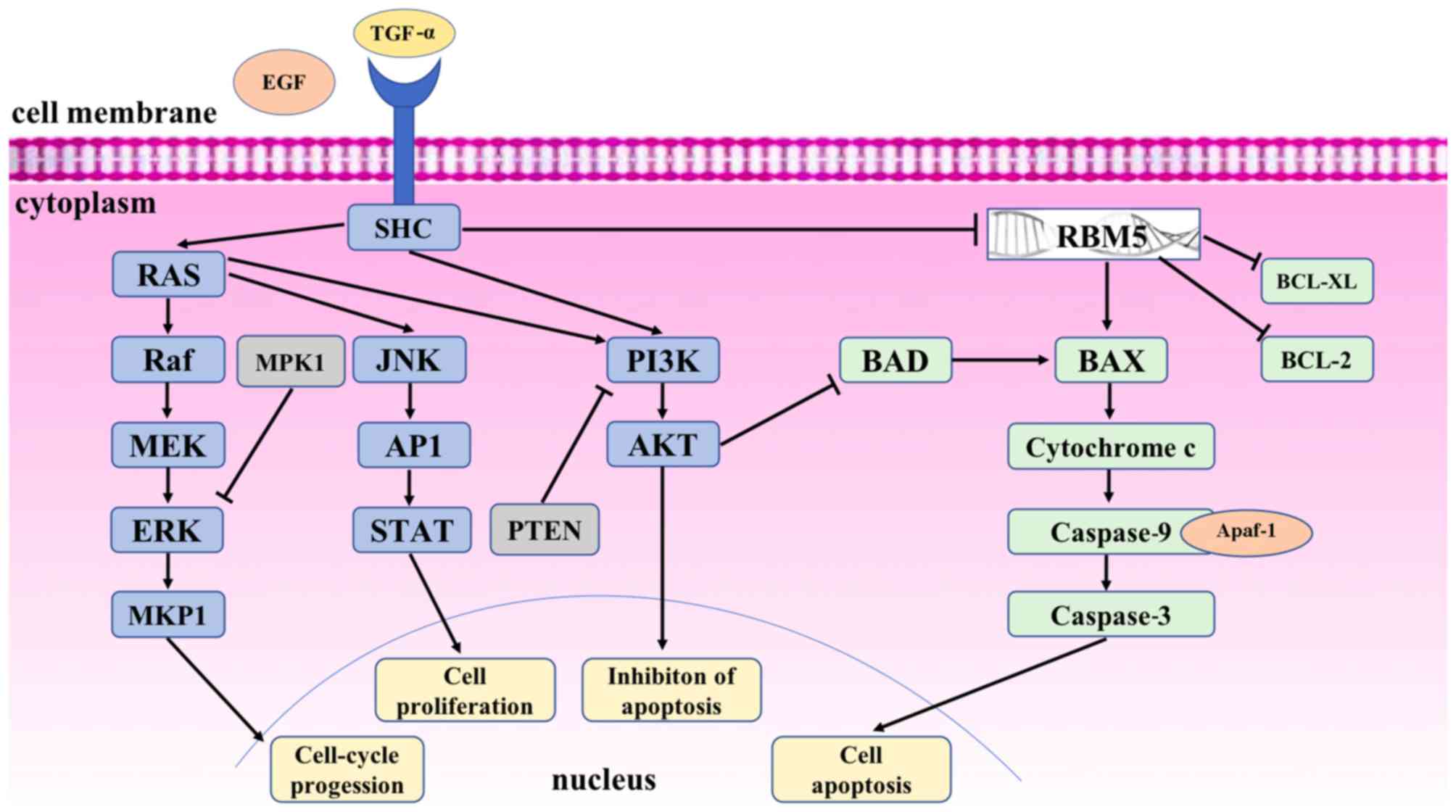
Lung cancer is one of the most common cancer types
and one of the leading causes of cancer-associated mortality
worldwide. In 2012, lung cancer accounted for ~13% of all cancer
cases and 26% of cancer-associated mortality, according to recent
data (1). In China alone, there were
733,000 new lung cancer cases diagnosed in 2011 (17% of all new
cancer cases), and 600,200 lung cancer-associated mortalities (22%
of all cancer-associated mortalities) (2). Histologically, lung cancer is classified
as small cell lung cancer (SCLC) and non-small cell lung cancer
(NSCLC), the latter of which accounts for up to 85% of all lung
cancer cases (3). NSCLC may be
further sub-categorized as adenocarcinoma, squamous cell carcinoma,
large cell carcinoma and numerous other less common types of
cancer, including pleomorphic, carcinoid tumor or undifferentiated
carcinoma; however, lung adenocarcinoma makes up 44% and lung
squamous cell carcinoma makes up 29% of all NSCLC cases clinically.
At present, ~79% of NSCLC patients are diagnosed at advanced stages
of the disease, when surgery is not a viable option (4). Thus, early detection, optimal tumor
resection and effective chemotherapy, radiotherapy, immunotherapy
and tumor-targeting therapy are important for the effective control
of NSCLC. Therefore, a better understanding of NSCLC carcinogenesis
and the underlying molecular mechanisms is key to developing novel
early diagnosis strategies and improving treatment responses for
NSCLC.
Lung carcinogenesis, like most human cancer types,
is a complex molecular process involving aberrant cell
proliferation (5) and apoptosis
(6), which leads to the
transformation of normal cells into malignant cells and subsequent
cell invasion and metastasis. Cell transformation occurs through
genetic mutations, loss of cell growth/critical genes, or
epigenetic alterations in genomic DNA that silence tumor suppressor
genes or activate oncogenes (7),
resulting in abnormal cell-cell communication (8), DNA repair (9), chromosome stability (10) and cell motility (6,11).
Recently, NSCLC was reported to exhibit abnormal expression of
epidermal growth factor receptor (EGFR) (12–14), c-Met
(15), thyroid transcription factor 1
(TTF-1) (16), phosphoinositide
3-kinase/Rac-α serine/threonine-protein
kinase/serine-threonine-protein kinase mTOR signaling (17), Ras-Raf-Mek-extracellular
signal-regulated kinase signaling (18,19) and
the echinoderm microtubule associated protein like 4-ALK receptor
tyrosine kinase fusion gene (20,21). In
addition, NSCLC may present with alterations in tumor suppressor
genes, including RB transcriptional corepressor 1 (RB), p16-RB
(16) and p14-MDM2
proto-oncogene-cellular tumor antigen p53 (p53) signaling (22), and other regulatory molecules,
including microRNAs (23) or
angiogenesis factors such as vascular endothelial growth factor
(24). However, despite marked
progress in understanding the molecular basis of human
tumorigenesis, including lung cancer, a number of crucial genes and
functions remain undefined. For example, RNA-binding protein 5
(RBM5) is localized at chromosome 3p21.3, a critical region
associated with lung carcinogenesis. RBM5 regulates cell growth,
cell cycle progression and apoptosis. Aberrant RBM5 protein
expression leads to the transformation of normal bronchial cells,
lung carcinogenesis, and alters the response of patients with lung
cancer to treatment (24). In this
review, the role of RBM5 in lung cancer is summarized.
RBM5, also referred to as g15, LUCA-15 and H37, was
initially cloned from a tumor suppressor gene (TSG) mapping area at
chromosome 3p21.3 (25). RBM5 cDNA
contains a full-length 815-amino acid open reading frame, with a
predicted protein weight of ~90 kDa (25). The RBM5 protein has two zinc finger
motifs, two RNA binding motifs and a bipartite nuclear signal. RBM5
localizes to the cell nucleus where it processes transcribed RNA,
due to its DNA/RNA binding function (26). Earlier studies reported that the
N-terminal of human RBM5 contains an RNA binding domain and RBM5
epitope marker (27), and that it had
a priority function involving the poly(G) RNA polymer in
vitro (28). At the C-terminal,
RBM5 contains multiple regions, including a rich glutamine domain
and a specific site for RNA and DNA binding proteins (28). RBM5 is widely expressed in various
human tissues, particularly during embryonic development and in the
adult thymus, although it is expressed at low levels in the fetal
thymus (27) and normal lung
(28). Another study reported a
series of splice variants in RBM5 (29). In a normal lung, expression levels of
the short transcript of RBM5 are higher compared with lung cancer
cell lines, suggesting that the short RBM5 transcript may
contribute to its tumor suppressor function in lung cancer
(28).
Biologically, RBM5 facilitates DNA/RNA binding to
process transcribed RNA, and regulates cell cycle progression and
apoptosis during sperm maturation, bone and cardiac cell
differentiation (30–32). Specifically, RBM5 may modulate the
alternative splicing of apoptosis-associated pre-mRNAs, including
caspase 2 (CASP2) and Fas cell surface death receptor (FAS/CD95),
to regulate cellular apoptosis (33,34). RBM5
may also upregulate the pro-apoptotic apoptosis regulator BAX
protein, reduce mitochondrial cytochrome c release into the cytosol
and activate caspase 9 and 3, whilst also downregulating the
anti-apoptotic apoptosis regulator BCL-2 (BCL-2) and BCL-2 like 1
proteins (30,35,36)
[Fig. 1; modified from (37)]. These data suggest that RBM5 may be
able to activate the mitochondrial apoptosis pathway. Indeed, RBM5
is able to manipulate the pre-mRNA splicing of multiple target
genes, including p53 (30,35,36,38–42).
Kobayashi et al (43) reported
that RBM5 expression enhanced p53 mRNA expression levels and
protein expression, whereas knockdown of RBM5 using RBM5 short
hairpin RNA inhibited p53 transcription and protein expression,
indicating that RBM5 regulates p53-mediated cell apoptosis.
Furthermore, RBM5 is able to regulate apoptosis and cell cycle
progression by increasing signal transducer and activator of
transcription 5B and bone morphogenetic protein 5 expression
levels, and reducing nuclear receptor coactivator 3, Pim-1
proto-oncogene serine/threonine kinase, baculoviral IAP repeat
containing 3, BCL-2, EGFR and cyclin dependent kinase 2 expression
levels (44–47). In addition, RBM5 was demonstrated to
inhibit cyclin A expression and RB phosphorylation, and thereby
regulate cell cycle progression and induce G1 arrest (30). Although initially cloned from a
TSG-mapping area at chromosome 3p21.3, RBM5 was previously
dismissed as a TSG due to the lack of RBM5 mutations and its
expression in the majority of lung cancers. Furthermore, there are
multiple RBM5 protein isoforms, each of which differentially
regulates apoptosis, leading to its inconsistent role as a tumor
suppressor gene (48); however, a
previous study did confirm its tumor suppressor function in lung
cancer and other cancer types (49).
In eukaryotic cells, gene expression is almost
completely regulated through mRNA splicing, and selective mRNA
splicing ensures the diversity of functional proteins in cells
(50). Thus, the accuracy and
effectiveness of mRNA splicing are essential for maintaining
homeostasis in eukaryotic cells. Defects in mRNA splicing are
associated with various human diseases (2,51–53). In this regard, RBM5 is involved in the
selective mRNA splicing of apoptosis and cell cycle-associated
genes (see above). For example, Fushimi et al (34) demonstrated that RBM5 regulated CASP2
splicing and expression in order to promote tumor suppression,
whereas alternative splicing of CASP2 led to a loss of tumor
suppressor activity. Thus, modulation of mRNA splicing regulators,
like RBM5, may provide a novel therapeutic strategy to control
human cancer. Bonnal et al (33) demonstrated that RBM5 was involved in
recognizing the mRNA 3′-splice site in order to regulate the
alternative splicing of apoptosis-associated mRNAs and their
isoforms (including Fas receptor) in angiogenesis and apoptosis.
RBM5 is unable to influence the early events of mRNA splicing for
FAS at exon 6; however, RBM5 is able to inhibit the transition from
the pre-spliceosome around FAS exon 6 into the mature spliceosome
between the flanking FAS introns to induce DNA sequence-specific
pairing in the distal mRNA splicing site. Jin et al
(54) reported that RBM5
overexpression significantly induced exon 4 skipping of
activation-induced cytidine deaminase by suppressing intron 3
splicing. This inhibitory effect required a weak mRNA 3′-splice
site. As a result, RBM5 is able interfere with the binding of
splicing factor U2AF 65 kDa subunit to polypyrimidines at the mRNA
3′-splice site in vitro (50).
Taken together, the selective functions and alterations of RBM5 may
alter the cell cycle and apoptosis, resulting in human
tumorigenesis.
Furthermore, previous studies demonstrated that
allele loss in a 370 kb region at chromosome 3p21.3 was the
earliest alteration detected in pre-malignant lesions of lung
cancer, or even in the histologically normal lung epithelium of
tobacco smokers (63). Thus, Timmer
et al (25) performed a
comparative genomic structure and expression pattern analysis of
this chromosomal region and identified RBM5. Specifically, RBM5 was
involved in EGFR downregulation to prevent lung cancer cell
proliferation, angiogenesis, invasion and metastasis (64,65).
One potential EGFR binding partner, the
proto-oncoprotein human epidermal growth factor receptor 2
(HER2)/ErbB2, was reported to be overactive in a small percentage
of patients with SCLC and non-smoker-associated NSCLC cases
(66,67). These activating mutations of EGFR,
KRAS and HER2 are mutually exclusive events in lung cancer
(68,69). Notably, HER2 overexpression affects
the alternative splicing of RBM5 (48). In light of these advances in lung
cancer research, it may be speculated that future studies on RBM5
and its potential tumor suppressor activity should consider
histological subtypes as well as tobacco smoking history and the
mutation status of RBM5 in lung cancer initiation and/or
progression. A previous in vitro study reported that RBM5
downregulation and activation of the Wnt/β-catenin signaling
pathway are involved in cigarette smoke extract-induced lung
epithelial injury, and that RBM5 functions as an upstream molecule
to downregulate Wnt/β-catenin signaling (70). Interestingly, RBM5 knockout mice
develop lung cancer at similar rates compared with those in wild
type mice following exposure to nicotine-derived nitrosamine
ketone. Loss of RBM5 expression leads to more aggressive lung
cancer. Thus, reduced RBM5 expression and tobacco use increase the
risk of an aggressive lung cancer phenotype (64).
Lung cancer pathogenesis is multifactorial and
results from the interaction between genetic and environmental
factors. At the molecular level, genetic alterations are the most
direct causes of lung cancer, and lung cancer development is
associated with the deletion of tumor suppressor gene loci,
including 3p21.3 (52,26), which may be observed in >90% of
SCLC and in 50–80% of NSCLC cases (71). RBM5 expression levels are low in
Ras-transformed rat embryonic fibroblasts (72), human vestibular schwannoma cells
(73), human prostate cancer
(74), ovarian cancer (74,75), human
breast cancer tissue (48), human
pancreatic cancer tissues (76) and
lung cancer (77). Overexpression of
RBM5 suppresses the growth of prostate cancer cells in vitro
(74,75), and RBM5 expression is associated with
lung cancer histological subtypes and tobacco use (78).
Although RBM5 expression is frequently reduced in
lung, renal and breast cancer (79),
RBM5 is not deleted in the majority of lung cancer cases (80). The reduced levels of RBM5 mRNA and
protein in NSCLC compared with levels in normal lung tissues are
associated with increased EGFR and KRAS expression levels, which
are associated with tobacco use, advanced tumor stage and lymph
node metastasis (81). Another study
reported that RBM5 expression levels were significantly reduced in
lung squamous cell tissues and were further associated with
deletions at chromosome 3p21.3 and tobacco use (71,78),
whereas three out of nine patients with lung adenocarcinoma did not
have significant decreases in RBM5 mRNA expression levels (lung
adenocarcinoma development may be associated with tobacco use,
among which 50% of cases are associated with chromosome 3p21.3
deficiency) (78). Furthermore, Oh
et al (82) reported that RBM5
expression was generally lower in lung cancer compared with normal
lung tissues. Thus, detection of RBM5 expression may be a useful
tumor marker for lung cancer.
RBM5 expression is able to suppress the growth of
mouse fibrosarcoma cells or lung adenocarcinoma cells in nude mouse
models (30,82,83).
Notably, a number of genes were mapped to the common deletion
region of chromosome 3p21.3, including RBM5 (25), FUS RNA binding protein (84), Ras association domain family member 1
(85), semaphorin 3B (86), semaphorin 3F (49), hyaluronidase 1 (49) and calcium voltage-gated channel
auxiliary subunit α2δ2 (87), and had
the ability to modulate lung cancer cell apoptosis. Reduced
expression levels of RBM5, as one of nine downregulated genes in
this 17-gene metastatic signature for solid tumors (including lung
cancer) in humans and mice, was considered important for the
development and/or progression of a wide range of human cancer
types (88,89). Indeed, a recent study demonstrated
that downregulation of RBM5 expression levels may be the key step
in malignant lung cell transformation, and that RBM5 is responsible
for inhibiting cell cycle progression and inducing apoptosis, in
addition to suppressing tumor cell transformation-associated
events, including angiogenesis, in SCLC cells (90). Furthermore, at the gene level, a
constitutively activated RAS mutant protein (G12V) was demonstrated
to be responsible for RBM5 downregulation in rat embryonic
fibroblasts (72). Therefore, RBM5
may be a tumor marker for SCLC, and targeting RBM5 may be a
potential novel and effective therapeutic option for controlling
SCLC.
Thus far, the published data indicate that RBM5 is a
lung cancer regulatory protein; however, the detection of various
RBM5 isoforms may also be used to the determine association between
lung cancer histological subtype and tobacco use, or even RBM5
mutation status (40). Moreover,
further research on RBM5 alterations associated with other genes in
the transforming growth factor signaling pathway is warranted
(37). For example, RBM5 was able to
post-transcriptionally regulate RBM10 expression by directly
interacting with specific RBM10 splice variants (91).
As discussed above, RBM5 mRNA and protein expression
levels are significantly reduced in different human cancer types,
including lung cancer; thus, targeting RBM5 may be a novel
therapeutic strategy for treating lung cancer. Indeed, a previous
study revealed that cisplatin-resistant lung adenocarcinoma
A549/DDP cells expressed decreased levels of RBM5 compared with
parental A549 cells (41).
Furthermore, knockdown of RBM5 expression with small interfering
RNA in parental A549 cells reduced cisplatin-induced apoptosis. By
contrast, exogenous RBM5 expression using a plasmid carrying RBM5
cDNA enhanced the sensitivity of A549/DDP cells to cisplatin
treatment (41). In addition,
RBM5-enhanced chemosensitivity to cisplatin was associated with
cytochrome c release into the cytosol and subsequent activation of
CASP9 and CASP3 (38). RBM5
expression inhibits the growth of human lung cancer cells by
inducing cell cycle arrest and apoptosis (30). A previous study demonstrated the
importance of RBM5 protein expression in normal lung cells and the
consequences of RBM5 deletion in SCLC development and progression
(90). RBM5 expression slowed the
growth of SCLC cells in vitro and increased the sensitivity
of tumor cells to cisplatin. Moreover, RBM5 expression inhibited
SCLC cell cycle progression and reduced tumor cell membrane
integrity (increase in apoptosis) following treatment with
cisplatin. In this regard, reduced RBM5 expression was observed in
95% of SCLC cases, indicating the importance of altered RBM5
expression levels in SCLC development (76). Thus, a therapeutic strategy involving
RBM5 and/or its direct target genes or pathways may be a very
effective approach. In addition, detection of RBM5 expression might
be useful for predicting response to chemotherapy in patients with
lung cancer. The mechanism by which RBM5 affects gefitinib
resistance in lung adenocarcinoma is currently being investigated
(Xu et al, unpublished data).
Environmental factors, including tobacco use, may
interact with human genes to cause cancer development and
progression. As indicated in 2000 by Hanahan and Weinberg (6), there are six essential hallmarks of
cancer cells: i) Self-sufficiency in growth signals; ii)
insensitivity to growth-inhibitory signals; iii) evasion of
apoptosis; iv) limitless replicative potential; v) sustained
angiogenesis; and vi) tissue invasion and metastasis. RBM5
possesses at least two of these hallmarks. Thus, further
investigations into RBM5 alterations, including chromosomal
deletion, loss of heterozygosity, DNA methylation and/or gene-gene
interactions, may lead to a better understanding of how RBM5
functions in lung cancer, leading to the development of a novel
therapeutic strategy for the treatment of lung cancer. Further
research on RBM5 may provide a novel mechanism to induce RBM5
expression or activate its downstream gene pathways. Moreover,
detection of RBM5 expression, or its isoforms, may lead to early
diagnosis of lung cancer and improve prognosis of patients with
lung cancer.
Not applicable.
This review was supported in part by a grant from
Nature Science Foundation of China (grant no. 81472169).
Not applicable.
YX and PG participated in writing the original draft
and in the design of this review; ZS participated in writing the
original draft; JL, QW and GM researched the relevant literature;
YZ participated in writing and editing the manuscript; WY managed
the references and participated in the design of this review; JZ
participated in the design of the review and in acquiring funding.
All authors contributed substantially to the writing of this
review. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Cancer Society, Cancer Facts
& Figures, 2016. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.htmlJanuary
20–2018
|
|
4
|
Carrato A, Vergnenègre A, Thomas M,
McBride K, Medina J and Cruciani G: Clinical management patterns
and treatment outcomes in patients with non-small cell lung cancer
(NSCLC) across Europe: EPICLIN-Lung study. Curr Med Res Opin.
30:447–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hunt T and Nasmyth K: Cell multiplication.
Curr Opin Cell Biol. 9:765–767. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mueller BM, Yu YB and Laug WE:
Overexpression of plasminogen activator inhibitor 2 in human
melanoma cells inhibits spontaneous metastasis in scid/scid mice.
Proc Natl Acad Sci USA. 92:205–209. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirschi KK, Xu CE, Tsukamoto T and Sager
R: Gap junction genes Cx26 and Cx43 individually suppress the
cancer phenotype of human mammary carcinoma cells and restore
differentiation potential. Cell Growth Differ. 7:861–870.
1996.PubMed/NCBI
|
|
9
|
Sancar A: DNA repair in humans. Annu Rev
Genet. 29:69–105. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanawalt PC: Transcription-coupled repair
and human disease. Science. 266:1957–1958. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ichihara S, Toyooka S, Fujiwara Y, Hotta
K, Shigematsu H, Tokumo M, Soh J, Asano H, Ichimura K, Aoe K, et
al: The impact of epidermal growth factor receptor gene status on
gefitinib-treated Japanese patients with non-small-cell lung
cancer. Int J Cancer. 120:1239–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soh J, Okumura N, Lockwood WW, Yamamoto H,
Shigematsu H, Zhang W, Chari R, Shames DS, Tang X, MacAulay C, et
al: Oncogene mutations, copy number gains and mutant allele
specific imbalance (MASI) frequently occur together in tumor cells.
PLoS One. 4:e74642009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei Y, Zou Z, Becker N, Anderson M,
Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, et
al: EGFR-mediated Beclin 1 phosphorylation in autophagy
suppression, tumor progression, and tumor chemoresistance. Cell.
154:1269–1284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Menges CW, Kadariya Y, Altomare D,
Talarchek J, Neumann-Domer E, Wu Y, Xiao GH, Shapiro IM, Kolev VN,
Pachter JA, et al: Tumor suppressor alterations cooperate to drive
aggressive mesotheliomas with enriched cancer stem cells via a
p53-miR-34a-c-Met axis. Cancer Res. 74:1261–1271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toyooka S, Mitsudomi T, Soh J, Aokage K,
Yamane M, Oto T, Kiura K and Miyoshi S: Molecular oncology of lung
cancer. Gen Thorac Cardiovasc Surg. 59:527–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo Y, Du J and Kwiatkowski DJ: Molecular
dissection of AKT activation in lung cancer cell lines. Mol Cancer
Res. 11:282–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Collisson EA, Trejo CL, Silva JM, Gu S,
Korkola JE, Heiser LM, Charles RP, Rabinovich BA, Hann B, Dankort
D, et al: A central role for RAF→MEK→ERK signaling in the genesis
of pancreatic ductal adenocarcinoma. Cancer Discov. 2:685–693.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16 Suppl
2:S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suda K, Tomizawa K, Yatabe Y and Mitsudomi
T: Lung cancers unrelated to smoking: Characterized by single
oncogene addiction? Int J Clin Oncol. 16:294–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu SG, Kuo YW, Chang YL, Shih JY, Chen YH,
Tsai MF, Yu CJ, Yang CH and Yang PC: EML4-ALK translocation
predicts better outcome in lung adenocarcinoma patients with
wild-type EGFR. J Thorac Oncol. 7:98–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paci M, Rapicetta C and Maramotti S: New
biomarkers for lung cancer. Expert Opin Med Diagn. 4:201–224. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen J, Liu Z, Todd NW, Zhang H, Liao J,
Yu L, Guarnera MA, Li R, Cai L, Zhan M and Jiang F: Diagnosis of
lung cancer in individuals with solitary pulmonary nodules by
plasma microRNA biomarkers. BMC Cancer. 11:3742011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Claesson-Welsh L: Blood vessels as targets
in tumor therapy. Ups J Med Sci. 117:178–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Timmer T, Terpstra P, van den Berg A,
Veldhuis PM, Ter Elst A, Voutsinas G, Hulsbeek MM, Draaijers TG,
Looman MW, Kok K, et al: A comparison of genomic structures and
expression patterns of two closely related flanking genes in a
critical lung cancer region at 3p21.3. Eur J Hum Genet. 7:478–486.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daigo Y, Nishiwaki T, Kawasoe T, Tamari M,
Tsuchiya E and Nakamura Y: Molecular cloning of a candidate tumor
suppressor gene, DLC1, from chromosome 3p21.3. Cancer Res.
59:1966–1972. 1999.PubMed/NCBI
|
|
27
|
Aravind L and Koonin EV: G-patch: A new
conserved domain in eukaryotic RNA-processing proteins and type D
retroviral polyproteins. Trends Biochem Sci. 24:342–344. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Drabkin HA, West JD, Hotfilder M, Heng YM,
Erickson P, Calvo R, Dalmau J, Gemmill RM and Sablitzky F:
DEF-3(g16/NY-LU-12), an RNA binding protein from the 3p21.3
homozygous deletion region in SCLC. Oncogene. 18:2589–2597. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sutherland LC, Rintala-Maki ND, White RD
and Morin CD: RNA binding motif (RBM) proteins: A novel family of
apoptosis modulators? J Cell Biochem. 94:5–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh JJ, Razfar A, Delgado I, Reed RA,
Malkina A, Boctor B and Slamon DJ: 3p21.3 tumor suppressor gene
H37/Luca15/RBM5 inhibits growth of human lung cancer cells through
cell cycle arrest and apoptosis. Cancer Res. 66:3419–3427. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loiselle JJ and Sutherland LC:
Differential downregulation of Rbm5 and Rbm10 during skeletal and
cardiac differentiation. In Vitro Cell Dev Biol Anim. 50:331–339.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Bryan MK, Clark BJ, McLaughlin EA,
D'Sylva RJ, O'Donnell L, Wilce JA, Sutherland J, O'Connor AE,
Whittle B, Goodnow CC, et al: RBM5 is a male germ cell splicing
factor and is required for spermatid differentiation and male
fertility. PLoS Genet. 9:e10036282013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonnal S, Martinez C, Förch P, Bachi A,
Wilm M and Valcárcel J: RBM5/Luca-15/H37 regulates Fas alternative
splice site pairing after exon definition. Mol Cell. 32:81–95.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fushimi K, Ray P, Kar A, Wang L,
Sutherland LC and Wu JY: Up-regulation of the proapoptotic caspase
2 splicing isoform by a candidate tumor suppressor, RBM5. Proc Natl
Acad Sci USA. 105:15708–15713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mourtada-Maarabouni M, Sutherland LC and
Williams GT: Candidate tumour suppressor LUCA-15 can regulate
multiple apoptotic pathways. Apoptosis. 7:421–432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sutherland LC, Lerman M, Williams GT and
Miller BA: LUCA-15 suppresses CD95-mediated apoptosis in Jurkat T
cells. Oncogene. 20:2713–2719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sutherland LC, Wang K and Robinson AG:
RBM5 as a putative tumor suppressor gene for lung cancer. J Thorac
Oncol. 5:294–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li P, Wang K, Zhang J, Zhao L, Liang H,
Shao C and Sutherland LC: The 3p21.3 tumor suppressor RBM5
resensitizes cisplatin-resistant human non-small cell lung cancer
cells to cisplatin. Cancer Epidemiol. 36:481–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mourtada-Maarabouni M, Sutherland LC,
Meredith JM and Williams GT: Simultaneous acceleration of the cell
cycle and suppression of apoptosis by splice variant delta-6 of the
candidate tumour suppressor LUCA-15/RBM5. Genes Cells. 8:109–119.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rintala-Maki ND and Sutherland LC:
LUCA-15/RBM5, a putative tumour suppressor, enhances multiple
receptor-initiated death signals. Apoptosis. 9:475–484. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sutherland KD, Lindeman GJ, Choong DY,
Wittlin S, Brentzell L, Phillips W, Campbell IG and Visvader JE:
Differential hypermethylation of SOCS genes in ovarian and breast
carcinomas. Oncogene. 23:7726–7733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sutherland LC, Edwards SE, Cable HC,
Poirier GG, Miller BA, Cooper CS and Williams GT: LUCA-15-encoded
sequence variants regulate CD95-mediated apoptosis. Oncogene.
19:3774–3781. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kobayashi T, Ishida J, Musashi M, Ota S,
Yoshida T, Shimizu Y, Chuma M, Kawakami H, Asaka M, Tanaka J, et
al: p53 transactivation is involved in the antiproliferative
activity of the putative tumor suppressor RBM5. Int J Cancer.
128:304–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mourtada-Maarabouni M, Keen J, Clark J,
Cooper CS and Williams GT: Candidate tumor suppressor
LUCA-15/RBM5/H37 modulates expression of apoptosis and cell cycle
genes. Exp Cell Res. 312:1745–1752. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shao C, Yang B, Zhao L, Wang S, Zhang J
and Wang K: Tumor suppressor gene RBM5 delivered by attenuated
Salmonella inhibits lung adenocarcinoma through diverse apoptotic
signaling pathways. World J Surg Oncol. 11:1232013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shao C, Zhao L, Wang K, Xu W, Zhang J and
Yang B: The tumor suppressor gene RBM5 inhibits lung adenocarcinoma
cell growth and induces apoptosis. World J Surg Oncol. 10:1602012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Su Z, Yin J, Zhao L, Li R, Liang H, Zhang
J and Wang K: Lentiviral vector-mediated RBM5 overexpression
downregulates EGFR expression in human non-small cell lung cancer
cells. World J Surg Oncol. 12:3672014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rintala-Maki ND, Goard CA, Langdon CE,
Wall VE, Traulsen KE, Morin CD, Bonin M and Sutherland LC:
Expression of RBM5-related factors in primary breast tissue. J Cell
Biochem. 100:1440–1458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zabarovsky ER, Lerman MI and Minna JD:
Tumor suppressor genes on chromosome 3p involved in the
pathogenesis of lung and other cancers. Oncogene. 21:6915–6935.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Graveley BR: Alternative splicing:
Increasing diversity in the proteomic world. Trends Genet.
17:100–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nissim-Rafinia M and Kerem B: Splicing
regulation as a potential genetic modifier. Trends Genet.
18:123–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Z, Lo HS, Yang H, Gere S, Hu Y,
Buetow KH and Lee MP: Computational analysis and experimental
validation of tumor-associated alternative RNA splicing in human
cancer. Cancer Res. 63:655–657. 2003.PubMed/NCBI
|
|
53
|
Zhou Z, Licklider LJ, Gygi SP and Reed R:
Comprehensive proteomic analysis of the human spliceosome. Nature.
419:182–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jin W, Niu Z, Xu D and Li X: RBM5 promotes
exon 4 skipping of AID pre-mRNA by competing with the binding of
U2AF65 to the polypyrimidine tract. FEBS Lett. 586:3852–3857. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
U.S. Department of Health and Human
Services: Smoking, Tobacco, and Cancer Program (1985–1989 Status
Report). Public Health Service, USA. 1990.
|
|
56
|
Hecht SS: Cigarette smoking: Cancer risks,
carcinogens, and mechanisms. Langenbecks Arch Surg. 391:603–613.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Harvey RG: Polycyclic aromatic
hydrocarbons. Chemistry and Carcinogenicity. Cambridge University
Press; Cambridge: pp. 3961991
|
|
58
|
Beland FA, Cain LG, Felton JS, et al:
Chemical Carcinogenesis and Mutagenesis I. Springer-Verlag. 33–572.
1990.
|
|
59
|
Bartsch H: DNA adducts in human
carcinogenesis: Etiological relevance and structure-activity
relationship. Mutat Res. 340:67–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mass MJ, Jeffers AJ, Ross JA, Nelson G,
Galati AJ, Stoner GD and Nesnow S: Ki-ras oncogene mutations in
tumors and DNA adducts formed by benz[j]aceanthrylene and
benzo[a]pyrene in the lungs of strain A/J mice. Mol Carcinog.
8:186–192. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Venkatachalam S, Denissenko MF, Alvi N and
Wani AA: Rapid activation of apoptosis in human promyelocytic
leukemic cells by (+/-)-anti-benzo[a]pyrene diol epoxide induced
DNA damage. Biochem Biophys Res Commun. 197:722–729. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Denissenko MF, Pao A, Tang M and Pfeifer
GP: Preferential formation of benzo[a]pyrene adducts at lung cancer
mutational hotspots in P53. Science. 274:430–432. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wistuba II, Lam S, Behrens C, Virmani AK,
Fong KM, LeRiche J, Samet JM, Srivastava S, Minna JD and Gazdar AF:
Molecular damage in the bronchial epithelium of current and former
smokers. J Natl Cancer Inst. 89:1366–1373. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jamsai D, Watkins DN, O'Connor AE,
Merriner DJ, Gursoy S, Bird AD, Kumar B, Miller A, Cole TJ, Jenkins
BJ, et al: In vivo evidence that RBM5 is a tumour suppressor in the
lung. Sci Rep. 7:163232017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Prabhu VV and Devaraj N: Regulating RNA
binding Motif 5 gene expression-a novel therapeutic target for lung
cancer. J Environ Pathol Toxicol Oncol. 36:99–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Canoz O, Ozkan M, Arsav V, Er O, Coskun
HS, Soyuer S and Altinbas M: The role of c-erbB-2 expression on the
survival of patients with small-cell lung cancer. Lung.
184:267–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hirsch FR, Franklin WA, Veve R,
Varella-Garcia M and Bunn PA Jr: HER2/neu expression in malignant
lung tumors. Semin Oncol. 29:51–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bae NC, Chae MH, Lee MH, Kim KM, Lee EB,
Kim CH, Park TI, Han SB, Jheon S, Jung TH and Park JY: EGFR, ERBB2,
and KRAS mutations in Korean non-small cell lung cancer patients.
Cancer Genet Cytogenet. 173:107–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Buttitta F, Barassi F, Fresu G, Felicioni
L, Chella A, Paolizzi D, Lattanzio G, Salvatore S, Camplese PP,
Rosini S, et al: Mutational analysis of the HER2 gene in lung
tumors from Caucasian patients: Mutations are mainly present in
adenocarcinomas with bronchioloalveolar features. Int J Cancer.
119:2586–2591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hao YQ, Su ZZ, Lv XJ, Li P, Gao P, Wang C,
Bai Y and Zhang J: RNA-binding motif protein 5 negatively regulates
the activity of Wnt/β-catenin signaling in cigarette smoke-induced
alveolar epithelial injury. Oncol Rep. 33:2438–2444. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lerman MI and Minna JD: The 630-kb lung
cancer homozygous deletion region on human chromosome 3p21.3:
Identification and evaluation of the resident candidate tumor
suppressor genes. The International Lung Cancer Chromosome 3p21.3
Tumor Suppressor Gene Consortium. Cancer Res. 60:6116–6133.
2000.PubMed/NCBI
|
|
72
|
Edamatsu H, Kaziro Y and Itoh H: LUCA15, a
putative tumour suppressor gene encoding an RNA-binding nuclear
protein, is down-regulated in ras-transformed Rat-1 cells. Genes
Cells. 5:849–858. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Welling DB, Lasak JM, Akhmametyeva E,
Ghaheri B and Chang LS: cDNA microarray analysis of vestibular
schwannomas. Otol Neurotol. 23:736–748. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhao L, Li R, Shao C, Li P, Liu J and Wang
K: 3p21.3 tumor suppressor gene RBM5 inhibits growth of human
prostate cancer PC-3 cells through apoptosis. World J Surg Oncol.
10:2472012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim YS, Hwan JD, Bae S, Bae DH and Shick
WA: Identification of differentially expressed genes using an
annealing control primer system in stage III serous ovarian
carcinoma. BMC Cancer. 10:5762010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Peng J, Valeshabad AK, Li Q and Wang Y:
Differential expression of RBM5 and KRAS in pancreatic ductal
adenocarcinoma and their association with clinicopathological
features. Oncol Lett. 5:1000–1004. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Oh JJ, Taschereau EO, Koegel AK, Ginther
CL, Rotow JK, Isfahani KZ and Slamon DJ: RBM5/H37 tumor suppressor,
located at the lung cancer hot spot 3p21.3, alters expression of
genes involved in metastasis. Lung Cancer. 70:253–262. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Toyooka S, Maruyama R, Toyooka KO,
McLerran D, Feng Z, Fukuyama Y, Virmani AK, Zochbauer-Muller S,
Tsukuda K, Sugio K, et al: Smoke exposure, histologic type and
geography-related differences in the methylation profiles of
non-small cell lung cancer. Int J Cancer. 103:153–160. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bechara EG, Sebestyén E, Bernardis I,
Eyras E and Valcárcel J: RBM5, 6, and 10 differentially regulate
NUMB alternative splicing to control cancer cell proliferation. Mol
Cell. 52:720–733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wistuba II, Behrens C, Virmani AK, Mele G,
Milchgrub S, Girard L, Fondon JW III, Garner HR, McKay B, Latif F,
et al: High resolution chromosome 3p allelotyping of human lung
cancer and preneoplastic/preinvasive bronchial epithelium reveals
multiple, discontinuous sites of 3p allele loss and three regions
of frequent breakpoints. Cancer Res. 60:1949–1960. 2000.PubMed/NCBI
|
|
81
|
Liang H, Zhang J, Shao C, Zhao L, Xu W,
Sutherland LC and Wang K: Differential expression of RBM5, EGFR and
KRAS mRNA and protein in non-small cell lung cancer tissues. J Exp
Clin Cancer Res. 31:362012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Oh JJ, West AR, Fishbein MC and Slamon DJ:
A candidate tumor suppressor gene, H37, from the human lung cancer
tumor suppressor locus 3p21.3. Cancer Res. 62:3207–3213.
2002.PubMed/NCBI
|
|
83
|
ter Elst A, Hiemstra BE, van der Vlies P,
Kamminga W, van der Veen AY, Davelaar I, Terpstra P, te Meerman GJ,
Gerbens F, Kok K, et al: Functional analysis of lung tumor
suppressor activity at 3p21.3. Genes Chromosomes Cancer.
45:1077–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ji L and Roth JA: Tumor suppressor FUS1
signaling pathway. J Thorac Oncol. 3:327–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Agathanggelou A, Bièche I, Ahmed-Choudhury
J, Nicke B, Dammann R, Baksh S, Gao B, Minna JD, Downward J, Maher
ER and Latif F: Identification of novel gene expression targets for
the Ras association domain family 1 (RASSF1A) tumor suppressor gene
in non-small cell lung cancer and neuroblastoma. Cancer Res.
63:5344–5351. 2003.PubMed/NCBI
|
|
86
|
Castro-Rivera E, Ran S, Thorpe P and Minna
JD: Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast
cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci
USA. 101:11432–11437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Carboni GL, Gao B, Nishizaki M, Xu K,
Minna JD, Roth JA and Ji L: CACNA2D2-mediated apoptosis in NSCLC
cells is associated with alterations of the intracellular calcium
signaling and disruption of mitochondria membrane integrity.
Oncogene. 22:615–626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Qiu TH, Chandramouli GV, Hunter KW,
Alkharouf NW, Green JE and Liu ET: Global expression profiling
identifies signatures of tumor virulence in MMTV-PyMT-transgenic
mice: Correlation to human disease. Cancer Res. 64:5973–5981. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ramaswamy S, Ross KN, Lander ES and Golub
TR: A molecular signature of metastasis in primary solid tumors.
Nat Genet. 33:49–54. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
90
|
Loiselle JJ, Roy JG and Sutherland LC:
RBM5 reduces small cell lung cancer growth, increases cisplatin
sensitivity and regulates key transformation-associated pathways.
Heliyon. 2:e002042016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Loiselle JJ, Roy JG and Sutherland LC:
RBM10 promotes transformation-associated processes in small cell
lung cancer and is directly regulated by RBM5. PLoS One.
12:e01802582017. View Article : Google Scholar : PubMed/NCBI
|