Introduction
Desmoplastic melanoma (DM) was first reported in
1971 as a rare morphological variant of melanoma composed of
spindle melanocytes and abundant collagen (1). Subsequently, its histological
definition was further expanded into two subtypes: ‘Pure’ DM, which
is a uniform desmoplasia throughout the entire tumor, and ‘mixed’
DM, which is a desmoplasia in combination with other malignant cell
types (2–4). The fibroblastic component of the
tumoral stroma in DM is crucial to define its desmoplastic behavior
(5).
DM differs from traditional melanomas in clinical
presentation (6). The diagnosis of
DM is challenging as DM predominantly presents as atypical and
amelanotic lesions rather than a pigmented nevus (7). Compared with conventional melanomas, DM
is locally aggressive, has a high incidence of local recurrence and
a low incidence of regional metastasis (8). DM is more prevalent in older subjects
and on sun-exposed areas of skin, particularly the head and neck
region, and affects men more than women (9,10). As
with other melanomas, surgical treatment with wide local excision
is the first-line therapy option and adjuvant radiation treatment
may be used for advanced lesions (6,11).
According to previously reported statistics based on
Surveillance, Epidemiology, and End Results (SEER) studies in the
USA, DM accounts for ~4% of all melanoma cases and its incidence
rate was ~2×10−6%, which steadily increased between 1992
and 2013 (12,13). However, understanding concerning DM
behavior, clinical outcomes and prognostic factors is limited to
several case reports and a small number of institutional reviews
(5,8,12–14). To
the best of our knowledge, no large case studies have reported the
general demographic and clinicopathological features or
disease-specific prognostic factors of DM. Thus, a retrospective
analysis of clinical cases using data from the SEER program was
performed in the present study.
Materials and methods
Data sources
Data from the present study is publicly available
from the SEER program (seer.cancer.gov; National Cancer Institute; National
Institute of Health, Bethesda, MD, USA), which collects incidence
and survival data of patients with malignant tumors through 18
population-based cancer registries and represents ~34% of the
population of the USA (12,13). Patients with a primary diagnosis of
DM were identified using the third edition of the International
Classification of Diseases for Oncology (ICD-O-3; code: 8745/3)
(15). Cases were excluded if
treatment or outcome data were unavailable for survival analysis.
Overall data were obtained using SEER*Stat software (version 8.3.4;
seer.cancer.gov/data/; National Cancer
Institute; National Institute of Health).
Statistical analysis
Overall statistical analysis was performed using
SPSS for Windows (version 23.0; IBM Corp., Armonk, IL, USA). A
χ2 test was used to examine bivariate associations
between categorical variables. Melanoma-specific and all-cause
mortality rates were investigated. The primary endpoint was
considered to be the date of DM-associated mortality. The time
point between the date of diagnosis and the date of DM-associated
mortality was defined as the disease-specific survival (DSS).
Kaplan-Meier survival analyses with log-rank tests were used to
estimate survival. Furthermore, Cox proportional hazards regression
was used to estimate the hazard ratio. All statistical tests were
two-tailed. P-values were two-sided. P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographic and clinicopathological
characteristics
The primary aim of the present study was to
determine the general demographics, incidence and tumor-specific
clinicopathological features of DM. Table I summarizes the clinical and disease
characteristics of the patients with DM patients. In brief, data
collected between 1973 and 2107 on a total of 3,657 patients with
DM were retrieved from SEER registries in the present study. The
total cohort consisted of 2,476 males and 1,181 females, with a
male-to-female ratio of ~2:1. The median age was 68 years (range,
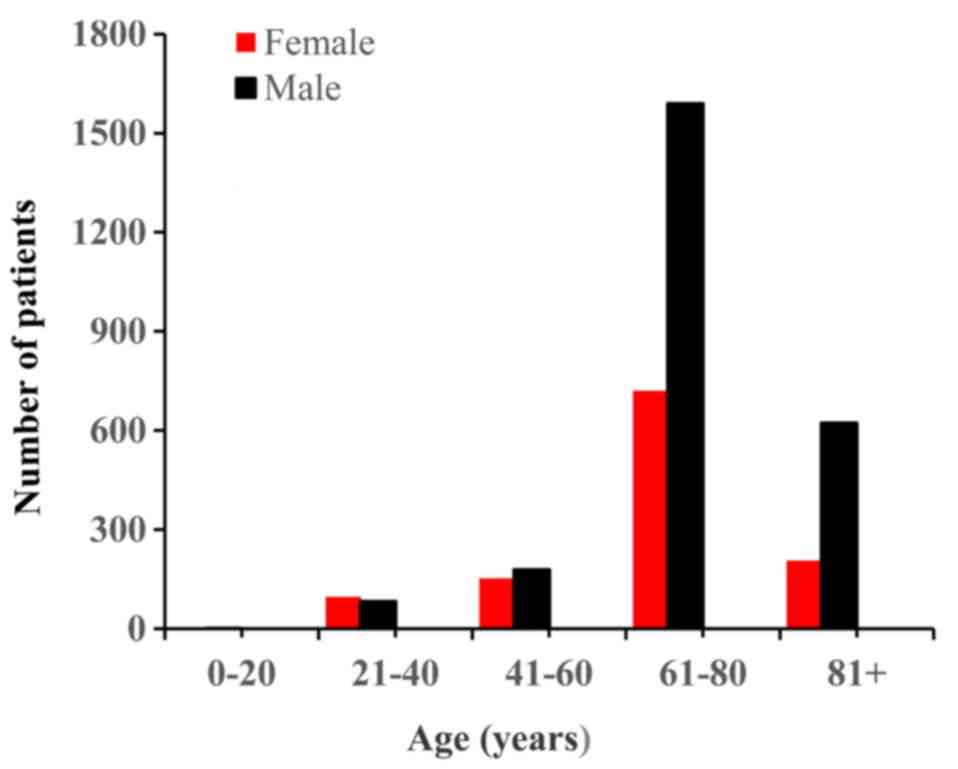
6–101 years). The age and sex distributions are presented in
Fig. 1. Regarding the ethnicity
distribution, 97% of the patients were Caucasian, and the remaining
patients were of African descent or other. The data demonstrated
that 3,635 cases of DM had originated from the skin, 13 cases from
the nose and mouth, 3 cases from internal organs and 6 cases from
other sites. Of the total number of cases, the pathological
differentiation status in 3,611 patients was unknown (Table I), according to the American Joint
Committee on Cancer (AJCC)/Union of International Cancer Control
pathological grade system (16).
Surgical treatment was the only recorded treatment modality. A
total of 3,517 patients received surgical treatment.
 | Table I.Baseline characteristics of
desmoplastic melanoma cases in the SEER database. |
Table I.
Baseline characteristics of
desmoplastic melanoma cases in the SEER database.
|
| Overall survival | Melanoma-specific
survival |
|---|
|
|
|
|
|---|
| Characteristics | Alive | Dead | P-value | Alive | Dead | P-value |
|---|
| Age, years |
|
| <0.001 |
|
| – |
|
≤68 | 1,278 | 320 |
| 689 | 137 |
|
|
>68 | 947 | 1,112 |
| 0 | 0 |
|
| Sex |
|
| <0.001 |
|
| <0.001 |
|
Female | 807 | 374 |
| 321 | 41 |
|
|
Male | 1,418 | 1,058 |
| 368 | 96 |
|
| Ethnicity |
|
| 0.026 |
|
| 0.995 |
|
Caucasian | 2,149 | 1,403 |
| 655 | 130 |
|
| African
descent | 15 | 9 |
| 10 | 2 |
|
|
Other | 61 | 20 |
| 24 | 5 |
|
| Tumor site |
|
| 0.014 |
|
| 0.548 |
|
Internal organs | 1 | 2 |
| 1 | 0 |
|
| Nose
and mouth | 7 | 6 |
| 5 | 0 |
|
|
Skin | 2,217 | 1,418 |
| 683 | 137 |
|
|
Other | 0 | 6 |
| 0 | 0 |
|
| Grade |
|
| 0.216 |
|
| 0.451 |
| I | 1 | 2 |
| 0 | 0 |
|
| II | 6 | 3 |
| 2 | 0 |
|
|
III | 10 | 15 |
| 2 | 1 |
|
| IV | 5 | 4 |
| 1 | 1 |
|
|
Unknown | 2,203 | 1,408 |
| 686 | 135 |
|
| AJCC stage |
|
| <0.001 |
|
| <0.001 |
| I | 663 | 178 |
| 187 | 15 |
|
| II | 792 | 383 |
| 184 | 26 |
|
|
III | 68 | 44 |
| 23 | 11 |
|
| IV | 25 | 50 |
| 12 | 3 |
|
| T stage |
|
| <0.001 |
|
| 0.073 |
| T0 | 6 | 8 |
| 2 | 0 |
|
| T1 | 365 | 109 |
| 107 | 11 |
|
| T2 | 376 | 106 |
| 104 | 8 |
|
| T3 | 387 | 171 |
| 94 | 10 |
|
| T4 | 455 | 275 |
| 109 | 25 |
|
| TX | 116 | 64 |
| 33 | 4 |
|
| N stage |
|
| <0.001 |
|
| 0.012 |
| N0 | 1,572 | 628 |
| 405 | 44 |
|
| N1 | 47 | 38 |
| 19 | 7 |
|
| N2 | 22 | 15 |
| 6 | 2 |
|
| NX | 64 | 52 |
| 19 | 5 |
|
| M stage |
|
| <0.001 |
|
| <0.001 |
| M0 | 1,654 | 660 |
| 465 | 59 |
|
| M1 | 23 | 51 |
| 169 | 53 |
|
| MX | 28 | 22 |
| 1 | 0 |
|
| SEER stage |
|
| <0.001 |
|
| <0.001 |
|
Localized | 1,486 | 710 |
| 465 | 59 |
|
|
Regional | 603 | 562 |
| 169 | 53 |
|
|
Distant | 4 | 7 |
| 1 | 0 |
|
|
Unstaged | 77 | 50 |
| 33 | 9 |
|
| Treatment |
|
| 0.017 |
|
| 0.075 |
|
Non-surgery | 67 | 69 |
| 19 | 3 |
|
|
Surgery | 2,156 | 1,361 |
| 670 | 133 |
|
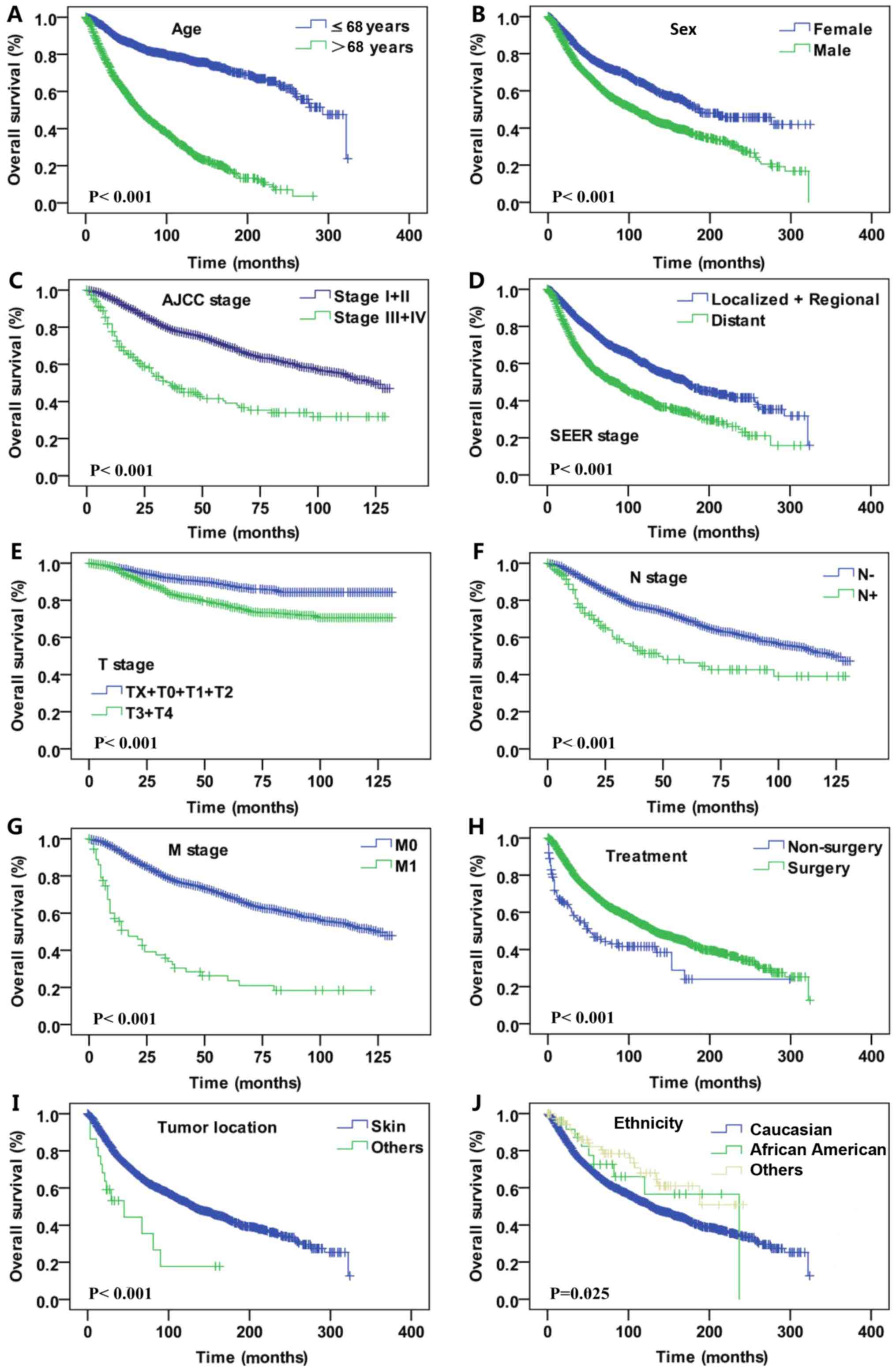
Survival outcomes
Kaplan-Meier analysis was utilized for time-to-event
analysis of overall survival (OS) and DSS rates. OS analysis was
performed by stratifying different demographic and
clinicopathological features of DM. Statistically significant
differences were identified with regard to sex (female vs. male,
P<0.001), age (≤68 vs. >68 years, P<0.001), AJCC stage
(I+II vs. III+IV, P<0.001), SEER historic tumor stage (localized
+ regional vs. distant metastasis tumor, P<0.001), T stage
(TX+T1+T2 vs. T3+T4, P<0.001; based on the Tumor-Node-Metastasis
staging system (16): TX, T stage
unknown; T0, no evidence of primary tumors; T1, tumor thickness
≤1.00 mm; T2, tumor thickness 1.01 mm-2.0 mm; T3, tumor thickness:
2.01 mm-4.0 mm; T4, tumor thickness ≥4.0 mm.), N stage (lymph node
negative vs. lymph node positive, P<0.001), M stage (M0 vs. M1,
P<0.001), treatment (surgery vs. non-surgery, P<0.001), tumor
location (skin vs. other site, P<0.001) and ethnicity (Caucasian
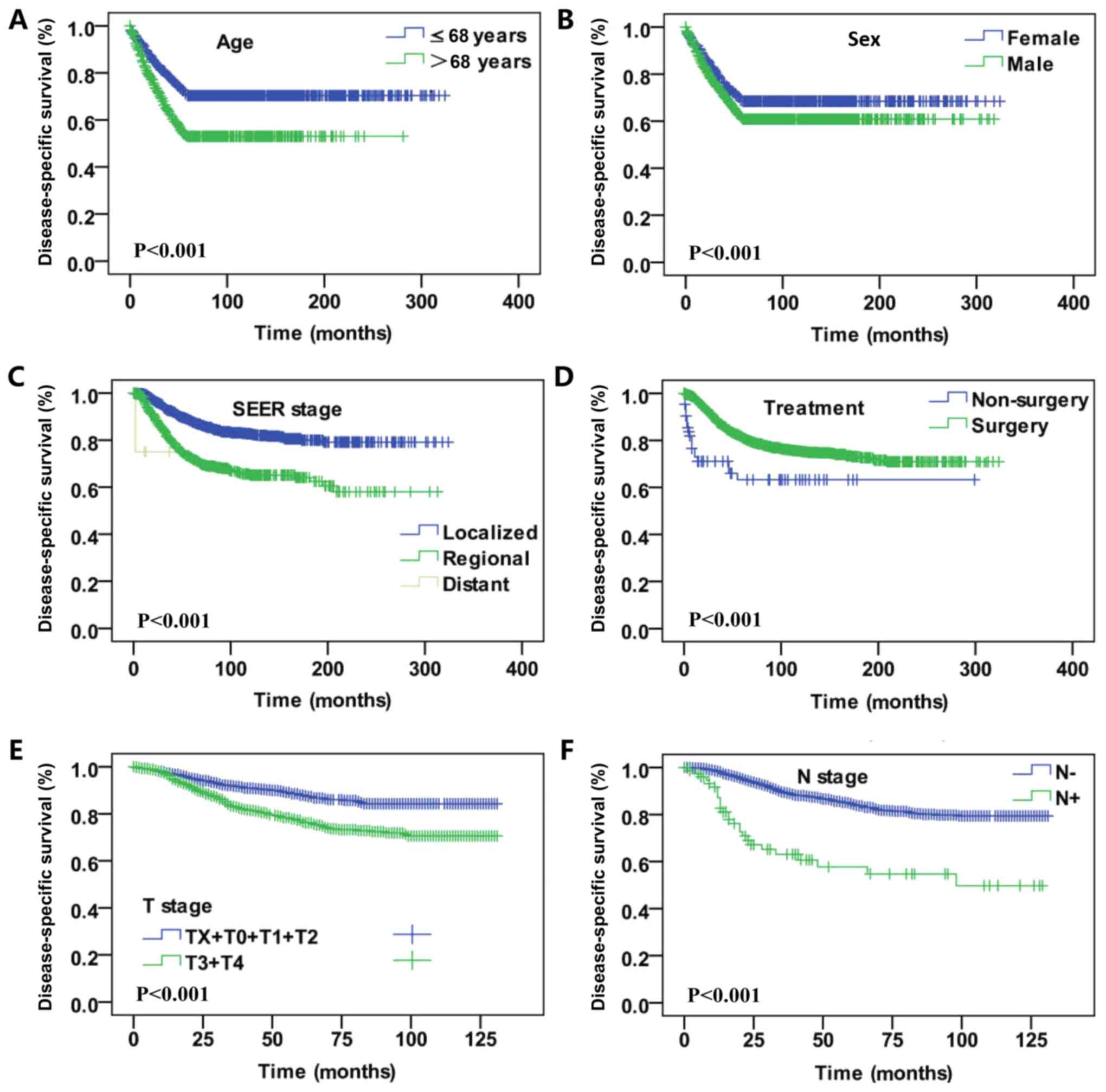
vs. African descent vs. other, P<0.001; Fig. 2). In the DSS analysis, significant
differences were also identified regarding sex (female vs. male,
P<0.001), age (≤68 vs. >68 years old, P<0.001), SEER
historic tumor stage (localized + regional vs. distant metastasis
tumor, P<0.001), treatment (surgery vs. non-surgery,
P<0.001), T stage (TX+T1+T2 vs. T3+T4, P<0.001) and N stage
(lymph node negative vs. lymph node positive, P<0.001; Fig. 3).
 | Figure 2.Kaplan-Meier curves for overall
survival rate. Kaplan-Meier curves for overall survival according
to (A) age, (B) sex, (C) AJCC stage, (D) SEER historic tumor stage,
(E) T stage, (F) N stage, (G) M stage, (H) treatment, (I) tumor
location and (J) ethnicity. T, tumor; N, node; M, metastasis; SEER,
Surveillance, Epidemiology and End Results AJCC, American Joint
Committee on Cancer. |
Prognostic factors
A Cox proportional hazards regression model was
constructed to evaluate predictors of OS and DSS (Table II). Univariate analysis of OS
revealed the risk of mortality was significantly higher for
patients that were aged >68 years old (P<0.001), male
(P<0.001), had an AJCC stage of II, III or IV (P<0.001), an N
stage of NX, N1 or N2 (NX stage, P<0.001; N1 stage, P<0.001;
and N2 stage, P=0.002) and an M stage of M1 (P<0.001).
Univariate analysis of DSS indicated the risk of melanoma-induced
mortality was significantly higher for patients that were aged
>68 years old, male, had an AJCC stage of II, III or IV, an N
stage of NX, N1 or N2 and an M stage of M1 (P<0.001).
 | Table II.Univariate Cox regression analysis of
clinicopathological parameters in desmoplastic melanoma for DSS and
OS. |
Table II.
Univariate Cox regression analysis of
clinicopathological parameters in desmoplastic melanoma for DSS and
OS.
|
| OS | DSS |
|---|
|
|
|
|
|---|
| Parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
≤68 | 1.0
(Reference) |
| 1.0
(Reference) |
|
|
>68 | 4.595
(4.043–5.223) | <0.001 | 3.083
(2.517–3.777) | <0.001 |
| Ethnicity |
|
|
|
|
|
Caucasian | 1.0
(Reference) |
| 1.0
(Reference) |
|
| African
descent | 0.747
(0.388–1.438) | 0.382 | 0.880
(0.329–4.129) | 0.800 |
|
Other | 0.566
(0.364–0.880) | 0.011 | 0.452
(0.187–1.093) | 0.078 |
| Sex |
|
|
|
|
|
Female | 1.0
(Reference) |
| 1.0
(Reference) |
|
|
Male | 1.639
(1.456–1.844) | <0.001 | 1.808
(1.448–2.258) | <0.001 |
| Tumor location |
|
|
|
|
|
Internal organs | 1.0
(Reference) |
| 1.0
(Reference) |
|
| Nose
and mouth | 0.793
(0.160–3.934) | 0.777 | 0.615
(0.056–6.788) | 0.691 |
|
Skin | 0.464
(0.116–1.858) | 0.278 | 0.311
(0.044–2.218) | 0.244 |
| Other
site | 2.839
(0.573–14.079) | 0.201 | 35.024
(3.047–402.601) | 0.004 |
| Grade |
|
|
|
|
| I | 1.0
(Reference) |
| 1.0
(Reference) |
|
| II | 0.650
(0.109–3.892) | 0.637 | 0.485
(0.030–7.761) | 0.609 |
|
III | 2.563
(0.568–11.212) | 0.211 | 1.791
(0.209–15.343) | 0.595 |
| IV | 1.060
(0.194–5.788) | 0.946 | 1.045
(0.095–11.252) | 0.971 |
|
Unknown | 0.719
(0.180–2.879) | 0.641 | 0.468
(0.066–3.333) | 0.449 |
| AJCC stage |
|
|
|
|
| I | 1.0
(Reference) |
| 1.0
(Reference) |
|
| II | 1.693
(1.417–2.022) | <0.001 | 2.031
(1.422–2.901) | <0.001 |
|
III | 2.570
(1.847–3.577) | <0.001 | 5.693
(3.444–9.412) | <0.001 |
| IV | 6.210
(4.533–8.509) | <0.001 | 11.207
(6.571–19.113) | <0.001 |
| T stage |
|
|
|
|
| T0 | 1.0
(Reference) |
| 1.0
(Reference) |
|
| T1 | 0.281
(0.137–0.577) | 0.001 | 1.800
(0.414–7.831) | 0.433 |
| T2 | 0.252
(0.123–0.517) | <0.001 | 0.452
(0.239–0.855) | 0.015 |
| T3 | 0.384
(0.189–0.781) | 0.008 | 0.484
(0.2630.891) | 0.020 |
| T4 | 0.521
(0.258–1.052) | 0.069 | 0.779
(0.441–1.379) | 0.392 |
| TX | 0.428
(0.205–0.891) | 0.024 | 1.329
(0.783–2.254) | 0.292 |
| N stage |
|
|
|
|
| N0 | 1.0
(Reference) |
| 1.0
(Reference) |
|
| N1 | 1.958
(1.41–2.717) | <0.001 | 3.297
(2.050–5.301) | <0.001 |
| N2 | 2.255
(1.351–3.764) | <0.001 | 5.364
(2.632–10.929) | <0.001 |
| NX | 1.791
(1.350–2.377) | <0.001 | 2.591
(1.612–4.615) | <0.001 |
| M stage |
|
|
|
|
| M0 | 1.0
(Reference) | <0.001 | 1.0
(Reference) |
|
| M1 | 4.533
(3.407–6.033) | <0.001 | 7.114
(4.527–11.181) | <0.001 |
| MX | 1.185
(0.774–1.813) | 0.434 | 0.773
(0.287–2.084) | 0.611 |
| SEER stage |
|
|
|
|
|
Localized | 1.0
(Reference) |
| 1.0
(Reference) |
|
|
Regional | 1.816
(1.625–2.029) | <0.001 | 2.345
(1.890–2.9120) | <0.001 |
|
Distant | 10.773
(5.098–22.767) | <0.001 | 8.951
(1.249–64.162) | 0.029 |
|
Unstaged | 1.212
(0.910–1.615) | 0.189 | 1.127
(0.595–2.134) | 0.714 |
| Treatment |
|
Non-surgery | 1.0
(Reference) |
| 1.0
(Reference) |
|
|
Surgery | 0.498
(0.391–0.634) | <0.001 | 0.428
(0.273–0.671) | <0.001 |
In the multivariate analysis, age >68 years old
(OS and DSS, P<0.001), male sex (OS, P<0.001; DSS, P=0.005),
AJCC stage II and III (OS for stage II and III, P<0.001; DSS for
stage II, P=0.009; and DSS for stage III, P<0.001) and SEER
historic tumor stage (OS, P<0.001; DSS, P=0.009) were associated
with poorer OS and DSS rates. Notably, surgical treatment was
associated with favorable DDS and OS rates (OS, P<0.001S; DSS,
P=0.015; Table III).
 | Table III.Multivariate Cox regression analysis
of clinicopathological parameters in desmoplastic melanoma for DSS
and OS. |
Table III.
Multivariate Cox regression analysis
of clinicopathological parameters in desmoplastic melanoma for DSS
and OS.
|
| OS | DSS |
|---|
|
|
|
|
|---|
| Parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
≤68 | 1.0
(Reference) |
| 1.0
(Reference) |
|
|
>68 | 4.225
(3.454–5.242) | <0.001 | 3.055
(2.204–4.235) | <0.001 |
| Sex |
|
|
|
|
|
Female | 1.0
(Reference) |
| 1.0
(Reference) |
|
|
Male | 1.465
(1.207–1.777) | <0.001 | 1.673
(1.169–2.392) | 0.005 |
| AJCC stage |
|
|
|
|
| I | 1.0
(Reference) |
| 1.0
(Reference) | <0.001 |
| II | 1.434
(1.174–1.750) | <0.001 | 1.716
(1.145–2.572) | 0.009 |
|
III | 2.305
(1.578–3.367) | <0.001 | 4.180
(2.252–7.756) | <0.001 |
| IV | 0.001
(0.000–1.037) | 0.948 | 0.000
(0.000–5.328) | 0.965 |
| SEER historic
stage |
|
|
|
|
|
Localized | 1.0
(Reference) |
| 1.0
(Reference) |
|
|
Regional | 1.467
(1.215–1.770) | <0.001 | 1.615
(1.127–2.315) | 0.009 |
|
Distant | 1.937
(0.528–3.392) |
0.932 | 1.827
(0.392–5.874) | 0.952 |
| Treatment |
|
|
|
|
|
Non-surgery | 1.0
(Reference) |
| 1.0
(Reference) |
|
|
Surgery | 0.317
(0.199–0.504) | <0.001 | 0.234
(0.073–0.751) | 0.015 |
Discussion
DM is a rare variant of melanoma that can be easily
misdiagnosed. Clinically, the appearance of DM is often nonspecific
and amelanotic (5,17). Histologically, DM can mimic a range
of benign and malignant neoplasms with spindle cells and fibrous
stroma (7). Dermoscopy and
reflectance confocal microscopy are useful tools for the
identification of DM, though immunohistochemical panels are needed
for the final diagnosis (18,19). The
diagnostic criteria of DM have become more consistent, and the
misdiagnosis of DM has decreased in patients (2,20).
However, due to the rarity of DM, its clinical and prognostic
characteristics have yet to be completely elucidated.
The present study indicated that the presentation of
DM was associated with increased age. Notably, the incidence of DM
was highest in the 6-8th decade of life, with predominance in
males. Furthermore, the present findings revealed that DM primarily
originated from the skin. Of note, people of Caucasian ethnicity
accounted for the majority of the study population. This is in
accordance with previous studies (5,8,12–14,21). In
previous studies, the male-to-female ratio was 2.3–3.7, and the
trend of incidence in males was suggested to be greater (12,13). The
current large population study of DM also revealed a predominance
of DM in males.
One of the main aims of the present study was to
identify prognostic factors in patients with DM. The results
demonstrated that sex and age were independent prognostic factors
for DSS and OS. These results are in accordance with previous
studies (12,13). However, other studies reported that
the sex and age of patients with DM were associated with poorer OS,
but not poorer DSS (14,22). The inconsistency in results may be
due to the substantial limitation of the study population. As DM
primarily occurred in older people, comorbidities should also be
taken into consideration, which could not be obtained from the SEER
Program in the present study.
Since DM has a low rate of nodal metastasis,
investigators have suggested that routine sentinel node biopsy may
be not necessary (6,23–25).
Conversely, certain studies have indicated that DM sentinel lymph
node biopsies may have a higher positive rate than previous
thought, thus sentinel node biopsy should be considered (26,27). In
addition, previous studies revealed that DM did not share the same
traditional prognostic factors with traditional malignant melanoma,
and nodal positivity did not predict survival (6). However, other researchers have proposed
that the potential for regional nodal involvement in patients with
DM must be considered from its diagnosis to surveillance for
recurrence, particularly in ‘mixed’ DM (9,28,29). An
explanation for these contradictory views may be that the subtypes
of DM, including ‘pure’ or ‘mixed’ DM, could impact on the clinical
behaviors and prognosis differently (22,30). In
the present study, the percentage of lymph node metastasis was ~5%,
and in the multivariate Cox regression analysis the N stage was not
an independent prognostic indicator. Therefore, the present results
indicate that sentinel node biopsy may be not useful for DM.
Previous studies have demonstrated that DM had a
propensity for local recurrence and distant metastasis,
particularly with regard to the ‘pure’ DM subtype (8,23,25).
Furthermore, local recurrence was observed to be associated with an
increased risk of systemic metastatic disease (31). In the current study, the proportion
of M1+MX stage tumor was ≤5%, and M1 stage was associated with
poorer OS and DSS rates, according to univariate analysis.
Furthermore, advanced AJCC and SEER stages were associated with
poorer OS and DSS rates. This is in accordance with previous
studies (9,13). These data support the idea that
detection of DM at its early stage is difficult and missed
diagnosis can impact the overall prognosis (7,32).
Delayed diagnosis of DM is likely due to its relative rarity and
atypical clinical presentation (8,17).
Previous studies have suggested that surgical
margins are critical in the management of DM local recurrence and
that wide surgical resection margins are required (6,9,33). In the present study, a total of 3,517
patients received surgical treatment; however, data on the surgical
margins were absent in the SEER database. The results indicated
that surgical treatment was associated with favorable DDS and OS.
This is in accordance with previous results (13).
In conclusion, use of the National Cancer Institute
SEER registries in the present study extended the current knowledge
of DM. The large number of patients enabled description of the
demographic and clinicopathological features and disease-specific
prognostic factors of DM. Compared with other studies, a notable
strength of the present study was its robust long-term follow-up
assessment of survival provided by the SEER database. However,
there were several limitations of the current study. Notably, the
study could not differentiate between the DM subtypes. This was
because the SEER registry is coded according to the final diagnosis
obtained from a pathology report and only applied the ICD-O-3
morphology code for all types of DM. In addition, not all cases had
complete information, and these missing data undoubtedly weaken the
strength of the current investigation. As aforementioned, certain
important prognostic data, including pathological grade, surgical
types, margin status and adjuvant therapies, were either absent or
incomplete in the SEER database. Therefore, the influences of these
factors on the overall prognosis could not be assessed. In
addition, the patients with DM represented an older population and
there was a lack of comorbidity data, which may significantly
affect treatment protocol and outcomes.
In conclusion, to the best of our knowledge, the
present study is the first to report on a large case series
concerning the demographics, clinicopathological features and
disease-specific prognostic factors of DM. The results demonstrated
that DM primarily occurred in Caucasians, with a predominance in
males, and the highest incidence occurred in the 6-8th decades of
life. Age, sex, AJCC stage, SEER historic stage and surgical
treatment were identified as independent prognostic factors for DSS
and OS rates.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Scientific
Research Foundation of Shanghai Stomatological Hospital, Fudan
University (Shanghai, China; grant no. SSDCZ-2016-01).
Availability of data and materials
The datasets generated during the present study are
available in the official software SEER*Stat v.8.3.4 repository
(https://seer.cancer.gov/data/).
Authors' contributions
ZX and PS were major contributors in writing the
manuscript. PS designed the experiments, wrote the original draft
and revised the manuscript. ZX was responsible for analysis of the
data and revising the manuscript. XL and FY collected and
interpreted patient data. AW was responsible for planning,
organizing, checking the data and the manuscript throughout the
project. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Due to the retrospective nature of this study, it
was granted an exemption in writing by the University of Fudan
Institutional Review Board (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DM
|
desmoplastic melanoma
|
|
OS
|
overall survival
|
|
DSS
|
disease-specific survival
|
|
SEER
|
Surveillance, Epidemiology and End
Results
|
|
AJCC
|
American Joint Committee on Cancer
|
References
|
1
|
Conley J, Lattes R and Orr W: Desmoplastic
malignant melanoma (a rare variant of spindle cell melanoma).
Cancer. 28:914–936. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weissinger SE, Keil P, Silvers DN, Klaus
BM, Möller P, Horst BA and Lennerz JK: A diagnostic algorithm to
distinguish desmoplastic from spindle cell melanoma. Mod Pathol.
27:524–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Magro CM, Crowson AN and Mihm MC: Unusual
variants of malignant melanoma. Mod Pathol. 19 (Suppl 2):S41–S70.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reed RJ and Leonard DD: Neurotropic
melanoma. A variant of desmoplastic melanoma. Am J Surg Pathol.
3:301–311. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manfredini M, Pellacani G, Losi L,
Maccaferri M, Tomasi A and Ponti G: Desmoplastic melanoma: A
challenge for the oncologist. Future Oncol. 13:337–345. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wasif N, Gray RJ and Pockaj BA:
Desmoplastic melanoma-the step-child in the melanoma family? J Surg
Oncol. 103:158–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mărgăritescu I and Chiriţă AD:
Desmoplastic melanoma-challenges in the diagnosis and management of
a rare cutaneous tumor. Rom J Morphol Embryol. 55:947–952.
2014.PubMed/NCBI
|
|
8
|
Pace CS, Kapil JP, Wolfe LG, Kaplan BJ and
Neifeld JP: Desmoplastic melanoma: Clinical behavior and management
implications. Eplasty. 16:e32016.PubMed/NCBI
|
|
9
|
Posther KE, Selim MA, Mosca PJ, Stanley
WE, Johnson JL, Tyler DS and Seigler HF: Histopathologic
characteristics, recurrence patterns, and survival of 129 patients
with desmoplastic melanoma. Ann Surg Oncol. 13:728–739. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lens MB, Newton-Bishop JA and Boon AP:
Desmoplastic malignant melanoma: A systematic review. Br J
Dermatol. 152:673–678. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oliver DE, Patel KR, Switchenko J, Parker
D, Lawson DH, Delman KA, Kudchadkar RR and Khan MK: Roles of
adjuvant and salvage radiotherapy for desmoplastic melanoma.
Melanoma Res. 26:35–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan F, Strohl A, Allen PD and Doerr TD:
Desmoplastic melanoma of the head and neck: Incidence and survival,
1992–2013. Otolaryngol Head Neck Surg. 157:648–656. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng Z, Wu X, Chen V, Velie E and Zhang Z:
Incidence and survival of desmoplastic melanoma in the United
States, 1992–2007. J Cutan Pathol. 38:616–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han D, Han G, Zhao X, Rao NG, Messina JL,
Marzban SS, Sarnaik AA, Cruse CW, Sondak VK and Zager JS:
Clinicopathologic predictors of survival in patients with
desmoplastic melanoma. PLoS One. 10:e01197162015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fritz A, Percy C, Jack A, Shanmugarathan
S, Sobin L, Parkin DM and Whelan S: International classification of
diseases for oncology (ICD-O-3). World Health Organization;
2000
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Machado I, Llombart B, Cruz J, Traves V,
Requena C, Nagore E, Parafioriti A, Monteagudo C and Llombart-Bosch
A: Desmoplastic melanoma may mimic a cutaneous peripheral nerve
sheath tumor: Report of 3 challenging cases. J Cutan Pathol.
44:632–638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plaza JA, Bonneau P, Prieto V, Sangueza M,
Mackinnon A, Suster D, Bacchi C, Estrozi B, Kazakov D, Kacerovska
D, et al: Desmoplastic melanoma: An updated immunohistochemical
analysis of 40 cases with a proposal for an additional panel of
stains for diagnosis. J Cutan Pathol. 43:313–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maher NG, Solinas A, Scolyer RA, Puig S,
Pellacani G and Guitera P: Detection of desmoplastic melanoma with
dermoscopy and reflectance confocal microscopy. J Eur Acad Dermatol
Venereol. 31:2016–2024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lawrence NF, Hammond MR, Frederick DT, Su
Y, Dias-Santagata D, Deng A, Selim MA, Mahalingam M, Flaherty KT
and Hoang MP: Ki-67, p53, and p16 expression, and G691S RET
polymorphism in desmoplastic melanoma (DM): A clinicopathologic
analysis of predictors of outcome. J Am Acad Dermatol. 75:595–602.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lorcy S, Koeppel MC, Richard MA, Grob JJ,
Berbis P and Morand JJ: Desmoplastic melanoma: a study of 23 cases
at 3 centres in the Bouches-du-Rhone region. Ann Dermatol Venereol.
141:656–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murali R, Zannino D, Synnott M, McCarthy
SW, Thompson JF and Scolyer RA: Clinical and pathological features
of metastases of primary cutaneous desmoplastic melanoma.
Histopathology. 58:886–895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sims JR, Wieland CN, Kasperbauer JL, Moore
EJ and Price DL: Head and neck desmoplastic melanoma: Utility of
sentinel node biopsy. Am J Otolaryngol. 38:537–541. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohebati A, Ganly I, Busam KJ, Coit D,
Kraus DH, Shah JP and Patel SG: The role of sentinel lymph node
biopsy in the management of head and neck desmoplastic melanoma.
Ann Surg Oncol. 19:4307–4313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murali R, Shaw HM, Lai K, McCarthy SW,
Quinn MJ, Stretch JR, Thompson JF and Scolyer RA: Prognostic
factors in cutaneous desmoplastic melanoma: A study of 252
patients. Cancer. 116:4130–4138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Egger ME, Huber KM, Dunki-Jacobs EM,
Quillo AR, Scoggins CR, Martin RC II, Stromberg AJ, McMasters KM
and Callender GG: Incidence of sentinel lymph node involvement in a
modern, large series of desmoplastic melanoma. J Am Coll Surg.
217:37–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Broer PN, Walker ME, Goldberg C, Buonocore
S, Braddock DT, Lazova R, Narayan D and Ariyan S: Desmoplastic
melanoma: A 12-year experience with sentinel lymph node biopsy. Eur
J Surg Oncol. 39:681–685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dunne JA, Wormald JC, Steele J, Woods E,
Odili J and Powell BW: Is sentinel lymph node biopsy warranted for
desmoplastic melanoma? A systematic review. J Plast Reconstr
Aesthet Surg. 70:274–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pawlik TM, Ross MI, Prieto VG, Ballo MT,
Johnson MM, Mansfield PF, Lee JE, Cormier JN and Gershenwald JE:
Assessment of the role of sentinel lymph node biopsy for primary
cutaneous desmoplastic melanoma. Cancer. 106:900–906. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Busam KJ, Mujumdar U, Hummer AJ, Nobrega
J, Hawkins WG, Coit DG and Brady MS: Cutaneous desmoplastic
melanoma: Reappraisal of morphologic heterogeneity and prognostic
factors. Am J Surg Pathol. 28:1518–1525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jaroszewski DE, Pockaj BA, DiCaudo DJ and
Bite U: The clinical behavior of desmoplastic melanoma. Am J Surg.
182:590–595. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trayanova E, Chokoeva AA, Patterson JW,
Wollina U, Lotti T and Tchernev G: Desmoplastic malignant melanoma
associated with pigmented ocular tumor: Second documented
problematic case from the board of adcrstr-association for
dermatohistopathologic control, re-evaluation and subsequent
therapeutic recommendations. J Biol Regul Homeost Agents 29 (1
Suppl). S59–S64. 2015.
|
|
33
|
Maurichi A, Miceli R, Camerini T, Contiero
P, Patuzzo R, Tragni G, Crippa F, Romanidis K, Ruggeri R, Carbone A
and Santinami M: Pure desmoplastic melanoma: A melanoma with
distinctive clinical behavior. Ann Surg. 252:1052–1057. 2010.
View Article : Google Scholar : PubMed/NCBI
|