Introduction
Melanoma is a malignancy of melanocytes, which
originate from pigment-producing cells initially derived from the
neuroectoderm that can be found throughout the skin, iris and
rectum (1). An epidemiological study
has demonstrated that ultraviolet radiation exposure and severe
sunburns are the major risk factors for developing melanoma
(2). The cutaneous form of the
disease is common in the Western world; 76,380 new cases and 10,130
cases of cancer-associated mortality were attributed to melanoma in
the United States in 2016 (3), and
it caused the majority (75%) of cases of skin cancer-associated
mortality. It has been reported that the global incidence rate of
melanoma is 15–25/100,000 individuals (4), with an increase in melanoma-associated
mortality every year. Following traditional therapy, the 5-year
survival rate of patients with metastatic melanoma is consistently
<10% in the majority of cases (5). Additionally, until 2010 no randomized
clinical trial could provide evidence that the survival rate can be
improved for those with advanced-stage metastatic melanoma
(1). Therefore, investigating
effective agents or adjuvant therapies for the treatment of
melanoma is a continuing concern.
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is one
of the ingredients in capsicum, the active component of peppers.
While chili peppers are extensively used in food as a pungent spice
(6), China is one of the first
countries to use peppers as a medicine. Capsaicin is a major active
compound from chili peppers and has numerous beneficial roles in
humans, which are similar with the practice of Traditional Chinese
Medicine. In Traditional Chinese Medicine, peppers are used to
treat various diseases, including abdominal pain, colds and
rheumatism. Previous studies have documented that capsaicin has
anti-inflammatory, analgesic, anesthetic and detoxification effects
(7–10). Furthermore, a previous study has
reported that capsaicin is a potential agonist of transient
receptor potential cation channel subfamily V member 1, and may
exert its effects through the receptor-dependent pathway and the
receptor-independent pathway (6). In
addition, by inducing apoptosis and autophagy in numerous types of
malignant cell lines, studies have revealed that capsaicin has an
inhibitory effect on tumor growth, which demonstrates that
capsaicin has potential anticancer effects (11), including in prostate cancer cells
(12), human nasopharyngeal
carcinoma (13), colon
adenocarcinoma (14,15) and hepatocellular carcinoma (16). However, to the best of our knowledge,
no study has investigated whether capsaicin could induce apoptosis
and autophagy in human melanoma cells.
Apoptosis and autophagy are two types of programmed
cell death (PCD). Cell death can be classified through
morphological differences as a hallmark of cancer. Apoptosis is
characterized by cell shrinkage, dynamic membrane blebbing, nuclear
condensation and fragmentation, and loss of adhesion to neighboring
cells or the extracellular matrix (17). Inducing cell apoptosis is considered
a reasonable and promising chemopreventive or chemotherapeutic
strategy for tumor cells (18).
Autophagy, or type II PCD, is a cellular self-catabolic process:
Cytoplasmic constituents are isolated in double membrane vesicles,
through the lysosome-dependent machinery, and fused by lysosomes
where they are degraded (19).
Autophagy is a tightly regulated strategy developed by cells to
provide energy and nutrients by digesting long-lived or damaged
cytosolic proteins and organelles. As a novel cancer therapy,
targeting PCD through modulation of autophagy could be an effective
approach for alleviating treatment resistance in
apoptosis-defective tumor cells (20). Under certain circumstances, autophagy
constitutes a stress adaptation that suppresses apoptosis (21). This is a complex functional
association between apoptosis and autophagy, which serves critical
functions in cancer development and progression.
The aim of the present study was to determine
whether it is possible for capsaicin to suppress human melanoma
cells in vitro, and to investigate the effect of capsaicin
on induction of apoptosis and autophagy in human melanoma cells.
Furthermore, the functional association between apoptosis and
autophagy was investigated.
Materials and methods
Chemicals and reagents
Capsaicin (purity >98%) was purchased from
Shanghai Yuanye Bio-technology Co., Ltd. (Shanghai, China).
Dulbecco's modified Eagle's medium (DMEM), trypsin, fetal bovine
serum (FBS) and dimethyl sulfoxide (DMSO) were purchased from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). EDTA-free
trypsin was purchased from HyClone; GE Healthcare Life Sciences
(Logan, UT, USA). A fluorescein isothiocyanate (FITC)-Annexin V kit
was obtained from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China). Propidium iodide (PI) was purchased from Takara Bio, Inc.
(Otsu, Japan). 3-methyladenine (3-MA) was purchased from Selleck
Chemicals (Houston, TX, USA). Antibodies against cleaved poly
(ADP-ribose) polymerase (cleaved-PARP) (cat no. 5625), cleaved
caspase-3 (cat no. 9664), microtubule-associated proteins 1A/1B
light chain 3B (LC3B) (cat no. 3868), beclin 1 (cat no. 3495) and
GAPDH (cat no. 5174) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA).
Cell lines and cell culture
The human melanoma A375 and C8161 cell lines were
obtained from Peking Union Cell Resource Center (Beijing, China).
The cells were cultured in DMEM supplemented with 1%
penicillin-streptomycin and 10% FBS in a humidified atmosphere with
5% CO2 at 37°C. A375 and C8161 cells in the treatment
group were treated with different concentrations of capsaicin (0,
25, 50, 100, 200 and 400 Μm) at 37°C for 24 or 48 h, whereas cells
in the control group were treated with equivalent volumes of
DMSO.
Cell viability assay
The effect of capsaicin on cell viability was
measured using a Cell Counting kit-8 (CCK8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay. A375 and C8161 cells
(100 µl) were seeded in a 96-well plate at a density of
2×104 cells/ml and were incubated overnight at 37°C in
an atmosphere containing 95% air and 5% CO2.
Subsequently, the cells were treated with various concentrations of
capsaicin (25, 50, 100, 200 and 400 µM). After 24 or 48 h, 10 µl
CCK8 solution was added to each well for 2 h at 37°C according to
the manufacturer's protocol. The absorbance at 450 nm was measured
using the iMark microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Apoptosis analysis by flow
cytometry
A375 cells (5×104 cells/ml) were placed
in 6-well culture plates and treated with capsaicin at indicated
concentrations (0, 50, 100, and 200 µM) or DMSO for 24 h. Following
capsaicin treatment, the cells were harvested by EDTA-free trypsin,
washed twice with cold PBS and resuspended in binding buffer at a
concentration of 1×106 cells/ml. Subsequently, cells
were incubated with FITC-conjugated Annexin V and PI (5 µl each)
for 15 min at room temperature in the dark. Subsequently, the
samples were analyzed using a flow cytometer r3.1 (FACSCalibur; BD
Biosciences, San Jose, CA, USA).
LysoTracker® red
staining
A375 cells were seeded in 6-well plates (1 ml per
well) at a density of 5×105 cells/ml and treated with or
without 100 µM capsaicin for 24 h at 37°C. Subsequently, the cells
were incubated with 50 nM LysoTracker® Red DND-99
(Invitrogen; Thermo Fisher Scientific, Inc.) in the dark for 30 min
at 37°C. Immunofluorescence images were captured using a confocal
laser scanning microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Green fluorescent protein (GFP)-LC3
puncta assay
The formation of fluorescent puncta of
autophagosomes was analyzed in cells transfected with GFP-LC3. A375
cells were cultured in 6-well plates at a density of
5×105 cells/ml and transfected with 2 µg/ml GFP-LC3
plasmid (Genomeditech (Shanghai) Co., Ltd., Shanghai, China), using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. After
24 h post-transfection, the cells were treated with or without 100
µM capsaicin for 24 h at 37°C. Immunofluorescence images were
captured using a fluorescence microscope.
Western blot analysis
A375 cells were cultured in 6-well plates at a
density of 5×105 cells/ml and treated with capsaicin (0,
50, 100 and 200 µM) for 24 h at 37°C. Cells were lysed in ice-cold
radioimmunoprecipitation assay buffer (Beyotime Biotechnology Co.
Ltd., Shanghai, China) containing a protease and phosphatase
inhibitor cocktail for 30 min on ice, centrifuged at 13,000 × g for
15 min at 4°C and the supernatant was collected. A Pierce
bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.) was used to quantify the protein concentration.
Subsequently, 10% SDS-PAGE was performed. Equivalent amounts of
protein (40 µg) were loaded, separated and transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). After blocking in 5% non-fat milk for 1 h at room
temperature, the membranes were incubated with primary antibodies
(1:1,000; cleaved-PARP, cleaved caspase-3, LC3B, beclin 1 and
GAPDH) at 4°C overnight. Subsequently, the membranes were washed
and incubated with horseradish peroxidase-conjugated secondary
antibody (cat. no. 7074s; Cell Signaling Technology, Inc.;
dilution, 1:5,000) for 1 h at room temperature. Proteins were
detected using an enhanced chemiluminescence kit (EMD
Millipore).
Statistical analysis
All data represent at least three independent
experiments and are expressed as the means ± standard deviation.
Student's t-test was used to compare differences between two
groups, and one-way ANOVA analysis of variance and Dunnett's post
hoc test were used for calculating the significance between
different groups. All statistical analyses were performed using
SPSS v19.0 software (IBM Corp., Armonk, NY, USA). *P<0.05 was
considered to indicate a statistically significant difference.
Results
Capsaicin inhibits A375 and C8161 cell
viability
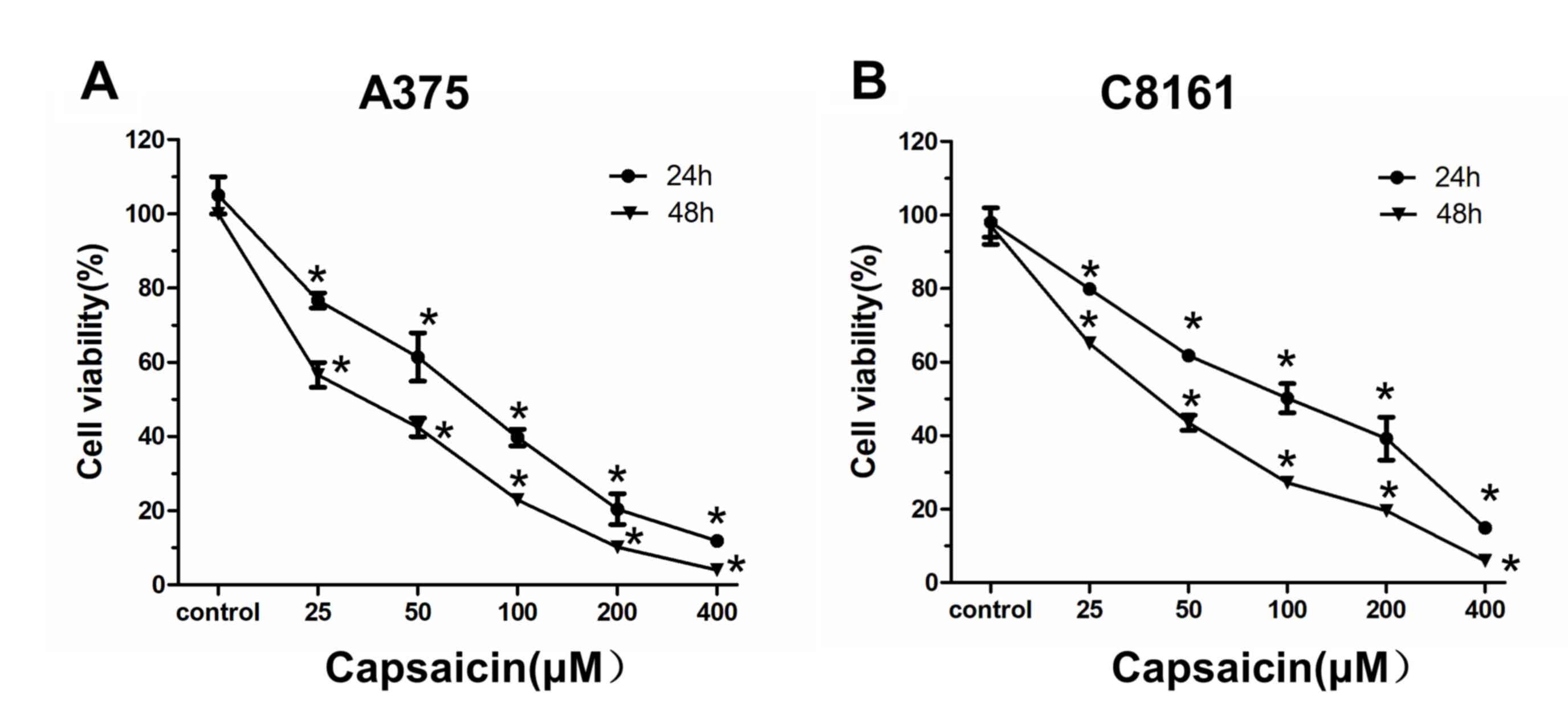
The impact of the inhibitory effects of capsaicin in
human melanoma cells was investigated. A375 and C8161 cells were
treated with various concentrations of capsaicin (25, 50, 100, 200
and 400 µM) for 24 or 48 h. Subsequently, cell viability was
measured by the CCK8 assay. As shown in Fig. 1, the viability of melanoma cells was
significantly decreased following capsaicin treatment, which
indicated that capsaicin caused cell viability inhibition in a
time- and dose-dependent manner.
Capsaicin induces apoptosis of human
melanoma cells
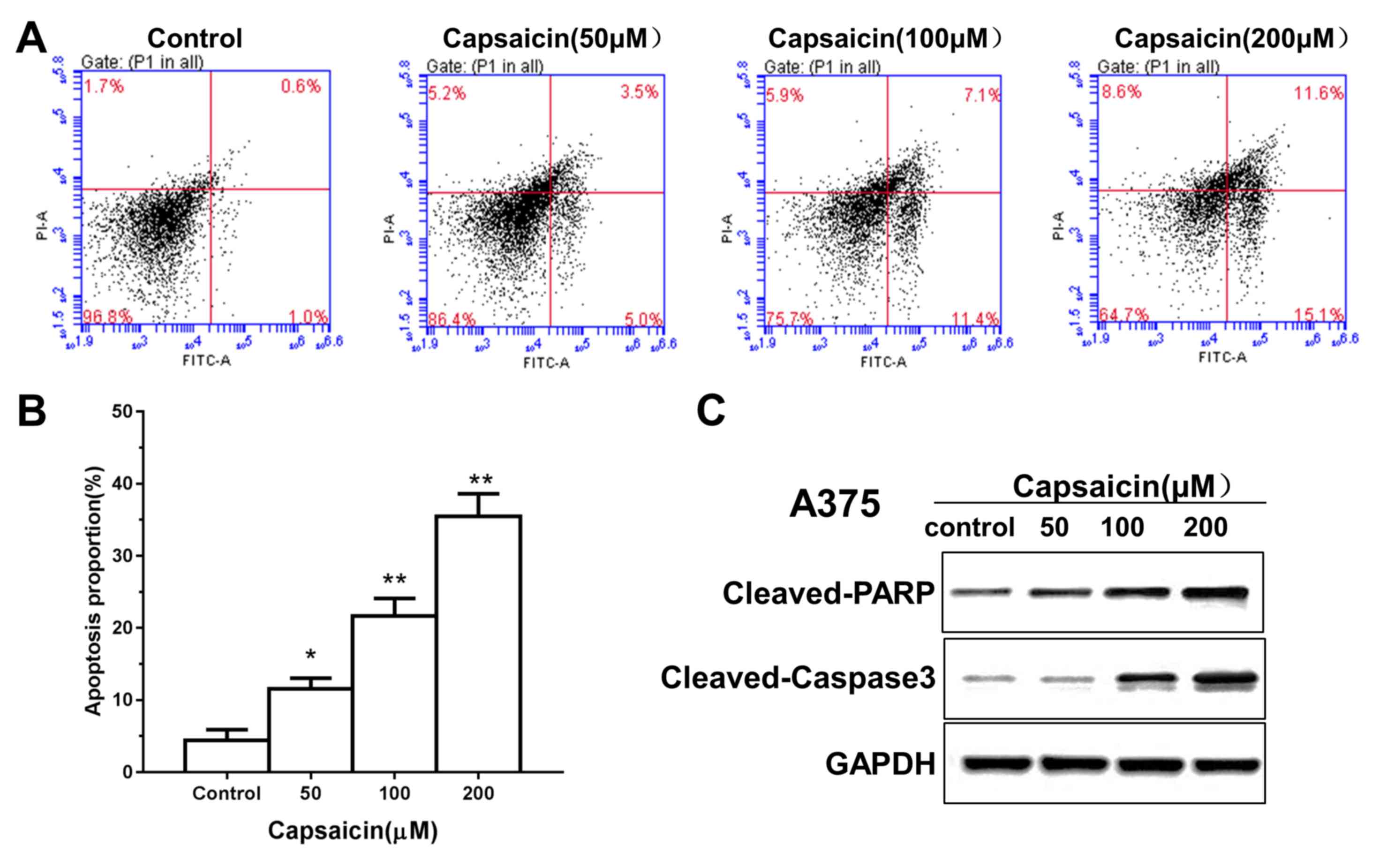
Flow cytometry was performed to ascertain whether
capsaicin induced apoptosis, which may mediate the inhibitory
effects of capsaicin on human melanoma cell viability. The results
demonstrated that treatment of melanoma cells with capsaicin
resulted in a dose-dependent increase in apoptotic cells (Fig. 2A and B). Furthermore, specific
signaling proteins involved in apoptosis were investigated by
western blotting. As shown in Fig.
2C, the expression levels of cleaved caspase-3 and cleaved PARP
were increased in the A375 and C8161 cells following capsaicin
treatment. These data indicated that capsaicin promoted
caspase-dependent apoptosis to decrease A375 and C8161 cell
viability.
Capsaicin activates autophagy in human
melanoma cells
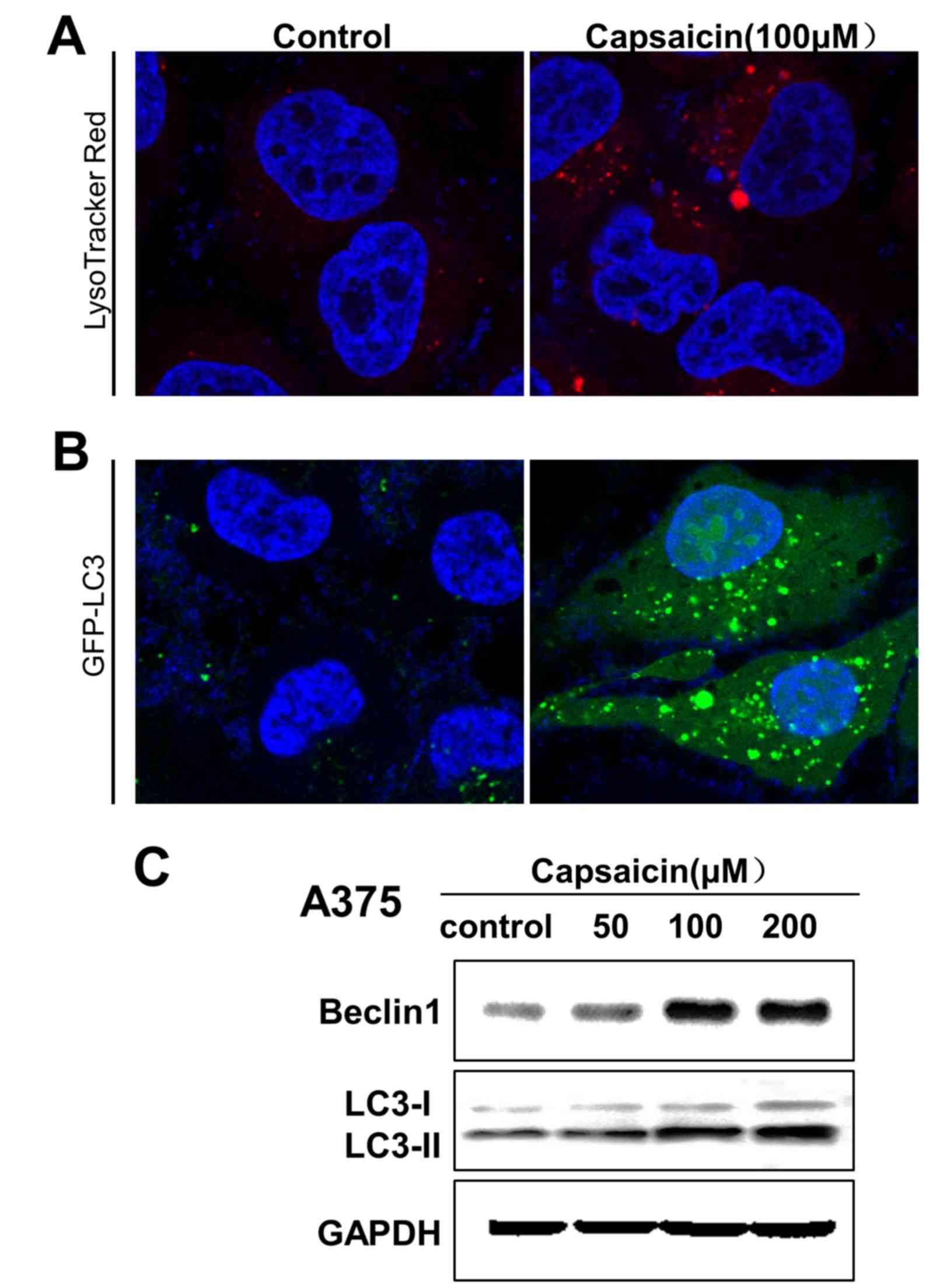
To determine whether capsaicin may trigger autophagy
in melanoma cells, the LysoTracker® Red and GFP-LC3
puncta transfection methods were used to label cellular acidic
compartments and analyze the formation of fluorescent puncta of
autophagosomes, respectively. The results revealed that upon
exposure to capsaicin (100 µM) for 24 h, the fluorescence intensity
in melanoma cells exhibited a clear increase (Fig. 3A). Additionally, capsaicin treatment
resulted in an increase in GFP-LC3 puncta formation in melanoma
cells (Fig. 3B). To verify the
aforementioned findings, autophagy-associated protein levels in the
cytoplasm were assessed by western blotting. As shown in Fig. 3C, the expression levels of LC3B and
beclin 1 in melanoma cells were increased in a
concentration-dependent manner following capsaicin treatment, which
indicated that capsaicin may induce autophagy in melanoma cells via
the upregulation of autophagy-associated protein expression
levels.
Capsaicin-induced autophagy inhibits
apoptosis to protect cell survival
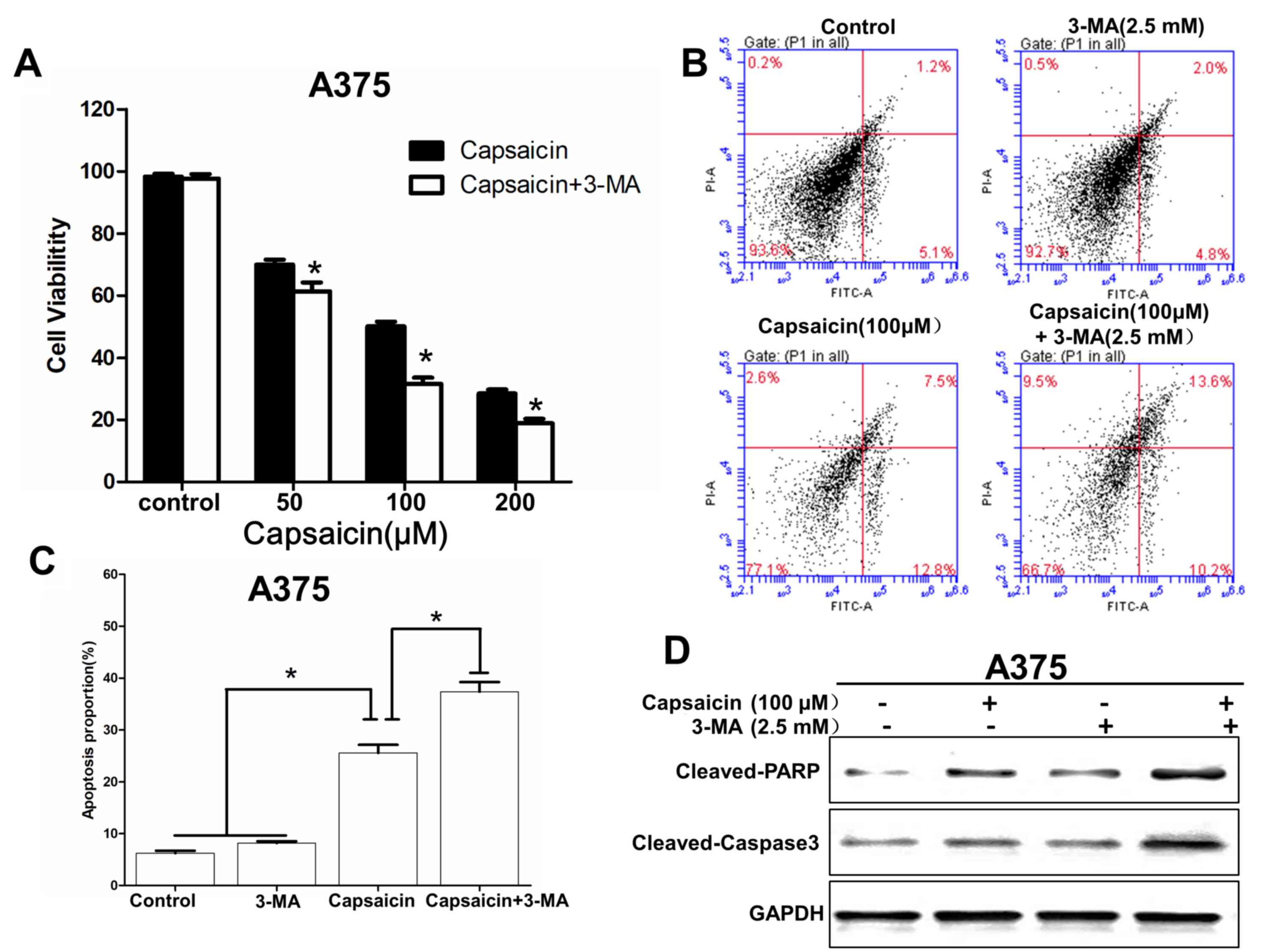
It has been reported that autophagy has a dual role
in cancer, serving as an antagonist to block apoptotic cell death
or to protect cell survival (20).
Therefore, an autophagy inhibitor, 3-MA (2.5 mM), was used to
investigate the role of autophagy in melanoma cells. The results
revealed that following treatment with 3-MA, the inhibitory effect
of capsaicin on cell viability were increased (Fig. 4A). Additionally, in response to
autophagy inhibition, the A375 cells turned to apoptosis following
capsaicin treatment, as demonstrated by the flow cytometry assay
(Fig. 4B and C). Western blotting
data revealed that the expression levels of cleaved caspase-3 and
cleaved PARP were increased following capsaicin and 3-MA treatment
(Fig. 4D). In conclusion,
capsaicin-induced apoptosis was increased by the inhibition of
autophagy, which indicated that the autophagy induced by capsaicin
may suppress apoptosis to protect cell survival.
Discussion
Melanoma is the most aggressive and
treatment-resistant type of skin cancer (22) that arises from malignant
transformation of melanocytes. The development of melanocytes is
modulated by the receptor tyrosine kinase c-KIT and
microphthalmia-associated transcription factor (23). Additionally, a 2002 genome-wide
screen revealed that B-Raf proto-oncogene, serine/threonine kinase
(BRAF) point mutations are highly frequent in melanoma and less
frequent in other types of cancer (24). The adenosine triphosphate-competitive
BRAF inhibitors, vemurafenib and dabrafenib, are targeted therapy
options with substantial efficacy against melanoma, and provide
similar clinical benefits (25,26).
However, vemurafenib and dabrafenib only produce rapid initial
disease stabilization in melanoma (27). The emergence of drug resistance and
disease progression, along with regressions on BRAF inhibitors,
leads to the majority of patients to relapse within 1 year
(28). Traditional treatment by
chemotherapy or immunotherapy is only useful in the advanced
stages. Over the last decade, several novel therapies for treating
melanoma have emerged. A number of drugs can target specifically
mutated melanoma cells, block or trigger important signaling
pathways, or unblock immune checkpoints (29). Although there has been considerable
progress in the melanoma-targeted therapy and immunotherapy field,
there remains a great challenge to prevent the emergence of
resistance (22). Capsaicin is a
naturally occurring alkaloid and its biological effects have been
extensively studied (30). Previous
studies have demonstrated that capsaicin has a variety of
pharmacological activities, including anti-itching (31), anti-inflammatory (9) and analgesic (32) effects. Additionally, capsaicin has
attracted widespread interest due to its antitumor effects
(11,33,34) and
it has been reported to exert notable effects on human melanoma
cells. A previous study demonstrated that capsaicin could inhibit
B16-F10 melanoma cell migration via the phosphatidylinositol
3-kinase/protein kinase B/Rac1 signaling pathway (35). Additional effects of capsaicin have
been reported, including the reduction of proliferation and
viability of cancer cells, by causing G2/M phase arrest
and resulting in activation of caspase-3, −8 and −9, which are
associated with apoptosis (13,36).
Therefore, an intriguing area in the field is the study of natural
dietary phytochemicals, including capsaicin, for safe and effective
treatment of melanoma.
In the present study, the human melanoma A375 and
C8161 cell lines were selected to analyze the chemotherapeutic
capacity of capsaicin for human melanoma. The data revealed that
capsaicin may suppress cell proliferation, induce apoptosis and
induce autophagy via the upregulation of expression levels of
cleaved caspase-3, cleaved PARP, beclin 1 and two types of LC3B
proteins in human melanoma cells. Additionally, the results of the
present study revealed that inhibiting autophagy may strengthen the
negative effect of capsaicin on melanoma cell viability, and
indicated that capsaicin-induced autophagy may protect cell
survival.
Apoptosis and autophagy jointly determine cellular
fate (37). PCD that culminates in
rapid cell loss is required to maintain homeostasis and is involved
in the host response to pathogens. This process can be regulated or
unregulated, and apoptosis is known as the regulated type (38). The regulation of apoptosis is divided
into two major pathways: The extrinsic (death receptor-mediated)
pathway and intrinsic (mitochondria-mediated) pathway, which are
triggered by plasma-membrane receptors with soluble molecules or by
various mitochondrial stimuli, respectively (39). Caspase-3, one of the 13
aspartate-specific cysteine proteases in the caspase family, serves
a central role in the execution of apoptosis, and is primarily
responsible for the cleavage of PARP during cell death (40). Previous studies have reported that
cell apoptosis is triggered by caspase-dependent or
caspase-independent pathways with the former being the most common
(18,36,39,40).
Cleavage of PARP is considered a central indicator of apoptosis
(41). Our results indicated that
capsaicin induced apoptosis in human melanoma cells through
activation of caspase-3 and PARP.
In this study, following capsaicin treatment, the
proportion of LC3B-I, LC3B-II and beclin 1 in human melanoma cells
was increased as determined by immunoblotting. The present study
demonstrated that capsaicin could induce autophagy, which is the
phenomenon of cell ‘self-eating’ (42). The mechanism of autophagy is
typically a stress adaptation in response to various stress
stimuli, including starvation, hypoxia, endoplasmic reticulum
stress and oxidative stress (43).
It has been demonstrated that capsaicin could increase levels of
the autophagy markers LC3-II and autophagy related 5, enhancing p62
and Fap-1 degradation, and increasing caspase-3 activity to induce
autophagy and apoptosis, respectively (13,42).
Previously, the dual roles of autophagy in cancer have been
verified, namely, protecting cell survival or contributing to cell
death (44,45). Autophagy functions as a recycling
process, being principally cytoprotective, recycling long-lived
proteins, and defending cells from damaged organelles and protein
aggregates. In contrast, excessive autophagy can induce
cytotoxicity, which promotes cell death. When flawed apoptosis is
induced in normal cells or cancer cells, or apoptosis blocks tumor
development, autophagy may act as a backup strategy to stop cell
processes or a strategy to promote other cell death mechanisms
(46). In the present study,
inhibition of autophagy by 3-MA increased the expression levels of
cleaved caspase-3 and cleaved PARP, which may lead to an increase
in melanoma cell death. These findings suggested that, in human
melanoma cells, capsaicin-induced autophagy may serve a prosurvival
role by suppressing apoptosis. Therefore, autophagy cannot only be
considered a tumor-inhibiting process but may also be considered a
tumor-promoting process. In summary, agents that inhibit autophagy
could be used as adjuvants for capsaicin and may be used for
antitumor treatment in the future.
In conclusion, capsaicin was demonstrated to exert a
negative effect on cancer cell viability, and induced apoptosis of
human melanoma A375 and C8161 cells via the activation of cleaved
caspase-3 and PARP. Additionally, capsaicin-triggered autophagy
contributed to cell survival by suppressing apoptosis of melanoma
cells. However, in order for capsaicin to become a drug candidate
for melanoma treatment, further issues need to be clarified: The
precise dose-effect association between capsaicin and triggered
autophagy; whether there could be a ‘double-edged sword’ effect of
capsaicin-triggered autophagy when culturing melanoma cells with
more specific concentrations of capsaicin; the mechanism and
pathway by which capsaicin activates apoptosis and autophagy; and
whether capsaicin exerts favorable antitumor effects in
vivo. These issues will be investigated in a future study.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science Foundation
of Shandong Province (grant no. S20160921).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC performed the experiments, drafted the manuscript
and analyzed the data. ML assisted with the data analysis. XW was
responsible for the study design and final approval of the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schadendorf D, Fisher DE, Garbe C,
Gershenwald JE, Grob JJ, Halpern A, Herlyn M, Marchetti MA,
McArthur G, Ribas A, et al: Melanoma. Nat Rev Dis Primers.
1:150032015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leiter U and Garbe C: Epidemiology of
melanoma and nonmelanoma skin cancer-the role of sunlight. Adv Exp
Med Biol. 624:89–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei W, Ehlerding EB, Lan X, Luo Q and Cai
W: PET and SPECT imaging of melanoma: The state of the art. Eur J
Nucl Med Mol Imaging. 45:132–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schadendorf D and Hauschild A: Melanoma in
2013: Melanoma-the run of success continues. Nat Rev Clin Oncol.
11:75–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srinivasan K: Biological activities of red
pepper (Capsicum annuum) and its pungent principle capsaicin: A
review. Crit Rev Food Sci Nutr. 56:1488–1500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ludy MJ, Moore GE and Mattes RD: The
effects of capsaicin and capsiate on energy balance: Critical
review and meta-analyses of studies in humans. Chem Senses.
37:103–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel S, Trueman D, Bentley A, Poole C and
Chambers C: Cost-effectiveness of capsaicin 8% patch (Qutenza(tm))
compared with pregabalin for the treatment of patients with
peripheral neuropathic pain (Pnp) in Scotland. Value Health.
17:A5312014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fattori V, Hohmann MS, Rossaneis AC,
Pinho-Ribeiro FA and Verri WA: Capsaicin: Current understanding of
its mechanisms and therapy of pain and other pre-clinical and
clinical uses. Molecules. 21(pii): E8442016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bernstein JA, Davis BP, Picard JK, Cooper
JP, Zheng S and Levin LS: A randomized, double-blind, parallel
trial comparing capsaicin nasal spray with placebo in subjects with
a significant component of nonallergic rhinitis. Ann Allergy Asthma
Immunol. 107:171–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Díaz-Laviada I and Rodríguez-Henche N: The
potential antitumor effects of capsaicin. Prog Drug Res.
68:181–208. 2014.PubMed/NCBI
|
|
12
|
Ramos-Torres Á, Bort A, Morell C,
Rodríguez-Henche N and Díaz-Laviada I: The pepper's natural
ingredient capsaicin induces autophagy blockage in prostate cancer
cells. Oncotarget. 7:1569–1583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin YT, Wang HC, Hsu YC, Cho CL, Yang MY
and Chien CY: Capsaicin induces autophagy and apoptosis in human
nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR
pathway. Int J Mol Sci. 18(pii): E13432017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang KM, Pyo JO, Kim GY, Yu R, Han IS, Ju
SA, Kim WH and Kim BS: Capsaicin induces apoptosis by generating
reactive oxygen species and disrupting mitochondrial transmembrane
potential in human colon cancer cell lines. Cell Mol Biol Lett.
14:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang R, Xia Y, Wang Z, Zheng J, Chen Y,
Li X, Wang Y and Ming H: Serum long non coding RNA MALAT-1
protected by exosomes is up-regulated and promotes cell
proliferation and migration in non-small cell lung cancer. Biochem
Biophys Res Commun. 490:406–414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang SP, Chen JC, Wu CC, Chen CT, Tang
NY, Ho YT, Lo C, Lin JP, Chung JG and Lin JG: Capsaicin-induced
apoptosis in human hepatoma HepG2 cells. Anticancer Res.
29:165–174. 2009.PubMed/NCBI
|
|
17
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jun HS, Park T, Lee CK, Kang MK, Park MS,
Kang HI, Surh YJ and Kim OH: Capsaicin induced apoptosis of B16-F10
melanoma cells through down-regulation of Bcl-2. Food Chem Toxicol.
45:708–715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenberg-Lerner A and Kimchi A: The
paradox of autophagy and its implication in cancer etiology and
therapy. Apoptosis. 14:376–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang ZJ, Chee CE, Huang S and Sinicrope
FA: The role of autophagy in cancer: Therapeutic implications. Mol
Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lo JA and Fisher DE: The melanoma
revolution: From UV carcinogenesis to a new era in therapeutics.
Science. 346:945–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin JY and Fisher DE: Melanocyte biology
and skin pigmentation. Nature. 445:843–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong J, Phelps RG, Qiao R, Yao S, Benard
O, Ronai Z and Aaronson SA: BRAF oncogenic mutations correlate with
progression rather than initiation of human melanoma. Cancer Res.
63:3883–3885. 2003.PubMed/NCBI
|
|
25
|
Flaherty KT, Puzanov I, Kim KB, Ribas A,
McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K and
Chapman PB: Inhibition of mutated, activated BRAF in metastatic
melanoma. N Engl J Med. 363:809–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hauschild A, Grob JJ, Demidov LV, Jouary
T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr,
Kaempgen E, et al: Dabrafenib in BRAF-mutated metastatic melanoma:
A multicentre, open-label, phase 3 randomised controlled trial.
Lancet. 380:358–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: Improved survival with vemurafenib in melanoma with BRAF V600E
mutation. N Engl J Med. 364:2507–2516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ribas A, Gonzalez R, Pavlick A, Hamid O,
Gajewski TF, Daud A, Flaherty L, Logan T, Chmielowski B, Lewis K,
et al: Combination of vemurafenib and cobimetinib in patients with
advanced BRAF (V600)-mutated melanoma: A phase 1b study. Lancet
Oncol. 15:954–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dvořánková B, Szabo P, Kodet O, Strnad H,
Kolář M, Lacina L, Krejčí E, Naňka O, Šedo A and Smetana K Jr:
Intercellular crosstalk in human malignant melanoma. Protoplasma.
254:1143–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toh CC, Lee TS and Kiang AK: The
pharmacological actions of capsaicin and analogues. Br J Pharmacol
Chemother. 10:175–182. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sekine R, Satoh T, Takaoka A, Saeki K and
Yokozeki H: Anti pruritic effects of topical crotamiton, capsaicin,
and a corticosteroid on pruritogen-induced scratching behavior. Exp
Dermatol. 21:201–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao HT, Lee HJ, Ho YC and Chiou LC:
Capsaicin in the periaqueductal gray induces analgesia via
metabotropic glutamate receptor-mediated endocannabinoid retrograde
disinhibition. Br J Pharmacol. 163:330–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Clark R and Lee SH: Anticancer properties
of capsaicin against human cancer. Anticancer Res. 36:837–843.
2016.PubMed/NCBI
|
|
34
|
Chapa-Oliver AM and Mejía-Teniente L:
Capsaicin: From plants to a cancer-suppressing agent. Molecules.
21(pii): E9312016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shin DH, Kim OH, Jun HS and Kang MK:
Inhibitory effect of capsaicin on B16-F10 melanoma cell migration
via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp
Mol Med. 40:486–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin CH, Lu WC, Wang CW, Chan YC and Chen
MK: Capsaicin induces cell cycle arrest and apoptosis in human KB
cancer cells. BMC Complement Altern Med. 13:462013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Z, Ouyang L, Peng H and Zhang WZ:
Oridonin: Targeting programmed cell death pathways as an
anti-tumour agent. Cell Prolif. 45:499–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hail N Jr, Carter BZ, Konopleva M and
Andreeff M: Apoptosis effector mechanisms: A requiem performed in
different keys. Apoptosis. 11:889–904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mukhopadhyay S, Panda PK, Sinha N, Das DN
and Bhutia SK: Autophagy and apoptosis: Where do they meet?
Apoptosis. 19:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kulms D and Schwarz T: Molecular
mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol
Photomed. 16:195–201. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu YP, Dong FX, Chai X, Zhu S, Zhang BL
and Gao DS: Role of autophagy in capsaicin-induced apoptosis in
U251 glioma cells. Cell Mol Neurobiol. 36:737–743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Radogna F, Dicato M and Diederich M:
Cancer-type-specific crosstalk between autophagy, necroptosis and
apoptosis as a pharmacological target. Biochem Pharmacol. 94:1–11.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li M, Tan J, Miao Y, Lei P and Zhang Q:
The dual role of autophagy under hypoxia-involvement of interaction
between autophagy and apoptosis. Apoptosis. 20:769–777. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fu D, Yu JY, Yang S, Wu M, Hammad SM,
Connell AR, Du M, Chen J and Lyons TJ: Survival or death: A dual
role for autophagy in stress-induced pericyte loss in diabetic
retinopathy. Diabetologia. 59:2251–2261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ravegnini G, Sammarini G, Nannini M,
Pantaleo MA, Biasco G, Hrelia P and Angelini S: Gastrointestinal
stromal tumors (GIST): Facing cell death between autophagy and
apoptosis. Autophagy. 13:452–463. 2017. View Article : Google Scholar : PubMed/NCBI
|