St Gallen risk
factor grading system (11). In this
grading system, age is one of the most valuable factors, which
suggests that similar to estrogen receptor (ER) status and lymph
node status, age is fundamental in predicting BC prognosis.
Previous studies have predominantly focused on the
clinicopathological features of BC in young patients (3,12).
However, to the best of our knowledge, a survival model remains to
be established. The current study investigated a number of factors,
including T stage, N stage, pathological type, grade, surgical
type, neoadjuvant chemotherapy, age and molecular subtype, for
predicting survival in young patients with BC. The study aimed to
assess an array of clinicopathological variables that are
potentially associated with visceral metastasis-free survival
(VFS), DFS and OS. In addition, the ultimate aim of the study was
to establish and validate prediction models for survival outcomes
in young patients with BC.
Patients and methods
Definition of a young patient with
BC
The definition of a young patient with BC varies
among previous studies. Previously, the upper age limit has ranged
from 35 (13) to 40 years (14,15). The
current study defined young BC as patients ≤40 years old at
preliminary diagnosis.
Study population
A total of 351 females with primary BC who were
diagnosed at ≤40 years old and treated at the Cancer Hospital of
Shantou University Medical College (Guangdong, China) between April
2009 and May 2014 were included in the current study. The inclusion
criteria were: i) female; ii) breast cancer confirmed by
pathological diagnosis; and iii) age ≤40 years old. Patients with
distant metastasis at primary diagnosis and patients with a
follow-up time <6 months were excluded. The mean age of the
patients was 35.74 years with a range of 19 to 40 years. Every
patient had undergone mammographic and/or ultrasound radiological
imaging, a chest radiograph or computed tomography scan of the
chest, Doppler ultrasound examination or a computed tomography scan
of the abdomen, a complete blood count test and blood biochemistry
assays to evaluate the primary tumor stage and the appropriate
treatment. Bone scans and brain magnetic resonance imaging were
performed if patients experienced bone pain, central nervous
symptoms or exhibited a locally advanced stage of BC. Patients with
primary resectable tumors received a mastectomy or
breast-conserving surgery with axillary lymph node dissection or
sentinel lymph node biopsy. A core needle biopsy was performed in a
standardized manner when the surgeon identified that a tumor was
inoperable. Neoadjuvant chemotherapy was administered to patients
with initially inoperable tumors, the majority of which were stages
T3/T4 and/or N2/N3 according to the 7th edition of the American
Joint Committee on Cancer staging system (16), to increase the possibility of radical
surgeries later on. The requirement of adjuvant chemotherapy and
the protocol of the chemotherapy treatment were guided by the St.
Gallen BC guidelines (11).
Clinical and pathological data were collected from
patient records. Histopathological features of surgical resection
specimens included tumor type and size, histological grade,
evidence of lymphovascular invasion and axillary nodal status. ER,
progesterone receptor (PR), HER-2, Ki-67 and other markers were
stained in the majority of the biopsy and resection specimens.
Adjuvant radiotherapy, chemotherapy, endocrine treatment and
targeted treatment were recorded. In addition, other basic
information, including age of menarche, fertility status, hepatitis
B virus (HBV) infection and family history were recorded. Follow-up
information was obtained from patient records. The median follow-up
time was 38.3 months (range, 6.0–106.6 months).
Written informed consent was obtained from all
participants for the use of clinicopathological data. The current
study was approved by the Ethics Committee of the Cancer Hospital
of Shantou University Medical College.
Classification of survival and
molecular subtypes
VFS was defined as the time from radical surgery to
visceral metastasis, excluding local relapse and metastasis of the
lymph nodes and bones. DFS was defined as the time from radical
surgery to disease relapse or metastasis, including visceral
metastasis. OS was defined as the time from diagnosis to mortality
from any cause. Molecular subtypes were differentiated according to
the status of ER, PR and HER-2, as determined by
immunohistochemistry (IHC). As the cut-off value of Ki-67 has not
previously been determined (17) and
since testing for Ki-67 was not routinely performed in the study
period, the current study did not use Ki-67 for the classification
of molecular subtypes. The molecular subtypes were defined as
follows: The luminal A subtype, which was HER-2−,
ER+ and/or PR+; the luminal B subtype, which
was HER-2+, ER+ and/or PR+; the
HER-2+ subtype, which was HER-2+,
ER− and PR−; and the triple-negative subtype,
which was HER-2, ER and PR. HER-2 positivity was defined as HER-2
gene amplification in a fluorescence in situ hybridization
test or HER-2 protein stained as ‘+++’ in IHC, as described
previously (18).
Statistical analysis
All statistical analyses were performed using SPSS
software (version 13.0; SPSS Inc., Chicago, IL, USA) and R software
(version 3.3.0; www.r-project.org). The univariate analysis for
assessing the prognostic factors was performed using the
Kaplan-Meier method with a log-rank test. Variables associated with
survival (P<0.05) were selected for multivariate Cox
regression analysis using forward stepwise selection. Nomograms
were then generated to illustrate the effect of the prognostic
factors on DFS, VFS and OS. Risk scores were created based on Cox
regression coefficients. Each patient was assigned a risk score
that was a linear combination of the values of the independent
prognostic factors weighted by their respective Cox regression
coefficients (19). Internal
validation of the prediction models was performed by evaluating the
accuracy of the risk score on the prognosis of 200, 250 and 300
patients who were randomly selected from the total 351 patients.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Univariate survival analysis for
predicting DFS, VFS and OS in young patients with BC
To preliminarily determine the potential prognostic
factors, univariate survival analysis was performed for VFS, DFS
and OS. The median follow-up time was 38.3 months and the median
values for VFS, DFS and OS were 38.0, 33.5 and 38.2 months,
respectively. The variables included in the analysis were age, T
stage, N stage, M stage, site of involvement, pathological type,
differentiation grade, molecular subtype, surgical type,
neoadjuvant chemotherapy, adjuvant radiation, age of menarche,
fertility status, HBV infection and family history.
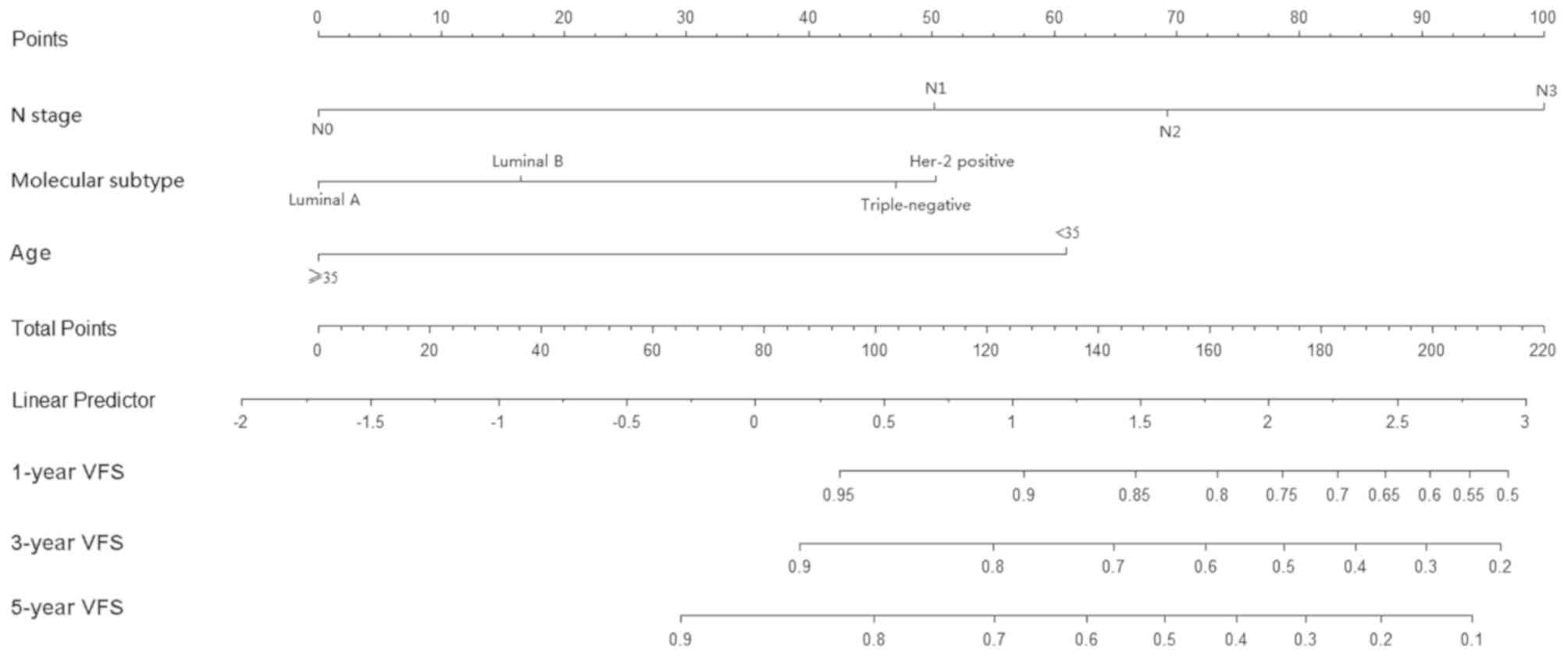
The 1-, 3- and 5-year VFS rates were 94.5, 87.6 and
80.6%, respectively. The 1-, 3- and 5-year DFS rates were 89.8,
76.2 and 64.6%, respectively. The 1-, 3- and 5-year OS rates were
98.2, 87.4 and 73.3%, respectively. Survival rates for different
clinicopathological features were analyzed and tested with
Kaplan-Meier analysis and a log-rank test (Tables I–III). This analysis identified that for
VFS, N stage (P=0.004), molecular subtype (P=0.007), age (P=0.005),
T stage (P=0.014), pathological type (P=0.029) and neoadjuvant
chemotherapy (P=0.020) were statistically significant variables.
For DFS, N stage (P=0.002) and molecular subtype (P=0.001) were
statistically significant. For OS, T stage (P=0.029), N stage
(P=0.006), M stage (P=0.002), molecular subtype (P=0.006), surgical
type (P<0.001) and neoadjuvant chemotherapy (P=0.005) were
statistically significant variables.
 | Table I.Clinicopathological characteristics
of patients and the associated 1-, 3- and 5-year VFS rates. |
Table I.
Clinicopathological characteristics
of patients and the associated 1-, 3- and 5-year VFS rates.
|
|
| VFS, % |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases, n (%) | 1-year | 3-year | 5-year | P-value |
|---|
| Age, years |
|
|
|
| 0.005 |
|
<35 | 108 (30.8) | 93.4 | 82.8 | 67.0 |
|
|
≥35 | 243 (69.2) | 95.0 | 89.6 | 85.3 |
|
| T stage |
|
|
|
| 0.014 |
|
Tis | 1 (0.3) |
|
|
|
|
| T1 | 64 (18.2) | 96.4 | 89.0 | 81.8 |
|
| T2 | 163 (46.4) |
|
|
|
|
| T3 | 52 (14.8) | 88.2 | 79.5 | 74.2 |
|
| T4 | 25 (7.1) |
|
|
|
|
|
Unknown | 46 (13.1) |
|
|
|
|
| N stage |
|
|
|
| 0.004 |
| N0 | 144 (41.0) | 97.2 | 93.7 | 92.2 |
|
| N1 | 80 (22.8) | 96.2 | 87.1 | 74.2 |
|
| N2 | 57 (16.2) | 94.6 | 88.3 | 71.9 |
|
| N3 | 56 (16.0) | 83.5 | 73.3 | 73.3 |
|
|
Unknown | 14 (4.0) |
|
|
|
|
| M stage |
|
|
|
| 0.544 |
| M0 | 339 (96.6) | 94.6 | 87.5 | 80.4 |
|
|
M1a | 6 (1.7) | 83.3 | 83.3 | 0.0 |
|
|
Unknown | 6 (1.7) |
|
|
|
|
| Site of
involvement |
|
|
|
| 0.596 |
|
Left | 177 (50.4) | 94.8 | 89.4 | 81.2 |
|
|
Right | 166 (47.3) | 93.8 | 86.2 | 80.8 |
|
|
Bilateral | 8 (2.3) | 100.0 | 75.0 | 75.0 |
|
| Pathological
type |
|
|
|
| 0.029 |
|
IDC | 290 (82.6) | 93.3 | 85.3 | 76.7 |
|
|
ILC | 11 (3.1) | 100.0 | 100.0 | 100.0 |
|
|
DCIS | 22 (6.3) | 100.0 | 100.0 | 100.0 |
|
|
Other | 27 (7.7) | 100.0 | 96.0 | 96.0 |
|
|
Unknown | 1 (0.3) |
|
|
|
|
| Grade |
|
|
|
| 0.063 |
| I | 15 (4.3) | 100.0 | 100.0 | 100.0 |
|
| II | 103 (29.3) | 92.0 | 88.5 | 86.2 |
|
|
III | 96 (27.4) | 93.7 | 78.2 | 70.6 |
|
|
Unknown | 137 (39.0) |
|
|
|
|
| Molecular
subtype |
|
|
|
| 0.007 |
| Luminal
A | 161 (45.9) | 98.1 | 90.9 | 85.9 |
|
| Luminal
B | 40 (11.4) | 97.4 | 92.0 | 78.7 |
|
|
HER-2+ | 47 (13.4) | 80.6 | 70.8 | 66.6 |
|
|
Triple-negative | 65 (18.5) | 90.7 | 83.0 | 75.4 |
|
|
Unknown | 38 (10.8) |
|
|
|
|
| Surgical type |
|
|
|
| 0.120 |
|
Modified radical
mastectomy | 276 (78.6) | 93.0 | 86.3 | 78.2 |
|
|
Breast-conserving surgery | 58 (16.5) | 100.0 | 92.6 | 92.6 |
|
|
Mastectomy and SLNB | 12 (3.4) | 100.0 | 100.0 | 100.0 |
|
| Simple
resectionb | 5 (1.4) | 100.0 | 75.0 | −c |
|
| Neoadjuvant
chemotherapy |
|
|
|
| 0.020 |
|
Yes | 46 (13.1) | 86.9 | 76.5 | 69.6 |
|
| No | 305 (86.9) | 95.6 | 89.2 | 82.1 |
|
| Adjuvant
radiation |
|
|
|
| 0.399 |
|
Yes | 190 (54.1) | 92.9 | 84.5 | 80.3 |
|
| No | 161 (45.9) | 96.3 | 91.1 | 81.3 |
|
| Age of menarche,
years |
|
|
|
| 0.934 |
|
≤15 | 252 (71.8) | 95.1 | 88.3 | 79.2 |
|
|
>15 | 50 (14.2) | 94.0 | 86.6 | 86.6 |
|
|
Unknown | 49 (14.0) |
|
|
|
|
| Fertility
status |
|
|
|
| 0.566 |
|
Yes | 323 (92.0) | 94.3 | 87.6 | 80.5 |
|
| No | 27 (7.7) | 96.3 | 87.4 | 80.1 |
|
|
Unknown | 1 (0.3) |
|
|
|
|
| HBV infection |
|
|
|
| 0.477 |
|
Yes | 9 (2.6) | 88.9 | 74.1 | 74.1 |
|
| No | 342 (97.4) | 94.6 | 87.9 | 80.7 |
|
| Family history |
|
|
|
| 0.143 |
| BC | 8 (2.3) | 100.0 | 100.0 | 50.0 |
|
| Other
cancer types | 13 (3.7) | 100.0 | 64.1 | 64.1 |
|
| No | 330 (94.0) | 94.1 | 88.2 | 82.2 |
|
 | Table III.Clinicopathological characteristics
of patients and the associated 1-, 3- and 5-year OS rates. |
Table III.
Clinicopathological characteristics
of patients and the associated 1-, 3- and 5-year OS rates.
|
|
| OS, % |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases, n (%) | 1-year | 3-year | 5-year | P-value |
|---|
| Age, years |
|
|
|
| 0.387 |
|
<35 | 108 (30.8) | 96.2 | 84.6 | 71.5 |
|
|
≥35 | 243 (69.2) | 99.6 | 88.4 | 75.9 |
|
| T stage |
|
|
|
| 0.029 |
|
Tis | 1 (0.3) |
|
|
|
|
| T1 | 64 (18.2) | 99.5 | 90.5 | 80.2 |
|
| T2 | 163 (46.4) |
|
|
|
|
| T3 | 52 (14.8) | 94.7 | 80.8 | 73.7 |
|
| T4 | 25 (7.1) |
|
|
|
|
|
Unknown | 46 (13.1) |
|
|
|
|
| N stage |
|
|
|
| 0.006 |
| N0 | 144 (41.0) | 98.6 | 93.2 | 84.2 |
|
| N1 | 80 (22.8) | 98.7 | 89.7 | 81.5 |
|
| N2 | 57 (16.2) | 98.2 | 85.9 | 64.6 |
|
| N3 | 56 (16.0) | 98.1 | 78.2 | 64.9 |
|
|
Unknown | 14 (4.0) |
|
|
|
|
| M stage |
|
|
|
| 0.002 |
| M0 | 339 (96.6) | 98.5 | 88.9 | 75.9 |
|
|
M1a | 6 (1.7) | 100.0 | 50.0 | −c |
|
|
Unknown | 6 (1.7) |
|
|
|
|
| Site of
involvement |
|
|
|
| 0.439 |
|
Left | 177 (50.4) | 98.2 | 90.6 | 77.3 |
|
|
Right | 166 (47.3) | 98.8 | 83.9 | 71.4 |
|
|
Bilateral | 8 (2.3) | 100.0 | 75.0 | 75.0 |
|
| Pathological
type |
|
|
|
| 0.289 |
|
IDC | 290 (82.6) | 98.2 | 86.2 | 71.8 |
|
|
ILC | 11 (3.1) | 100.0 | 100.0 | 75.0 |
|
|
DCIS | 22 (6.3) | 100.0 | 95.0 | 95.0 |
|
|
Other | 27 (7.7) | 100.0 | 86.6 | 86.6 |
|
|
Unknown | 1 (0.3) |
|
|
|
|
| Grade |
|
|
|
| 0.103 |
| I | 15 (4.3) | 100.0 | 100.0 | 100.0 |
|
| II | 103 (29.3) | 98.0 | 86.3 | 82.3 |
|
|
III | 96 (27.4) | 97.9 | 81.3 | 69.1 |
|
|
Unknown | 137 (39.0) |
|
|
|
|
| Molecular
subtype |
|
|
|
| 0.006 |
| Luminal
A | 161 (45.9) | 100.0 | 90.8 | 78.9 |
|
| Luminal
B | 40 (11.4) | 97.4 | 94.9 | 81.9 |
|
|
HER-2+ | 47 (13.4) | 97.8 | 71.8 | 57.1 |
|
|
Triple-negative | 65 (18.5) | 95.2 | 81.5 | 65.3 |
|
|
Unknown | 38 (10.8) |
|
|
|
|
| Surgical type |
|
|
|
| <0.001 |
|
Modified radical
mastectomy | 276 (78.6) | 98.1 | 86.8 | 72.9 |
|
|
Breast-conserving surgery | 58 (16.5) | 100.0 | 95.3 | 90.8 |
|
|
Mastectomy and SLNB | 12 (3.4) | 100.0 | 88.9 | −c |
|
| Simple
resectionb | 5 (1.4) | 100.0 | 0.0 | 0.0 |
|
| Neoadjuvant
chemotherapy |
|
|
|
| 0.005 |
|
Yes | 46 (13.1) | 93.3 | 70.9 | 63.0 |
|
| No | 305 (86.9) | 99.3 | 89.8 | 76.3 |
|
| Adjuvant
radiation |
|
|
|
| 0.559 |
|
Yes | 190 (54.1) | 98.9 | 90.0 | 74.9 |
|
| No | 161 (45.9) | 98.1 | 84.2 | 74.3 |
|
| Age of menarche,
years |
|
|
|
| 0.193 |
|
≤15 | 252 (71.8) | 98.3 | 88.6 | 76.6 |
|
|
>15 | 50 (14.2) | 98.0 | 83.8 | 69.8 |
|
|
Unknown | 49 (14.0) |
|
|
|
|
| Fertility
status |
|
|
|
| 0.849 |
|
Yes | 323 (92.0) | 98.4 | 87.4 | 73.4 |
|
| No | 27 (7.7) | 96.2 | 86.5 | 71.9 |
|
|
Unknown | 1 (0.3) |
|
|
|
|
| HBV infection |
|
|
|
| 0.592 |
|
Yes | 9 (2.6) | 100.0 | 70.0 | 70.0 |
|
| No | 342 (97.4) | 98.2 | 87.8 | 73.4 |
|
| Family history |
|
|
|
| 0.986 |
| BC | 8 (2.3) | 100.0 | 100.0 | 66.7 |
|
| Other
cancer types | 13 (3.7) | 100.0 | 88.9 | 74.1 |
|
| No | 330 (94.0) | 98.1 | 87.0 | 73.5 |
|
Multivariate survival analysis for
predicting VFS, DFS and OS in young patients with BC
To further analyze the prognostic factors for VFS,
DFS and OS, multivariate survival analysis was performed. Variables
revealed as statistically significant by Kaplan-Meier analysis
(P<0.05) were selected for Cox regression analysis to identify
independent factors. As presented in Table IV, the variables analyzed for VFS
were as follows: N stage (P<0.001); molecular subtype (P=0.027);
and age (P<0.001). As presented in Table V, the variables analyzed for DFS
included: N stage (P=0.004) and molecular subtype (P=0.002). As
presented in Table VI, the variables
analyzed for OS were as follows: N stage (P=0.029), molecular
subtype (P=0.006) and neoadjuvant chemotherapy (P=0.006). Nomograms
were created to illustrate the effect of the prognostic factors on
VFS, DFS and OS using multivariate Cox regression coefficients
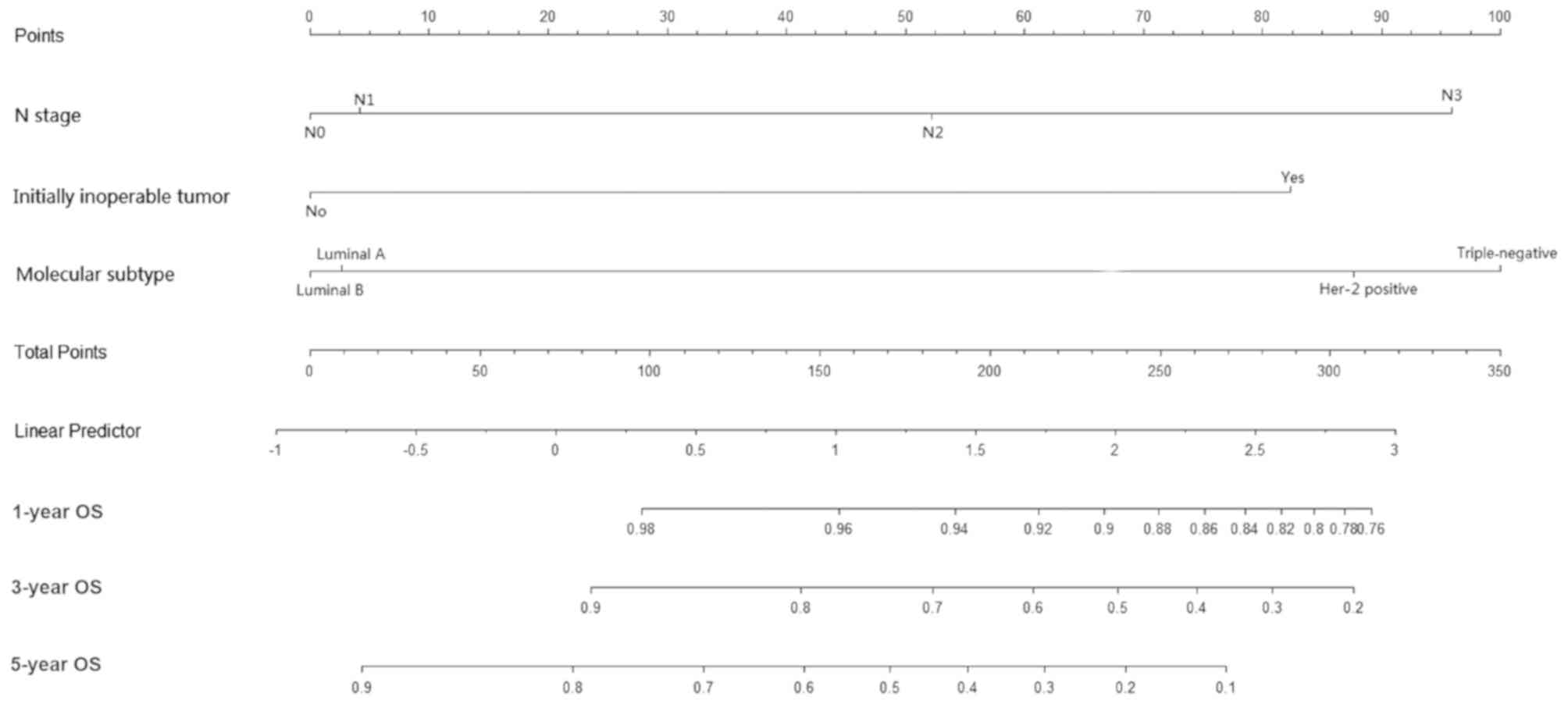
(Figs. 1–3).
 | Table IV.Cox regression analysis for
predicting visceral metastasis-free survival. |
Table IV.
Cox regression analysis for
predicting visceral metastasis-free survival.
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Characteristic | HR | P-value | Lower | Upper |
|---|
| N stage |
| <0.001 |
|
|
|
N1/N0 | 2.977 | 0.025 | 1.148 | 7.722 |
|
N2/N0 | 4.477 | 0.003 | 1.641 | 12.211 |
|
N3/N0 | 8.695 | <0.001 | 0.296 | 22.937 |
| Molecular
subtype |
| 0.027 |
|
|
| Luminal
B/luminal A | 1.426 | 0.536 | 0.463 | 4.390 |
|
HER-2/luminal A | 2.965 | 0.007 | 1.342 | 6.552 |
|
Triple-negative/luminal A | 2.763 | 0.017 | 1.201 | 6.353 |
| Age, years |
|
<35/≥35 | 3.739 | <0.001 | 1.905 | 7.338 |
 | Table V.Cox regression analysis for
predicting disease-free survival. |
Table V.
Cox regression analysis for
predicting disease-free survival.
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Characteristic | HR | P-value | Lower | Upper |
|---|
| N stage |
| 0.004 |
|
|
|
N1/N0 | 1.742 | 0.082 | 0.933 | 3.253 |
|
N2/N0 | 2.295 | 0.009 | 1.230 | 4.284 |
|
N3/N0 | 3.041 | 0.001 | 1.621 | 5.704 |
| Molecular
subtype |
| 0.002 |
|
|
| Luminal
B/luminal A | 1.846 | 0.090 | 0.908 | 3.751 |
|
HER-2/luminal A | 3.030 | <0.001 | 1.707 | 5.379 |
|
Triple-negative/luminal A | 1.944 | 0.029 | 1.071 | 3.528 |
 | Table VI.Cox regression analysis for
predicting overall survival. |
Table VI.
Cox regression analysis for
predicting overall survival.
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Characteristic | HR | P-value | Lower | Upper |
|---|
| N stage |
| 0.029 |
|
|
|
N1/N0 | 1.052 | 0.920 | 0.392 | 2.822 |
|
N2/N0 | 1.888 | 0.193 | 0.725 | 4.921 |
|
N3/N0 | 3.210 | 0.009 | 1.347 | 7.653 |
| Molecular
subtype |
| 0.006 |
|
|
| Luminal
B/luminal A | 0.968 | 0.959 | 0.276 | 3.399 |
|
HER-2/luminal A | 2.809 | 0.009 | 1.290 | 6.119 |
|
Triple-negative/luminal A | 3.262 | 0.003 | 1.504 | 7.075 |
| Neoadjuvant
chemotherapy |
|
|
|
|
|
Yes/no | 2.722 | 0.006 | 1.336 | 5.543 |
Risk scores for predicting survival
outcomes in young patients with BC
Based on the regression analysis, prediction models
for VFS, DFS and OS were generated through the calculations of risk
scores, previously established by Shukla et al (19). Each patient was assigned a risk score;
a linear combination of the values of the independent prognostic
factors weighted by their respective Cox regression coefficients.
Risk scores for VFS were calculated as follows: Risk score = 1.091
× N stage (N1/N0) + 1.499 × N stage (N2/N0) + 2.163 × N stage
(N3/N0) + 0.355 × molecular subtype (luminal B/luminal A) + 1.087 ×
molecular subtype (HER-2/luminal A) + 1.016 × molecular subtype
(triple-negative/luminal A) + 1.319 × age (<35/≥35). Risk scores
for DFS were calculated as follows: Risk score = 0.555 × N stage
(N1/N0) + 0.831 × N stage (N2/N0) + 1.112 × N stage (N3/N0) + 0.613
× molecular subtype (luminal B/luminal A) + 1.109 × molecular
subtype (HER-2/luminal A) + 0.665 × molecular subtype
(triple-negative/luminal A). Risk scores for OS were calculated as
follows: Risk score = 0.050 × N stage (N1/N0) + 0.636 × N stage
(N2/N0) + 1.166 × N stage (N3/N0) - 0.033 × molecular subtype
luminal B/luminal A) + 1.033 × molecular subtype (HER-2/luminal A)
+ 1.182 × molecular subtype (triple-negative/luminal A) + 1.001 ×
neoadjuvant chemotherapy (yes/no).
Internal validation of the prediction models was
conducted by evaluating the effect of the risk score on the
prognosis of patients. A total of 200, 250 and 300 cases were
randomly selected 10 times from the total 351 cases and univariate
Cox proportional hazard regression analysis was performed. As
presented in Tables VII–IX, the range of the HR was 1.692–2.239 for
VFS with P≤0.005, 1.910–2.879 for DFS with P≤0.003 and 1.938–2.652
for OS with P≤0.003. Therefore, risk scores and nonograms were
demonstrated to be reliable for predicting VFS, DFS and OS time in
young patients with BC.
 | Table VII.Internal validation of risk scores
for predicting visceral metastasis-free survival in randomly
sampled patients by Cox regression analysis. |
Table VII.
Internal validation of risk scores
for predicting visceral metastasis-free survival in randomly
sampled patients by Cox regression analysis.
|
| 200 cases | 250 cases | 300 cases |
|---|
|
|
|
|
|
|---|
| Subset no. | P-value | HR | P-value | HR | P-value | HR |
|---|
| 1 | 0.002 | 1.836 | <0.001 | 1.861 | <0.001 | 2.113 |
| 2 | <0.001 | 1.759 | <0.001 | 2.388 | <0.001 | 2.239 |
| 3 | <0.001 | 2.025 | <0.001 | 1.834 | <0.001 | 1.942 |
| 4 | <0.001 | 2.157 | <0.001 | 1.812 | <0.001 | 1.846 |
| 5 | <0.001 | 2.041 | 0.002 | 1.666 | <0.001 | 2.109 |
| 6 | 0.005 | 1.692 | <0.001 | 1.915 | <0.001 | 1.942 |
| 7 | <0.001 | 1.860 | <0.001 | 2.371 | <0.001 | 1.968 |
| 8 | <0.001 | 2.003 | <0.001 | 2.146 | <0.001 | 1.990 |
| 9 | <0.001 | 1.902 | 0.001 | 1.656 | <0.001 | 1.823 |
| 10 | 0.001 | 1.764 | <0.001 | 2.177 | <0.001 | 1.786 |
 | Table IX.Internal validation of risk scores
for predicting overall survival in randomly sampled patients by Cox
regression analysis. |
Table IX.
Internal validation of risk scores
for predicting overall survival in randomly sampled patients by Cox
regression analysis.
|
| 200 cases | 250 cases | 300 cases |
|---|
|
|
|
|
|
|---|
| Subset no. | P-value | HR | P-value | HR | P-value | HR |
|---|
| 1 | 0.003 | 1.938 | <0.001 | 2.208 | <0.001 | 2.253 |
| 2 | <0.001 | 2.442 | <0.001 | 2.986 | <0.001 | 2.141 |
| 3 | <0.001 | 2.323 | <0.001 | 2.071 | <0.001 | 2.338 |
| 4 | <0.001 | 2.652 | <0.001 | 2.243 | <0.001 | 2.052 |
| 5 | <0.001 | 2.496 | <0.001 | 2.007 | <0.001 | 2.269 |
| 6 | <0.001 | 2.090 | <0.001 | 2.181 | <0.001 | 2.369 |
| 7 | <0.001 | 2.243 | <0.001 | 2.396 | <0.001 | 2.175 |
| 8 | <0.001 | 2.273 | <0.001 | 2.516 | <0.001 | 2.353 |
| 9 | 0.001 | 2.106 | <0.001 | 2.365 | <0.001 | 2.03 |
| 10 | <0.001 | 2.241 | <0.001 | 2.249 | <0.001 | 2.039 |
Discussion
China has a high prevalence of young patients with
BC, who exhibit a poor prognosis (2).
A number of studies have demonstrated that age (3,6,8,9,20) and molecular subtype (4,7) are
associated with survival in these patients, in addition to a larger
tumor size, higher incidence of lymph node involvement (4,5) and higher
incidence of poorly differentiated tumors (4,5). However,
to the best of our knowledge, a prediction model for these patients
has not been established. Nomograms are widely used to present
prediction models for a number of cancer types (21–23). Due
to their distinctness and clarity, nomograms are useful for
patients to understand the prognosis of their disease and for
doctors to decide the most appropriate treatment protocol.
Nomograms have been generated for BC to predict the outcome of
patients who have undergone neoadjuvant chemotherapy (24) and of patients with advanced tumors
(21). In addition, nomograms have
been established to predict axillary lymph node status (25) and loco-regional recurrence (26), thus assisting surgeons with the
decision of surgical type. The current study created and displayed
survival models as nomograms to predict the outcome of young
patients with BC.
In the current study, the prediction model for DFS
included two independent variables, N stage and molecular subtype,
which was consistent with a previous study (8). N stage represented the tumor burden and
the capacity of metastasis, while the molecular subtype represented
the biological characteristics of the tumor. Patients with the
luminal A subtype exhibited the longest DFS time, while patients
with the HER-2+ subtype exhibited the worst prognosis. A
significant difference was identified in the DFS between these
molecular subtypes, as demonstrated in previous studies (8,27).
Notably, to the best of our knowledge, the current
study is the first to introduce the concept of VFS for breast
cancer, which is defined as the time from radical surgery to the
first visceral metastasis or mortality. Previous studies have
typically used the concept of distant recurrence-free survival
(DDFS) (28), which is defined as the
time from radical surgery to the first distant metastasis or
mortality. The difference between DDFS and VFS is the metastatic
sites. Bone metastasis and distant lymph node metastasis are
included in DDFS, but not in VFS. Savci-Heijink et al
(29) reported that BC cases without
visceral metastasis exhibited improved survival rates compared with
those with visceral metastasis. It was identified that patients
with local relapse, lymph node metastasis and bone metastasis
exhibited improved survival rates compared with patients with
visceral metastasis. Therefore, the current study assumed that VFS
was a valuable measurement for prognostic prediction. The current
study identified that VFS was associated with molecular subtype, N
stage and age, but not local relapse, bone metastasis and lymph
node metastasis. This result differed from the prediction model for
DFS time, as age at diagnosis was identified as an independent
predictor for VFS time. Previous studies revealed that a younger
age is associated with a more aggressive cancer that is more likely
to metastasize to visceral organs (3–6)
Additionally, a previous study demonstrated that age is an
independent predictor of DFS and OS time (30). The current study also demonstrated
that age (<35 years) was negatively associated with VFS.
Furthermore, molecular subtype has previously been
associated with patterns of metastasis (29,31).
Patients with certain molecular subtypes, including ER−
and HER-2+ subtypes, have been associated with visceral
metastasis, while patients with an ER+ subtype have been
associated with bone metastasis (29,31,32). The
current study revealed that patients with the luminal A subtype
experienced the longest VFS time, while patients with the
HER-2+ subtype experienced the shortest VFS time and the
highest frequency of visceral metastasis. The unfavorable outcome
of patients with the HER-2+ subtype may partially be due
to the low percentage of patients in this group who experienced
targeted treatment. However, by July 2017 >75,000 patients with
HER-2+ breast cancer in China benefited from the
Herceptin Patient Assistance Program and received targeted
treatment (unpublished data), which may increase their survival
rates.
The current study identified that N stage, molecular
subtype and neoadjuvant chemotherapy were associated with OS. N
stage and molecular subtype have been associated with OS in
previous studies (8,28,29,31).
However, a significant association between OS and neoadjuvant
chemotherapy was also identified in the current study. To the best
of our knowledge, this result has not previously been reported. In
the current study, only 1 patient received neoadjuvant chemotherapy
prior to breast conservation surgery. The remaining 45 cases
received neoadjuvant chemotherapy due to the presence of initially
inoperable tumors. The prediction model demonstrated that patients
with a HER-2+ subtype, an advanced N stage or an
initially inoperable tumor exhibited unfavorable OS.
According to the survival analysis, nomograms were
created and risk scores (19) were
calculated based on the Cox regression coefficients for VFS, DFS
and OS time. Internal validation was performed in patients randomly
sampled from the total population. This validation demonstrated
that the risk scores were associated with VFS, DFS and OS time.
This suggests that the nomograms constructed following Cox
regression analysis were reliable. However, the lack of a
validation cohort is a limitation of the current study. Future
studies should collect a larger number of cases to further validate
the nomograms.
In conclusion, the current study constructed and
validated survival models displayed as nomograms to predict VFS,
DFS and OS time in young patients with BC using retrospective data
from patients <40 years old at diagnosis. In addition, the
concept of VFS was introduced. Molecular subtype and N stage were
identified as independent predictors for VFS, DFS and OS time. Age
at diagnosis was revealed to independently predict VFS and
neoadjuvant chemotherapy was identified as an unfavorable factor
for OS. Risk scores based on these survival models were established
for young patients with BC. These survival models were validated
and the current study recommends their use in the survival analysis
of young patients with BC in the future.
Acknowledgements
The authors would like to thank Professor William Au
from Shantou University Medical College (Shantou, China) for
providing assistance in editing the original manuscript.
Funding
The current study was supported by the Shantou
Health and Technology Program (grant no. 123).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and FZ designed the study. FZ conducted the
statistical analysis. HL and FZ analyzed and interpreted the data.
HL, FZ, DZ and LW were involved in the data acquisition. HL, FZ, DZ
and LW wrote the manuscript. All authors have read and approved the
final submitted manuscript. HL takes final responsibility.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants for the use of clinicopathological information. The
current study was approved by the Ethics Committee of the Cancer
Hospital of Shantou University Medical College (Guangdong,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
VFS
|
visceral metastasis-free survival
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HBV
|
hepatitis B virus
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
DeSantis CE, Bray F, Ferlay J,
Lortet-Tieulent J, Anderson BO and Jemal A: International variation
in female breast cancer incidence and mortality rates. Cancer
Epidemiol Biomarkers Prev. 24:1495–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:E279–E289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang LC, Yin WJ, Di GH, Shen ZZ and Shao
ZM: Unfavourable clinicopathologic features and low response rate
to systemic adjuvant therapy: Results with regard to poor survival
in young Chinese breast cancer patients. Breast Cancer Res Treat.
122:95–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zabicki K, Colbert JA, Dominguez FJ, Gadd
MA, Hughes KS, Jones JL, Specht MC, Michaelson JS and Smith BL:
Breast cancer diagnosis in women <or=40 versus 50 to 60 years:
Increasing size and stage disparity compared with older women over
time. Ann Surg Oncol. 13:1072–1077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goksu SS, Tastekin D, Arslan D, Gunduz S,
Tatli AM, Unal D, Salim D, Guler T and Coskun HS: Clinicopathologic
features and molecular subtypes of breast cancer in young women
(age </=35). Asian Pac J Cancer prev. 15:6665–6668. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tjokrowidjaja A, Lee CK, Houssami N and
Lord S: Metastatic breast cancer in young women: A population-based
cohort study to describe risk and prognosis. Intern Med J.
44:764–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keegan TH, DeRouen MC, Press DJ, Kurian AW
and Clarke CA: Occurrence of breast cancer subtypes in adolescent
and young adult women. Breast Cancer Res. 14:R552012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen HL, Ding A and Wang FW: Prognostic
effect analysis of molecular subtype on young breast cancer
patients. Chin J Cancer Res. 27:428–436. 2015.PubMed/NCBI
|
|
9
|
Liedtke C, Rody A, Gluz O, Baumann K,
Beyer D, Kohls EB, Lausen K, Hanker L, Holtrich U, Becker S and
Karn T: The prognostic impact of age in different molecular
subtypes of breast cancer. Breast Cancer Res Treat. 152:667–673.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Azim HA Jr, Nguyen B, Brohee S, Zoppoli G
and Sotiriou C: Genomic aberrations in young and elderly breast
cancer patients. BMC Med. 13:2662015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldhirsch A, Wood WC, Gelber RD, Coates
AS, Thürlimann B and Senn HJ; 10th St, : Gallen conference:
Progress and promise: Highlights of the international expert
consensus on the primary therapy of early breast cancer 2007. Ann
Oncol. 18:1133–1144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Plichta JK, Rai U, Tang R, Coopey SB,
Buckley JM, Gadd MA, Specht MC, Hughes KS, Taghian AG and Smith BL:
Factors associated with recurrence rates and long-term survival in
women diagnosed with breast cancer ages 40 and younger. Ann Surg
Oncol. 23:3212–3220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fredholm H, Eaker S, Frisell J, Holmberg
L, Fredriksson I and Lindman H: Breast cancer in young women: Poor
survival despite intensive treatment. PLoS One. 4:e76952009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hearne BJ, Teare MD, Butt M and Donaldson
L: Comparison of nottingham prognostic index and adjuvant online
prognostic tools in young women with breast cancer: Review of a
single-institution experience. BMJ open. 5:e0055762015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karihtala P, Winqvist R, Bloigu R and
Jukkola-Vuorinen A: Long-term observational follow-up study of
breast cancer diagnosed in women </=40 years old. Breast.
19:456–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alco G, Bozdogan A, Selamoglu D, Pilanci
KN, Tuzlali S, Ordu C, Igdem S, Okkan S, Dincer M, Demir G and
Ozmen V: Clinical and histopathological factors associated with
Ki-67 expression in breast cancer patients. Oncol Lett.
9:1046–1054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Saboorian MH, Frenkel E, Hynan L,
Gokaslan ST and Ashfaq R: Laboratory assessment of the status of
Her-2/neu protein and oncogene in breast cancer specimens:
Comparison of immunohistochemistry assay with fluorescence in situ
hybridisation assays. J Clin Pathol. 53:374–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shukla S, Pia Patric IR, Thinagararjan S,
Srinivasan S, Mondal B, Hegde AS, Chandramouli BA, Santosh V,
Arivazhagan A and Somasundaram K: A DNA methylation prognostic
signature of glioblastoma: Identification of NPTX2-PTEN-NF-kappaB
nexus. Cancer Res. 73:6563–6573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang LC, Jin X, Yang HY, He M, Chang H,
Shao ZM and Di GH: Luminal B subtype: A key factor for the worse
prognosis of young breast cancer patients in China. BMC Cancer.
15:2012015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee CK, Hudson M, Stockler M, Coates AS,
Ackland S, Gebski V, Lord S, Friedlander M, Boyle F and Simes RJ: A
nomogram to predict survival time in women starting first-line
chemotherapy for advanced breast cancer. Breast cancer Res Treat.
129:467–476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vernerey D, Huguet F, Vienot A, Goldstein
D, Paget-Bailly S, Van Laethem JL, Glimelius B, Artru P, Moore MJ,
André T, et al: Prognostic nomogram and score to predict overall
survival in locally advanced untreated pancreatic cancer (PROLAP).
Br J Cancer. 115:281–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang W, Zhang L, Jiang G, Wang Q, Liu L,
Liu D, Wang Z, Zhu Z, Deng Q, Xiong X, et al: Development and
validation of a nomogram for predicting survival in patients with
resected non-small-cell lung cancer. J Clin Oncol. 33:861–869.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keam B, Im SA, Park S, Nam BH, Han SW, Oh
DY, Kim JH, Lee SH, Han W, Kim DW, et al: Nomogram predicting
clinical outcomes in breast cancer patients treated with
neoadjuvant chemotherapy. J Cancer Res Clin Oncol. 137:1301–1308.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu SQ, Zeng HC, Zhang F, Chen C, Huang
WH, Pleijhuis RG, Wu JD, van Dam GM and Zhang GJ: A nomogram to
predict the probability of axillary lymph node metastasis in early
breast cancer patients with positive axillary ultrasound. Sci Rep.
6:211962016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Witteveen A, Vliegen IM, Sonke GS, Klaase
JM, MJ IJ and Siesling S: Personalisation of breast cancer
follow-up: A time-dependent prognostic nomogram for the estimation
of annual risk of locoregional recurrence in early breast cancer
patients. Breast Cancer Res Treat. 152:627–636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song N, Choi JY, Sung H, Jeon S, Chung S,
Park SK, Han W, Lee JW, Kim MK, Lee JY, et al: Prediction of breast
cancer survival using clinical and genetic markers by tumor
subtypes. PLoS One. 10:e01224132015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hennigs A, Riedel F, Gondos A, Sinn P,
Schirmacher P, Marmé F, Jäger D, Kauczor HU, Stieber A, Lindel K,
et al: Prognosis of breast cancer molecular subtypes in routine
clinical care: A large prospective cohort study. BMC Cancer.
16:7342016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Savci-Heijink CD, Halfwerk H, Hooijer GK,
Horlings HM, Wesseling J and van de Vijver MJ: Retrospective
analysis of metastatic behaviour of breast cancer subtypes. Breast
Cancer Res Treat. 150:547–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Y, Dong X, Li R, Song J and Zhang D:
Correlation between clinical-pathologic factors and long-term
follow-up in young breast cancer patients. Transl Oncol. 8:265–272.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kast K, Link T, Friedrich K, Petzold A,
Niedostatek A, Schoffer O, Werner C, Klug SJ, Werner A, Gatzweiler
A, et al: Impact of breast cancer subtypes and patterns of
metastasis on outcome. Breast Cancer Res Treat. 150:621–629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bartmann C, Diessner J, Blettner M,
Häusler S, Janni W, Kreienberg R, Krockenberger M, Schwentner L,
Stein R, Stüber T, et al: Factors influencing the development of
visceral metastasis of breast cancer: A retrospective multi-center
study. Breast. 31:66–75. 2017. View Article : Google Scholar : PubMed/NCBI
|