Ewing sarcoma (EWS) is a small-round-blue-cell tumor
that is derived from primordial mesenchymal stem cells, which often
originate from the bone marrow (1).
The incidence of EWS is one case in one million people in the US
(1). The current standard first-line
chemotherapy for EWS includes vincristine, doxorubicin,
cyclophosphamide (VDC), ifosfamide and etoposide (IE), also termed
VDC/IE (2,3), or vincristine, ifosfamide, doxorubicin
and etoposide (VIDE) (4). The use of
these chemotherapy regimens has resulted in the 5-year survival
rate increasing from 59 to 78% in children and young adolescents,
and from 20 to 60% in adults (5).
However, there is currently no standardized second-line treatment
for recurrent or refractory EWS. Various methods, including
classical cytotoxic agents, targeted therapy, such as
anti-angiogenesis small molecular tyrosine kinase inhibitors
(aaTKIs), and immunotherapy, such as check-point inhibitors, have
been tested in these progressed cases. Unfortunately, the prognosis
for these patients remains poor (5,6). The
majority of phase I trials for these methods have demonstrated
acceptable safety profiles, but have failed to reach the primary
endpoint in the phase II trials. In the last two decades, only one
phase II trial testing these new drugs has progressed to phase III;
however, there is no published data available. Until now, there has
not been a standard second-line regimen following progression from
the first-line treatment. As a rare disease with a number of
different treatment options, it can be time-consuming for doctors
to obtain useful information. In the present study, the outcomes of
various treatment regimens for relapsed or refractor Ewing sarcoma,
the optimal sequence of drugs following VDC/IE or VIDE treatment,
and the promising management techniques expected in future trials
were investigated. The records of phase II and phase I/II clinical
trials in the last 15 years were reviewed according to PRISMA
methodology (7).
Four data sources were initially searched using the
following search terms: i) (Condition or disease ‘Ewing sarcoma’ OR
‘Ewing family of tumors’) AND (phase ‘Phase 2’) AND (study start
from ‘01/01/2003’ to ‘10/01/2018’) on ClinicalTrials.gov; ii) (‘Ewing sarcoma’ OR ‘Ewing
family of tumors’) AND (‘Phase 2’ OR ‘Phase II’) AND
(date-publication ‘2003:2018’) on PubMed; iii) (‘Ewing sarcoma’ OR
‘Ewing family of tumors’) AND (trial phase ‘Phase two’) AND (data
range ‘2003-01-01’ to ‘2018-10-01’) on Clinicaltrialsregister.eu
(EudraCT); and iv) ‘Ewing sarcoma’ in the abstracts available on
the American Society of Clinical Oncology (ASCO) website. The final
search was performed on October 15, 2018. As there were no phase
III trials with published results available using the
aforementioned search strategy, only phase II trials were included
in the present study. There was only one phase III trial identified
that is currently recruiting, which opened in April 2018 (no.
NCT03495921); a multicenter, 1:1 randomized phase III study of
intradermal autologous Vigil immunotherapy in combination with
irinotecan and temozolomide.
After the initial screening, the following
eligibility criteria were used in further investigation: i)
Patients had recurrent disease or their cancer was deemed
refractory to previous first-line chemotherapy (VDC/IE or VIDE);
ii) trials focused on EWS patients, or had one EWS stratum; iii)
antitumor activity was assessed using a primary or secondary
endpoint; and iv) language was limited to English. The
aforementioned four data sources were searched sequentially.
Finally, duplications among or inside each database were
removed.
The systematic search in each database was performed
by two different individuals. Disagreements were resolved by
discussion. The following information was extracted from each
trial: i) General information, including date, identification
number, principle investigators and centers; ii) drug information,
including name and dose; iii) trial design, including phase,
randomization, population, study status and statistical design; iv)
participant enrollment, including the estimated and effective
enrollment in each stage (for multiple-stage design), age, mean
time from initial diagnosis to protocol enrollment and prior lines
of systemic anticancer therapy; and v) endpoints, including the
criteria of response, patients evaluated for efficacy, response
rate and survival rate. Response to therapy was recorded as
complete response, partial response, stable disease and progression
of disease. The objective response rate (ORR) was defined as the
rate of complete response and partial response. The records of
phase II and phase I/II clinical trials in the last 15 years were
reviewed according to PRISMA methodology (7).
Interventions were classified into four groups: i)
Classical cytotoxic chemotherapy, either alone or in combination
with other cytotoxic drugs; ii) targeted therapy, including TKIs
that target different molecules or pathways, either alone or in
combination with cytotoxic drugs; iii) immunotherapy, including
monoclonal antibodies, immune checkpoint blockade and antitumor
viruses, either alone or in combination with the previous two
groups; and iv) other therapy. For phase I/II trials, only
participants in the phase II part were analyzed.
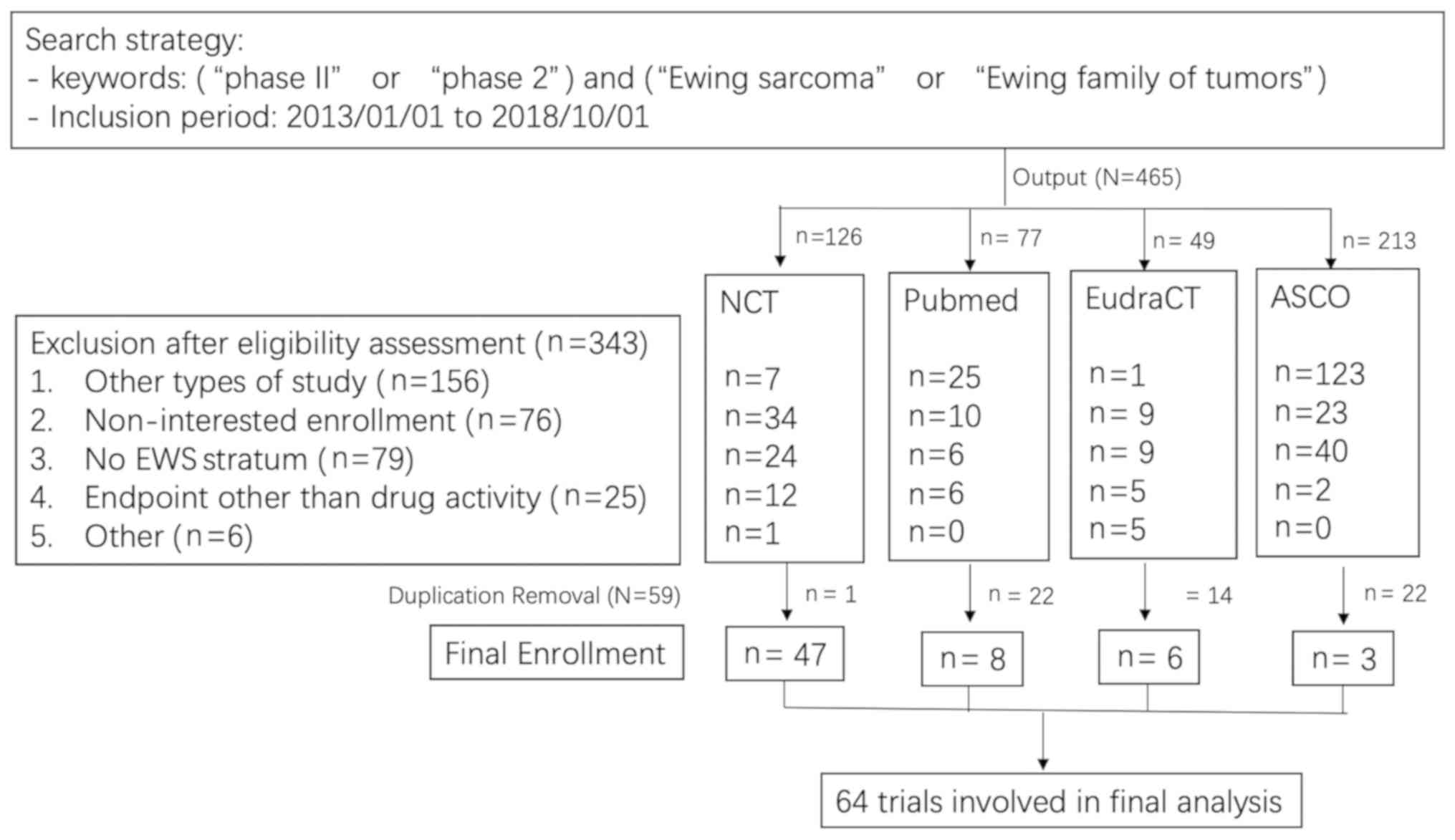
Study selection. Overall, 465 trials were identified
following the initial screening (Fig.
1). The first step involved an eligibility assessment, and 343
trials were excluded for the following reasons: i) The studies were
not phase II clinical trials (n=156), that is, they were phase I
clinical trials (n=55), retrospective clinical trials (n=6), case
reports (n=12), literature reviews or meta-analyses (n=49),
preclinical studies (n=33) or papers presenting methodologies
(n=1); ii) non-interested enrollment (n=76), including trials for
patients with chemo-naïve metastatic disease (n=57) and trials for
other diseases (n=19); iii) there was no EWS stratum available
(n=79); iv) endpoints were used that did not include the antitumor
activity of the drugs (n=25), including local control of
radiotherapy (n=6), engraftment (n=2) and toxicity (n=17); and v)
others (n=6), including one trial that closed before enrolling any
participants and five trials that were reported in languages other
than English.
The second step involved the removal of duplications
(n=59). Duplicate trials were removed sequentially in order of
ClinicalTrials.gov (n=1), PubMed (n=22), EudraCT
(n=14) and ASCO (n=22). One trial was registered twice on
ClinicalTrials.gov (no. NCT00154388 and
NCT00031915) with the final result was reported in one paper
(8). Finally, 64 trials were
included in the present study (Fig.
1).
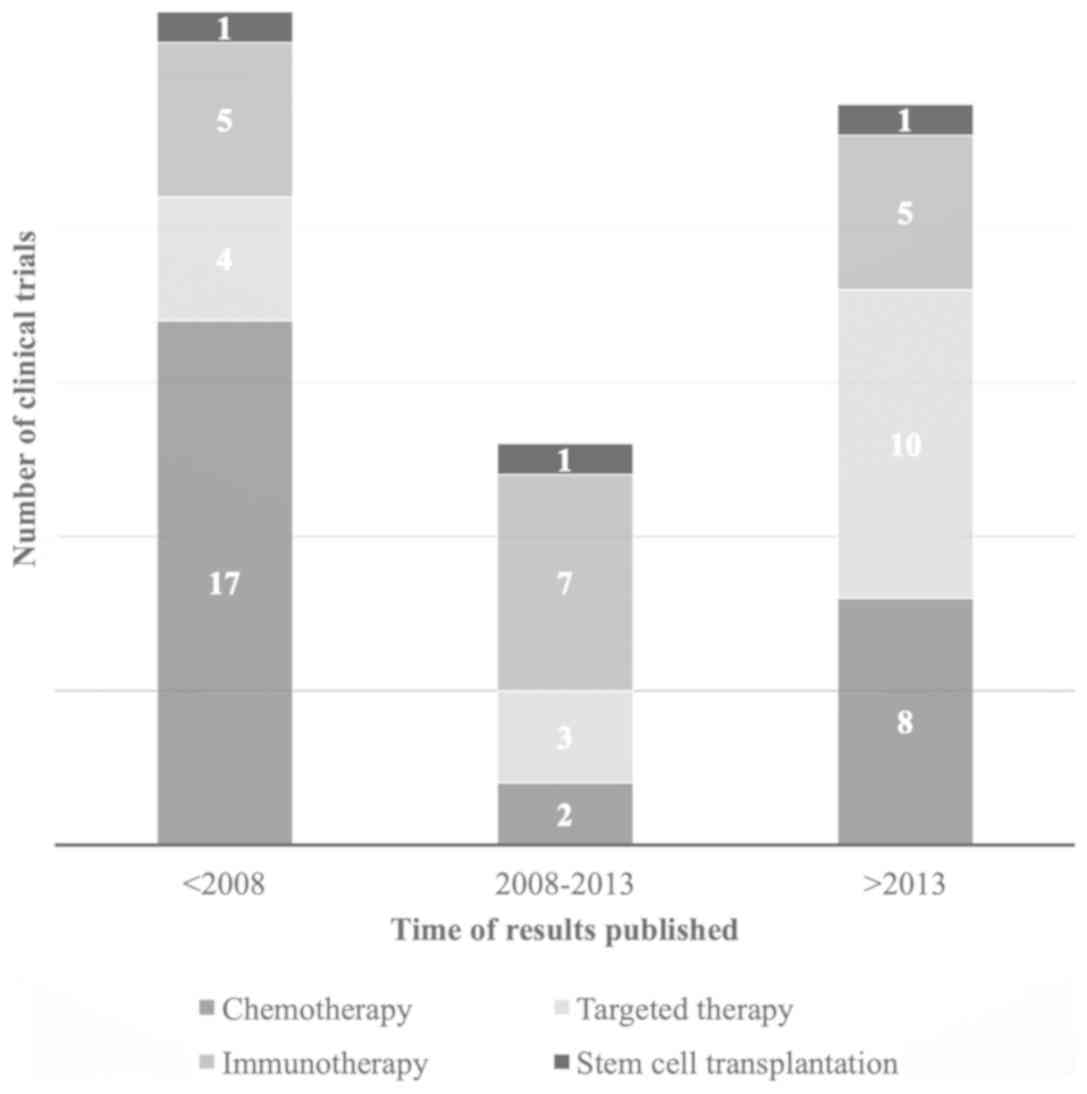
The general characteristics of the 64 trials
included in the present study are summarized in Table I. They were classified into four
groups: Chemotherapy (n=27), targeted therapy (n=17), immunotherapy
(n=17) and stem cell transplantation (n=3; Fig. 2). Of the 64 trials, 37 were completed
(at least EWS stratum was completed) and had published results with
an abstract (n=10) or full-text (n=27) available. The ORR was
assessed in 36 trials, which were then further analyzed.
There were eight trials that used targeted therapy
in the present study and six drugs were assessed. The majority of
these trials did not reach their primary endpoints in phase I and
failed to enter phase II (Table
III). Only one trial using regorafenib demonstrated a clinical
response, with an ORR of 11% (15).
There were nine trials enrolled in the present study
that used immunotherapy, in which IGF-1R was administrated as
monotherapy (n=6) or in combination with temsirolimus (n=3). The
best result was identified in the combination group (ORR, 29%)
(16). However, all the other eight
trials revealed a poor ORR of ≤15%. Five of the nine trials closed
before entering phase II due to a lack of efficacy (Table IV).
Although the participants were strictly limited to
recurrent or refractory EWS, conflicting results were observed for
the same drug or regimen. For trabectedin, a promising result was
reported in an ASCO abstract (13)
with an ORR of 15%, whereas in 2012 another trial revealed no
response (ORR, 0%) (17). The same
dose and response criteria were used in each trial. A similar
phenomenon was identified in irinotecan, where the ORR varied from
0 (18), to 38 (10), to 71% (9). All three trials utilized the World
Health Organization criteria to assess objective response rates
(ORR). However, different irinotecan administration strategies were
utilized in these three trials, from 50 mg/m2/dose for 5
days, repeated every 3 weeks; to 20 mg/m2/dose for 5
days per week for 2 weeks, repeated every 4 weeks; to 16
mg/m2/dose for 5 days per week for 2 weeks, repeated
every 3 weeks. The combination of cixutumumab and temsirolimus was
administered with the same variations, and an ORR of 12% (or 29% if
a regression of 20–30% was recorded as minor response) was reported
in adults in 2012 (16), 15% in
adults in 2013 (19) and 0% in
children and young adults in 2015 (20). The same dose of cixutumumab was used
in the three trials, with the only difference being a lower dose of
temsirolimus of 8–10 mg/m2 (equivalent to an adult flat
dose of 14 mg) in children and young adults, compared with a 25 mg
flat dose in adults. Furthermore, over half of the adults required
a decreased dose amount due to toxicity levels, and 29% of them
required a second reduction (19).
The mean time from the initial diagnosis to
recurrence or progression varied from 19 to 43 months (21,22).
With available data, almost all participants had more than two
lines of prior systemic anticancer therapy, except in the
cisplatin/etoposide trial (12) and
in one of the cixutumumab trials (16). The median prior line of systemic
therapy varied among trials (range 1–6).
In the 36 trials that reported their results and
used ORR as an endpoint, response evaluation criteria in solid
tumors (RECIST) was the most commonly used criteria (27 trials),
including 13 that used RECIST version 1.1 (23), nine that used RECIST version 1.0
(24), four that used a non-specific
version of RECIST and one that used RECIST version 1.1 and the
World Health Organization (WHO) criteria (25,26) at
the same time and observed no difference in the outcome from
different criteria. For the remaining nine trials, seven used the
WHO criteria alone, one used the Choi criteria (27) and one was not available.
According to the registration system, two trials
started enrolling participants 10 years ago; however, no published
results were available. One trial investigated exatecan (no.
NCT00055952), which started in January 2003, and the other
investigated hematopoietic stem cell transplantations (no.
NCT00998361), which started in June 2009. There was no specific
reason given for the unpublished results (Table V).
Trials registered in the domestic clinical trials
registration system were not screened. There were five trials
registered in languages other than English, which were then
excluded.
Several trials assessing new drugs are still ongoing
and the results have not yet been reported, including targeted
therapy (aaTKIs, PI3K/mTOR and poly(ADP-ribose) polymerase) and
immunotherapy (checkpoint blockade, oncolytic virus; Table V).
Duplicated studies were identified and removed
following abstract and/or full text screening.
The present study investigated what can be learned
from prospective phase II trials, and what can be expected from
ongoing clinical trials. A comprehensive systematic review was
performed with the aim of determining the optimal sequence of drugs
following the use of VDC/IE or VIDE.
Cytotoxic chemotherapy. New drugs and regimens have
been investigated more recently, but the most promising results
still came from chemotherapy (e.g., irinotecan) based on available
data. In addition to phase II trials (9,10,18),
retrospective studies have provided more evidence on
irinotecan/temozolomide (IT), which had ORRs as high as 34, 68 and
55% (28,29), and a median time to progression of
5.5 (30) and 3.0 months (29). At first, two patients showed an
initial response but relapsed following withdrawal of the drug for
5 and 6 months, respectively (28).
After recommencing the same IT regimen, the two patients achieved a
second PR; one that lasted for at least another 15 cycles and the
other another 22 cycles (28). On
the basis of the success of IT, more clinicians use it as the first
choice of treatment following the failure of VDC/IE or VIDE.
As for targeted therapy, classical agents arising
from leukemia regimens, such as imatinib or dasatinib, did not
exhibit any activity in patients with EWS. Only regorafenib, which
has a stronger anti-angiogenesis effect, demonstrated promising
clinical activity in patients with EWS. Further trials for other
types of aaTKI, including pazopanib, cabozantinib and apatinib,
which have shown some activity in other types of sarcoma (31–33), are
ongoing and the results of which are anticipated. For patients who
were refractory to first-line chemotherapy, pazopanib was reported
to be effective in a set of case series (34–37).
Early results from the cabozantinib trial (no. NCT02243605) in
patients with EWS look promising, and an ORR of 28.1% in 32
patients was observed, as well as a high tumor burden reduction
rate of 71% (38). For apatinib,
which is also a strong aaTKI (39),
an ORR of 70% (7/10) was observed in an off-label set of patients
with EWS (33). Based on these data,
it was concluded that aaTKIs require further investigation.
Except for monotherapy, preclinical studies have
demonstrated the synergistic antiproliferative and pro-apoptotic
activity of irinotecan or topotecan and aaTKIs in vitro, and
the improvement of the in vivo anticancer activity on
angiogenesis, endothelial and cancer cells, such as pancreatic
(40) and ovarian cancer cells
(41). Based on the non-overlapped
adverse effects of irinotecan (42,43) and
aaTKIs (44,45), these studies suggested a possible
translation of this combination into the clinic. A phase I study of
axitinib and irinotecan combined with 5-fluorouracil and leucovorin
in patients with advanced colorectal cancer described an acceptable
toxicity profile (46). Another
phase I trial that used a triplet combination of pazopanib,
irinotecan and cetuximab in patients with refractory metastatic
colorectal cancer also provided evidence for a manageable safety
profile (47). Based on this
evidence, trials have been designed that use IT in combination with
aaTKIs to maximize antitumor activity (no. NCT03416517).
Immunotherapy based on anti-insulin-like growth
factor 1 receptor (IGF-1R) antibody was somewhat disappointing.
Preclinical studies have revealed the IGF-1R pathway as promising
new targets for EWS (48,49) and these observations have led to
several clinical studies. However, given the non-optimal results
from these trials, almost all health providers have stopped further
investigation on IGF-1R antibody. Efforts have been made to look
for biomarkers and narrow down the population who may benefit from
the use of IGF-1R antibody. A multi-center study classified
patients into different subtypes based on IGF-1R expression via
immunohistochemistry (19), but
there was no overall effect on outcome. Although in patients with
EWS who were IGF-1R-negative had improved median PFS, it may be
explained by the less aggressive biological behavior rather than
real response to therapy.
Another type of immunotherapy with checkpoint
blockade remains ongoing. Tumor mutation burden is considered an
important factor for immune checkpoint blockade therapy (50,51).
However, from the view of biological nature and genomic landscape,
EWS does not belong to hyper-mutated tumors with a mutation
frequency of <10 mutation/Mb (52), and only EWS-ETS gene rearrangements
were identified in the majority of tumors (53,54). The
role of the immune checkpoint blockade remains to be defined by
well-designed clinical trials.
The time to recurrence is the most important
prognostic factor for patients with recurrent EWS. Patients who
relapsed >2 years from the initial diagnosis had a 5-year
survival of 30%, compared with 7% for patients that relapsed within
2 years (5,6). Patients in different trials experienced
recurrence at different time points and may impact final
oncological outcomes.
Different criteria have been used to assess drug
response. The WHO criteria, RECIST 1.0 (a simplified version of the
WHO criteria) and its newer version, RECIST 1.1, continue to be
based on changes in tumor size. All these three criteria have a
similar evaluation power for solid tumors (25,55). In
the 37 trials with published results that were investigated in the
present study, 36 used at least one of the three aforementioned
criteria and provided a fair comparison among the trials. In the
dasatinib trial (56), the Choi
criteria were selected as the tumor response criteria, which the
authors believed was associated with improved outcome in patients
with gastrointestinal stromal tumors that were treated with TKIs
(57). The significant differences
observed between the Choi and RECIST criteria were due to the
addition of change in tumor density in computed tomography scans
and a smaller magnitude of change in tumor size to score response.
From that point, more responses were scored using the Choi
criteria, although only one partial response was recorded in all 17
participants with EWS (56).
Abundant trials assessing new drugs are still
ongoing and no results have been reported yet (Table V). Although classical targeted drugs
such as imatinib and IGF-1R antibody demonstrated no activity in
patients with EWS, aaTKIs appear more promising from the early
revealed data, either as monotherapy or in combination with
cytotoxic drugs. Therefore, more evidence is required to draw a
robust conclusion for the new drugs.
Although abundant new drugs for targeted therapy and
immunotherapy have been tested in the last 15 years, the best
response came from traditional cytotoxic chemotherapy, particularly
irinotecan-based regimens. Targeted therapy with aaTKIs either
alone or in combination with chemotherapy require further
investigation. Currently, immunotherapy is not recommended for
off-label use.
Not applicable.
The present study was funded by the Beijing
Municipal Science and Technology Project (grant no.
Z181100001718054).
Not applicable.
JX and LX performed the systematic search. XS and SD
reviewed the original phase 2 trial studies. WG designed the study.
XT designed the data extraction sheet and final tables, provided
supportive data from COG and ESMO meetings and revised the
manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Jiang S, Wang G, Chen J and Dong Y:
Comparison of clinical features and outcomes in patients with
extraskeletal vs skeletal Ewing sarcoma: An SEER database analysis
of 3,178 cases. Cancer Manag Res. 10:6227–6236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grier HE, Krailo MD, Tarbell NJ, Link MP,
Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers
PA, et al: Addition of ifosfamide and etoposide to standard
chemotherapy for Ewing's sarcoma and primitive neuroectodermal
tumor of bone. N Engl J Med. 348:694–701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrari S, Mercuri M, Rosito P, Mancini A,
Barbieri E, Longhi A, Rimondini S, Cesari M, Ruggieri P, Di Liddo M
and Bacci G: Ifosfamide and actinomycin-D, added in the induction
phase to vincristine, cyclophosphamide and doxorubicin, improve
histologic response and prognosis in patients with non metastatic
Ewing's sarcoma of the extremity. J Chemother. 10:484–491. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Juergens C, Weston C, Lewis I, Whelan J,
Paulussen M, Oberlin O, Michon J, Zoubek A, Juergens H and Craft A:
Safety assessment of intensive induction with vincristine,
ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of
Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr
Blood Cancer. 47:22–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leavey PJ, Mascarenhas L, Marina N, Chen
Z, Krailo M, Miser J, Brown K, Tarbell N, Bernstein ML, Granowetter
L, et al: Prognostic factors for patients with Ewing sarcoma (EWS)
at first recurrence following multi-modality therapy: A report from
the Children's oncology group. Pediatr Blood Cancer. 51:334–338.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stahl M, Ranft A, Paulussen M, Bölling T,
Vieth V, Bielack S, Görtitz I, Braun-Munzinger G, Hardes J, Jürgens
H and Dirksen U: Risk of recurrence and survival after relapse in
patients with Ewing sarcoma. Pediatr Blood Cancer. 57:549–553.
2001. View Article : Google Scholar
|
|
7
|
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA; PRISMA-P Group,
: Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4:12015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chugh R, Wathen JK, Maki RG, Benjamin RS,
Patel SR, Meyers PA, Priebat DA, Reinke DK, Thomas DG, Keohan ML,
et al: Phase II multicenter trial of imatinib in 10 histologic
subtypes of sarcoma using a bayesian hierarchical statistical
model. J Clin Oncol. 27:3148–3153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dumont SN, Trent JC, Patel S, Araujo DM,
Dumont AG and Benjamin RS: A phase II study of low-dose protracted
irinotecan in patients with advanced sarcomas. J Clin Oncol.
29:100642011. View Article : Google Scholar
|
|
10
|
Bisogno G, Riccardi R, Ruggiero A,
Arcamone G, Prete A, Surico G, Provenzi M, Bertolini P, Paolucci P
and Carli M: Phase II study of a protracted irinotecan schedule in
children with refractory or recurrent soft tissue sarcoma. Cancer.
106:703–707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Winkle P, Angiolillo A, Krailo M,
Cheung YK, Anderson B, Davenport V, Reaman G and Cairo MS:
Ifosfamide, carboplatin, and etoposide (ICE) reinduction
chemotherapy in a large cohort of children and adolescents with
recurrent/refractory sarcoma: The Children's cancer group (CCG)
experience. Pediatr Blood Cancer. 44:338–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Owens C, Laurence V, Benboubker L,
Defachelles AS, Cupissol D, Rubie H, Brisse H, Rey A, Ollivier L,
Couanet D, et al: Phase II study of cisplatin and oral VP16 in
patients with refractory or relapsed Ewing sarcoma. Cancer
Chemother Pharmacol. 71:399–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dileo P, Grosso F, Casanova M, Jimeno J,
Marsoni S, Podda RS, Ferrari S, Bertulli R and Casali PG:
Trabectedin (T) in metastatic Ewing's family tumors (EFT) patients
(pts) progressing after standard chemotherapy. J Clin Oncol.
25:100402007.
|
|
14
|
Zwerdling T, Krailo M, Monteleone P, Byrd
R, Sato J, Dunaway R, Seibel N, Chen Z, Strain J and Reaman G;
Children's Oncology Group, : Phase II investigation of docetaxel in
pediatric patients with recurrent solid tumors: A report from the
Children's oncology group. Cancer. 106:1821–1828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Attia S, Bolejack V, Ganjoo KN, George S,
Agulnik M, Rushing DA, Loggers ET, Livingston MB, Wrig JA, Chawla
SP, et al: A phase II trial of regorafenib (REGO) in patients (pts)
with advanced Ewing sarcoma and related tumors (EWS) of soft tissue
and bone: SARC024 trial results. J Clin Oncol. 35:110052017.
View Article : Google Scholar
|
|
16
|
Naing A, LoRusso P, Fu S, Hong DS,
Anderson P, Benjamin RS, Ludwig J, Chen HX, Doyle LA and Kurzrock
R: Insulin growth factor-receptor (IGF-1R) antibody cixutumumab
combined with the mTOR inhibitor temsirolimus in patients with
refractory Ewing's sarcoma family tumors. Clin Cancer Res.
18:2625–2631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baruchel S, Pappo A, Krailo M, Baker KS,
Wu B, Villaluna D, Lee-Scott M, Adamson PC and Blaney SM: A phase 2
trial of trabectedin in children with recurrent rhabdomyosarcoma,
Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: A
report from the Children's oncology group. Eur J Cancer.
48:579–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bomgaars LR, Bernstein M, Krailo M, Kadota
R, Das S, Chen Z, Adamson PC and Blaney SM: Phase II trial of
irinotecan in children with refractory solid tumors: A Children's
oncology group study. J Clin Oncol. 25:4622–4627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwartz GK, Tap WD, Qin LX, Livingston
MB, Undevia SD, Chmielowski B, Agulnik M, Schuetze SM, Reed DR,
Okuno SH, et al: Cixutumumab and temsirolimus for patients with
bone and soft-tissue sarcoma: A multicentre, open-label, phase 2
trial. Lancet Oncol. 14:371–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wagner LM, Fouladi M, Ahmed A, Krailo MD,
Weigel B, DuBois SG, Doyle LA, Chen H and Blaney SM: Phase II study
of cixutumumab in combination with temsirolimus in pediatric
patients and young adults with recurrent or refractory sarcoma: A
report from the Children's oncology group. Pediatr Blood Cancer.
62:440–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Minard-Colin V, Ichante JL, Nguyen L, Paci
A, Orbach D, Bergeron C, Defachelles AS, André N, Corradini N,
Schmitt C, et al: Phase II study of vinorelbine and continuous low
doses cyclophosphamide in children and young adults with a relapsed
or refractory malignant solid tumour: good tolerance profile and
efficacy in rhabdomyosarcoma-a report from the Société Française
des Cancers et leucémies de l'Enfant et de l'adolescent (SFCE). Eur
J Cancer. 48:2409–2416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jones RL, Ferrari S, Blay JY, Navid F,
Lardelli P, Alfaro V, Siguero M, Soman N and Chawla SP: A Phase II
multicenter, open-label, clinical and pharmokinetic trial of
PM00104 in patients with advanced Ewing family of tumors. Invest
New Drugs. 32:171–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duffaud F and Therasse P: New guidelines
to evaluate the response to treatment in solid tumors. Bull Cancer.
87:881–886. 2000.(In French). PubMed/NCBI
|
|
25
|
Park JO, Lee SI, Song SY, Kim K, Kim WS,
Jung CW, Park YS, Im YH, Kang WK, Lee MH, et al: Measuring response
in solid tumors: Comparison of RECIST and WHO response criteria.
Jpn J Clin Oncol. 33:533–537. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi H, Charnsangavej C, Faria SC,
Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA and
Benjamin RS: Correlation of computed tomography and positron
emission tomography in patients with metastatic gastrointestinal
stromal tumor treated at a single institution with imatinib
mesylate: Proposal of new computed tomography response criteria. J
Clin Oncol. 25:1753–1759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palmerini E, Jones RL, Setola E, Picci P,
Marchesi E, Luksch R, Grignani G, Cesari M, Longhi A, Abate ME, et
al: Irinotecan and temozolomide in recurrent Ewing sarcoma: an
analysis in 51 adult and pediatric patients. Acta Oncol.
57:958–964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raciborska A, Bilska K, Drabko K, Chaber
R, Pogorzala M, Wyrobek E, Polczyńska K, Rogowska E,
Rodriguez-Galindo C and Wozniak W: Vincristine, irinotecan, and
temozolomide in patients with relapsed and refractory Ewing
sarcoma. Pediatr Blood Cancer. 60:1621–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kurucu N, Sari N and Ilhan IE: Irinotecan
and temozolamide treatment for relapsed Ewing sarcoma: A
single-center experience and review of the literature. Pediatr
Hematol Oncol. 32:50–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mir O, Brodowicz T, Italiano A, Wallet J,
Blay JY, Bertucci F, Chevreau C, Piperno-Neumann S, Bompas E, Salas
S, et al: Safety and efficacy of regorafenib in patients with
advanced soft tissue sarcoma (REGOSARC): A randomised,
double-blind, placebo-controlled, phase 2 trial. Lancet Oncol.
17:1732–1742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie L, Guo W, Wang Y, Yan T, Ji T and Xu
J: Apatinib for advanced sarcoma: Results from multiple
institutions' off-label use in China. BMC Cancer. 18:3962018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alcindor T: Response of refractory Ewing
sarcoma to pazopanib. Acta Oncol. 54:1063–1064. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamamoto Y, Nozawa M, Shimizu N, Minami T,
Yoshimura K and Uemura H: Pazopanib for recurrent extraosseous
Ewing's sarcoma of the retroperitoneum. Int J Urol. 21:1183–1184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Attia S, Okuno SH, Robinson SI, Webber NP,
Indelicato DJ, Jones RL, Bagaria SP, Jones RL, Sherman C, Kozak KR,
et al: Clinical activity of pazopanib in metastatic extraosseous
Ewing sarcoma. Rare Tumors. 7:59922015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mori Y, Kinoshita S, Kanamori T, Kataoka
H, Joh T, Iida S, Takemoto M, Kondo M, Kuroda J and Komatsu H: The
successful treatment of metastatic extraosseous Ewing sarcoma with
pazopanib. Intern Med. 57:2753–2757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Italiano A, Penel N, Toulmonde M, Bompas
E, Piperno-Neumann S, Pulido M, Entz-Werle N, Le Cesne A, Chevreau
CM, Duffaud F, et al: LBA67-Cabozantinib in Patients With Advanced
Osteosarcomas and Ewing sarcomas: A French Sarcoma Group (FSG)/US
National Cancer Institute phase II collaborative study. From ESMO
2018 congress, Proffered Paper session. https://cslide.ctimeetingtech.com/library/esmo/browse/search/2AuE#2Ea3302NBOctober
19–2018
|
|
39
|
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang
S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis
through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death
Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Canu B, Fioravanti A, Orlandi P, Di
Desidero T, Ali G, Fontanini G, Di Paolo A, Del Tacca M, Danesi R
and Bocci G: Irinotecan synergistically enhances the
antiproliferative and proapoptotic effects of axitinib in vitro and
improves its anticancer activity in vivo. Neoplasia. 13:217–229.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hashimoto K, Man S, Xu P, Cruz-Munoz W,
Tang T, Kumar R and Kerbel RS: Potent preclinical impact of
metronomic low-dose oral topotecan combined with the antiangiogenic
drug pazopanib for the treatment of ovarian cancer. Mol Cancer
Ther. 9:996–1006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mego M, Chovanec J, Vochyanova-Andrezalova
I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B,
Beniak J, Medvecova L, et al: Prevention of irinotecan induced
diarrhea by probiotics: A randomized double blind, placebo
controlled pilot study. Complement Ther Med. 23:356–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kimura K, Yamano T, Igeta M, Imada A,
Jihyung S, Babaya A, Hamanaka M, Kobayashi M, Tsukamoto K, Noda M,
et al: UGT1A1 polymorphisms in rectal cancer associated with the
efficacy and toxicity of preoperative chemoradiotherapy using
irinotecan. Cancer Sci. 109:3934–3942. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Wit M, Boers-Doets CB, Saettini A,
Vermeersch K, de Juan CR, Ouwerkerk J, Raynard SS, Bazin A and
Cremolini C: Prevention and management of adverse events related to
regorafenib. Support Care Cancer. 22:837–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Milling RV, Grimm D, Krüger M, Grosse J,
Kopp S, Bauer J, Infanger M and Wehland M: Pazopanib, cabozantinib,
and vandetanib in the treatment of progressive medullary thyroid
cancer with a special focus on the adverse effects on hypertension.
Int J Mol Sci. 19(pii): E32582018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sharma S, Abhyankar V, Burgess RE, Infante
J, Trowbridge RC, Tarazi J, Kim S, Tortorici M, Chen Y and Robles
RL: A phase I study of axitinib (AG-013736) in combination with
bevacizumab plus chemotherapy or chemotherapy alone in patients
with metastatic colorectal cancer and other solid tumors. Ann
Oncol. 21:297–304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bennouna J, Deslandres M, Senellart H, de
Labareyre C, Ruiz-Soto R, Wixon C, Botbyl J, Suttle AB and Delord
JP: A phase I open-label study of the safety, tolerability, and
pharmacokinetics of pazopanib in combination with irinotecan and
cetuximab for relapsed or refractory metastatic colorectal cancer.
Invest New Drugs. 33:138–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Garofalo C, Mancarella C, Grilli A, Manara
MC, Astolfi A, Marino MT, Conte A, Sigismund S, Carè A, Belfiore A,
et al: Identification of common and distinctive mechanisms of
resistance to different anti-IGF-IR agents in Ewing's sarcoma. Mol
Endocrinol. 26:1603–1616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Manara MC, Landuzzi L, Nanni P, Nicoletti
G, Zambelli D, Lollini PL, Nanni C, Hofmann F, Garcia-Echeverria C,
Picci P and Scotlandi K: Preclinical in vivo study of new
insulin-like growth factor-I receptor-specific inhibitor in Ewing's
sarcoma. Clin Cancer Res. 13:1322–1330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chan TA, Yarchoan M, Jaffee E, Swanton C,
Quezada SA, Stenzinger A and Peters S: Development of tumor
mutation burden as an immunotherapy biomarker: Utility for the
oncology clinic. Ann Oncol. 30:44–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tong M, Wang J, He W, Wang Y, Pan H, Li D
and Zhang H: Predictive biomarkers for tumor immune checkpoint
blockade. Cancer Manag Res. 10:4501–4507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Campbell BB, Light N, Fabrizio D, Zatzman
M, Fuligni F, de Borja R, Davidson S, Edwards M, Elvin JA, Hodel
KP, et al: comprehensive analysis of hypermutation in human cancer.
Cell. 171:1042–1056 e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Crompton BD, Stewart C, Taylor-Weiner A,
Alexe G, Kurek KC, Calicchio ML, Kiezun A, Carter SL, Shukla SA,
Mehta SS, et al: The genomic landscape of pediatric Ewing sarcoma.
Cancer Discov. 4:1326–1341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sand LG, Szuhai K and Hogendoorn PC:
Sequencing overview of ewing sarcoma: A journey across genomic,
epigenomic and transcriptomic landscapes. Int J Mol Sci.
16:16176–16215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aras M, Erdil TY, Dane F, Gungor S, Ones
T, Dede F, Inanir S and Turoglu HT: Comparison of WHO, RECIST 1.1,
EORTC, and PERCIST criteria in the evaluation of treatment response
in malignant solid tumors. Nucl Med Commun. 37:9–15.
2016.PubMed/NCBI
|
|
56
|
Schuetze SM, Wathen JK, Lucas DR, Choy E,
Samuels BL, Staddon AP, Ganjoo KN, von Mehren M, Chow WA, Loeb DM,
et al: SARC009: Phase 2 study of dasatinib in patients with
previously treated, high-grade, advanced sarcoma. Cancer.
122:868–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ronot M, Bouattour M, Wassermann J, Bruno
O, Dreyer C, Larroque B, Castera L, Vilgrain V, Belghiti J, Raymond
E and Faivre S: Alternative Response Criteria [Choi, European
association for the study of the liver, and modified Response
Evaluation Criteria in Solid Tumors (RECIST)] versus RECIST 1.1 in
patients with advanced hepatocellular carcinoma treated with
sorafenib. Oncologist. 19:394–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Beaty O III, Berg S, Blaney S,
Malogolowkin M, Krailo M, Knight R, Schaiquevich P, Stewart C, Chen
Z, Nelson M, et al: A phase II trial and pharmacokinetic study of
oxaliplatin in children with refractory solid tumors: A Children's
oncology group study. Pediatr Blood Cancer. 55:440–445. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Subbiah V, Sankhala KK, Ratan R, Garcia
ES, Boni V, Gill T, Villalobos VM, Chawla SP, Lardelli P, Siguero
M, et al: Efficacy and safety of lurbinectedin (PM1183) in Ewing
sarcoma: Final results from a phase 2 study. J Clin Oncol.
36:115192018. View Article : Google Scholar
|
|
60
|
Michelagnoli M, Whelan J and Forsyth S;
OTIS Trial Management Group, Site Investigators, : A phase II study
to determine the efficacy and safety of oral treosulfan in patients
with advanced pre-treated Ewing sarcoma ISRCTN11631773. Pediatr
Blood Cancer. 62:158–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hawkins DS, Bradfield S, Whitlock JA,
Krailo M, Franklin J, Blaney SM, Adamson PC and Reaman G: Topotecan
by 21-day continuous infusion in children with relapsed or
refractory solid tumors: A Children's oncology group study. Pediatr
Blood Cancer. 47:790–794. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fox E, Patel S, Wathen JK, Schuetze S,
Chawla S, Harmon D, Reinke D, Chugh R, Benjamin RS and Helman LJ:
Phase II study of sequential gemcitabine followed by docetaxel for
recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally
recurrent chondrosarcoma: Results of Sarcoma Alliance for Research
Through Collaboration Study 003. Oncologist. 17:3212012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jacobs S, Fox E, Krailo M, Hartley G,
Navid F, Wexler L, Blaney SM, Goodwin A, Goodspeed W, Balis FM, et
al: Phase II trial of ixabepilone administered daily for five days
in children and young adults with refractory solid tumors: A report
from the Children's oncology group. Clin Cancer Res. 16:750–754.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
DuBois SG, Krailo MD, Lessnick SL, Smith
R, Chen Z, Marina N, Grier HE and Stegmaier K; Children's Oncology
Group, : Phase II study of intermediate-dose cytarabine in patients
with relapsed or refractory Ewing sarcoma: A report from the
Children's oncology group. Pediatr Blood Cancer. 52:324–327. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Warwick AB, Malempati S, Krailo M, Melemed
A, Gorlick R, Ames MM, Safgren SL, Adamson PC and Blaney SM: Phase
2 trial of pemetrexed in children and adolescents with refractory
solid tumors: A Children's oncology group study. Pediatr Blood
Cancer. 60:237–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Grohar PJ, Glod J, Peer CJ, Sissung TM,
Arnaldez FI, Long L, Figg WD, Whitcomb P, Helman LJ and Widemann
BC: A phase I/II trial and pharmacokinetic study of mithramycin in
children and adults with refractory Ewing sarcoma and EWS-FLI1
fusion transcript. Cancer Chemother Pharmacol. 80:645–652. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chao J, Budd GT, Chu P, Frankel P, Garcia
D, Junqueira M, Loera S, Somlo G, Sato J and Chow WA: Phase II
clinical trial of imatinib mesylate in therapy of KIT and/or
PDGFRalpha-expressing Ewing sarcoma family of tumors and
desmoplastic small round cell tumors. Anticancer Res. 30:547–552.
2010.PubMed/NCBI
|
|
68
|
Bond M, Bernstein ML, Pappo A, Schultz KR,
Krailo M, Blaney SM and Adamson PC: A phase II study of imatinib
mesylate in children with refractory or relapsed solid tumors: A
Children's oncology group study. Pediatr Blood Cancer. 50:254–258.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Choy E, Butrynski JE, Harmon DC, Morgan
JA, George S, Wagner AJ, D'Adamo D, Cote GM, Flamand Y, Benes CH,
et al: Phase II study of olaparib in patients with refractory Ewing
sarcoma following failure of standard chemotherapy. BMC Cancer.
14:8132014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
University of Oxford: Phase II trial of
Linsitinib (anti-IGFR/IR) in patients with relapsed and/or
refractory Ewing Sarcoma. 2015.https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-000616-28/resultsNovember
2–2018
|
|
71
|
Children's Oncology Group, : Alisertib in
treating young patients with recurrent or refractory solid tumors
or leukemia. 2017.https://clinicaltrials.gov/ct2/show/results/NCT01154816November
2–2018
|
|
72
|
Juergens H, Daw NC, Geoerger B, Ferrari S,
Villarroel M, Aerts I, Whelan J, Dirksen U, Hixon ML, Yin D, et al:
Preliminary efficacy of the anti-insulin-like growth factor type 1
receptor antibody figitumumab in patients with refractory Ewing
sarcoma. J Clin Oncol. 29:4534–4540. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tap WD, Demetri G, Barnette P, Desai J,
Kavan P, Tozer R, Benedetto PW, Friberg G, Deng H, McCaffery I, et
al: Phase II study of ganitumab, a fully human anti-type-1
insulin-like growth factor receptor antibody, in patients with
metastatic Ewing family tumors or desmoplastic small round cell
tumors. J Clin Oncol. 30:1849–1856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pappo AS, Patel SR, Crowley J, Reinke DK,
Kuenkele KP, Chawla SP, Toner GC, Maki RG, Meyers PA, Chugh R, et
al: R1507, a monoclonal antibody to the insulin-like growth factor
1 receptor, in patients with recurrent or refractory Ewing sarcoma
family of tumors: Results of a phase II sarcoma alliance for
research through collaboration study. J Clin Oncol. 29:4541–4547.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Anderson PM, Bielack SS, Gorlick RG,
Skubitz K, Daw NC, Herzog CE, Monge OR, Lassaletta A, Boldrini E,
Pápai Z, et al: A phase II study of clinical activity of SCH 717454
(robatumumab) in patients with relapsed osteosarcoma and Ewing
sarcoma. Pediatr Blood Cancer. 63:1761–1770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Malempati S, Weigel B, Ingle AM, Ahern CH,
Carroll JM, Roberts CT, Reid JM, Schmechel S, Voss SD, Cho SY, et
al: Phase I/II trial and pharmacokinetic study of cixutumumab in
pediatric patients with refractory solid tumors and Ewing sarcoma:
A report from the Children's oncology group. J Clin Oncol.
30:256–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Schöffski P, Adkins D, Blay JY, Gil T,
Elias AD, Rutkowski P, Pennock GK, Youssoufian H, Gelderblom H,
Willey R and Grebennik DO: An open-label, phase 2 study evaluating
the efficacy and safety of the anti-IGF-1R antibody cixutumumab in
patients with previously treated advanced or metastatic soft-tissue
sarcoma or Ewing family of tumours. Eur J Cancer. 49:3219–3228.
2013. View Article : Google Scholar : PubMed/NCBI
|