Introduction
Brain metastasis (BM) is a common complication of
malignant cancers in adulthood (1)
and serves as a major cause of cancer-associated cases of mortality
(2,3).
Lung cancer, particularly non-small cell lung cancer (NSCLC)
(4), is the most common primary
cancer that metastasizes to the brain. The percent of patients with
NSCLC that suffer from BM is ~10% at the onset of illness (5), with another 25–40% demonstrating
advancing BM during the disease course (6). Although the precise occurrence is
unknown, it is predicted that 35–50% of all patients with NSCLC are
afflicted with BM, based on various studies (4,7). Lung
adenocarcinoma (LAD) is a common NSCLC with a frequent incidence of
developing brain metastases and accounts for over 50% of all NSCLC
brain metastases (1). Once lung
cancer cells have spread to the brain, the patient has a very poor
prognosis. The median overall survival of untreated and treated
brain metastases patients is 4–11 weeks and 4–15 months,
respectively (8,9). Prior investigations have established
that there are two mechanisms underlying the involvement of LAD
metastasis to the brain, including activation of the Wnt/T-cell
factor signal pathway (with homeobox B9 and lymphoid enhancer
binding factor 1 expression markedly associated with metastatic
ability) and the upregulation of plasminogen activator (PA)
inhibitory serpins (10,11). Recently, Singh et al (12) revealed that Sparc/osteonectin, cwcv,
and kazal-like domains proteoglycan 1 and twist family bHLH
transcription factor 2 are necessary moderators of brain
metastasis-initiating cells in LAD and play a key role in the
metastatic process in the brain.
MicroRNAs (miRNAs or miRs) are non-coding single
stranded nucleotides between 19–24 nucleotides in length (13), which may be important factors in the
regulation of tumor invasion and metastasis, including that of LAD,
by regulating gene expression (14).
Previous studies have identified that miR-95, miR-378 and miR-145
may regulate certain key pathways in the process of lung cancer
cell metastasis to the brain (15–17).
However, there are few reports of miRNAs associated with LAD
metastasis.
A previous study has identified differentially
expressed genes (DEGs) between primary LAD and metastatic brain
tumors by cDNA microarray (18). The
results of this previous study suggest that those DEGs, including
genes coding for cytoskeletal proteins, cellular antigens and
plasma membrane proteins, may serve important roles in cell-cell
interactions. However, tumor cell metastasis to the brain is a
complicated multi-step process and other important molecules that
may be involved, and how they regulate the mechanisms of
metastasis, remain largely unknown. Therefore, the current study
analyzed the GSE14108 and GSE10245 microarray datasets to identify
DEGs in primary LADs and BM. Furthermore, the current study
performed enrichment analysis and protein-protein interaction (PPI)
network construction. Additionally, the hub genes were combined
with the TargetScan database to construct a miRNA-target regulatory
network. These comprehensive bioinformatics methods provided an
opportunity to identify effective biomarkers for the prognosis of
LAD metastasis and to better illuminate the underlying molecular
pathogenesis of BM.
Materials and methods
Data resources
The raw data from the GSE14108 and GSE10245
microarray datasets were downloaded from the Gene Expression
Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo/) database. From the
datasets, 19 LAD-BM samples (GSE14108) (19) and 40 LAD samples (GSE10245) (20) were used in the current study. The
platform used for the detection of these microarray data was the
GPL570 Human Genome U133 Plus 2.0 Array (Affymetrix; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Pre-treatment and DEG analysis
Raw microarray data were pre-processed using the
Affy package (version 1.48.0; http://bioconductor.org/packages/release/bioc/html/affy.html);
robust multi-array average (RMA) was used for background adjustment
and the linear scaling method was applied for normalization. Then,
the standardized data were summarized based on the Perfect
Match-Mismatch difference model (21). The intensity of gene expression was
calculated from the hybridization signal of the probe-set
(containing multiple probes). When multiple probes corresponded to
the same gene, the average value of probe expression was considered
the gene expression value. Using the limma package of R software
(version 3.26.9; http://bioconductor.org/packages/release/bioc/html/limma.html),
DEGs were identified by comparing the LAD-BM samples with the LAD
samples (22). All DEGs were selected
according to the following criteria: |log2 (fold-change)| ≥1.5 and
a false discovery rate of adjusted P<0.001.
Functional enrichment analysis of
DEGs
To identify the potential biological processes that
were affected, the Bioconductor package ‘Cluster Profiler’ of R
software (version 3.2.2; http://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html)
(23,24) was used to classify the enriched Gene
Ontology (GO) terms. Information in the Kyoto Encyclopedia of Genes
and Genomes (KEGG) (http://www.genome.jp/kegg/pathway.html) database was
used for the pathway enrichment analysis of DEGs (25). P<0.05 was considered to indicate a
statistically significant selection of GO terms and KEGG
pathways.
PPI network analysis of the DEGs
The PPI networks of the DEGs were constructed
utilizing the Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING; string-db.org)
database (26) and visualized using
Cytoscape software (version 3.6.1; http://www.cytoscape.org/). From 787,896 pairs of
human protein interactions containing 16,730 genes, DEG-containing
interactions were obtained. STRING (https://string-db.org) utilized a combined score (0–1)
(27) to assess reliability.
Spearman's correlation coefficient was implemented to assess the
edge scores, which evaluated the probability of two co-expressed
gene pairs in the current study. Each protein was regarded as a
node in the network, and the degree of a node was regarded as the
number of interactions with other nodes. Hub genes were nodes with
≥50 degrees.
Establishment of the miRNA-target
regulatory network
The TargetScore (version 1.12.0) R package (28) was used to predict biological
miRNA-gene interactions based on the TargetScan database
(http://www.targetscan.org). The current
study combined ‘targetscan context+score’ with ‘probabilities of
conserved targeting’ of the hub gene to calculate the gene
‘targetscore’ value, utilizing a variational Bayesian-Gaussian
mixture model (28). The miRNAs were
identified as miRNA-gene interactions according to the criterion
‘targetscore’ >0.4. The miRNA-gene regulatory network was
subsequently visualized using Cytoscape software (version
3.6.1).
Results
Identification of DEGs in LAD-BM
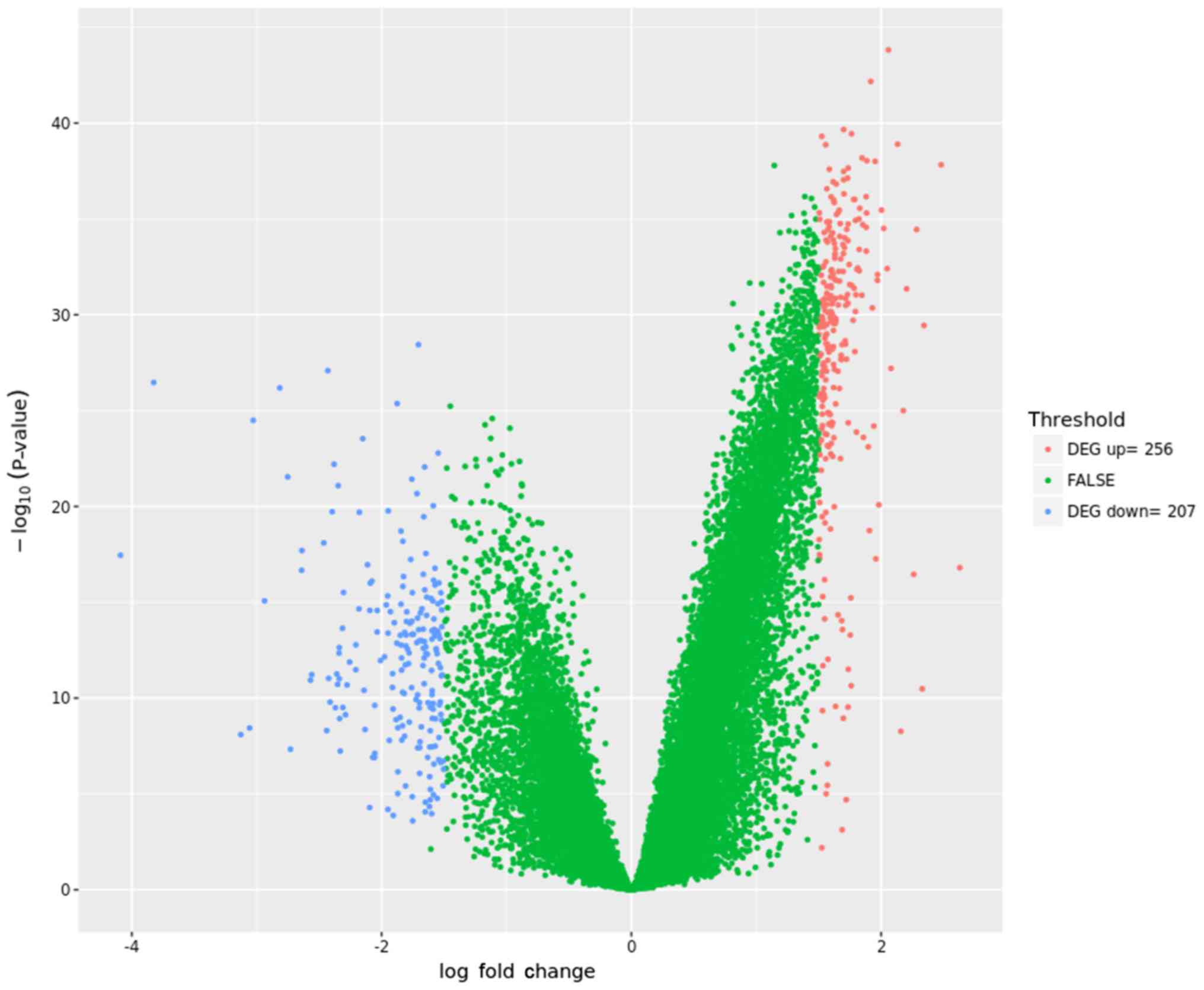
Overall, the current study identified 463 DEGs (256
upregulated and 207 downregulated) between the LAD-BM and LAD
samples. A volcano plot (Fig. 1)
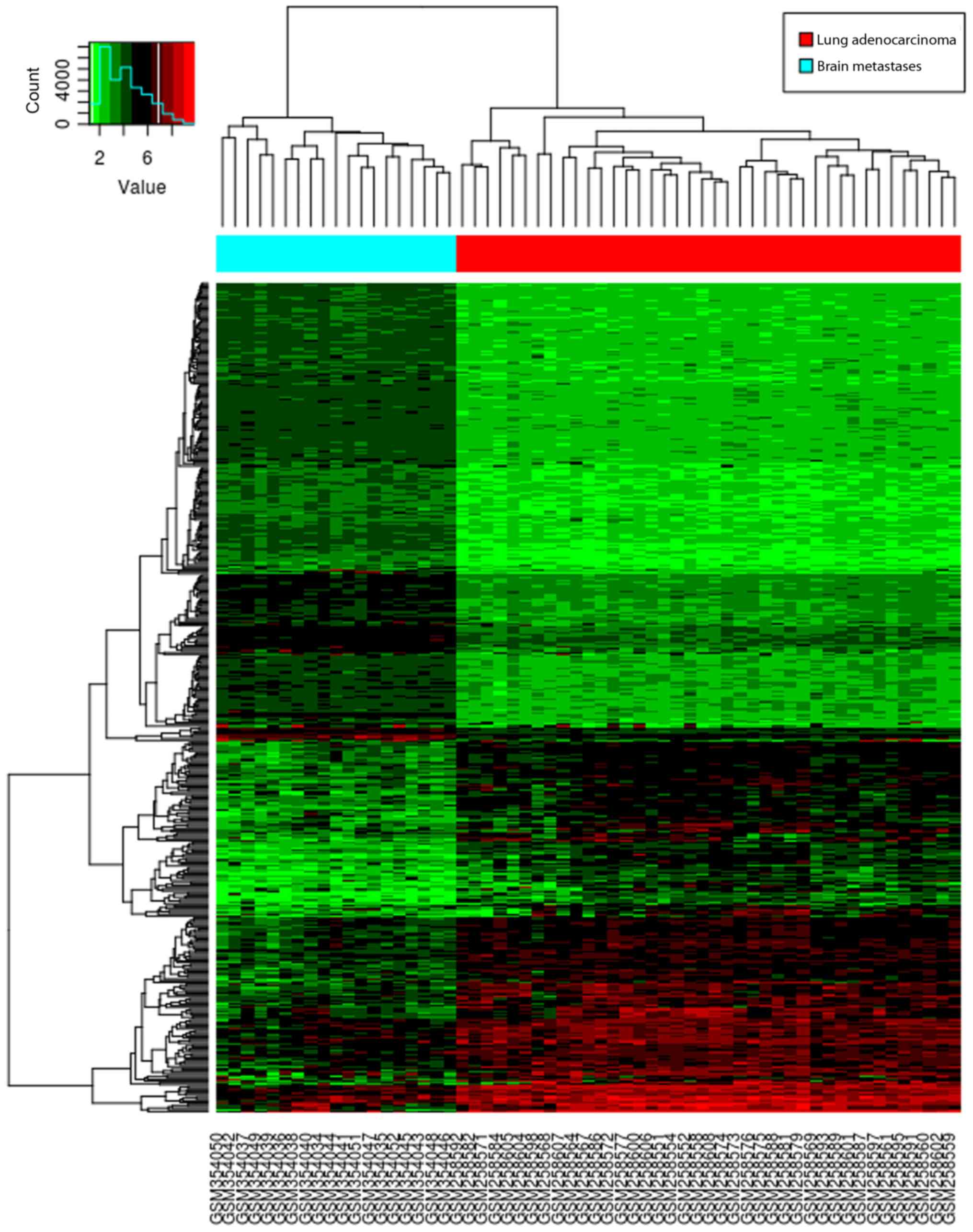
demonstrated the distribution of DEGs, and a heat map is presented
in Fig. 2.
GO terms and KEGG pathways of
DEGs
In total, 65 GO terms and 23 KEGG pathways were
enriched in DEGs according to the criteria P<0.05. The 20 most
significantly enriched GO terms are presented in Table I. The majority of the enriched GO
terms contained ‘chemokine-mediated signaling pathway’, ‘collagen
catabolic process’ and ‘blood vessel morphogenesis’. Certain
enriched GO terms also included ‘angiogenesis’, ‘extracellular
matrix organization’ and ‘extracellular structure organization’.
The enriched KEGG pathways of the DEGs are listed in Table II. The majority of the enriched KEGG
pathways, including ‘cytokine-cytokine receptor interaction’ and
‘chemokine signaling pathway’ were directly involved in the process
of lung cancer metastasis to the brain.
 | Table I.Top 20 most significantly enriched GO
terms for biological processes of differentially expressed genes in
brain metastases samples compared with lung adenocarcinoma. |
Table I.
Top 20 most significantly enriched GO
terms for biological processes of differentially expressed genes in
brain metastases samples compared with lung adenocarcinoma.
| GO ID | GO name | DEG number of genes
involved | P-value |
|---|
| GO:0070098 | Chemokine-mediated
Signaling pathway | 13 |
3.65×10−9 |
| GO:0030574 | Collagen catabolic
process | 12 |
2.77×10−8 |
| GO:0048514 | Blood vessel
morphogenesis | 30 |
2.80×10−8 |
| GO:0006959 | Humoral immune
response | 18 |
6.75×10−8 |
| GO:0044236 | Multicellular
organismal metabolic process | 15 |
6.94×10−8 |
| GO:0044243 | Multicellular
organismal catabolic process | 12 |
7.55×10−8 |
| GO:0002685 | Regulation of
leukocyte migration | 15 |
1.12×10−7 |
| GO:0001525 | Angiogenesis | 26 |
1.35×10−7 |
| GO:0030198 | Extracellular
matrix organization | 25 |
1.45×10−7 |
| GO:0043062 | Extracellular
structure organization | 25 |
1.52×10−7 |
| GO:0070661 | Leukocyte
proliferation | 20 |
3.00×10−7 |
| GO:0002688 | Regulation of
leukocyte chemotaxis | 12 |
3.02×10−7 |
| GO:0030595 | Leukocyte
chemotaxis | 16 |
3.15×10−7 |
| GO:0050920 | Regulation of
chemotaxis | 15 |
3.27×10−7 |
| GO:0060326 | Cell
chemotaxis | 18 |
4.57×10−7 |
| GO:0032963 | Collagen metabolic
process | 13 |
4.88×10−7 |
| GO:0048247 | Lymphocyte
chemotaxis | 9 |
5.20×10−7 |
| GO:0050795 | Regulation of
behavior | 17 |
6.23×10−7 |
| GO:0044259 | multicellular
organismal macromolecule metabolic process | 13 |
7.89×10−7 |
| GO:0002548 | Monocyte
chemotaxis | 9 |
1.39×10−6 |
 | Table II.The 10 most significantly enriched
signaling pathways of differentially expressed genes. |
Table II.
The 10 most significantly enriched
signaling pathways of differentially expressed genes.
| KEGG pathway
no. | Signaling
pathway | DEGs involved | DEG number of genes
involved | P-value |
|---|
| hsa04060 | Cytokine-cytokine
receptor interaction | PDGFRA, CCL2, IL7R,
CCL18, CXCR4, HGF, CXCL12 and others | 23 |
1.99×1008 |
| hsa04145 | Phagosome | CTSS, HLA-DRA,
FCGR2B, HLA-DPA1, HLA-DQB1, NOS1, COMP and others | 13 |
3.13×1005 |
| hsa05323 | Rheumatoid
arthritis | IL17A, CCL2,
TNFSF13B, HLA-DRA, HLA-DPA1, CXCL12, MMP1 and others | 10 |
2.55×1005 |
| hsa05150 | Staphylococcus
aureus infection | C1R, C1S, FCGR2A,
FPR3, HLA-DRA, FCGR2B, HLA-DPA1, HLA-DQB1 | 8 |
2.69×1005 |
| hsa04672 | Intestinal immune
network for IgA production | CXCR4, TNFSF13B,
HLA-DRA, HLA-DPA1, CXCL12, TNFRSF17, HLA-DQB1 | 7 |
7.61×1005 |
| hsa05320 | Autoimmune thyroid
disease | IFNA14, IFNA7,
HLA-DRA, HLA-DPA1, HLA-DQB1, GZMB | 6 | 0.001016 |
| hsa05164 | Influenza A | CCL2, SOCS3, CASP1,
HLA-DRA, HLA-DPA1, CCL5, TNFSF10, HLA-DQB1 and others | 11 | 0.001679 |
| hsa04062 | Chemokine signaling
pathway | CCL2, GNG2, CCL18,
CXCR4, CXCL12, CCL5, CXCL9, CCL19, CCL8, CXCL13, CXCL11 | 11 | 0.002944 |
| hsa04940 | Type I diabetes
mellitus | GAD2, HLA-DRA,
HLA-DPA1, HLA-DQB1, GZMB | 5 | 0.002649 |
| hsa05152 | Tuberculosis | IFNA14, IFNA7,
FCGR2A, CTSS, HLA-DRA, FCGR2B, HLA-DPA1 and others | 10 | 0.005802 |
PPI network of DEGs
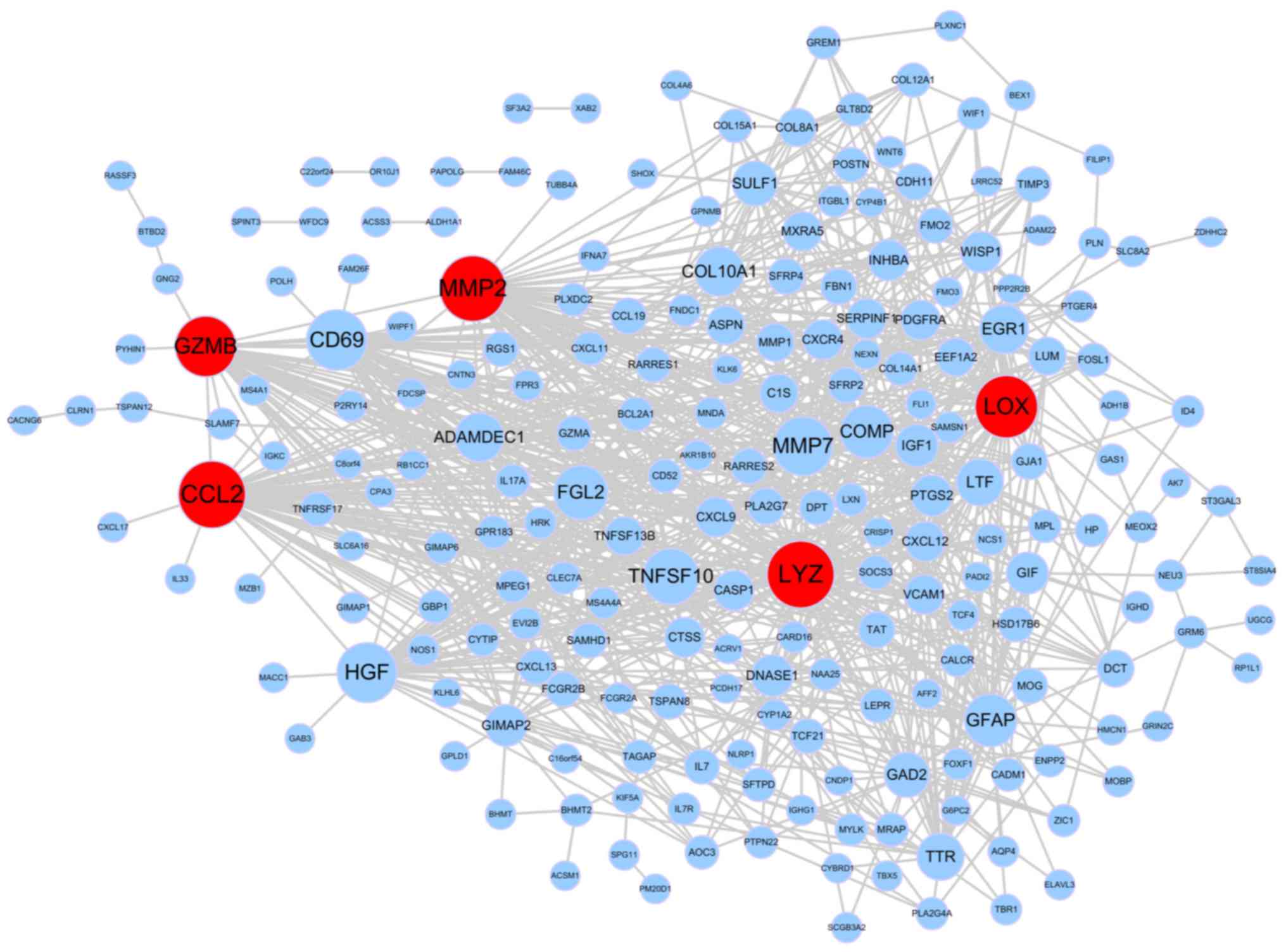
Using the STRING database, a PPI network was
constructed and is presented in Fig.
3. It included 942 pairs and 317 nodes. A total of five hub
genes were identified, including CCL2 (degree=60), LYZ (degree=60),
MMP-2, (degree=58), LOX (degree=53) and GZMB (degree=50).
miRNA-target gene regulatory
network
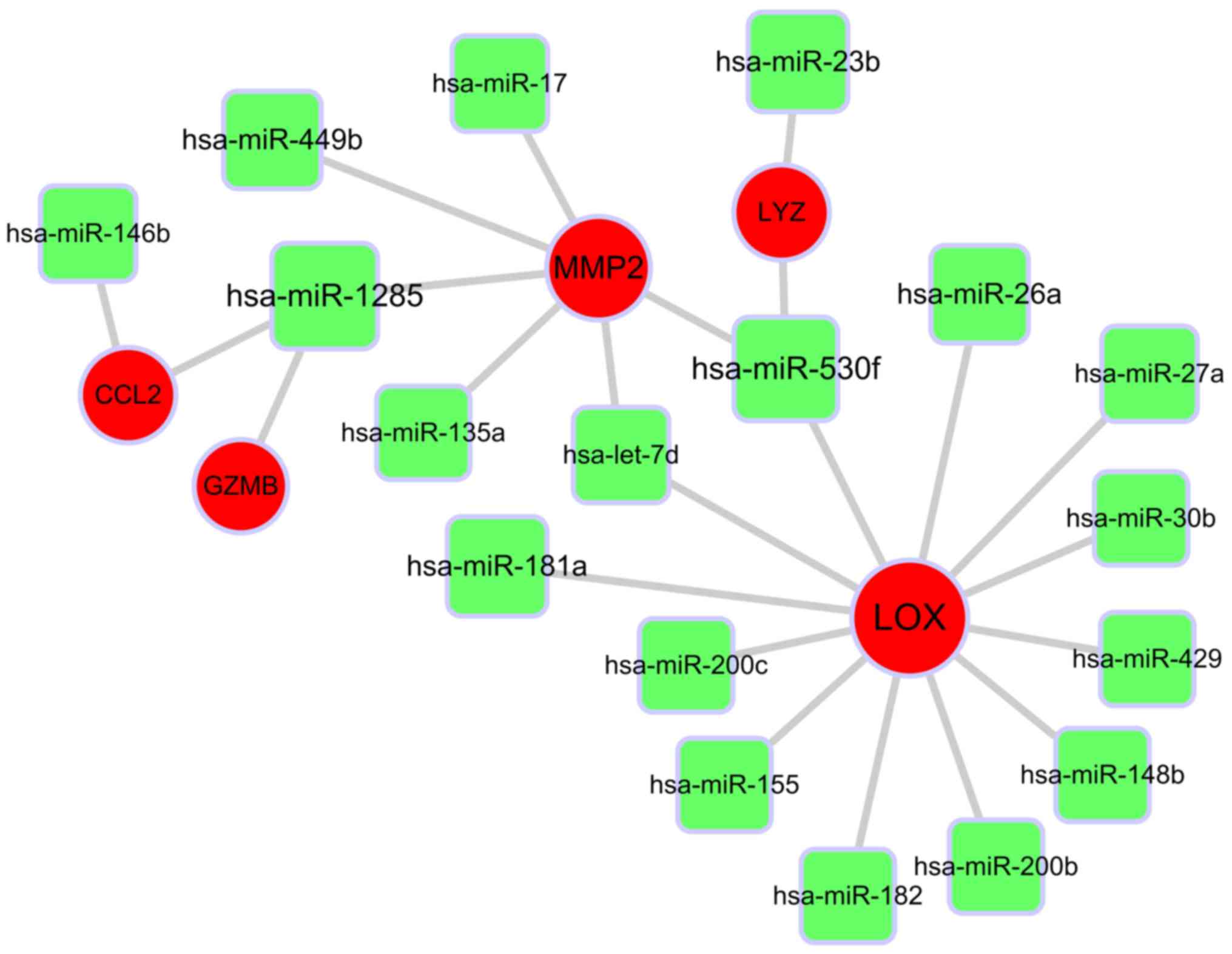
The current study focused on the five hub genes, and
further assessed their miRNA-target associations. The miRNA-target
regulatory network was based on interactions in the TargetScan
database. A miRNA-gene-regulated network was established, including
23 connections and five hub genes, which are presented in Fig. 4. MMP-2 and LOX were significant
targets that were identified by and interacted with many miRNAs.
MMP-2 was predicted to be the target of the following six miRNAs:
hsa-miR-17, hsa-miR-449b, hsa-miR-135a, hsa-miR-530f, hsa-miR-1285
and hsa-let-7d. LOX was predicted to be the target of the following
12 miRNAs: hsa-miR-181a, hsa-miR-155, hsa-miR-26a, hsa-miR-148b,
hsa-miR-530f, hsa-miR-182, hsa-miR-200c, hsa-miR-429, hsa-miR-200b,
hsa-let-7d, hsa-miR-30b and hsa-miR-27a.
Discussion
In the current study, the significantly enriched GO
terms of DEGs contained ‘chemokine-mediated signaling pathway’,
‘collagen catabolic process’ and ‘blood vessel morphogenesis’. As
demonstrated by Table II, the
enriched KEGG pathways were tumor-associated biological processes,
including ‘cytokine-cytokine receptor interaction’ and ‘chemokine
signaling pathway’. The overlapping DEGs, including CCL2, CXCL12
and CXCR4, which were mainly enriched in the GO terms and KEGG
pathways, were primarily involved in chemokine-associated signal
transduction. Numerous studies have previously demonstrated that
chemokines serve a pivotal role in cancer invasion, angiogenesis
and metastases (29–31). Emerging studies have revealed that the
CXCR4/CXCL12 signaling axis is involved in tumor cell migration and
angiogenesis, facilitating the formation of BM (32–34).
Hartmann et al (35)
identified that CXCR4 cooperates with integrins to mediate adhesion
and chemo-resistance in small cell lung cancer cells. CXCR4 and
CXCL12, which are overexpressed in brain metastases, have been
identified to be correlated with brain-specific metastasis and poor
prognosis in NSCLC patients (36).
Furthermore, the proteolysis of interstitial collagen has been
recognized as a contributing factor that participates in tumor cell
invasion and metastasis (37).
Collagen is one of the chief structural proteins of the
extracellular matrix (ECM) and provides a major obstacle impeding
cancer cell migration. Thus, it has been speculated that collagen
catabolism serves an important role in facilitating the spread and
invasion of cancer cells to host organs (38). As demonstrated in Table I, the second most enriched GO term was
‘collagen catabolic process’. Indeed, collagen degradation can be
observed histologically at the periphery of certain aggressive
tumors (39). Thus, the remodeling of
the ECM, which is caused by the degradation of collagen, serves an
important role in tumor invasion and migration.
The PPI networks based on topology analysis revealed
five potential key genes, including CCL2, LYZ, MMP2, LOX and GZMB,
which may play important roles in lung cancer BM. CCL2 encodes the
chemokine (C-C motif) ligand 2, which interplays with its receptor,
C-C chemokine receptor type 2 (CCR2), and subsequently promotes
tumor progression and metastasis caused by the Snail-induced
epithelial-to-mesenchymal transition (EMT) (40). Previous research has reported that the
release of CCL2 is induced by activating protease-activated
receptor 2 through matrix metalloproteinase-1 expression, which
initiates the activation of the thromboxane A2 receptor in LAD
cells (41). Furthermore, activation
of the thromboxane A2 receptor also increases the expression of
CCL2 and then recruits macrophages, thereby stimulating human LAD
cell invasion (42). In addition, the
CCL2/CCR2 axis cooperates with interleukin-6 to enhance EMT by
activating signal transducer and activator of transcription 3-Twist
signaling, and then boosts lung cancer progression and metastasis
(43).
The matrix metalloprotease (MMP) family includes a
series of functionally similar enzyme molecules that degrade
protein substrates based on a highly conserved mechanism (44). MMP-2, also known as gelatinase A, has
the ability to degrade matrix proteins including gelatin, type IV
and type V collagens and elastin (45). MMP-2 is involved in tumor metastasis
as it degrades vascular basement membranes (46). Recently, a study demonstrated that the
epigenetic activation of MMP-2 induced by the interaction of
megakaryocytic leukemia 1 with histone methyltransferase SET1 can
also facilitate the migration and invasion of ovarian tumor cells
(47).
The LOX gene encodes lysyloxidase, which is a
secretory copper-dependent amine oxidase (48). Previous studies have identified that
the overexpression of LOX can also promote tumor cell
proliferation, invasion and metastasis in various cancer types
(49–51). The downregulation of LOX markedly
enhances the expression of E-cadherin and decreases the expression
of vimentin (49), increasing the
tendency of tumor cells to metastasize. Furthermore, Wilgus et
al (52) confirmed that the high
expression of LOX is associated with invasion and poor prognosis in
patients with LAD.
The most well-known function of lysozyme, encoded by
the LYZ gene, is anti-infection (53). Previous research has revealed that the
expression of LYZ is associated with an unfavorable prognosis and
may be a potential prognostic factor in male breast cancer
(54). However, the relationship
between LYZ and lung cancer is still not clear.
Additionally, granzyme B is a cytotoxic
T-lymphocyte-associated serine esterase. The inhibition of granzyme
B and interferon-γ activates the transforming growth factor-β/Smad
pathway and then inhibits T-cell mediated cancer clearance in
vivo, emphasizing the role of the perforin/granzyme pathway in
cancer clearance (55).
Interestingly, in specific settings, granzyme B and perforin are
associated with the regulatory T cell-mediated inhibition of cancer
clearance in vivo (56).
However, Smyth et al (57)
discovered that granzyme A and B are not required for cytotoxic T
cell- and natural killer cell-induced cancer rejection, including
spontaneous and experimental cancers. Therefore, more convincing
studies are required to confirm the function of granzymes in cancer
immune surveillance and rejection.
A miRNA-target regulatory network was constructed,
and many potential miRNAs were identified. MMP-2 and LOX were the
leading targets identified by and interacting with multiple miRNAs.
MMP-2 and LOX were predicted to be targets of hsa-let-7d and
hsa-miR-530f. So far, the function of hsa-miR-530f is unclear.
Hsa-let-7d reportedly inhibits cancer pathogenesis (58). Furthermore, let-7d may inhibit cancer
cell migration, invasion and metastasis, by directly targeting
PBX3, COL3A1 and CCL7 (58,59). Meanwhile, a recent study reported that
let-7d may suppress proliferation and invasion of trophoblast
cells, by targeting MMP-2 (60).
However, to the best of our knowledge, the targeting of MMP-2 by
hsa-let-7d has not yet been reported in lung cancer. In the current
study, MMP-2 and LOX were predicted to be targets of hsa-let-7d,
implying that hsa-let-7d may target the two genes during lung
cancer metastasis.
The current study also had specific limitations.
Certainly, the current results may be more meaningful if matched
cases were used to validate the expression of selected genes.
Clinically, the most common primary origin of BM is the lung
(61), and LAD is usually
characterized by the early development of BM, even though certain
primary tumors may be observed without symptoms (62). Unfortunately, the current study did
not have the opportunity to obtain enough BM and matched primary
tumors to analyze the expression of selected genes in tumor
patients. Studies aimed at identifying candidate genes that lead to
tumor metastasis are difficult to perform, particularly using human
tumor samples. This is due to the unpredictable time point of tumor
BM and the difficulty in obtaining tumor tissue samples from
patients who have never received chemotherapy or radiotherapy,
which is required to ensure that the analysis and interpretation of
the results is not confused with drug or radiation-induced changes
in gene expression (63).
Furthermore, for many years, patients with BM have not generally
been considered candidates for surgical intervention, and
neurosurgeons have been reluctant to surgically treat these
patients due to the wide dissemination of cancer and limited
survival (64). Prospective or
multi-center cooperation may be necessary to collect a well-defined
BM group and matched primary tumor cases for further comparative
analysis of candidate genes (65).
Comprehensive bioinformatics analyses of gene expression profiles
based on public databases (e.g. GEO and TCGA) have yielded
important insights into potential prognostic biomarkers for tumors
(66–68). The current study re-analyzed
microarray data from 19 LAD-BM samples and 40 primary LAD samples
in the GEO database. All DEGs in LAD-BM compared with the control
group were identified via a bioinformatics-based method.
Furthermore, the current study performed GO term and pathway
enrichment analyses, and PPI network construction. The current
study also combined the DEG data with information on miRNAs in the
TargetScan database to predict miRNA-target interactions. These
analyses aided the identification of key genes associated with BM,
including CCL2, LYZ, MMP2, LOX and GZMB, and the essential miRNA,
hsa-let-7d. Through these comprehensive bioinformatical methods,
the current study may contribute to understanding the molecular
mechanism underlying BM, facilitating the identification of
potential gene targets for the diagnosis and treatment of patients
with BM.
In conclusion, many DEGs and miRNAs were regarded as
potential biomarkers for the prognosis of LAD metastasis in the
present study. These DEGs were mainly associated with
chemokine-mediated signaling pathways. In addition, MMP-2 and LOX
were predicted to be targets of hsa-let-7d. However, these
predictive results require additional experiments to confirm their
function.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study were obtained from the GEO, KEGG, STRING and TargetScan
databases.
Authors' contributions
HS and ZH were involved in the conception and design
of the research and drafting the manuscript. WP participated in the
acquisition of data. HS and ZL performed the analysis and
interpretation of data. WP was involved in the statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nayak L, Lee EQ and Wen PY: Epidemiology
of brain metastases. Curr Oncol Rep. 14:48–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin NU and Winer EP: Brain metastases: The
HER2 paradigm. Clin Cancer Res. 13:1648–1655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yau T, Swanton C, Chua S, Sue A, Walsh G,
Rostom A, Johnston SR, O'Brien ME and Smith IE: Incidence, pattern
and timing of brain metastases among patients with advanced breast
cancer treated with trastuzumab. Acta Oncol. 45:196–201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hubbs JL, Boyd JA, Hollis D, Chino JP,
Saynak M and Kelsey CR: Factors associated with the development of
brain metastases. Cancer. 116:5038–5046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schuette W: Treatment of brain metastases
from lung cancer: Chemotherapy. Lung Cancer. 45 Suppl 2:S253–S257.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barnholtz-Sloan JS, Sloan AE, Davis FG,
Vigneau FD, Lai P and Sawaya RE: Incidence proportions of brain
metastases in patients diagnosed (1973 to 2001) in the metropolitan
detroit cancer surveillance system. J Clin Oncol. 22:2865–2872.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi AA, Digumarthy SR, Temel JS, Halpern
EF, Kuester LB and Aquino SL: Does initial staging or tumor
histology better identify asymptomatic brain metastases in patients
with non-small cell lung cancer? J Thorac Oncol. 1:205–210. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sundström JT, Minn H, Lertola KK and
Nordman E: Prognosis of patients treated for intracranial
metastases with whole-brain irradiation. Ann Med. 30:296–299. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Besse B, Le Moulec S, Mazières J,
Senellart H, Barlesi F, Chouaid C, Dansin E, Bérard H, Falchero L,
Gervais R, et al: Bevacizumab in patients with nonsquamous
non-small cell lung cancer and asymptomatic, untreated brain
metastases (BRAIN): A nonrandomized, phase II study. Clin Cancer
Res. 21:1896–1903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nguyen DX, Chiang AC, Zhang XH, Kim JY,
Kris MG, Ladanyi M, Gerald WL and Massagué J: WNT/TCF signaling
through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis.
Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valiente M, Obenauf AC, Jin X, Chen Q,
Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E and Massagué
J: Serpins promote cancer cell survival and vascular cooption in
brain metastasis. Cell. 156:1002–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh M, Venugopal C, Tokar T, Brown KR,
McFarlane N, Bakhshinyan D, Vijayakumar T, Manoranjan B, Mahendram
S, Vora P, et al: RNAi screen identifies essential regulators of
human brain metastasis-initiating cells. Acta Neuropathol.
134:923–940. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castro D, Moreira M, Gouveia AM, Pozza DH
and De Mello RA: MicroRNAs in lung cancer. Oncotarget.
8:81679–81685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang SJ, Lee HW, Kim HR, Song HJ, Lee DH,
Lee H, Shin CH, Joung JG, Kim DH, Joo KM and Kim HH: Overexpression
of microRNA-95-3p suppresses brain metastasis of lung
adenocarcinoma through downregulation of cyclin D1. Oncotarget.
6:20434–20448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen LT, Xu SD, Xu H, Zhang JF, Ning JF
and Wang SF: MicroRNA-378 is associated with non-small cell lung
cancer brain metastasis by promoting cell migration, invasion and
tumor angiogenesis. Med Oncol. 29:1673–1680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao C, Xu Y, Zhang Y, Tan W, Xue J, Yang
Z, Zhang Y, Lu Y and Hu X: Downregulation of miR-145 contributes to
lung adenocarcinoma cell growth to form brain metastases. Oncol
Rep. 30:2027–2034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kikuchi T, Daigo Y, Ishikawa N, Katagiri
T, Tsunoda T, Yoshida S and Nakamura Y: Expression profiles of
metastatic brain tumor from lung adenocarcinomas on cDNA
microarray. Int J Oncol. 28:799–805. 2006.PubMed/NCBI
|
|
19
|
Kuner R, Muley T, Meister M, Ruschhaupt M,
Buness A, Xu EC, Schnabel P, Warth A, Poustka A, Sültmann H and
Hoffmann H: Global gene expression analysis reveals specific
patterns of cell junctions in non-small cell lung cancer subtypes.
Lung Cancer. 63:32–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lüke F, Blazquez R, Yamaci RF, Lu X,
Pregler B, Hannus S, Menhart K, Hellwig D, Wester HJ, Kropf S, et
al: Isolated metastasis of an EGFR-L858R-mutated NSCLC of the
meninges: The potential impact of CXCL12/CXCR4 axis in EGFRmut
NSCLC in diagnosis, follow-up and treatment. Oncotarget.
9:18844–18857. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C and Wong WH: Model-based analysis of
oligonucleotide arrays: Expression index computation and outlier
detection. Proc Natl Acad Sci USA. 98:31–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu G, Wang LG, Han Y and He QY:
ClusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu G, Wang LG, Yan GR and He QY: DOSE: An
R/Bioconductor package for disease ontology semantic and enrichment
analysis. Bioinformatics. 31:608–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahn T, Lee E, Huh N and Park T:
Personalized identification of altered pathways in cancer using
accumulated normal tissue data. Bioinformatics. 30:422–429. 2014.
View Article : Google Scholar
|
|
26
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
P, et al: The STRING database in 2011: Functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39:D561–D568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Weng Y, Zhang Y, Yan X, Guo L, Wang
J, Song X, Yuan Y, Chang FY and Wang CL: Systematic expression
profiling analysis mines dys-regulated modules in active
tuberculosis based on re-weighted protein-protein interaction
network and attract algorithm. Microb Pathog. 107:48–53. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Goldenberg A, Wong KC and Zhang Z: A
probabilistic approach to explore human miRNA targetome by
integrating miRNA-overexpression data and sequence information.
Bioinformatics. 30:621–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keeley EC, Mehrad B and Strieter RM: CXC
chemokines in cancer angiogenesis and metastases. Adv Cancer Res.
106:91–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bachelder RE, Wendt MA and Mercurio AM:
Vascular endothelial growth factor promotes breast carcinoma
invasion in an autocrine manner by regulating the chemokine
receptor CXCR4. Cancer Res. 62:7203–7206. 2002.PubMed/NCBI
|
|
31
|
Akishima-Fukasawa Y, Nakanishi Y, Ino Y,
Moriya Y, Kanai Y and Hirohashi S: Prognostic significance of
CXCL12 expression in patients with colorectal carcinoma. Am J Clin
Pathol. 132:202–10; quiz 307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fokas E, Steinbach JP and Rödel C: Biology
of brain metastases and novel targeted therapies: Time to translate
the research. Biochim Biophys Acta. 1835:61–75. 2013.PubMed/NCBI
|
|
33
|
Lee BC, Lee TH, Avraham S and Avraham HK:
Involvement of the chemokine receptor CXCR4 and its ligand stromal
cell-derived factor 1alpha in breast cancer cell migration through
human brain microvascular endothelial cells. Mol Cancer Res.
2:327–338. 2004.PubMed/NCBI
|
|
34
|
Hinton CV, Avraham S and Avraham HK: Role
of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to
the brain. Clin Exp Metastasis. 27:97–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hartmann TN, Burger JA, Glodek A, Fujii N
and Burger M: CXCR4 chemokine receptor and integrin signaling
co-operate in mediating adhesion and chemoresistance in small cell
lung cancer (SCLC) cells. Oncogene. 24:4462–4471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Salmaggi A, Maderna E, Calatozzolo C,
Gaviani P, Canazza A, Milanesi I, Silvani A, DiMeco F, Carbone A
and Pollo B: CXCL12, CXCR4 and CXCR7 Expression in brain
metastases. Cancer Biol Ther. 8:1608–1614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fields GB: Interstitial collagen
catabolism. J Biol Chem. 288:8785–8793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Woolley DE: Collagenolytic mechanisms in
tumor cell invasion. Cancer Metastasis Rev. 3:361–372. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liotta LA, Thorgeirsson UP and Garbisa S:
Role of collagenases in tumor cell invasion. Cancer Metastasis Rev.
1:277–288. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kudo-Saito C, Shirako H, Ohike M,
Tsukamoto N and Kawakami Y: CCL2 is critical for immunosuppression
to promote cancer metastasis. Clin Exp Metastasis. 30:393–405.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X and Tai HH: Thromboxane A 2
receptor-mediated release of matrix metalloproteinase-1 (MMP-1)
induces expression of monocyte chemoattractant protein-1 (MCP-1) by
activation of protease-activated receptor 2 (PAR2) in A549 human
lung adenocarcinoma cells. Mol Carcinog. 53:659–666.
2014.PubMed/NCBI
|
|
42
|
Li X and Tai HH: Activation of thromboxane
A2 receptor (TP) increases the expression of monocyte
chemoattractant protein −1 (MCP-1)/chemokine (C-C motif) ligand 2
(CCL2) and recruits macrophages to promote invasion of lung cancer
cells. PLoS One. 8:e540732013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen W, Gao Q, Han S, Pan F and Fan W: The
CCL2/CCR2 axis enhances IL-6-induced epithelial-mesenchymal
transition by cooperatively activating STAT3-Twist signaling. Tumor
Biol. 36:973–981. 2015. View Article : Google Scholar
|
|
44
|
Lauer-Fields JL, Juska D and Fields GB:
Matrix metalloproteinases and collagen catabolism. Biopolymers.
66:19–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vu TH: Don't mess with the matrix. Nat
Genet. 28:202–203. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chakrabarti S and Patel KD: Matrix
metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp
Lung Res. 31:599–621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu W, Xu H, Fang M, Wu X and Xu Y: MKL1
links epigenetic activation of MMP2 to ovarian cancer cell
migration and invasion. Biochem Biophys Res Commun. 487:500–508.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang TH, Hsia SM and Shieh TM: Lysyl
oxidase and the tumor microenvironment. Int J Mol Sci. 18:E622016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kasashima H, Yashiro M, Kinoshita H,
Fukuoka T, Morisaki T, Masuda G, Sakurai K, Kubo N, Ohira M and
Hirakawa K: Lysyl oxidase is associated with the
epithelial-mesenchymal transition of gastric cancer cells in
hypoxia. Gastric Cancer. 19:431–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shih YH, Chang KW, Chen MY, Yu CC, Lin DJ,
Hsia SM, Huang HL and Shieh TM: Lysyl oxidase and enhancement of
cell proliferation and angiogenesis in oral squamous cell
carcinoma. Head Neck. 35:250–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Osawa T, Ohga N, Akiyama K, Hida Y,
Kitayama K, Kawamoto T, Yamamoto K, Maishi N, Kondoh M, Onodera Y,
et al: Lysyl oxidase secreted by tumour endothelial cells promotes
angiogenesis and metastasis. Br J Cancer. 109:2237–2247. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wilgus ML, Borczuk AC, Stoopler M,
Ginsburg M, Gorenstein L, Sonett JR and Powell CA: Lysyl oxidase: A
lung adenocarcinoma biomarker of invasion and survival. Cancer.
117:2186–2191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rubio CA: The natural antimicrobial enzyme
lysozyme is up-regulated in gastrointestinal inflammatory
conditions. Pathogens. 3:73–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Serra C, Vizoso F, Alonso L, Rodríguez JC,
González LO, Fernández M, Lamelas ML, Sánchez LM, García-Muñiz JL,
Baltasar A and Medrano J: Expression and prognostic significance of
lysozyme in male breast cancer. Breast Cancer Res. 4:R162002.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Thomas DA and Massagué J: TGF-beta
directly targets cytotoxic T cell functions during tumor evasion of
immune surveillance. Cancer Cell. 8:369–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cao X, Cai SF, Fehniger TA, Song J,
Collins LI, Piwnica-Worms DR and Ley TJ: Granzyme B and perforin
are important for regulatory T cell-mediated suppression of tumor
clearance. Immunity. 27:635–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Smyth MJ, Street SE and Trapani JA:
Cutting edge: Granzymes A and B are not essential for
perforin-mediated tumor rejection. J Immunol. 171:515–518. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ramberg H, Alshbib A, Berge V, Svindland A
and Taskén KA: Regulation of PBX3 expression by androgen and Let-7d
in prostate cancer. Mol Cancer. 10:502011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Su B, Zhao W, Shi B, Zhang Z, Yu X, Xie F,
Guo Z, Zhang X, Liu J, Shen Q, et al: Let-7d suppresses growth,
metastasis, and tumor macrophage infiltration in renal cell
carcinoma by targeting COL3A1 and CCL7. Mol Cancer. 13:2062014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dai X and Cai Y: Down-regulation of
microRNA let-7d inhibits the proliferation and invasion of
trophoblast cells in preeclampsia. J Cell Biochem. 119:1141–1151.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Al-Shamy G and Sawaya R: Management of
brain metastases: The indispensable role of surgery. J Neurooncol.
92:275–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hoffman PC, Mauer AM and Vokes EE: Lung
cancer. Lancet. 355:479–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Suzuki M and Tarin D: Gene expression
profiling of human lymph node metastases and matched primary breast
carcinomas: Clinical implications. Mol Oncol. 1:172–180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Johnson JD and Young B: Demographics of
brain metastasis. Neurosurg Clin N Am. 7:337–344. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Preusser M, Berghoff AS, Koller R,
Zielinski CC, Hainfellner JA, Liebmann-Reindl S, Popitsch N, Geier
CB, Streubel B and Birner P: Spectrum of gene mutations detected by
next generation exome sequencing in brain metastases of lung
adenocarcinoma. Eur J Cancer. 51:1803–1811. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhou S, Liu P, Jiang W and Zhang H:
Identification of potential target genes associated with the effect
of propranolol on angiosarcoma via microarray analysis. Oncol Lett.
13:4267–4275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang
Y, Jia L and Li S: Cancer Genome Atlas Research Network, Xie W and
Yang D: lncRNA epigenetic landscape analysis identifies EPIC1 as an
oncogenic lncRNA that interacts with MYC and promotes cell-cycle
progression in cancer. Cancer Cell. 33:706–720.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li S, Li H, Xu Y and Lv X: Identification
of candidate biomarkers for epithelial ovarian cancer metastasis
using microarray data. Oncol Lett. 14:3967–3974. 2017. View Article : Google Scholar : PubMed/NCBI
|