Introduction
Colon cancer is a common malignancy type of the
gastrointestinal tract (1). In 2012,
colorectal cancer in China was 16.9 per 100,000 in males and 11.6
per 100,000 in females, and the age-standardized mortality was 9.0
per 100,000 in males and 6.1 per 100,000 in females (1). Notable progress has been achieved in
colon cancer therapy; however, metastasis and recurrence of colon
cancer still influences the outcome of patients profoundly
(2). To improve patient prognosis,
it is necessary to determine more effective biomarkers for
diagnosis and treatment of colon cancer.
Inflammation is associated with the development of
multiple types of cancer, such as esophageal cancer and pancreatic
cancer (3,4). Previous studies have determined that
the novel pro-inflammatory cytokine interleukin (IL)-32 is
associated with the development of numerous types of cancer, such
as breast cancer (5) and esophageal
cancer (6). Additionally, IL-32 has
increased expression levels in gastric cancer (7), pulmonary adenocarcinoma (8), pancreatic adenocarcinoma (9) and esophageal cancer (6).
Paradoxically, a number of researchers consider that
overexpression of IL-32 has a tumor-suppressing effect (10–12). In
thyroid cancer, cytological experiments have demonstrated that the
number of BC-PAP and FTC133 apoptotic thyroid cancer cells
decreases following suppression of IL-32 expression, while
overexpression of IL-32 promotes apoptosis in cancer cells
(10). The apoptosis-inducing effect
of IL-32 has also been observed in HeLa cells, which originated
from a cervical cancer sample (11).
In a previous study, B16 melanoma cells were subcutaneously
injected into transgenic and non-transgenic mice overexpressing
IL-32. The volume and weight of the resulting tumor samples were
measured on day 26, and those in transgenic mice had significantly
reduced size and weight, compared with the non-transgenic mice
(12).
In the present study, the IL-32 expression in colon
cancer was assessed and its association with clinicopathological
parameters was investigated. To the best of our knowledge, the
present study provides the first evidence of the role of IL-32 in
the prognosis of patients with colon cancer.
Patients and methods
Patients and samples
In the present study, 60 colon cancer specimens
obtained from the Department of Pathology, The First Affiliated
Hospital of Henan University of Science and Technology (Luoyang,
China) between January 2006 and January 2007 were examined. The
patients included 35 males and 25 females with a mean age of 56.2
years (range, 42–71 years). Pathological data were studied
prospectively following surgery. The survival time was calculated
from the date of surgery to the follow-up deadline (August 2016) or
date of mortality. Two experienced pathologists from the Department
of Pathology, the First Affiliated Hospital of Henan University of
Science and Technology, were blinded to the results of the present
study. Clinicopathological parameters of these patients are
displayed in Table I. Tumor
differentiation and Dukes' stage were classified in accordance with
the report by Fukushima et al (13). Written informed consent was obtained
from each patient upon collection of the samples. The study
protocol was approved by the Human Ethics Review Committees of
Henan University of Science and Technology (approval no.
2013-PJ152).
 | Table I.Association between IL-32 expression
and clinicopathological variables of patients with colon
cancer. |
Table I.
Association between IL-32 expression
and clinicopathological variables of patients with colon
cancer.
|
|
| IL-32 expression |
|
|---|
|
|
|
|
|
|---|
| Variable | n | Negative | Positive | P-value |
|---|
| Sex |
|
|
| 0.794 |
| Male | 35 | 14 | 21 |
|
|
Female | 25 | 9 | 16 |
|
| Age (years) |
|
|
| 0.271 |
| ≤60 | 31 | 16 | 15 |
|
|
>60 | 29 | 7 | 22 |
|
| Tumor size (cm) |
|
|
| 0.015a |
| ≤5
cm | 38 | 10 | 28 |
|
| >5
cm | 22 | 13 | 9 |
|
| Tumor differentiation
(13) |
|
|
| 0.261 |
| Grade
1 | 20 | 10 | 10 |
|
| Grade 2
and 3 | 40 | 13 | 27 |
|
| Dukes' stage
(13) |
|
|
| 0.001a |
| A+B | 28 | 17 | 11 |
|
| C+D | 32 | 6 | 26 |
|
| Lymph node
metastasis |
|
|
| 0.298 |
| No | 26 | 12 | 14 |
|
| Yes | 34 | 11 | 23 |
|
Immunohistochemistry
Staining for IL-32 was performed using 10%
formalin-fixed, paraffin-embedded serial sections at 37°C. Sections
(4-µm thick) were cut from the selected paraffin blocks and
deparaffinized in dimethylbenzene. The slides were microwaved in
sodium citrate-hydrochloric acid buffer solution for 4 min for
antigen retrieval at 95°C and rehydration with phosphate buffered
saline (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4).
IL-32 was detected with a mouse polyclonal antibody (cat. no:
sc-517408, 1:150 dilution, Santa Cruz Biotechnology, Dallas, TX,
USA). Ki-67 (cat. no: GT209401) were purchased from Gene
Biotechnology (http://www.genetech.com.cn, Shanghai, China). The
antibody reaction was conducted at 37°C for 3 h. Labeling was
detected by adding biotinylated secondary antibodies (http://www.maxim.com.cn/sitecn/myzhjcxthsjh/981.html,
KIT-9710, Rabbit, 37°C for 0.5 h), avidin-biotin complex and
diaminobenzidine (all from Maxim-Bio, Fuzhou, China, http://www.maxim.com.cn/sitecn/myzhjcxthsjh/981.html).
Sections were then counterstained with 10% hematoxylin at 37°C for
0.5 h. Expression of IL-32 was scored according to the positive
percentage and staining intensity of the stained tumor cells. The
percent positivity was scored as ‘0’ (0-25%), ‘1’ (26-50%), ‘2’
(51-75%) and ‘3’ (>75%). The staining intensity was scored as
‘0’ (no staining), ‘1’ (weakly stained), ‘2’ (moderately stained)
and ‘3’ (strongly stained). If the product of multiplication
between staining intensity and the percentage of positive cells is
≥2, it was considered as immunoreaction positive (+). Ki-67 was
scored according the positive number of 100 cancer cells at high
magnification by a light microscope (Olympus Corporation, Tokyo,
Japan; magnification, ×400).
Western blot analysis
A total of 5 paired colon cancer and non-cancerous
colonic epithelium tissues were lysed (RIPA Buffer Beyotime
Institute of Biotechnology) and lysates were obtained by
centrifugation at 4°C (35,000 × g, 30 min). BCA Protein assay kit
was used to measure protein concentration. Following applying 30 µg
total protein to 10% SDS-PAGE, the total protein was transferred to
a polyvinylidene fluoride membrane. Following blocking (5% skimmed
milk, 37°C for 3 h), the membranes were incubated with IL-32
antibody at 4°C for 12 h (1:200 dilution; cat. no. sc-50001; Santa
Cruz Biotechnology), followed by horseradish peroxidase-conjugated
secondary antibody (Rabbit anti-goat IgG, ZB 2306, 1:5,000
dilution, incubation at 37°C for 2 h). Subsequently, IL-32
expression was detected by enhanced chemiluminescent substrate
(cat. no. 34580; Thermo Scientific, Inc.). GAPDH was served as the
internal control (1:150 dilution; cat. no. sc-0411; Santa Cruz
Biotechnology).
Statistical analysis
Statistical analyses were performed using SPSS 13.0
for Windows (SPSS, Inc., Chicago, IL, US). The association between
IL-32 and clinicopathological parameters was assessed using
Fisher's exact test. Results are presented as mean ± SD. Survival
curves were calculated using the Kaplan-Meier method and compared
by log-rank test. The Cox proportional hazards regression model was
performed for multivariate survival analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
IL-32 expression in non-cancerous
colonic epithelium and colon cancer tissues
To investigate the expression of IL-32 in colon
cancer, IL-32 staining in 60 colon cancer specimens was assessed
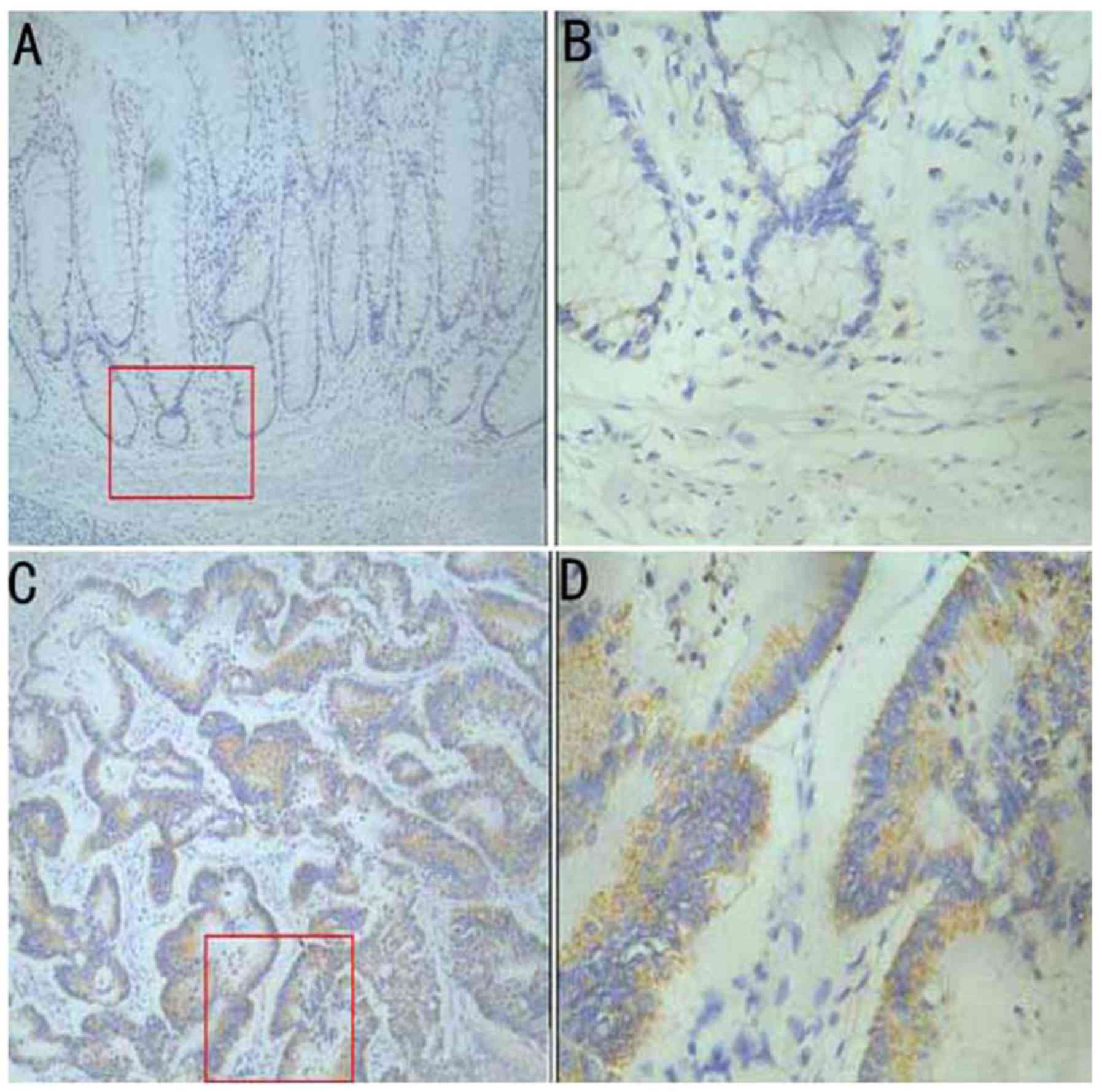
using immunohistochemistry. Immunohistochemical results indicated
weak immunoreactivity of IL-32 in non-cancerous colonic epithelium
(Fig. 1A and B); however, IL-32
staining was distributed in the cytoplasm of cancer cells (Fig. 1C and D). Of the 60 cases, 37 (61.67%)
samples exhibited positive staining of IL-32 in the cytoplasm.
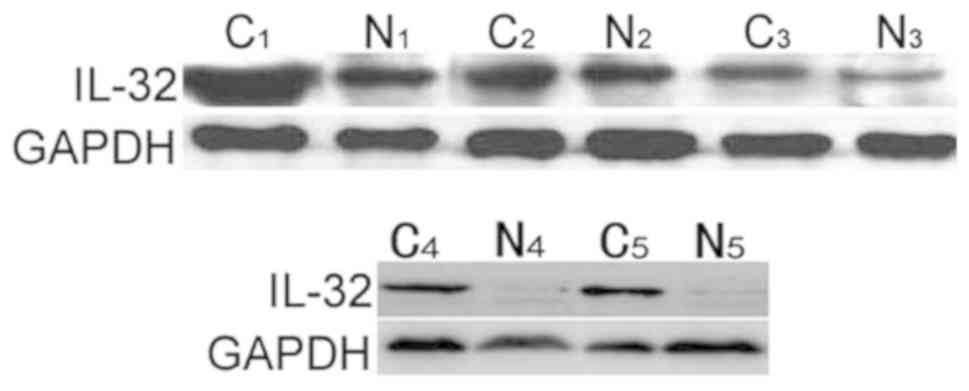
To further verify the results obtained from
immunohistochemistry, western blot analysis was performed to detect
the IL-32 expression in 5 paired colon cancer and non-cancerous
colonic epithelium tissues. The data demonstrated that there was
notably increased IL-32 expression in colon cancer tissues,
compared with complementary non-cancerous tissues (Fig. 2), which was consistent with the
immunohistochemical results.
Association between IL-32 expression
and proliferation index (PI)
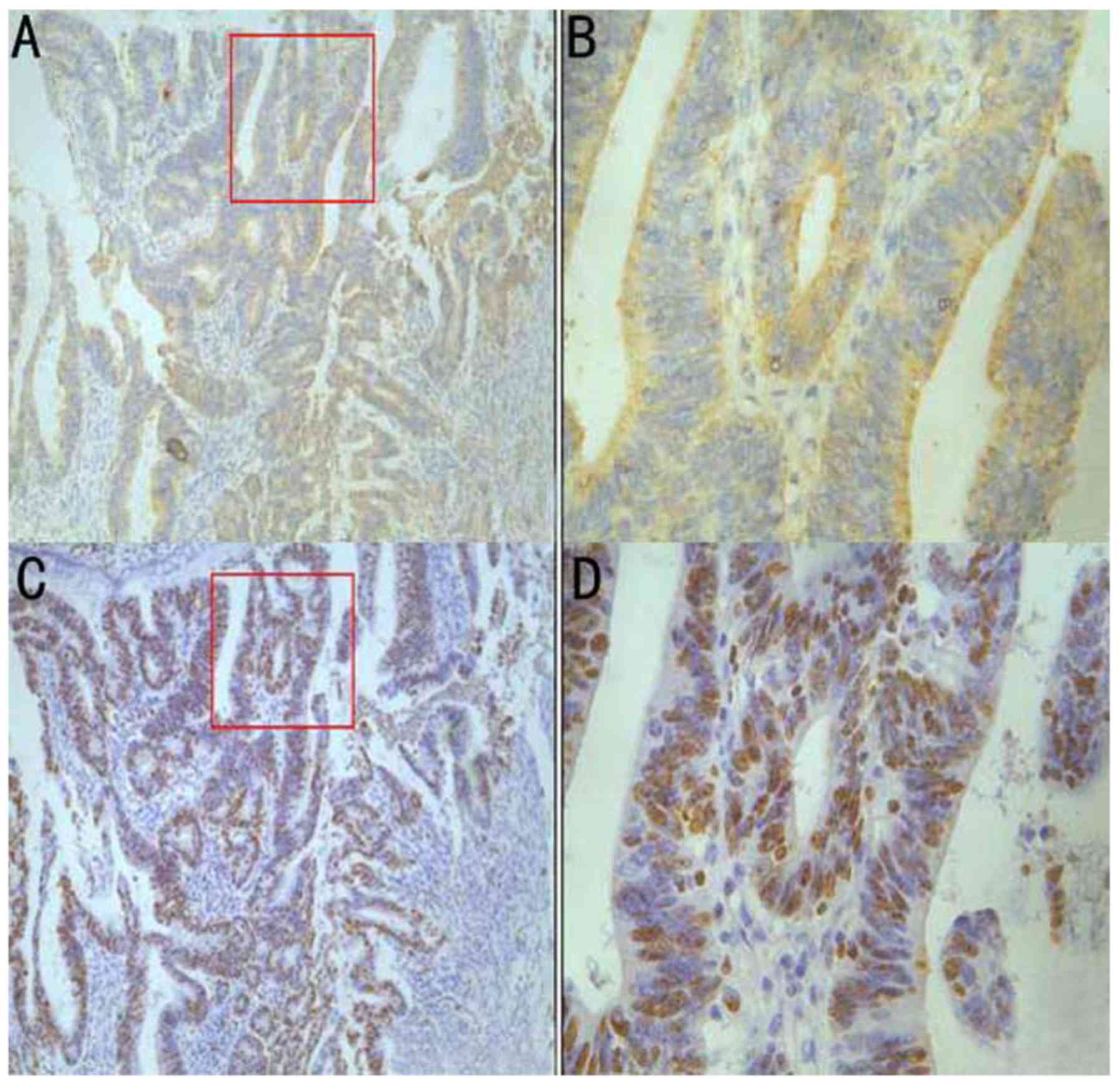
To investigate the role of IL-32 on proliferation of
colon cancer, the association between IL-32 and PI was evaluated.
As depicted in Fig. 3, there was
significantly increased PI in the group positive for IL-32
expression (64.5±9.2), compared with the IL-32 negative group
(33.5±5.8). These data demonstrated that IL-32 may be associated
with the proliferation of colon cancer cells.
Association between IL-32 expression
and clinicopathological factors of patients with colon cancer
As depicted in Table
I, there were 26 IL-32 positive samples with Dukes' stage C+D,
compared with only 6 for IL-32 negative samples. Furthermore,
positive IL-32 expression was significantly associated with tumor
size ≤5 cm, compared with negative IL-32 expression (P=0.015) and
this result confirmed that IL-32 may be associated with the
proliferation of colon cancer. No significant association was
observed between IL-32 expression and other variables, including
sex, age, tumor differentiation and lymph node metastasis
(P>0.05).
Association between IL-32 expression
and outcome of patients with colon cancer
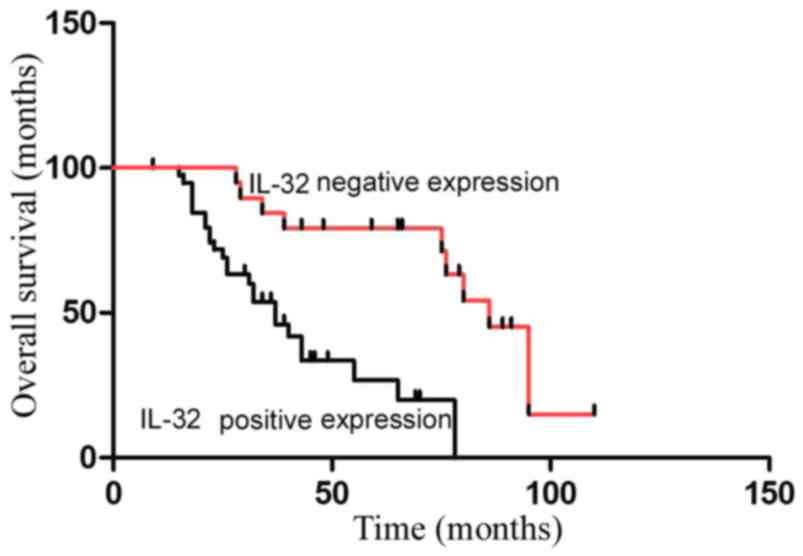
In the present study, the survival of 60 patients
with colon cancer, divided into IL-32-positive and -negative
groups, was investigated. Patients positive for IL-32 expression
had less favorable prognoses, compared with patients negative for
IL-32 expression, which indicates that IL-32 may be an independent
prognostic factor for colon cancer (IL-32-positive group vs.
IL-32-negative group: P<0.05; Fig.
4).
Multivariate analyses were performed to confirm that
IL-32 is an independent prognostic predictor for patients with
colon cancer. Cox proportional regression models together with
other pathological features, including sex, age, tumor size, Dukes'
stage, tumor differentiation and lymph node metastasis, were used.
As presented in Table II,
multivariate analysis indicated that IL-32 expression, Dukes'
stage, tumor differentiation and lymph node metastasis could be
independent prognostic factors in patients with colon cancer.
Notably, lymph node metastasis had the most significant parameter
(P=0.014), followed by IL-32 expression (P=0.026), Dukes' stage
(P=0.035) and tumor differentiation (P=0.042).
 | Table II.Univariate and multivariate analysis
of different prognostic parameters in patients with colon
cancer. |
Table II.
Univariate and multivariate analysis
of different prognostic parameters in patients with colon
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.467 | 0.681–3.852 | 0.252 |
|
|
|
| Sex | 1.346 | 0.797–1.368 | 0.559 |
|
|
|
| Tumor size | 0.786 | 0.293–2.863 | 0.825 |
|
|
|
| Dukes' stage
(13) | 3.679 | 1.863–7.469 | 0.009a | 2.964 | 1.373–7.069 | 0.035a |
| Tumor
differentiation (13) | 5.232 | 2.343–11.683 | 0.006a | 2.623 | 0.918–6.437 | 0.042a |
| Lymph node
metastasis | 6.394 | 2.375–17.643 | 0.001a | 4.156 | 1.228–9.748 | 0.014a |
| IL-32
expression | 6.735 | 2.602–15.741 | 0.002a | 3.486 | 1.672–8.122 | 0.026a |
Discussion
Inflammation promotes the development, invasion, and
metastasis of a number of cancer types, such as colorectal cancer
and nasopharyngeal carcinoma (14,15); the
novel pro-inflammatory cytokine IL-32 has been demonstrated to have
tumor-promoting and tumor-suppressing effects under certain
conditions (5,16–22).
Tsai et al (23) determined
that overexpression of IL-32 can cause gastric cancer cells to
transition to spindle cells and epithelial cells to transition to
mesenchymal cells, enhancing the ability of tumor cells to move and
inducing the expression levels of IL-8, vascular endothelial growth
factor, metalloproteinase (MMP)2 and MMP9 through the p-AKt/β
catenin pathway and the p-AKt/hypoxia inducible factor-α pathway.
Subsequently, this enhances the ability of the cancer cells to
invade other tissues and metastasize (23). Immunohistochemical staining of 120
patients with gastric cancer indicated that the strength of IL-32
staining in the tumor cytoplasm was notably increased, compared
with the surrounding non-neoplastic mucosal cells (23). Ishigami et al (7) investigated 181 patients with gastric
cancer and concluded consistent results to the aforementioned study
(23). Additionally, IL-32
expression determined to be associated with lymph node metastasis
and the progression of gastric cancer (7). Multiple-factor analysis demonstrated
that IL-32 is an independent prognostic factor for patients with
pulmonary adenocarcinoma (24).
In vitro investigation of pulmonary adenocarcinoma A549
cells indicated that IL-32 has the ability to activate nuclear
factor (NF)-κB and induce the generation of MMP2 and MMP9,
consequently promoting the ability of cancer cells to invade other
tissues (8).
However, Heinhuis et al (10) determined that IL-32β and IL-32γ
promote apoptosis in thyroid cancer cells by activating the
caspase-3/caspase8 pathway, and IL-32γ exhibited a more pronounced
ability to induce apoptosis due to it downregulating C-X-C motif
chemokine receptor 1 expression, blocking the survival signaling
pathway of IL-8 (10). Furthermore,
IL-32γ overexpression in the colon cancer cell line SW620 causes
suppression of cell proliferation along with reduced activity of
NF-κB and signal transducer and activator of transcription (STAT)3,
which indicates that IL-32γ can regulate tumor cell apoptosis and
tumor development by suppressing NF-κB and STAT3 signals (22).
In the present study, it was determined that IL-32
expression is increased in colon cancer tissues, compared with
non-tumoral colorectal tissues. Additionally, it was determined
that IL-32 expression is significantly associated with an earlier
Dukes' stage and a tumor size ≤5 cm. Therefore, IL-32 may
participate in the tumorigenesis of colon cancer. Using
multivariate Cox proportional hazards regression analysis, the role
of IL-32 in the prognosis of patients with colon cancer was
investigated. Consistent with data reported by Ishigami et
al (7) in gastric cancer, the
present results also indicated IL-32 staining to be associated with
shorter overall survival time by multiple-factor analysis, and it
could be considered as an independent prognostic factor for
patients with colon cancer.
To conclude, the present data demonstrated that
IL-32 expression participated in the progression of colon cancer.
IL-32 staining is also associated with unfavorable prognosis in
colon cancer; therefore, IL-32 could be considered as a novel
biomarker for predicting the prognosis of colon cancer, but further
confirmation is required in a larger cohort to provide novel cancer
treatments.
Acknowledgements
Not applicable.
Funding
The present study was funded by project of Science
and technology of the Henan Province (172102310406).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JMZ and YHA designed the study. JMZ, WW and YGF
analyzed the data. GLY collected and analyzed clinical samples, and
also was a major contributor in writing the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent from all patients and
ethical approval was obtained from the Human Ethics Review
Committees of Henan University of Science and Technology (approval
no. 2013-PJ152).
Patient consent for publication
Patients have provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y, Shi J, Huang H, Ren J, Li N and
Dai M: Burden of colorectal cancer in China. Zhonghua Liu Xing Bing
Xue Za Zhi. 36:709–714. 2015.(In Chinese). PubMed/NCBI
|
|
2
|
Kanwar SS1, Poolla A and Majumdar AP:
Regulation of colon cancer recurrence and development of
therapeutic strategies. World J Gastrointest Pathophysiol. 3:1–9.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu H, Ouyang W and Huang C: Inflammation,
a key event in cancer development. Mol Cancer Res. 4:221–233. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Chen F and Tang L: IL-32 promotes
breast cancer cell growth and invasiveness. Oncol Lett. 9:305–307.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yousif NG, Al-Amran FG, Hadi N, Lee J and
Adrienne J: Expression of IL-32 modulates NF-κB and p38 MAP kinase
pathways in human esophageal cancer. Cytokine. 61:223–227. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishigami S, Arigami T, Uchikado Y,
Setoyama T, Kita Y, Sasaki K, Okumura H, Kurahara H, Kijima Y,
Harada A, et al: IL-32 expression is an independent prognostic
marker for gastric cancer. Med Oncol. 30:4722013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng Q, Li S, Zhou Y, Ou W, Cai X, Zhang
L, Huang W, Huang L and Wang Q: Interleukin-32 contributes to
invasion and metastasis of primary lung adenocarcinoma via
NF-kappaB induced matrix metalloproteinases 2 and 9 expression.
Cytokine. 65:24–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishida A, Andoh A, Inatomi O and Fujiyama
Y: Interleukin-32 expression in the pancreas. J Biol Chem.
284:17868–17876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heinhuis B, Plantinga TS, Semango G,
Küsters B, Netea MG, Dinarello CA, Smit JWA, Netea-Maier RT and
Joosten LAB: Alternatively spliced isoforms of IL-32 differentially
influence cell death pathways in cancer cell lines. Carcinogenesis.
37:197–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goda C, Kanaji T, Kanaji S, Tanaka G,
Arima K, Ohno S and Izuhara K: Involvement of IL-32 in
activation-induced cell death in T cells. Int Immunol. 18:233–240.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oh JH, Cho MC, Kim JH, Lee SY, Kim HJ,
Park ES, Ban JO, Kang JW, Lee DH, Shim JH, et al: IL-32γ inhibits
cancer cell growth through inactivation of NF-κB and STAT3 signals.
Oncogene. 30:3345–3359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukushima Y, Iinuma H, Tsukamoto M,
Matsuda K and Hashiguchi Y: Clinical significance of microRNA-21 as
a biomarker in each Dukes' stage of colorectal cancer. Oncol Rep.
33:573–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itzkowitz SH and Yio X: Inflammation and
cancer IV. Colorectal cancer in inflammatory bowel disease: The
role of inflammation. Am J Physiol Gastrointest Liver Physiol.
287:G7–G17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semango G, Heinhuis B, Plantinga TS, Blokx
WAM, Kibiki G, Sonda T, Mavura D, Masenga EJ, Nyindo M, van der Ven
AJAM and Joosten LAB: Exploring the role of IL-32 in HIV-related
Kaposi sarcoma. Am J Pathol. 188:196–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Yang Y, Zhu Y, Li L, Chen F and
Zhang L: Polymorphisms and expression of IL-32: Impact on genetic
susceptibility and clinical outcome of lung cancer. Biomarkers.
22:165–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JS, Choi SY, Lee JH, Lee M, Nam ES,
Jeong AL, Lee S, Han S, Lee MS, Lim JS, et al: Interleukin-32β
stimulates migration of MDA-MB-231 and MCF-7 cells via the
VEGF-STAT3 signaling pathway. Cell Oncol (Dordr). 36:493–503. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suga H, Sugaya M, Miyagaki T, Kawaguchi M,
Fujita H, Asano Y, Tada Y, Kadono T and Sato S: The role of IL-32
in cutaneous T-cell lymphoma. J Invest Dermatol. 134:1428–1435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seo EH, Kang J, Kim KH, Cho MC, Lee S, Kim
HJ, Kim JH, Kim EJ, Park DK, Kim SH, et al: Detection of expressed
IL-32 in human stomach cancer using ELISA and immunostaining. J
Microbiol Biotechnol. 18:1606–1612. 2008.PubMed/NCBI
|
|
21
|
Nicholl MB, Chen X, Qin C, Bai Q, Zhu Z,
Davis MR and Fang Y: IL-32α has differential effects on
proliferation and apoptosis of human melanoma cell lines. J Surg
Oncol. 113:364–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park ES, Yoo JM, Yoo HS, Yoon DY, Yun YP
and Hong J: IL-32γ enhances TNF-α-induced cell death in colon
cancer. Mol Carcinog. 53 Suppl 1:E23–E35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai CY, Wang CS, Tsai MM, Chi HC, Cheng
WL, Tseng YH, Chen CY, Lin CD, Wu JI, Wang LH and Lin KH:
Interleukin-32 increases human gastric cancer cell invasion
associated with tumor progression and metastasis. Clin Cancer Res.
20:2276–2288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sorrentino C and Di Carlo E: Expression of
IL-32 in human lung cancer is related to the histotype and
metastatic phenotype. Am J Respir Crit Care Med. 180:769–779. 2009.
View Article : Google Scholar : PubMed/NCBI
|