Introduction
Prostate cancer (PCa) is one of the most common,
life-threatening malignancies among men worldwide (1). The incidence of PCa continues to rise,
particularly in developing countries (2,3). Although
a number of therapeutic strategies have been developed to treat PCa
(including surgery), there remains a high incidence of relapse
following primary therapy (4). The
involvement of numerous genes in the regulation of PCa is
increasingly apparent (5–7). However, the pathogenesis and progression
of this malignancy remains to be fully elucidated, and new
therapeutic targets require investigation.
MicroRNAs (miRNAs) are a class of 20–25 nt
non-coding RNA transcripts that negatively regulate gene expression
by directly binding to the 3′-untranslated region (UTR) of target
mRNAs (8). The altered expression of
miRNAs, and their contribution to the carcinogenesis and
progression of various human cancer types, including PCa, is well
characterized (9–11). miR-128 directly targets stem cell
regulatory factors, including polycomb complex protein BMI-1
(BMI-1), homeobox protein NANOG, and TGF-β receptor type-1 to
suppress PCa (12). Downregulation of
miR-221, miR-30d and miR-15a contributes to the pathogenesis of PCa
by targeting BMI-1 (13). Serum
analysis demonstrated that miR-103, miR-125b and miR-222 may serve
as biomarkers to monitor therapeutic efficacy in PCa (14).
In the present study, miR-744, which has been
implicated in various human cancer types (15–18), was
investigated; miR-744 increases the tumorigenicity of pancreatic
cancer by activating the Wnt/β-catenin pathway, and elevated serum
miR-744 predicts poor prognosis in patients with nasopharyngeal
carcinoma. miR-744 functions as a proto-oncogene in nasopharyngeal
carcinoma through transcriptional regulation of Rho
GTPase-activating protein 5 (ARHGAP5). However, little is known of
the biological function of miR-744 in PCa.
In the present study, the biological function and
molecular mechanism of miR-744 in PCa were investigated. It was
identified that miR-744 was upregulated in PCa tissue when compared
to the adjacent tissues. Silencing of miR-744 resulted in the
inhibition of cell growth, and an increased population of apoptotic
cells was identified following miR-744 knockdown. The knockdown of
miR-744 activated the adenosine monophosphate-activated protein
kinase (AMPK) signaling pathway, and to a lesser extent, mammalian
target of rapamycin (mTOR) signaling. The study provides evidence
of a critical role for miR-744 in PCa, and targeting miR-744 may
represent a potential target for PCa.
Materials and methods
Clinical tissues
All PCa tissues and adjacent tissues were collected
from surgical resections from patients with PCa at the China and
Japan Union Hospital of Jilin University (Changchun. China).
Informed consent was received from all patients, and the research
methodology was approved by the Ethics Committee of Jilin
University. All tissue samples were immediately frozen and
preserved in liquid nitrogen until use.
Cell culture
The PCa cell lines PC3 (ATCC® CRL-7934),
Du145 (ATCC® HTB-81), and PPC-1 (ATCC®
HTB-190) were obtained from The American Type Culture Collection
(Manassas, VA, USA). The non-tumorigenic human prostate epithelial
cell line 9 (NHP9) was procured from Dr. Tang's lab. Cells were
cultured in Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) containing 10% (v)
heat-inactivated fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
incubator with 5% (v/v) CO2.
Cell transfection
The sequences of the miR-744 mimic, its inhibitor,
and the corresponding negative control, were obtained from
GenePharma Co., Ltd. (Shanghai, China). A total of 2×103
PC3 cells were seeded into 35-mm plates and incubated for 24 h, and
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect miRNA-744 the cells. miRNAs
were used at a concentration of 50 nM. Following a 6 h incubation,
cells were transferred to complete medium, and cell lysates were
harvested 48 h post transfection. Their sequences were as follows:
Mimics-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
3′-ACGUGACACGUUCGGAGAATT-5′; miR-744, 5′-UGCGGGGCUAGGGCUAACAGCA-3′
and 3′-CUGUUAGCCCUAGCCCCGCAUU-5′; inhibitor-NC,
5′-CAGUACUUUUGUGUAGUACAA-3′ and miR-744 inhibitor,
5′-UGCUGUUAGCCCUAGCCCCGCA-3′.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from PCa tumor samples and
their corresponding adjacent tissue samples or from PCa cell lines
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.). All methods were performed according to the manufacturer's
protocol. RNA was reverse transcribed into cDNA using
Superscriptase II (Invitrogen; Thermo Fisher Scientific, Inc.).
qPCR was performed using the Power SYBR Green PCR master mix (Life
Technologies; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The thermocycling conditions were as
follows: 35 cycles of 95°C for 30 sec, 60°C for 30 sec, and 72°C
for 35 sec. The following primer sets were used to measure the
expression of LKB1: LKB1 forward, 5′-TGTCGGTGGGTATGGACAC-3′ and
reverse, 5′-CCTTGCCGTAAGAGCCTTCC-3′; GAPDH-forward,
5′-AGCCTCCCGCTTCGCTCTCT-3′ and reverse,
5′-GCGCCCAATACGACCAAATCCGT-3′. GAPDH was used for
normalization.
To detect miR-744 expression by qPCR, a
hairpin-it™ miRNA qPCR quantitation kit (GenePharma Co.,
Ltd, Shanghai, China) was used. In brief, total RNA was reverse
transcribed using miR-744 specific stem-loop RT primers, and levels
of miR-744 measured. A miR-744 specific molecular beacon probe was
used to ensure the accuracy of the real-time PCR data. The
2−ΔΔCq method was used to calculate relative expression
levels (9). The following primer sets
were used to measure the expression of LKB1 miR-744 Forward primer,
5′-ACACTCCAGCTGGGTGCGGGGCTAGGGCTAAC-3′; GAPDH-forward,
5′-AGCCTCCCGCTTCGCTCTCT-3′ and reverse,
5′-GCGCCCAATACGACCAAATCCGT-3′.
Antibodies and western blotting
PC3 cells were lysed in ice-cold
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing a protease inhibitor
cocktail (Roche Diagnostics, Basel, Switzerland). The protein
concentration of the lysates was measured using a bicinchoninic
acid assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal
amounts of each protein (40 µg) were separated by 12% SDS-PAGE and
transferred to a nitrocellulose membrane (Pall Life Sciences, Port
Washington, NY, USA). The membranes were blocked with 5% nonfat
milk for 2 h at room temperature, and incubated with primary
antibodies against β-actin (1:2,000; cat. no. 3700), cyclin D1
(1:1,000; cat. no. 2922), cyclin-dependent kinase 4 (CDK4) (1:500;
cat. no. 12790), proliferating cell nuclear antigen (PCNA)
(1:1,000; cat. no. 2586), AMPKα (1:500; cat. no. 5831), AMPKβ1
(1:1,000; cat. no. 12063), phospho-AMPKα (Thr172) (1:200; cat. no.
2535), phospho-AMPKβ1 (Ser182) (1:250; cat. no. 4186) TSC2 (1:500;
cat. no. 3990), phospho-TSC2 (Ser1387) (1:250; cat. no. 23402),
mTOR (1:1,000; cat. no. 2983), phospho-mTOR (Ser2448) (1:250
dilution; cat. no. 2971) acquired from Cell Signaling Technology,
Inc., (Danvers, MA, USA), and p21Cip1/Waf1 (1:500; cat.
no. 610233; BD Pharmingen; Becton, Dickinson and Company, Franklin
Lakes, NJ, USA) at 4°C, over night. After washing and incubating
with rabbit (cat. no. 926-32201) or mouse (cat. no. 926-32211)
secondary antibodies (1:10,000) dilution (LI-COR Biosciences,
Lincoln, NE, USA) for 1 h at room temperature, the blots were
imaged using the LI-COR imaging system, and band density was
quantified using Odyssey 3.0 software (both LI-COR Biosciences,
Lincoln, NE, USA) for each group, and normalized to β-actin.
Cell Counting kit-8 (CCK-8) cell
viability assay
PC3 cells were transfected with an miR-744 inhibitor
or negative control sequences respectively (outlined above). A
total of 24 h post-transfection, cells were transferred into
96-well plates at a density of 2×103 cells/well for a
further 48 h. Cell viability was assessed using the CCK-8 assay per
the manufacturer's protocol (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan).
Acridine orange/ethidium bromide
(AO/EB) fluorescence staining
PC3 cells (2×103) in the exponential
growth phase were cultured on sterile coverslips for 24 h, followed
by transfection with miR-744 inhibitor or negative control
sequences, and were incubated with AO/EB mix solution for 5 min per
the manufacturer's protocol (Beijing Solarbio Science &
Technology Co., Ltd, Beijing, China). Cell morphology was examined
using a fluorescence microscope at magnification ×200, and the
percentage of apoptotic cells was calculated using the following
formula: Apoptotic rate (%)=(number of apoptotic cells/number of
total cells) ×100.
Immunofluorescence assay
PC3 cells (8×102) were seeded onto cover
slips and transfected with miR-744 inhibitor or negative control
sequences. After 48 h, cells were fixed and incubated with Ki-67
antibody (cat. no. 11882, 1:200, Cell Signaling Technology, Inc.)
for 1 h and subsequently incubated with Alexa-488-Fluor (Thermo
Fisher Scientific, Inc.) at room temperature for 20 min in the
dark. Cells were then counterstained with DAPI (1:1,000) for 5 min
to stain the cell nucleus. All coverslips were mounted using
Prolong® diamond antifade mounting reagent (Applied
Biosystems; Thermo Fisher Scientific, Inc.).
Dual luciferase reporter assay
Wild-type (WT) LKB1 (LKB1-WT) and mutant LKB1
(LKB1-Mut) 3′-UTR were cloned into separate pMIR-REPORT Luciferase
vectors (Ambion; Thermo Fisher Scientific, Inc.). PC3 cells
(2×103) were harvested from six-well plates and treated
with the specific vectors and Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 48 h. Luciferase activity was
assessed by the Dual Luciferase-reporter 1000 assay system (Promega
Corporation, Madison, WI, USA). Renilla activity was used
for normalization.
Kaplan-Meier method
All clinical data and the Tier 3 RNASeqV2 mRNA
expression data were downloaded from https://cancergenome.nih.gov. Samples for patients
with a follow up time or time to mortality >0 days were kept for
analysis. For each gene, all samples were divided into two groups
(low and high expressing groups) based on median expression values.
Kaplan-Meier analysis was performed to test the significance
between the two groups.
Cox proportional hazards regression was also
performed with the coxph function from the R survival library
(https://cran.r-project.org/web/packages/survival/index.html).
Hazard ratios with 95% confidence intervals were obtained.
Statistical analysis
Data are presented as the mean ± standard error of
the mean from three independent experiments. Statistical analysis
was conducted using SPSS 19.0 software (IBM Corp., Armonk, NY, USA)
and illustrated using GraphPad Prism 5.0 (GraphPad Software, La
Jolla, CA, USA). Statistical significance was determined using the
Student's t-test to compare two groups or analysis of variance with
Tukey's post-hoc test to compare multiple groups. Paired Student's
t-test was used to analyze the results. Pearson's correlation was
used to assess the correlation between miR-744 and LKB1 expression.
P≤0.05 was considered to indicate a statistically
significant difference.
Results
miR-744 is upregulated in PCa and
promotes PCa cell growth
To verify the expression of miR-744 in PCa, tumor
tissue samples and their corresponding adjacent tissue samples were
isolated from 60 individual PCa patients, and levels of miR-744
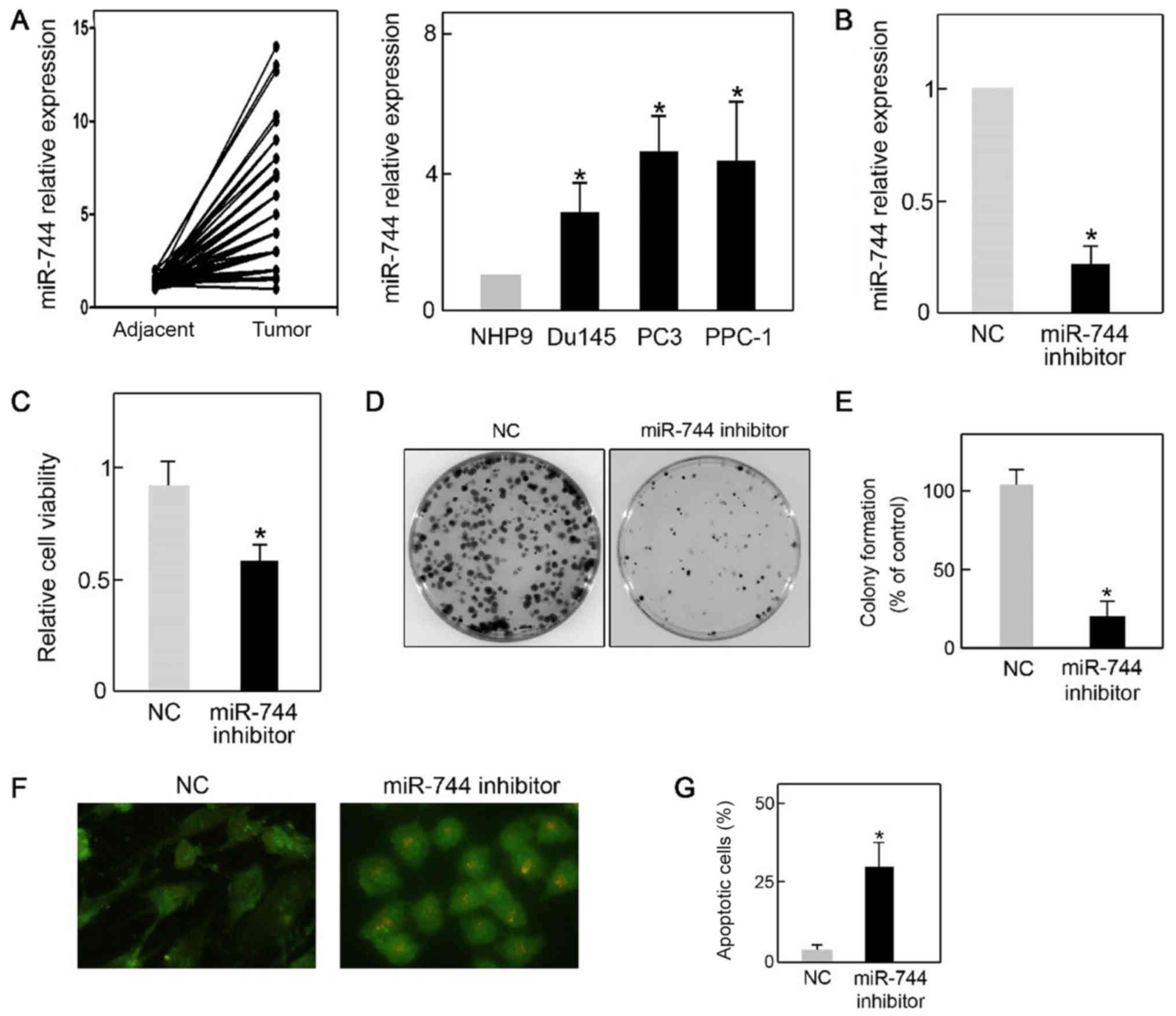
were quantified by qPCR. As presented in Fig. 1A, cancer tissues demonstrated a
significantly higher level of miR-744 expression when compared with
adjacent tissues, suggesting a potential role for miR-744 in PCa.
In agreement with this was the finding that following
quantification of miR-744 levels in three PCa cell lines (PC3,
Du145 and PPC-1), miR-744 levels were higher in PCa cells when
compared with the non-tumorigenic prostate epithelial cell line
NHP9 (Fig. 1A). However, PC3 cells
expressed the highest level of miR-744, when compared with the
other PCa cell lines. For this reason, PC3 cells were used for the
remainder of the in vitro study. To elucidate the biological
function of miR-744 in PCa, PC3 cells were transfected with miR-744
inhibitor or its negative control sequence (NC) respectively. Using
qPCR, it was confirmed that the miR-744 inhibitor successfully
reduced the level of miR-744 in PC3 cells (Fig. 1B). Furthermore, the capacity for cell
growth was assessed with the CCK-8 and colony formation assay. The
results demonstrated that cell viability was significantly reduced
by miR-744 knockdown (Fig. 1C).
miR-744 inhibitor transfection also resulted in a lower number of
colonies (Fig. 1D and E).
Unexpectedly, it was observed that silencing of miR-744 led to an
increased population of apoptotic cells as measured by AO/EB
staining (Fig. 1F and G). These data
demonstrated that miR-744 was overexpressed in PCa cells and
promoted PCa cell growth.
miR-744 downregulation inhibits cell
proliferation
To confirm the role of miR-744 in the regulation of
cell proliferation, PC3 cells were transfected with an miR-744
inhibitor and its NC respectively, and the cells assessed using
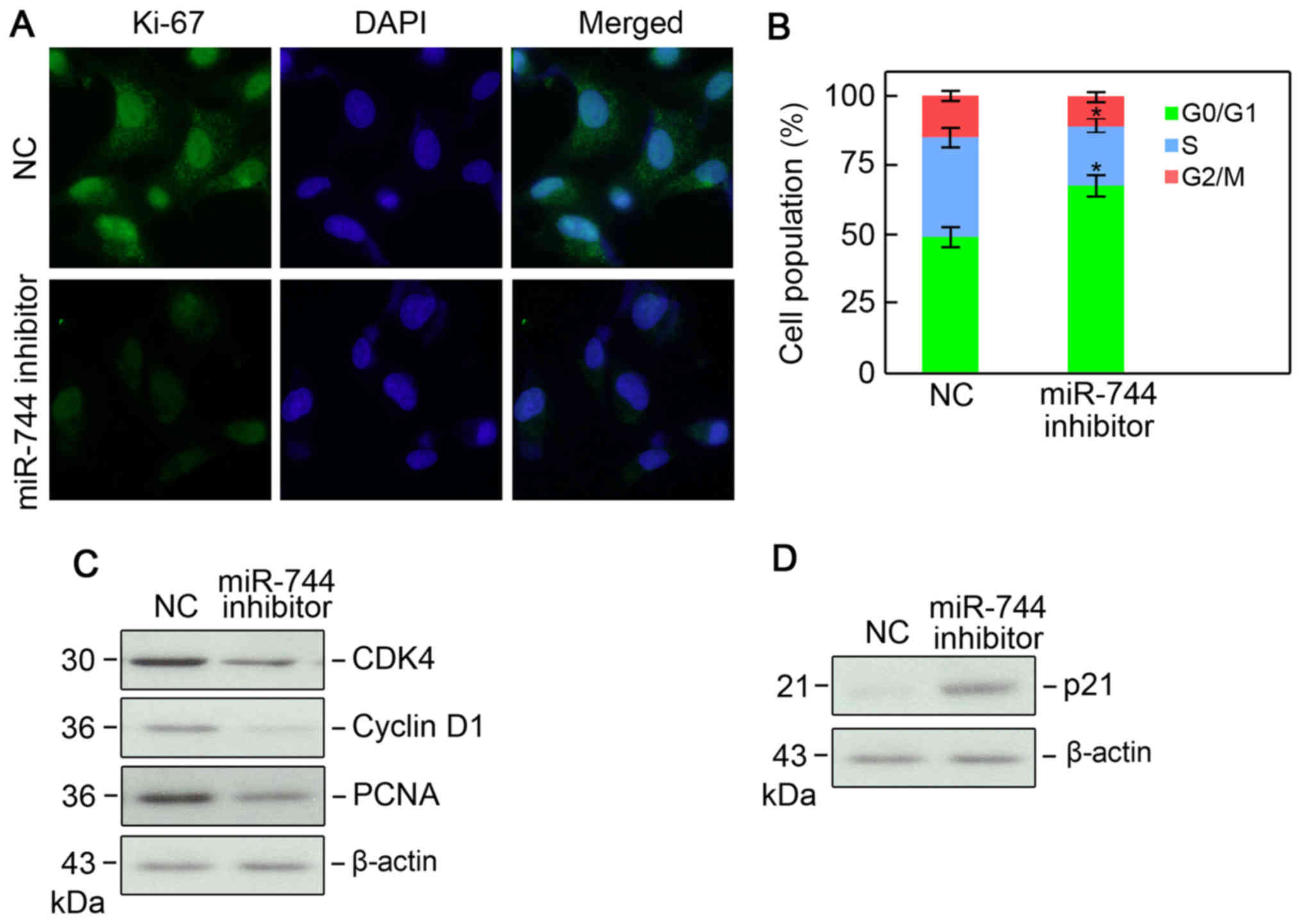
Ki-67 immunofluorescence. A lower Ki-67 immunofluorescence was
demonstrated in PC3 cells following miR-744 knockdown, indicating a
lower proliferative capacity (Fig.
2A). Cell cycle analysis using flow cytometry revealed that
silencing of miR-744 resulted in a larger population of cells in
the G1 phase of the cell cycle and a smaller population in the S
phase (Fig. 2B). Cell cycle
associated proteins, cyclin D1, CDK4, PCNA, and cyclin-dependent
kinase inhibitor p21 (p21) were measured by western blotting, and
the results demonstrated that levels of cyclin D1, CDK4, and PCNA
were all reduced, whereas the cell cycle inhibitor p21 was
increased (Fig. 2C and D). These data
indicated that miR-744 knockdown inhibits PC3 cell
proliferation.
miR-744 directly targets LKB1
To determine the association between miR-744 and
LKB1, the expression of LKB1 in PCa tissue samples and their
corresponding adjacent samples, and in PCa cell lines was confirmed
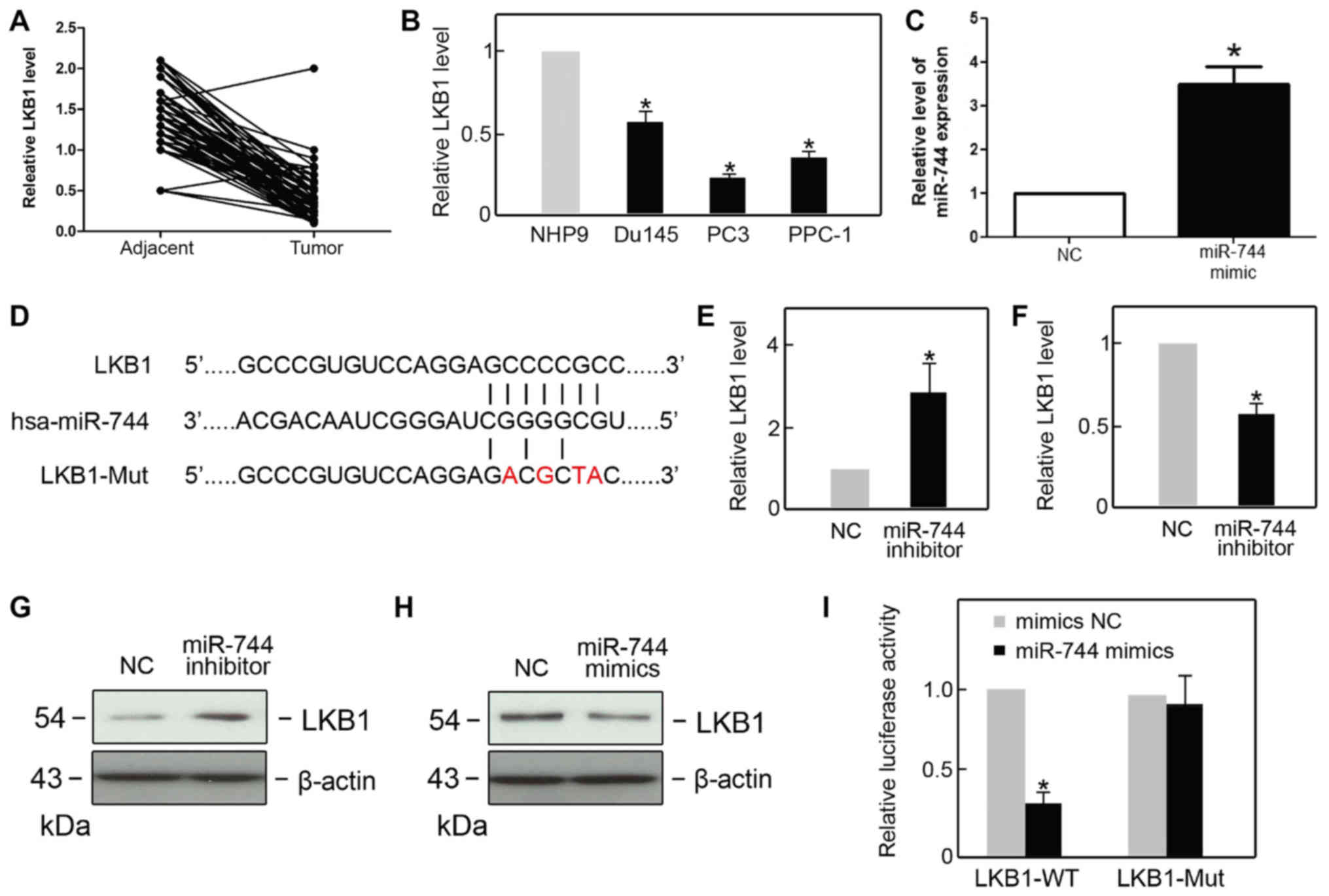
(Table I). Using qPCR, it was
revealed that the expression of LKB1 was lower in PCa tissues than
in the adjacent tissues (Fig. 3A).
Additionally, lower expression of LKB1 in PCa cell lines PC3, Du145
and PPC-1 was observed when compared with non-tumorigenic prostate
epithelial (NHP9) cells (Fig. 3B).
The expression of miR-744 was also assessed following transfection
with an miR-744 mimic. The expression of miR-744 was found to be
upregulated in the miR-744 mimic group compared with the NC group
(Fig. 3C). It is known that miRNA
regulates gene expression by targeting the 3′-UTR of target mRNA,
leading to its degradation or translational inhibition. By
consulting bioinformatics databases (www.targetScan.org), LKB1 was revealed to be a
potential target of miR-744 (Fig.
3D). The qPCR results demonstrated that LKB1 mRNA expression
level was negatively associated with the level of miR-744 (Fig. 3E and F). Additionally, the protein
levels of LKB1 showed a similar negative association pattern with
miR-744 to that of the mRNA expression levels (Fig. 3G and H). To address whether LKB1 was a
direct target of miR-744, a dual luciferase assay was performed.
Co-transfection with miR-744 mimics/LKB1-WT resulted in a reduction
in luciferase activity, whereas miR-744 mimics did not alter the
luciferase activity in the LKB1 mutant group (Fig. 3I). All results indicated that LKB1 is
a direct target of miR-744.
 | Table I.Patient clinical information. |
Table I.
Patient clinical information.
| Variable | No. patients
(n=60) |
|---|
| Age, years [median
(range)] | 45.3 (30–85) |
| Sex, female | 30 (50%) |
| Tumor size, ≤5
cm | 40 (66.7%) |
| Tumor size, >5
cm | 20 (33.3%) |
| Lymph node
involvement | 15 (25%) |
| Metastasis | 55 (negative)/5
(positive) |
miR-744 downregulation interferes with
AMPK signaling and inhibits cell growth
LKB1 is well established as a potent tumor
suppressor involved in the activation of the AMPK signaling
pathway. Following evidence that LKB1 is a direct target of
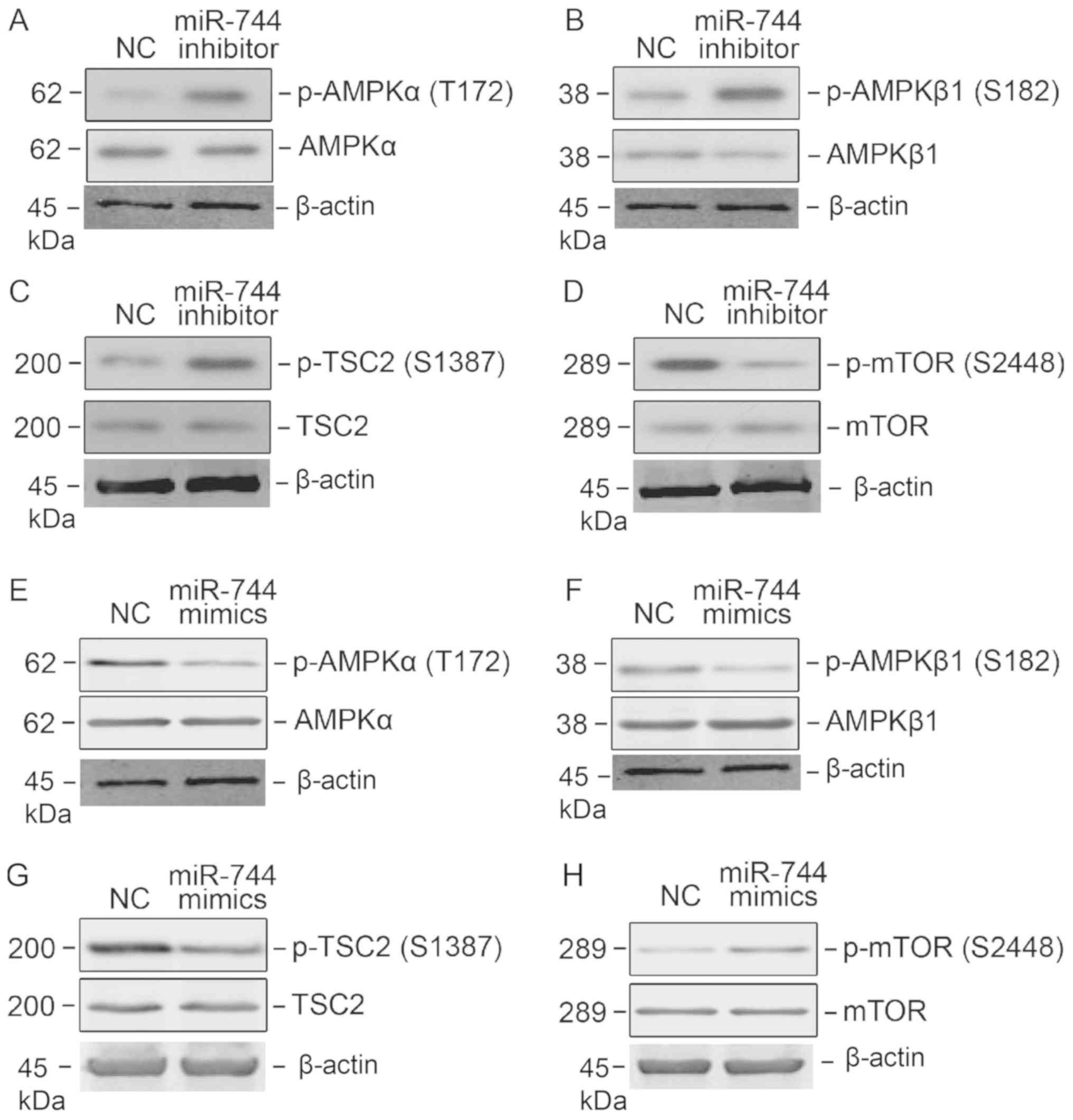
miR-744, it was hypothesized that miR-744 may regulate AMPK
signaling. Western blot analysis demonstrated that the
phosphorylation of T172 on AMPKα and S182 on AMPKβ was higher
following miR-744 knockdown, suggesting that the AMPK signaling
pathway was activated (Fig. 4A and
B). The tumor suppressor Tuberin (TSC2) is a downstream target
of AMPK signaling, and the activation of AMPK signaling by miR-744
knockdown resulted in TSC2 and S1387 phosphorylation (Fig. 4C). The mTOR signaling pathway may be
negatively regulated by AMPK signaling. Therefore, mTOR signaling
activity was determined following miR-744 knockdown. Notably,
knockdown reduced mTOR signaling in PC3 cells, as determined by its
phosphorylation of S2448 (Fig. 4D).
To strengthen the data, PC3 cells were transfected with miR-744
mimics and the activation of AMPK and mTOR signaling pathways
determined; miR-744 mimics successfully blocked the AMPK signaling
pathway, and activated mTOR signaling (Fig. 4E-H).
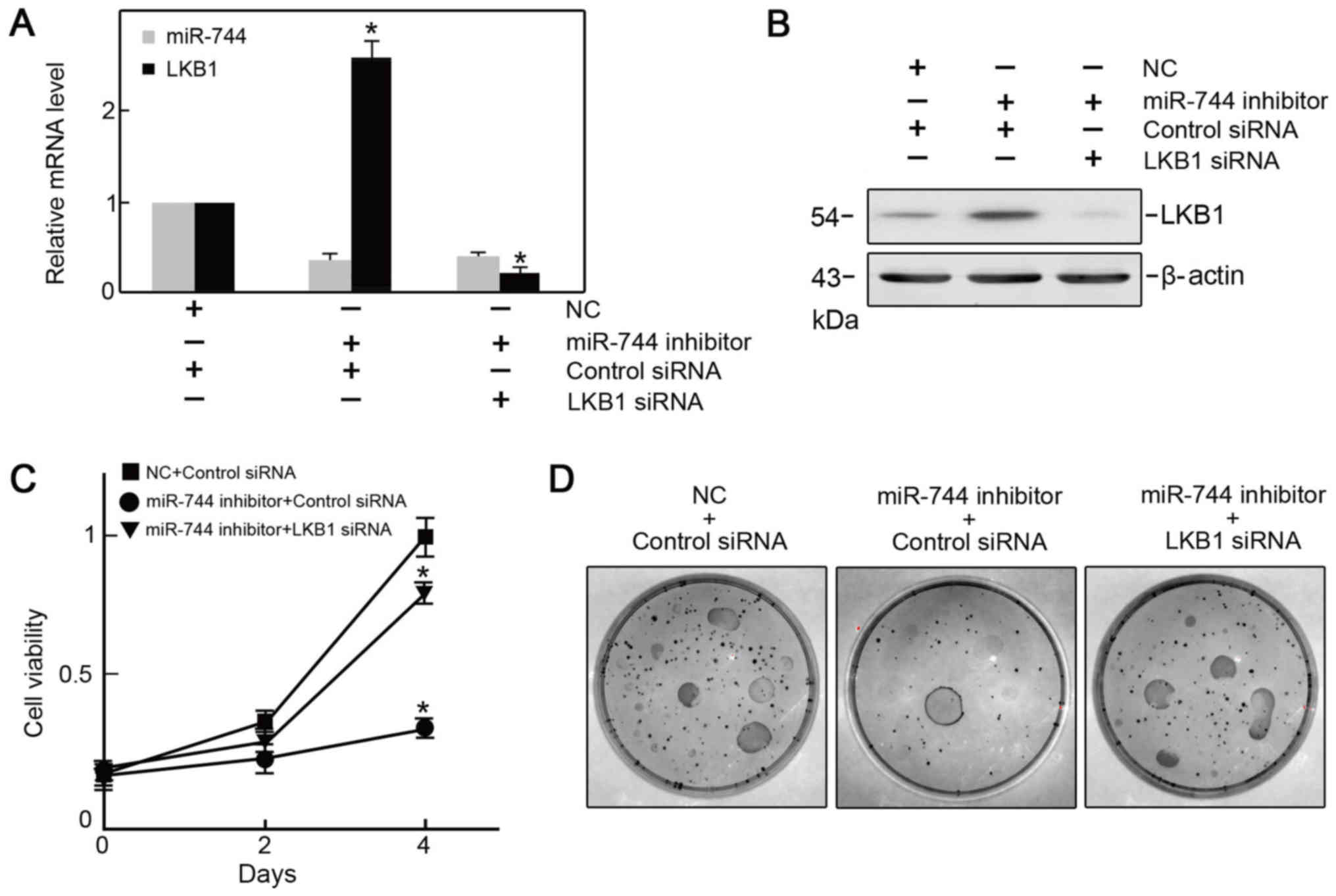
Knockdown of LKB1p reverses the effect
of miR-744 silencing in PC3 cells
To examine the association between miR-744 and LKB1
in PCa cells, the LKB1 expression vector was co-transfected with
miR-744. It was verified that LKB1 expression was restored by
co-transfection of the LKB1 expression vector (Fig. 5A and B), and it was revealed that the
viability of PC3 cells was preserved when analyzed with the CCK-8
and clonogenic assays (Fig. 5C and
D).
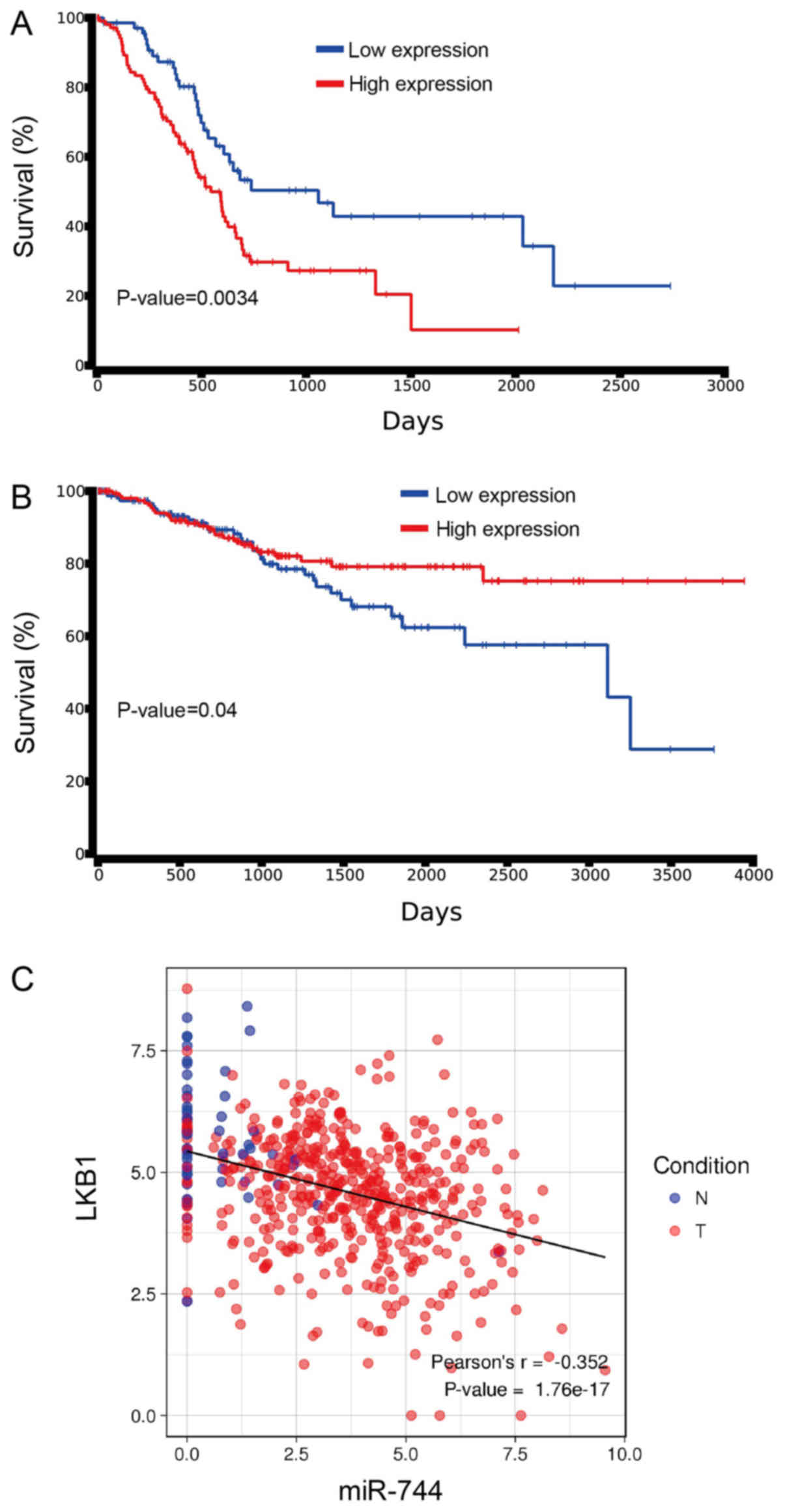
miR-744 and LKB1 are involved in the
survival of PCa patients
To investigate the potential role of miR-744 and
LKB1 in PCa survival, the Kaplan-Meier test was used to determine
the correlation between overall survival times in patients with
miR-744, and LKB1 expression in PCa patients, using data from the
TCGA database. Patient survival was lower with increased miR-744
expression, whereas survival was increased in those patients
exhibiting negative or low- miR-744 expression (Fig. 6A). However, with lower LKB1
expression, patient survival was significantly longer compared with
patients with higher LKB1 expression (Fig. 6B). A similar negative correlation was
observed between LKB1 and miR-744 in the TCGA PCa patient database
(Fig. 6C).
Discussion
PCa is clinically challenging to treat. Therefore,
understanding the molecular mechanisms of PCa carcinogenesis and
progression has become crucial for the development of effective new
therapies. Non-coding RNAs, particularly miRNAs, have opened up
novel research avenues for PCa (19,20). It
has been reported that expression levels of numerous miRNAs are
altered in PCa (21). This alteration
indicates a potential role for miRNAs in PCa progression.
Researchers have determined that miRNAs are involved in all
processes associated with cancer, including oncogenesis, migration,
invasion and angiogenesis (22–24). The
present study focused on miR-744 and investigated its biological
function and mechanism of action in PCa. It was identified that the
expression of miR-744 was upregulated in PCa tissues. Knockdown of
miR-744 produced PC3 cell growth inhibition, and increased
apoptosis. Furthermore, knockdown of miR-744 reduced the capability
of PC3 cells to proliferate. The involvement of miR-744 and LKB1 in
PCa patient survival was also demonstrated. It has been reported
that miR-744 promotes PCa progression through activation of
Wnt/β-catenin signaling (25). The
findings from the present study are consistent with those of the
previous studies, and have revealed a potential mechanism of
miR-744 in PCa progression, involving the suppression of LKB1.
To date, miRNAs have been known to suppress target
gene expression by binding to the 3′-UTR of target mRNAs and
initiating the degradation of target sequences or inhibiting
translation (26). Multiple genes,
such as tyrosine-protein phosphatase non-receptor type 1, ARHGAP5,
programmed cell death protein 4 and elongation factor 1-alpha 2
have been identified and validated as targets for miR-744 (17,27–29). The
present study demonstrates that the tumor suppressor LKB1 is a
potential target of miR-744. RT-qPCR and western blot analysis
confirmed that LKB1 expression negatively correlates with miR-744
levels. This expression pattern was consistent with the classical
miRNA regulation model. The dual luciferase assay further confirmed
that LKB1 is directly targeted by miR-744.
LKB1 is a well-established tumor suppressor that
activates the AMPK signaling pathway (30,31) by
directly phosphorylating T172 on AMPK, a requirement for its
activation (32). As LKB1 was
identified as a direct target of miR-744, activation of AMPK
signaling was further investigated following miR-744 knockdown.
Elevated phosphorylation of AMPK was observed, suggesting
activation of the AMPK signaling pathway. Crosstalk between the
mTOR and the AMPK signaling pathways is widely accepted (33). The AMPK signaling pathway may inhibit
mTOR signaling through TSC2, and thereby promote the arrest of
cancer cell proliferation (34,35).
Higher TSC2 phosphorylation and lower mTOR phosphorylation were
consistently detected in miR-744 silenced PC3 cells. These results
indicate that the mTOR signaling pathway is inhibited by miR-744
knockdown. Therefore, it was concluded that miR-744 may regulate
PCa cancer cell growth, at least through the AMPK signaling and
mTOR signaling pathways.
Taken together, these findings demonstrate a novel
insight into the biological function of miR-744 in PCa, and its
molecular mechanism of action. Our findings suggest that miR-744
may serve an oncogenic role in PCa, and that it may be a potential
therapeutic target for PCa treatment.
Acknowledgements
The authors would like to thank Dr G. Tang
(Department of Molecular Carcinogenesis, The University of Texas MD
Anderson Cancer Center) for his donation of human prostate
epithelial cell line 9 (NHP9).
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HYL and MZ designed the study and analyzed and
interpreted the cell experiment data and patient data regarding the
prostate cancer. HL and YZ performed the RT-qPCR experiments. MZ
and YZ were major contributors in writing the manuscript. All
authors read and approved the final manuscript. All authors were
involved in the conception of the study, read and approved the
manuscript and MZ and YZ agree to be accountable for all aspects of
the research in ensuring that the accuracy or integrity of any part
of the study are appropriately investigated and resolved.
Ethics approval and consent to
participate
Informed consent was received from all patients, and
the research methodology was approved by the Ethics Committee of
Jilin University (Changchun, China).
Patient consent for publication
All the patient, or parent, guardian or next of kin
(in case of deceased patients) have provided written informed
consent for the publication of any associated data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pound CR, Partin AW, Eisenberger MA, Chan
DW, Pearson JD and Walsh PC: Natural history of progression after
PSA elevation following radical prostatectomy. JAMA. 281:1591–1597.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie H, Li C, Dang Q, Chang LS and Li L:
Infiltrating mast cells increase prostate cancer chemotherapy and
radiotherapy resistances via modulation of p38/p53/p21 and ATM
signals. Oncotarget. 7:1341–1353. 2016.PubMed/NCBI
|
|
6
|
Sutherland SI, Pe Benito R, Henshall SM,
Horvath LG and Kench JG: Expression of phosphorylated-mTOR during
the development of prostate cancer. Prostate. 74:1231–1239. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galardi S, Mercatelli N, Farace MG and
Ciafrè SA: NF-kB and c-Jun induce the expression of the oncogenic
miR-221 and miR-222 in prostate carcinoma and glioblastoma cells.
Nucleic Acids Res. 39:3892–3902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
David R: Small RNAs: miRNA machinery
disposal. Nat Rev Mol Cell Biol. 14:4–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao R, Li C and Chai B: miRNA-144
suppresses proliferation and migration of colorectal cancer cells
through GSPT1. Biomed Pharmacother. 74:138–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue J, Chi Y, Chen Y, Huang S, Ye X, Niu
J, Wang W, Pfeffer LM, Shao ZM, Wu ZH and Wu J: MiRNA-621
sensitizes breast cancer to chemotherapy by suppressing FBXO11 and
enhancing p53 activity. Oncogene. 35:448–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lewis H, Lance R, Troyer D, Beydoun H,
Hadley M, Orians J, Benzine T, Madric K, Semmes OJ, Drake R, et al:
miR-888 is an expressed prostatic secretions-derived microRNA that
promotes prostate cell growth and migration. Cell Cycle.
13:227–239. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin M, Zhang T, Liu C, Badeaux MA, Liu B,
Liu R, Jeter C, Chen X, Vlassov AV and Tang DG: miRNA-128
suppresses prostate cancer by inhibiting BMI-1 to inhibit
tumor-initiating cells. Cancer Res. 74:4183–4195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xuan H, Xue W, Pan J, Sha J, Dong B and
Huang Y: Downregulation of miR-221, −30d and −15a contributes to
pathogenesis of prostate cancer by targeting Bmi-1. Biochemistry
(Mosc). 80:276–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh PK, Preus L, Hu Q, Yan L, Long MD,
Morrison CD, Nesline M, Johnson CS, Koochekpour S, Kohli M, et al:
Serum microRNA expression patterns that predict early treatment
failure in prostate cancer patients. Oncotarget. 5:824–840. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyamae M, Komatsu S, Ichikawa D,
Kawaguchi T, Hirajima S, Okajima W, Ohashi T, Imamura T, Konishi H,
Shiozaki A, et al: Plasma microRNA profiles: Identification of
miR-744 as a novel diagnostic and prognostic biomarker in
pancreatic cancer. Br J Cancer. 113:1467–1476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song MY, Pan KF, Su HJ, Zhang L, Ma JL, Li
JY, Yuasa Y, Kang D, Kim YS and You WC: Identification of serum
microRNAs as novel non-invasive biomarkers for early detection of
gastric cancer. PLoS One. 7:e336082012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Y, Zhu X, Wang J, Li N, Li D, Sakib
N, Sha Z and Song W: MiR-744 functions as a proto-oncogene in
nasopharyngeal carcinoma progression and metastasis via
transcriptional control of ARHGAP5. Oncotarget. 6:13164–13175.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou W, Li Y, Gou S, Xiong J, Wu H, Wang
C, Yan H and Liu T: MiR-744 increases tumorigenicity of pancreatic
cancer by activating Wnt/β-catenin pathway. Oncotarget.
6:37557–37569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin F, Lei S, Ma J, Shi L, Mao D and Zhang
S, Huang J, Liu X, Ding D, Zhang Y and Zhang S: Inhibitory effect
of jianpi-jiedu prescription-contained serum on colorectal cancer
SW48 cell proliferation by mTOR-P53-P21 signalling pathway. Zhong
Nan Da Xue Xue Bao Yi Xue Ban. 41:1128–1136. 2016.(In Chinese).
PubMed/NCBI
|
|
20
|
Jackson BL, Grabowska A and Ratan HL:
MicroRNA in prostate cancer: Functional importance and potential as
circulating biomarkers. BMC Cancer. 14:9302014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song C, Chen H, Wang T, Zhang W, Ru G and
Lang J: Expression profile analysis of microRNAs in prostate cancer
by next-generation sequencing. Prostate. 75:500–516. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei P, Qiao B, Li Q, Han X, Zhang H, Huo Q
and Sun J: microRNA-340 suppresses tumorigenic potential of
prostate cancer cells by targeting high-mobility group
nucleosome-binding domain 5. DNA Cell Biol. 35:33–43. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji H, Li Y, Jiang F, Wang X, Zhang J, Shen
J and Yang X: Inhibition of transforming growth factor beta/SMAD
signal by MiR-155 is involved in arsenic trioxide-induced
anti-angiogenesis in prostate cancer. Cancer Sci. 105:1541–1549.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guan H, Liu C, Fang F, Huang Y, Tao T,
Ling Z, You Z, Han X, Chen S, Xu B and Chen M: MicroRNA-744
promotes prostate cancer progression through aberrantly activating
Wnt/β-catenin signaling. Oncotarget. 8:14693–14707. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pencheva N and Tavazoie SF: Control of
metastatic progression by microRNA regulatory networks. Nat Cell
Biol. 15:546–554. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Han X, Tang Y, Wu Y, Qu B and
Shen N: miR-744 enhances type I interferon signaling pathway by
targeting PTP1B in primary human renal mesangial cells. Sci Rep.
5:129872015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng H, Qu J, Jin N, Xu J, Lin C, Chen Y,
Yang X, He X, Tang S, Lan X, et al: Feedback activation of leukemia
inhibitory factor receptor limits response to histone deacetylase
inhibitors in breast cancer. Cancer Cell. 30:459–473. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vislovukh A, Kratassiouk G, Porto E,
Gralievska N, Beldiman C, Pinna G, El'skaya A, Harel-Bellan A,
Negrutskii B and Groisman I: Proto-oncogenic isoform A2 of
eukaryotic translation elongation factor eEF1 is a target of
miR-663 and miR-744. Br J Cancer. 108:2304–2311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hezel AF and Bardeesy N: LKB1; linking
cell structure and tumor suppression. Oncogene. 27:6908–6919. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blagih J, Krawczyk CM and Jones RG: LKB1
and AMPK: Central regulators of lymphocyte metabolism and function.
Immunol Rev. 249:59–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shaw RJ, Kosmatka M, Bardeesy N, Hurley
RL, Witters LA, DePinho RA and Cantley LC: The tumor suppressor
LKB1 kinase directly activates AMP-activated kinase and regulates
apoptosis in response to energy stress. Proc Natl Acad Sci USA.
101:3329–3335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agarwal S, Bell CM, Rothbart SB and Moran
RG: AMP-activated protein kinase (AMPK) control of mTORC1 is p53-
and TSC2-independent in pemetrexed-treated carcinoma cells. J Biol
Chem. 290:27473–27486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jozwiak J, Jozwiak S, Grzela T and
Lazarczyk M: Positive and negative regulation of TSC2 activity and
its effects on downstream effectors of the mTOR pathway.
Neuromolecular Med. 7:287–296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen MB, Zhang Y, Wei MX, Shen W, Wu XY,
Yao C and Lu PH: Activation of AMP-activated protein kinase (AMPK)
mediates plumbagin-induced apoptosis and growth inhibition in
cultured human colon cancer cells. Cell Signal. 25:1993–2002. 2013.
View Article : Google Scholar : PubMed/NCBI
|