Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer death worldwide (1)
and it mostly develops in patients with chronic liver disease.
Hepatitis C virus (HCV) is one of the major causes of HCC, and the
prevalence of HCV-related HCC (HCV-HCC) has been decreasing
worldwide owing to recent advances in prevention, surveillance, and
treatment (2). Meanwhile, the
prevalence of non-viral HCC is increasing worldwide (3). Non-viral HCC can have various causes,
and alcoholic liver disease is a well-known risk factor (4). In addition, metabolic disorders, such
as obesity, non-alcoholic fatty liver disease (NAFLD), and type 2
diabetes mellitus, are thought to be associated with the increased
incidence of non-viral HCC (5,6) and
growing evidence suggests that aging; lifestyle factors, such as
smoking; hepatic fibrosis; and gamma-glutamyl transpeptidase levels
are also risk factors (7,8). Accordingly, a surveillance strategy for
non-viral HCC has been proposed (8).
Various factors are associated with HCC prognosis
and have been reported elsewhere in patients with HCV-HCC. Lower
serum albumin levels, presence of decompensated cirrhosis, higher
serum levels of α-fetoprotein (AFP) and des-γ-carboxy prothrombin
(DCP), and non-curative treatment have been associated with poor
prognosis (9,10). In patients with non-viral HCC, the
factors associated with disease-free survival have been
investigated. For instance, Hashimoto et al (11), showed that sex has an impact on
disease-free survival after surgery in patients with non-viral HCC
and Hiwatashi et al (12),
demonstrated that elevated serum bilirubin levels predict poor
disease-free survival after surgery. However, limited information
is available regarding prognosis and risk factors in patients with
non-viral HCC, especially in the advanced stages of HCC. Moreover,
no studies have investigated distinguishable differences in
prognosis between patients with non-viral HCC and HCV-HCC.
In the present exploratory research investigating
prognostic factors in non-viral HCC, we used an artificial
intelligence technique called data mining analysis. Two popular
approaches of data-mining analysis are random forest analysis and
decision tree algorithms. Random forest analysis identifies hidden
factors distinguishing between the case and control groups, with a
high level of predictive accuracy, even if no a priori hypothesis
has been imposed (13). Decision
tree algorithms reveal a series of classification rules by
identifying priorities, allowing clinicians to choose an option
that maximizes benefit for the patient (8). Random forest analysis has been applied
to reveal factors associated with survival in esophageal cancer
patients treated using chemo-radiation therapy (14), and in patients with metastatic
pancreatic adenocarcinoma (15).
Decision tree analysis has been used to evaluate prognostic factors
in patients with gastric cancer (16) and bile duct cancer (17). However, these techniques have never
been applied to identify prognostic factors in patients with
non-viral HCC.
The aims of this study are to investigate prognosis
of patients with non-viral HCC compared to that of patients with
HCV-HCC. We also performed an exploratory analysis to ascertain
distinguishable factors associated with prognosis between patients
with non-viral HCC and HCV-HCC.
Patients and methods
Study design and ethics
This was a retrospective study to compare prognosis
between patients with non-viral HCC and those with HCV-HCC. The
protocol conformed to the ethical guidelines of the 1975
Declaration of Helsinki and was granted prior approval by the
institutional review board of Kurume University. An opt-out
approach was used to obtain informed consent from the patients, and
personal information was protected during data collection.
Patients
We enrolled 794 consecutive adult patients with
either non-viral HCC (n=182) or HCV-HCC (n=612) who had been
treated at Kurume University Hospital between January 2005 and
December 2015. HCC was diagnosed on the basis of either
histological examination or a combination of serum tumor makers,
such as AFP and DCP, and imaging modalities, such as dynamic
computed tomography (CT) and dynamic magnetic resonance imaging
(MRI), as detailed in the Japanese Clinical Practice Guidelines for
HCC, published by The Japan Society of Hepatology (18). Non-viral HCC was defined as primary
HCC accompanied by negative results for serum hepatitis B surface
antigen and anti-HCV antibody. HCV-HCC was defined as primary HCC
accompanied by a positive result for serum anti-HCV antibody and a
negative result for hepatitis B surface antigen. The exclusion
criteria were as follows: i) age less than 20 years; ii) history of
treatment for HCC; iii) observational period less than 90 days, and
iv) positive result for hepatitis B surface antigen or history of
anti-viral treatment for chronic hepatitis B.
In the 182 patients with non-viral HCC, the
etiologies were as follows: alcoholic liver disease (n=84),
non-alcoholic steatohepatitis (n=9), normal liver (n=9), autoimmune
hepatitis (n=9), primary biliary cholangitis (n=4), and cryptogenic
liver disease (n=67).
Data collection
The following three items of categorical data were
collected at the time of HCC diagnosis: i) host factors, namely
age, sex, body mass index, alcohol intake of ≥60 g/day, <60
g/day, >20 g/day, or ≤20 g/day’ in ethanol amount), history of
diabetes mellitus, Child - Pughscore/class, platelet count, and
prothrombin activity, as well as serum levels of aspartate
aminotransferase (AST), alanine aminotransferase (ALT), albumin,
total bilirubin, and hepatitis B core antibody; ii) tumor factors,
namely size and number of HCCs, presence or absence of
macrovascular invasion (MVI), clinical staging
(tumor-node-metastasis [TNM] classification) based on the criteria
of the Liver Cancer Study Group of Japan, and serum levels of AFP,
AFP-L3, and DCP (19), and iii)
treatment factors, namely treatment modality (hepatic resection,
radiofrequency ablation [RFA], trans-arterial chemoembolization
[TACE], and hepatic arterial infusion chemotherapy [HAIC]). The
treatment strategies were based on the Japan Society of
Hepatology's clinical practice guidelines for HCC (18). For patients with advanced HCC,
beneficial effects of HAIC has been reported (20–23). In
our institution, HAIC was employed as a treatment option for
advanced HCC, and therapeutic strategy for HCC including the
indication of HAIC was determined by 10 hepatologists, all of whom
were board certified by the Japan Society of Hepatology.
Observational period
All the enrolled patients were followed up until
March 2016. The observational period was defined as the time span
from the first date of treatment for HCC to death or the end
date.
Comparison of survival
Overall survival in patients with non-viral HCC was
compared to that in patients with HCV-HCC. In addition, we
performed stratification analyses of survival according to HCC
treatment (hepatic resection/RFA/TACE/HAIC) and Child - PughClass
(A-C).
Statistical analysis
Data were expressed as a number, percentage, or mean
± SD (standard deviation). Differences between the two groups were
analyzed using the Mann-Whitney U-test and chi-squared test, as
appropriate. A Kaplan-Meier curve and log-rank test were used to
compare overall survival between the groups. P<0.05 was
considered to indicate a statistically significant difference.
Variables or profiles associated with the survival of patients with
HCC were analyzed using data mining techniques using the software
environment for statistical computing R (http://www.rproject.org/index.html). The statistical
methods are described in detail below.
Multivariate stepwise analysis
A Cox proportional hazards regression model was used
in a multivariate stepwise analysis to identify any independent
variables associated with the survival of patients with HCC. Data
were expressed as hazard ratio and 95% confidence intervals, as
previously described.8 Explanatory variables were selected in a
stepwise manner from factors listed in Table I.
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
| Variables | Non-viral HCC | HCV-HCC | P-value |
|---|
| N | 182 | 612 |
|
| Host factors |
|
|
|
| Age, years | 70.4 ± 8.93 | 70 ± 8.41 | 0.459 |
| Sex, female/male, n
(%) | 44 (24.2)/138
(75.8) | 238 (38.9)/374
(61.1) | <0.001 |
| Ethanol
consumption |
|
| <0.001 |
| <20
g/day, n (%) | 69 (37.9) | 439 (71.7) |
|
| 20 - 60
g/day, n (%) | 32 (17.6) | 37 (6) |
|
| ≥60
g/day, n (%) | 81 (44.5) | 136 (22.2) |
|
| Diabetes mellitus,
n (%) | 90 (49.5) | 176 (28.8) | <0.001 |
| Child - Pugh
class |
|
| 0.922 |
| A, n
(%) | 144 (79.1) | 487 (79.6) |
|
| B, n
(%) | 35 (19.2) | 112 (18.3) |
|
| C, n
(%) | 3 (1.6) | 13 (2.1) |
|
| Platelet count
(104/mm3) | 14 ± 8.11 | 11.6 ± 6.74 | <0.001 |
| AST (IU/l) | 45.2 ± 26.45 | 59.8 ± 28.79 | <0.001 |
| ALT (IU/l) | 39.1 ± 25.57 | 51.7 ± 30.78 | <0.001 |
| Albumin (g/dl) | 3.66 ± 0.499 | 3.59 ± 0.498 | 0.103 |
| Total bilirubin
(mg/dl) | 1.04 ± 0.57 | 0.98 ± 0.482 | 0.57 |
| PT activity
(%) | 84.7 ± 15.87 | 83.9 ± 14.12 | 0.596 |
| Tumor factors |
|
|
|
| AFP (ng/ml) | 5,169 ± 26,608 | 1,408 ± 12,953 | 0.15 |
| DCP (mAU/ml) | 6,247 ± 16,513 | 960 ± 4,912 | <0.001 |
| Tumor diameter
(mm) | 45.1 ± 33.1 | 26.8 ± 18.65 | <0.001 |
| No. of tumors | 2.7 ± 2.93 | 1.9 ± 1.78 | 0.021 |
| MVI, n (%) | 27 (14.8) | 29 (4.7) | <0.001 |
| HCC stage |
|
| <0.001 |
| I, n
(%) | 16 (8.8) | 199 (32.5) |
|
| II, n
(%) | 91 (50) | 244 (39.9) |
|
| III, n
(%) | 45 (24.7) | 134 (21.9) |
|
| IVA, n
(%) | 22 (12.1) | 22 (3.6) |
|
| IVB, n
(%) | 8 (4.4) | 13 (2.1) |
|
| Treatment for
HCC |
|
| <0.001 |
| Hepatic
resection, n (%) | 42 (23.1) | 139 (22.7) |
|
| RFA, n
(%) | 53 (29.1) | 339 (55.4) |
|
| TACE, n
(%) | 50 (27.5) | 95 (15.5) |
|
| HAIC, n
(%) | 37 (20.3) | 39 (6.4) |
|
Random forest analysis
Random forest analysis was used to identify factors
that distinguished prognosis between the non-viral HCC and HCV-HCC
groups, as previously described.8 The procedure employed for
building the random forest was as follows: Firstly, n tree models
were created using bootstrap samples that were randomly chosen from
the original dataset; Secondly, each classification or regression
tree model was grown with no pruning. Instead of determining the
best split among all potential predictors, we chose a random sample
of these variables (one-third of the variables) to consider as
potential splitting variables. Thus, the best split variable was
chosen from among these variables; Thirdly, new data were predicted
by aggregating the predictions of the n trees; Finally, the error
rate was estimated by predicting the data not in the bootstrap
sample (out-of-bag) using the tree grown with the bootstrap sample.
The variable importance, which reflects the relative contribution
of each variable to the model, was estimated by random permutation
of its value and subsequent recalculation of the predictive
accuracy of the model. Random forest analysis was conducted using
the R package.
Decision tree analysis using
prognostic score
To evaluate distinguishable prognostic factors of
survival period between patients with HCV-HCC and those with
non-viral HCC, decision tree analysis was performed using a
prognostic score that was based on the Cox regression model.
Firstly, all patients were classified into short survival or long
survival group based on median prognostic score. Next, the short
survival group of patients with HCV-HCC and the long survival group
of patients with non-viral HCC were defined as working group 1.
Conversely, the long survival group of HCV-HCC and the short
survival group of non-viral HCC were defined as working group 2
(Fig. S1).
We then performed a decision tree analysis to
ascertain distinguishable prognostic factors between working groups
1 and 2, which provided profiles of subgroups with different
prognosis among the HCV-HCC and non-viral HCC groups. Decision tree
analysis was conducted using the R package.
Results
Patients' characteristics
Patients' characteristics were summarized in
Table I. There was no significant
difference in age between the non-viral HCC and HCV-HCC groups;
however, the male ratio, alcohol consumption, and diabetes mellitus
rate were significantly higher in the non-viral HCC group than in
the HCV-HCC group (Table I).
Although Child - Pughclass did not differ significantly between the
two groups, there was a significant difference in HCC stage and HCC
treatment (Table I).
Overall survival
Overall survival rates were presented in Fig. S2. Overall survival of patients with
non-viral HCC was significantly shorter than that of patients with
HCV-HCC. Specifically, the median survival terms of patients with
non-viral HCC and HCV-HCC were 1,553 days and 2,304 days,
respectively. The 1-, 3-, and 5-year survival rates were 86.8%,
59.4%, and 43.8%, respectively, in patients with non-viral HCC and
93.3%, 76%, and 59.3%, respectively, in patients with HCV-HCC.
Univariate and multivariate analysis
of survival in patients with HCC
Univariate analysis for survival was summarized in
Table II. Non-viral HCC group, HCC
stage ≥II, Child - Pughclass B or C, and treatment for HCC were
independent risk factors for reduced survival (Table II). Multivariate analysis revealed
that, in the non-viral HCC group, HCC stage ≥III, Child - Pughclass
C, and treatment for HCC were independent risk factors for reduced
survival (Table III).
 | Table II.Univariate analysis of survival in
patients with HCC. |
Table II.
Univariate analysis of survival in
patients with HCC.
|
|
|
| 95% CI |
|
|---|
|
|
|
|
|
|
|---|
| Variables | Group/Unit | HR | Lower | Upper | P-value |
|---|
| Group | Non-viral | 1.7 | 1.32 | 2.19 | <0.0001 |
| Sex | Male | 1.1 | 0.87 | 1.38 | 0.4406 |
| Ethanol
consumption | 20–60 g/day | 1.13 | 0.76 | 1.69 | 0.5489 |
|
| ≥60 g/day | 1.37 | 1.08 | 1.74 | 0.0104 |
| Diabetes
mellitus | Presence | 1.05 | 0.83 | 1.32 | 0.6922 |
| Tumor diameter,
mm | 10 | 1.21 | 1.17 | 1.25 | <0.0001 |
| No. of tumors | 1 | 1.3 | 1.24 | 1.35 | <0.0001 |
| MVI | Presence | 2.84 | 1.98 | 4.08 | <0.0001 |
| HCC Stage | II | 1.75 | 1.27 | 2.42 | 0.0007 |
|
| III | 3.49 | 2.48 | 4.92 | <0.0001 |
|
| IVA | 5.96 | 3.79 | 9.36 | <0.0001 |
|
| IVB | 7.49 | 4.2 | 13.35 | <0.0001 |
| Child - Pugh
class | B | 2.32 | 1.81 | 2.98 | <0.0001 |
|
| C | 6.14 | 3.56 | 10.58 | <0.0001 |
| Treatment of
HCC | RFA | 1.99 | 1.41 | 2.8 | 0.0004 |
|
| TACE | 6.48 | 4.39 | 9.55 | <0.0001 |
|
| HAIC | 10.33 | 6.75 | 15.81 | <0.0001 |
| Platelet count,
104/mm3 | 1 | 0.98 | 0.96 | 1 | 0.0716 |
| AST, IU/L | 10 | 1.06 | 1.02 | 1.09 | 0.0015 |
| ALT, IU/L | 10 | 1 | 0.96 | 1.03 | 0.8491 |
| Albumin, g/dL | 0,1 | 0.9 | 0.88 | 0.92 | <0.0001 |
| Total bilirubin,
mg/dL | 0,1 | 1.08 | 1.06 | 1.1 | <0.0001 |
| PT activity, % | 10 | 0.83 | 0.76 | 0.9 | <0.0001 |
| AFP, ng/mL | 10 | 1 | 1 | 1 | <0.0001 |
| DCP, mAU/mL | 10 | 1 | 1 | 1 | <0.0001 |
 | Table III.Multivariate analysis of survival in
patients with HCC. |
Table III.
Multivariate analysis of survival in
patients with HCC.
|
|
|
| 95% CI |
|
|---|
|
|
|
|
|
|
|---|
| Variables | Group/Unit | HR | Lower | Upper | P-value |
|---|
| Group | Non-viral | 1.42 | 1.08 | 1.87 | 0.0131 |
| Tumor diameter,
mm | 10 | 1.08 | 1.01 | 1.15 | 0.0349 |
| No. of tumors | 1 | 1.15 | 1.09 | 1.22 | <0.0001 |
| MVI | None | 0.27 | 0.13 | 0.57 | 0.0006 |
| HCC stage | II | 1.39 | 0.98 | 1.96 | 0.0618 |
|
| III | 1.68 | 1.1 | 2.55 | 0.0154 |
|
| IVA | 6.64 | 2.98 | 14.76 | <0.0001 |
|
| IVB | 4.4 | 1.92 | 10.1 | 0.0005 |
| Child - Pugh
class | C | 2.03 | 1.1 | 3.74 | 0.0241 |
| Treatment of
HCC | RFA | 1.73 | 1.17 | 2.54 | 0.0057 |
|
| TACE | 2.45 | 1.56 | 3.84 | 0.0001 |
|
| HAIC | 2.28 | 1.19 | 4.36 | 0.0128 |
| Platelet count,
104/mm3 | 1 | 0.97 | 0.95 | 0.99 | 0.0074 |
| Albumin, g/dl | 0.1 | 0.95 | 0.92 | 0.97 | 0.0001 |
| Total bilirubin,
mg/dl | 0.1 | 1.03 | 1 | 1.05 | 0.0233 |
| AFP, ng/ml | 10 | 1 | 1 | 1 | 0.0040 |
Stratification analysis of survival
according to HCC treatment
Stratification analysis for survival according to
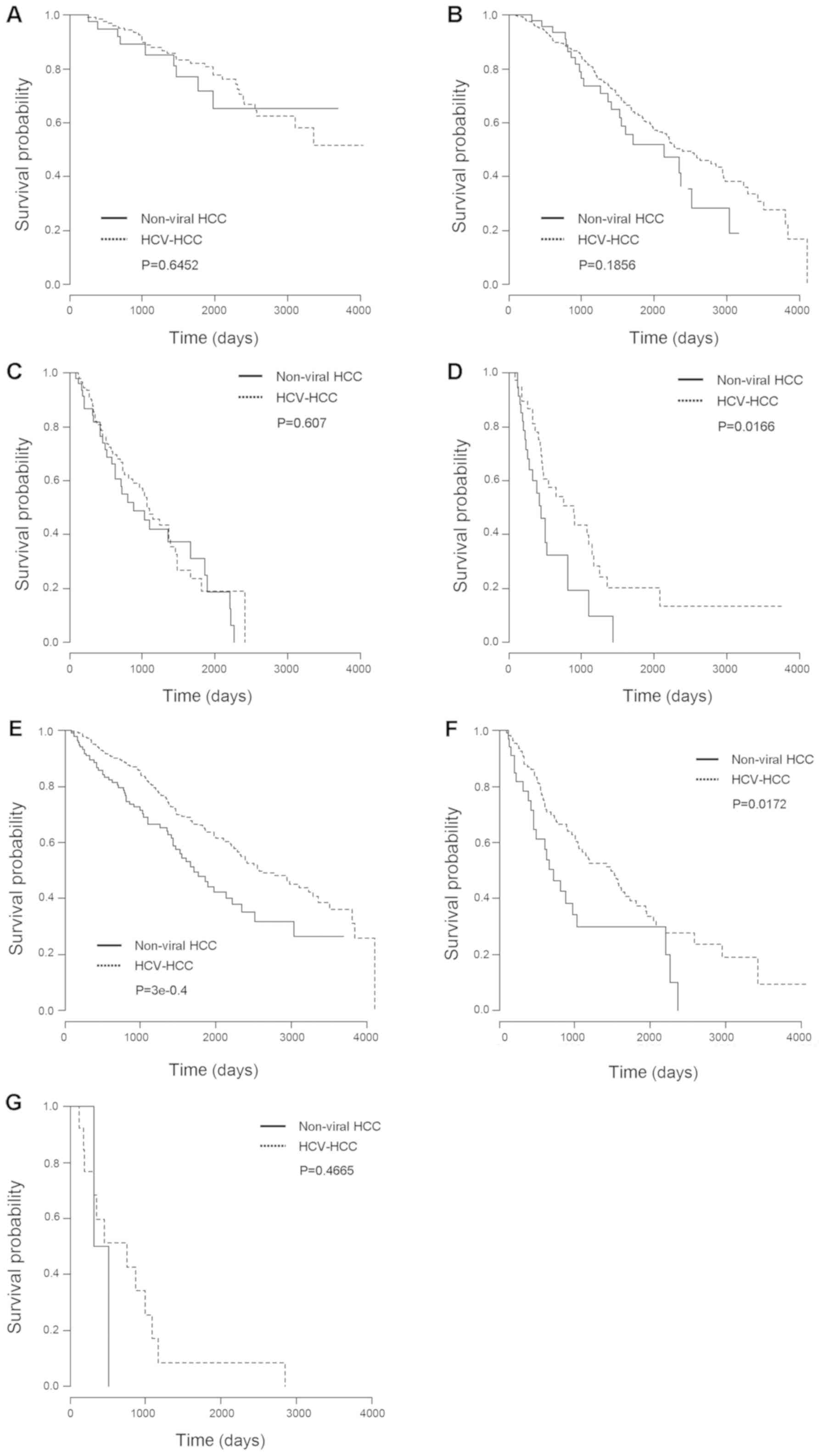
treatment for HCC was shown in Fig.
1A-D. Although there was no significant difference in survival
rate after hepatic resection, RFA and TACE between the non-viral
HCC and HCV-HCC groups (Fig. 1A-C),
survival rate after HAIC in the non-viral HCC group was
significantly lower than in the HCV-HCC group. In patients with
non-viral HCC treated using HAIC, the 1-, 3-, and 5-year survival
rates were 60%, 9.7%, and 0%, respectively, while the equivalent
rates were 78.1%, 39.9%, and 20.2%, respectively, in patients with
HCV-HCC (Fig. 1D).
Stratification analysis for survival
according to Child - Pughclass
Stratification analysis for survival according to
Child-Pugh class was shown in Fig.
1E-G. In Child - Pughclasses A and B, survival rate was
significantly lower in the non-viral HCC group than in the HCV-HCC
group (Fig. 1E and F). Specifically,
in patients with Child - Pughclass A, the 1-, 3-, and 5-year
survival rates were 89.4%, 67.9%, and 47.9%, respectively, in the
non-viral HCC group, and 95.5%, 81.8%, and 65.9%, respectively, in
the HCV-HCC group (Fig. 1E). In
patients with Child - Pughclass B, the 1-, 3-, and 5-year survival
rates were 78.4%, 29.8%, and 29.8%, respectively, in the non-viral
HCC group, and 88%, 58%, and 37.4%, respectively, in the HCV-HCC
group (Fig. 1F).
Random forest analysis for survival in
patients with HCC
Factors distinguishing between life and death in
patients with HCC were evaluated by a random forest analysis
(Table IV). In all patients, the
distinguishable factors, from the top, were number of tumors,
treatment for HCC, albumin, total bilirubin, and AFP (Table IV, left column). In the non-viral
HCC group, distinguishable factors, from the top, were number of
tumors, HCC stage, treatment for HCC, tumor diameter, and albumin
(Table IV, middle column). In the
HCV-HCC group, distinguishable factors, from the top, were albumin,
total bilirubin, AFP, treatment for HCC, and DCP (Table IV, right column).
 | Table IV.Random forest analysis of life and
death in patients with HCC. |
Table IV.
Random forest analysis of life and
death in patients with HCC.
| ALL (Variable
importance) | Non-viral HCC
(Variable importance) | HCV-HCC (Variable
importance) |
|---|
| 1 | No. of tumors
(1.000) | No. of tumors
(1.000) | Albumin
(1.000) |
| 2 | Treatment for HCC
(0.938) | HCC stage
(0.694) | Total bilirubin
(0.956) |
| 3 | Albumin
(0.704) | Treatment for HCC
(0.385) | AFP (0.913) |
| 4 | Total bilirubin
(0.615) | Tumor diameter
(0.246) | Treatment for HCC
(0.881) |
| 5 | AFP (0.549) | Albumin
(0.223) | DCP (0.719) |
| 6 | Tumor diameter
(0.513) | Platelet count
(0.218) | No. of tumors
(0.681) |
| 7 | DCP (0.504) | AFP (0.167) | Platelet count
(0.438) |
| 8 | Platelet count
(0.412) | Child - Pughclass
(0.118) | Age (0.413) |
| 9 | Child - Pughclass
(0.363) | AST (0.113) | Child - Pughclass
(0.344) |
| 10 | HCC stage
(0.363) | DCP (0.096) | Tumor diameter
(0.338) |
| 11 | Age (0.186) | Total bilirubin
(0.066) | HCC stage
(0.288) |
| 12 | ALT (0.133) | PT activity
(0.062) | AST (0.163) |
| 13 | AST (0.115) | Male (0.015) | PT activity
(0.119) |
| 14 | PT activity
(0.080) | Diabetes mellitus
(0.006) | ALT (0.019) |
| 15 | Non-viral HCC Group
(0.035) | Macrovascular
invasion (0.004) | Diabetes mellitus
(0.006) |
| 16 | Diabetes mellitus
(0.009) | Ethanol consumption
(0.000) | Macrovascular
invasion (0.000) |
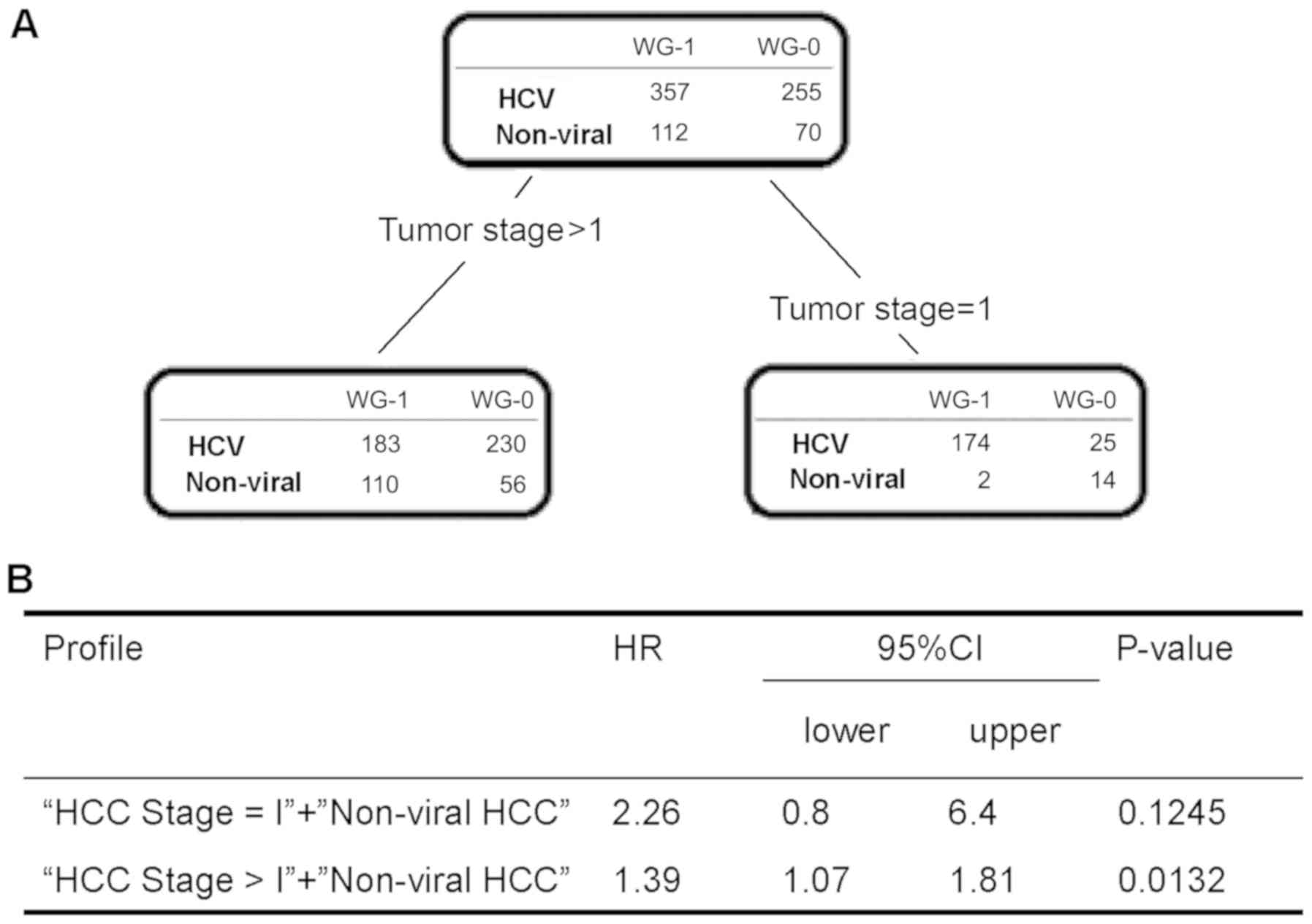
Decision tree analysis of the
characteristic differences in survival terms between HCV-HCC and
non-viral HCC
To evaluate distinguishable factors for survival
period between patients with HCV-HCC and those with non-viral HCC,
we performed a decision tree analysis using the linear-prediction
method. HCC stage I was identified as the most distinguishable
factor for survival period (Fig.
2A). In patients with HCC stage I, there was no significant
difference in survival period between patients with non-viral HCC
and those with HCV-HCC. Meanwhile, in patients with HCC stage
>I, the survival period of patients with non-viral HCC was
significantly shorter than that of patients with HCV-HCC (HR 1.39,
95% CI: 1.07–1.81, P= 0.0132; Fig.
2B).
Discussion
In the present study, we demonstrated that patients
with non-viral HCC have poorer prognosis than patients with
HCV-HCC. Random forest analysis for survival showed that ‘number of
tumors’ and ‘HCC stage’ were high-ranking factors in the non-viral
HCC group, while ‘serum albumin level’ and ‘serum total bilirubin
level’ were high-ranking factors in the HCV-HCC group. In addition,
decision tree analysis demonstrated that ‘HCC stage >I’ was a
distinguishable factor associated with prognosis between patients
with non-viral HCC and HCV-HCC. These data suggest that the
survival of patients with non-viral HCC may be shorter than that of
patients with HCV-HCC because it is difficult to detect non-viral
HCC at early stages.
In the present study, Child - Pughclass C, HCC stage
≥III, presence of tumor MVI, and treatment for HCC were independent
risk factors for prognosis of patients. Cabibbo et al
(24), performed a meta-analysis of
survival rates in 30 randomized clinical trials of HCC and reported
that impaired performance status and Child - Pughclass B or C were
independently associated with shorter survival of patients with
HCC. In addition, MVI is observed in up to 44% of patients with
advanced HCC (25), and MVI of the
hepatic and/or portal vein branches in particular is associated
with poorer prognosis than HCC without MVI (26). Moreover, treatment strategy for HCC
is a well-known independent prognostic factor (27–29).
These findings corroborate our results and suggest that the
patients enrolled in our study had similar characteristics to those
of previous studies. The results of the multivariate analysis were
supported by results of random forest analysis and decision-tree
analysis. However, there were high intercorrelations among
independent variables such as Child-Pugh score and serum levels of
albumin and bilirubin in a multiple regression model and we have to
be aware that multicollinearity may exist in the multivariate
analysis.
We demonstrated that the non-viral HCC is an
independent factor associated with survival. Overall survival of
patients with non-viral HCV was significantly shorter than that of
patients with HCV-HCC. In contrast, Wakiyama et al. reported no
significant differences in overall survival after hepatic resection
between patients with HCV-HCC and those with non-viral HCC
(30). Piscaglia et al
(31), performed a propensity score
analysis and also showed no significant difference in overall
survival between patients with nonalcoholic fatty liver
disease-related HCC and those with HCV-HCC. In the present study,
we performed a stratification analysis and revealed no significant
difference in overall survival between HCV-HCC and non-viral HCC in
patients treated with hepatic resection, RFA, and TACE. However, in
patients treated with HAIC, overall survival was significantly
shorter in the non-viral HCC group than in the HCV-HCC group.
Moreover, among patients with Child - Pughclass A and B, overall
survival was significantly shorter in the non-viral HCC group than
in the HCV-HCC group. These data suggest that prognostic factors
may differ between patients with non-viral HCC and those with
HCV-HCC. To further evaluate this difference, we performed
exploratory analyses, namely random forest analysis and decision
tree analysis.
To investigate factors that distinguish prognosis
between non-viral HCC and HCV-HCC groups, we performed a random
forest analysis and a decision tree analysis. Random forest
analysis for survival showed that ‘number of tumors’ and ‘HCC
stage’ were high-ranking factors in the non-viral HCC group, while
‘serum albumin level’ and ‘serum total bilirubin level’ were
high-ranking factors in the HCV-HCC group. Decision tree analysis
with consideration of survival period showed that ‘HCC stage >I’
was a distinguishing factor associated with the survival period
between patients with non-viral HCC and those with HCV-HCC. Thus,
both exploratory methods revealed that tumor factors were important
for prognosis of patients with non-viral HCC. Followings are 2
possible reasons for the importance of tumor factors in survival of
patients with non-viral HCC. First, non-viral HCC is diagnosed at a
more advanced stage (32–34). Second, a GALNT14 single nucleotide
polymorphism, rs9679162, has been reported to predict chemotherapy
response in HCC (35). Specifically,
the TT genotype of rs9679162 is less represented among patients
with viral HCC than among those with non-viral HCC. Moreover, the
TT genotype is significantly more prevalent among Japanese
individuals (35). Based on this
prognostic factor, early detection of non-viral HCC may prolong
survival of patients.
There are several limitations on this study. First,
this is not a multicenter prospective study. Second, BMI was not
measured in all patients and, therefore, we could not evaluate the
impact of BMI on prognosis of patients with non-viral HCC and
HCV-HCC. Third, there is a possibility that non-viral HCC patients
may include those with positive for hepatitis B core antibody.
Fourth, the non-viral HCC group was consisted of several etiologies
of liver diseases including alcoholic liver disease and
non-alcoholic steatohepatitis. Fifth, for patients with advanced
HCC, molecular targeted agents are first line treatment option for
advanced cancer. However, in our institution, HAIC was employed as
a treatment option for advanced HCC, since beneficial effects of
HAIC has been reported (20–23). Thus, a large-scale, long-term,
prospective study is required to improve prognosis of patients with
non-viral HCC. In addition, the natural course of liver diseases
differs depending on the etiology of liver disease. Moreover,
sustained virological response (SVR) can be achieved by direct
acting antivirals even in HCC patients nowadays. Accordingly,
prognostic profile should be analyzed according to each etiology of
liver disease including the SVR or non-SVR in the future
studies.
In conclusion, we demonstrated that patients with
non-viral HCC have poorer prognosis than those with HCV-HCC. Data
mining analysis revealed that tumor-related variables were
high-ranking prognostic factors in the non-viral HCC group. These
data suggest that the prognosis of patients with non-viral HCC may
be improved by the early detection of HCC through the
identification of high-risk factors.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by AMED (grant no.
JP18fk0210040).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YN and TK participated in study conception and
design, acquisition of data, interpretation of data, and drafting
of manuscript. RK, MN, and NT participated in acquisition of data.
SK and AK participated in analysis and interpretation of data. HK
and TT participated in study conception and design, and critical
revision.
Ethics approval and consent to
participate
The protocol conformed to the ethical guidelines of
the 1975 Declaration of Helsinki and was granted prior approval by
the institutional review board of Kurume University. An opt-out
approach was used to obtain informed consent from the patients, and
personal information was protected during data collection.
Patient consent for publication
Not applicable.
Conflicts of interest
Takumi Kawaguchi received lecture fees from
Mitsubishi Tanabe Pharma Corporation, MSD K.K., and Otsuka
Pharmaceutical Co., Ltd. The other authors have no conflicts of
interest.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
HCV-HCC
|
hepatitis C virus-related
hepatocellular carcinoma
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
AFP
|
alpha-fetoprotein
|
|
DCP
|
des-γ-carboxy prothrombin
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
AST
|
aspartate aminotransferase
|
|
ALT
|
alanine aminotransferase
|
|
MVI
|
macrovascular invasion
|
|
TNM
|
tumor-node-metastasis
|
|
RFA
|
radiofrequency ablation
|
|
TACE
|
rans-arterial chemoembolization
|
|
HAIC
|
hepatic arterial infusion
chemotherapy
|
|
SVR
|
sustained virological response
|
References
|
1
|
Bertuccio P, Turati F, Carioli G,
Rodriguez T, La Vecchia C, Malvezzi M and Negri E: Global trends
and predictions in hepatocellular carcinoma mortality. J Hepatol.
67:302–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cazzagon N, Trevisani F, Maddalo G,
Giacomin A, Vanin V, Pozzan C, Poggio PD, Rapaccini G, Nolfo AM,
Benvegnù L, et al Italian Liver Cancer (ITA.LI.CA) Group, : Rise
and fall of HCV-related hepatocellular carcinoma in Italy: A
long-term survey from the ITA.LI.CA centres. Liver Int.
33:1420–1427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taura N, Fukushima N, Yastuhashi H, Takami
Y, Seike M, Watanabe H, Mizuta T, Sasaki Y, Nagata K, Tabara A, et
al: The incidence of hepatocellular carcinoma associated with
hepatitis C infection decreased in Kyushu area. Med Sci Monit.
17:PH7–PH11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marot A, Henrion J, Knebel JF, Moreno C
and Deltenre P: Alcoholic liver disease confers a worse prognosis
than HCV infection and non-alcoholic fatty liver disease among
patients with cirrhosis: An observational study. PLoS One.
12:e01867152017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamed MA and Ali SA: Non-viral factors
contributing to hepatocellular carcinoma. World J Hepatol.
5:311–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hemkens LG, Grouven U, Bender R, Günster
C, Gutschmidt S, Selke GW and Sawicki PT: Risk of malignancies in
patients with diabetes treated with human insulin or insulin
analogues: A cohort study. Diabetologia. 52:1732–1744. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oda K, Uto H, Mawatari S and Ido A:
Clinical features of hepatocellular carcinoma associated with
nonalcoholic fatty liver disease: A review of human studies. Clin J
Gastroenterol. 8:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamada S, Kawaguchi A, Kawaguchi T,
Fukushima N, Kuromatsu R, Sumie S, Takata A, Nakano M, Satani M,
Tonan T, et al: Serum albumin level is a notable profiling factor
for non-B, non-C hepatitis virus-related hepatocellular carcinoma:
A data-mining analysis. Hepatol Res. 44:837–845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Degos F, Christidis C, Ganne-Carrie N,
Farmachidi JP, Degott C, Guettier C, Trinchet JC, Beaugrand M and
Chevret S: Hepatitis C virus related cirrhosis: Time to occurrence
of hepatocellular carcinoma and death. Gut. 47:131–136. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minami T, Tateishi R, Shiina S, Nakagomi
R, Kondo M, Fujiwara N, Mikami S, Sato M, Uchino K, Enooku K, et
al: Comparison of improved prognosis between hepatitis B- and
hepatitis C-related hepatocellular carcinoma. Hepatol Res.
45:E99–E107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hashimoto M, Tashiro H, Kobayashi T,
Kuroda S, Hamaoka M and Ohdan H: Clinical characteristics and
prognosis of non-B, non-C hepatocellular carcinoma: The impact of
patient sex on disease-free survival - A retrospective cohort
study. Int J Surg. 39:206–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiwatashi K, Ueno S, Sakoda M, Iino S,
Minami K, Yamasaki Y, Okubo K, Noda M, Kurahara H, Mataki Y, et al:
Problems of long survival following surgery in patients with
nonBNonC-HCC: Comparison with HBV and HCV related-HCC. J Cancer.
6:438–447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radice R, Ramsahai R, Grieve R, Kreif N,
Sadique Z and Sekhon JS: Evaluating treatment effectiveness in
patient subgroups: A comparison of propensity score methods with an
automated matching approach. Int J Biostat. 8:252012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Desbordes P, Ruan S, Modzelewski R, Pineau
P, Vauclin S, Gouel P, Michel P, Di Fiore F, Vera P and Gardin I:
Predictive value of initial FDG-PET features for treatment response
and survival in esophageal cancer patients treated with
chemo-radiation therapy using a random forest classifier. PLoS One.
12:e01732082017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diouf M, Filleron T, Pointet AL,
Dupont-Gossard AC, Malka D, Artru P, Gauthier M, Lecomte T,
Aparicio T, Thirot-Bidault A, et al: Prognostic value of
health-related quality of life in patients with metastatic
pancreatic adenocarcinoma: A random forest methodology. Qual Life
Res. 25:1713–1723. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohammadzadeh F, Noorkojuri H,
Pourhoseingholi MA, Saadat S and Baghestani AR: Predicting the
probability of mortality of gastric cancer patients using decision
tree. Ir J Med Sci. 184:277–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Min KW, Kim DH, Son BK, Kim EK, Ahn SB,
Kim SH, Jo YJ, Park YS, Seo J, Oh YH, et al: Invasion depth
measured in millimeters is a predictor of survival in patients with
distal bile duct cancer: Decision tree approach. World J Surg.
41:232–240. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kokudo N, Hasegawa K, Akahane M, Igaki H,
Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, et al:
Evidence-based clinical practice guidelines for hepatocellular
carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC
Guidelines). Hepatol Res. 45:452015. View Article : Google Scholar
|
|
19
|
Liver Cancer Study Group of Japan, . The
general rules for the clinical and pathological study of primary
liver cancer. Jpn J Surg. 19:98–129. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawaoka T, Aikata H, Kobayashi T, Uchikawa
S, Ohya K, Kodama K, Nishida Y, Daijo K, Osawa M, Teraoka Y, et al:
Comparison of hepatic arterial infusion chemotherapy between
5-fluorouracil-based continuous infusion chemotherapy and low-dose
cisplatin monotherapy for advanced hepatocellular carcinoma.
Hepatol Res. 48:1118–1130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saeki I, Yamasaki T, Maeda M, Hisanaga T,
Iwamoto T, Matsumoto T, Hidaka I, Ishikawa T, Takami T and Sakaida
I: Evaluation of the ‘assessment for continuous treatment with
hepatic arterial infusion chemotherapy’ scoring system in patients
with advanced hepatocellular carcinoma. Hepatol Res. 48:E87–E97.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsunematsu S, Suda G, Yamasaki K, Kimura
M, Takaaki I, Umemura M, Ito J, Sato F, Nakai M, Sho T, et al:
Combination of neutrophil-to-lymphocyte ratio and early
des-γ-carboxyprothrombin change ratio as a useful predictor of
treatment response for hepatic arterial infusion chemotherapy
against advanced hepatocellular carcinoma. Hepatol Res. 47:533–541.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakano M, Niizeki T, Nagamatsu H, Tanaka
M, Kuromatsu R, Satani M, Okamura S, Iwamoto H, Shimose S, Shirono
T, et al Kurume Liver Cancer Study Group of Japan, : Clinical
effects and safety of intra-arterial infusion therapy of cisplatin
suspension in lipiodol combined with 5-fluorouracil versus
sorafenib, for advanced hepatocellular carcinoma with macroscopic
vascular invasion without extra-hepatic spread: A prospective
cohort study. Mol Clin Oncol. 7:1013–1020. 2017.PubMed/NCBI
|
|
24
|
Cabibbo G, Enea M, Attanasio M, Bruix J,
Craxì A and Cammà C: A meta-analysis of survival rates of untreated
patients in randomized clinical trials of hepatocellular carcinoma.
Hepatology. 51:1274–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pirisi M, Avellini C, Fabris C, Scott C,
Bardus P, Soardo G, Beltrami CA and Bartoli E: Portal vein
thrombosis in hepatocellular carcinoma: Age and sex distribution in
an autopsy study. J Cancer Res Clin Oncol. 124:397–400. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Costentin CE, Ferrone CR, Arellano RS,
Ganguli S, Hong TS and Zhu AX: Hepatocellular carcinoma with
macrovascular invasion: Defining the optimal treatment strategy.
Liver Cancer. 6:360–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasegawa K, Kokudo N, Makuuchi M, Izumi N,
Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O, et al:
Comparison of resection and ablation for hepatocellular carcinoma:
A cohort study based on a Japanese nationwide survey. J Hepatol.
58:724–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murakami T, Ishimaru H, Sakamoto I, Uetani
M, Matsuoka Y, Daikoku M, Honda S, Koshiishi T and Fujimoto T:
Percutaneous radiofrequency ablation and transcatheter arterial
chemoembolization for hypervascular hepatocellular carcinoma: Rate
and risk factors for local recurrence. Cardiovasc Intervent Radiol.
30:696–704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al
Barcelona Liver Cancer Group, : Arterial embolisation or
chemoembolisation versus symptomatic treatment in patients with
unresectable hepatocellular carcinoma: A randomised controlled
trial. Lancet. 359:1734–1739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wakiyama S, Matsumoto M, Haruki K, Gocho
T, Sakamoto T, Shiba H, Futagawa Y, Ishida Y and Yanaga K: Clinical
features and outcome of surgical patients with non-B non-C
hepatocellular carcinoma. Anticancer Res. 37:3207–3213.
2017.PubMed/NCBI
|
|
31
|
Piscaglia F, Svegliati-Baroni G, Barchetti
A, Pecorelli A, Marinelli S, Tiribelli C and Bellentani S;
HCC-NAFLD Italian Study Group, : Clinical patterns of
hepatocellular carcinoma in nonalcoholic fatty liver disease: A
multicenter prospective study. Hepatology. 63:827–838. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jun TW, Yeh ML, Yang JD, Chen VL, Nguyen
P, Giama NH, Huang CF, Hsing AW, Dai CY, Huang JF, et al: More
advanced disease and worse survival in cryptogenic compared to
viral hepatocellular carcinoma. Liver Int. 38:895–902. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohsen W, Rodov M, Prakoso E, Charlton B,
Bowen DG, Koorey DJ, Shackel NA, McCaughan GW and Strasser SI:
Patients with non-viral liver disease have a greater tumor burden
and less curative treatment options when diagnosed with
hepatocellular carcinoma. World J Gastroenterol. 23:2763–2770.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kimura T, Kobayashi A, Tanaka N, Sano K,
Komatsu M, Fujimori N, Yamazaki T, Shibata S, Ichikawa Y, Joshita
S, et al: Clinicopathological characteristics of non-B non-C
hepatocellular carcinoma without past hepatitis B virus infection.
Hepatol Res. 47:405–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang KH, Yang PC and Yeh CT: Genotyping
the GALNT14 gene by joint analysis of two linked single nucleotide
polymorphisms using liver tissues for clinical and geographical
comparisons. Oncol Lett. 8:2215–2220. 2014. View Article : Google Scholar : PubMed/NCBI
|