Introduction
Lung cancer is among the most common malignant
tumors and has increasing rates of morbidity and mortality
worldwide (1). It is estimated that
~234,000 new lung cancer cases were diagnosed in 2018 in the United
States, and that ~154,000 people will succumb to lung cancer in
this year (2). The majority of
patients with lung cancer (85%) are diagnosed with non-small cell
lung cancer (NSCLC), and >80% of these patients have different
degrees of metastasis (3).
The most common sites for lung cancer metastasis are
the nervous system, bone, liver, respiratory system and adrenal
glands. Bone metastasis is the most common in patients with lung
adenocarcinoma (39%) (4). The
prognosis and survival rate of patients with advanced lung cancer
are very poor, and the survival rate is not satisfactory. The
median survival time of patients with stage IV NSCLC is 5 months
(5). Patients with stage IV NSCLC
with liver metastasis have the worst prognosis, <3 months
(5).
With advances in cancer treatment, molecular
targeted therapy and immunotherapy may provide alternatives to the
conventional surgery, radiotherapy and chemotherapy. However,
targeted therapy is not effective in people without epidermal
growth factor receptor (EGFR) mutations (6). The emergence of drug resistance in
tumor cells may lead to treatment failure in a patient population
that is suitable for targeted therapy (7). In addition, both targeted therapy and
immunotherapy may be economically unfeasible for patients with lung
cancer (8,9). Accordingly, cost-effective treatment
alternatives for patients with lung cancer are required.
The gold standard treatment for patients with NSCLC
with distant metastasis is a multidisciplinary comprehensive
treatment including chemoradiotherapy, immunotherapy, targeted
therapy and immunotherapy rather than surgery. Furák et al
(10) reported that the 5-year
survival rate of patients with NSCLC that did not undergo surgical
treatment was 5.8%. However, improvements in surgical techniques
have improved the 5-year survival rate and median survival of
patients with stage IV NSCLC (11–13).
Therefore, surgical treatment in patients with stage IV NSCLC may
be beneficial.
To date, there have been few large clinical
retrospective studies on the surgical treatment of patients with
stage IV NSCLC (11,12). Accordingly, the current study aimed
to investigate whether surgical treatment may improve the outcome
of patients with stage IV NSCLC, as well as to identify the factors
which influence the prognosis of patients. Relevant cases were
selected from the Surveillance, Epidemiology, and End Results
(SEER) (https://seer.cancer.gov/) program for
further analysis.
Materials and methods
Data collection
A total of 27,725 patients with stage IV NSCLC in
the United States diagnosed between January 1, 2010 and December
31, 2013 with distinct metastatic sites in bone, brain, lung and
liver and multiple metastases, that had received chemotherapy at
least once, were selected from the SEER program. Patients included
in this study were followed up between January 1, 2010 and November
31, 2016. Patients with incomplete or missing information were
excluded. The distant metastatic lesions included only bone, brain,
lung and liver. Other common sites, such as the pleura, adrenal
gland and gastrointestinal tract were not included. The inclusion
codes and criteria from the SEER database were are as follows: The
primary tumor type was coded as lung (063), the coding of tumor
pathological tissue classification was squamous cell neoplasms
(02), and adenomas and adenocarcinomas (05). The following patient
data were collected: i) Marital status; ii) ethnicity; iii) sex;
iv) age at diagnosis; v) survival time (months); vi) overall
survival (OS) and cancer-specific survival (CSS); vii) T stage;
viii) N stage; ix) surgery of the primary site; x) surgery of the
metastatic sites; xi) radiation therapy received; and xii) whether
there was bone (not including the bone marrow), brain (not
including the spinal cord or other parts of the central nervous
system), lung (not including the pleura or pleural fluid) or liver
metastasis. According to the SEER program definition, survival time
means the time between diagnosis and death or the last follow-up
time. OS is the time from the date of diagnosis to the death of any
cause. CSS is the time from the date of diagnosis to the date of
cancer-associated mortality. Surgery of the primary site describes
a surgical procedure that removes and/or destroys tissue of the
primary site performed as part of the therapy. Surgery of the
metastatic sites describes the surgical removal of distant lymph
node(s) or other tissue(s) or organ(s) beyond the primary site.
According to the definition of the 7th Edition of the American
Joint Committee on Cancer (AJCC) staging system (14), all the included patients with NSCLC
were stage IV patients
(T0-4N0-3M1), and the
histopathological types included adenocarcinoma and squamous cell
carcinoma. Patients with adenomas did not meet the above criteria
and therefore were excluded from this study.
Statistical analysis
The χ2 test was used to compare the
clinicopathological features of the patients included in the study
and determine whether there were differences between different
metastatic lesions. The Kaplan-Meier method was used to estimate
the survival function, and the differences were evaluated with the
log-rank test by pair comparison. Multivariate Cox regression
analysis was conducted to assess the association of specific
factors that impact overall survival (OS) and CSS. Additionally,
the 95% confidence interval (CI) for all hazard ratio (HRs)
estimates across all strata were calculated. P<0.05 was
considered to indicate a statistically significant difference. All
statistical operations were performed using SPSS software (version
22.0; IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 27,725 patients with NSCLC in the United
States diagnosed from January 1, 2010 to December 31, 2013 were
included in the current study. A total of 17,603 patients had one
metastatic lesion while 10,122 patients had ≥2 metastatic lesions.
The number of patients with only bone, brain, lung and liver
metastases was 5,989, 4,255, 5,717 and 1,642, respectively. The
number of patients with two, three and four metastatic lesions was
7,275, 2,389 and 458 respectively. The mean age of the patients was
67.51 years, with a median of 68 years (range, 13–102 years). A
total of 737, 1,761 and 146 patients received surgical intervention
for their primary lesion only, metastatic lesion only and both
primary and secondary lesions, respectively. However, the specific
surgical intervention for each patient was not recorded. The basic
information of the patients is presented in Table I.
 | Table I.Clinicopathological characteristics
of patients with metastatic non-small cell lung cancer. |
Table I.
Clinicopathological characteristics
of patients with metastatic non-small cell lung cancer.
| Variable | Bone
metastasis | Brain
metastasis | Liver
metastasis | Lung
metastasis | Multiple
metastasis | χ2
value | P-value |
|---|
| Age at
diagnosis |
|
≤60 | 1,483 | 1,493 | 335 | 1,101 | 3,087 | 5,842.255 | <0.001 |
|
>60 | 4,506 | 2,762 | 1,307 | 4,616 | 7,035 |
|
|
| Sex |
|
Female | 2,417 | 2,021 | 692 | 2,684 | 4,517 | 3,38.394 | <0.001 |
|
Male | 3,572 | 2,234 | 950 | 3,033 | 5,605 |
|
|
| Tumor type |
|
Squamous cell | 1,569 | 838 | 603 | 1,958 | 1,934 | 6,989.873 | <0.001 |
|
carcinomas |
|
Adenocarcinoma | 4,420 | 3,417 | 1,039 | 3,759 | 8,188 |
|
|
| Marital status |
|
Unmarried | 2,643 | 2,042 | 783 | 2,820 | 4,462 | 178.562 | <0.001 |
|
Married | 3,346 | 2,231 | 859 | 2,897 | 5,660 |
|
|
| Ethnicity |
|
Caucasian | 4,879 | 3,391 | 1,338 | 4,513 | 7,860 | 44,453.648 | <0.001 |
|
African-American | 716 | 573 | 214 | 759 | 1,284 |
|
|
|
Asian | 387 | 282 | 83 | 432 | 948 |
|
|
|
Australoid | 7 | 9 | 7 | 13 | 30 |
|
|
| T stage |
| 0 | 72 | 66 | 28 | 9 | 50 | 11,588.136 | <0.001 |
| 1 | 1,068 | 780 | 245 | 241 | 782 |
|
|
| 2 | 1,871 | 1,452 | 490 | 665 | 1,808 |
|
|
| 3 | 1,441 | 996 | 420 | 1,687 | 2,962 |
|
|
| 4 | 1,537 | 991 | 459 | 3,115 | 4,520 |
|
|
| N stage |
| 0 | 1,637 | 1,253 | 475 | 1,646 | 1,827 | 8,358.724 | <0.001 |
| 1 | 616 | 424 | 145 | 349 | 764 |
|
|
| 2 | 2,755 | 1,931 | 778 | 2,351 | 5,036 |
|
|
| 3 | 984 | 647 | 244 | 1,371 | 2,495 |
|
|
| Surgery of the
primary site |
| No | 5,847 | 4,006 | 1,596 | 5,403 | 9,990 | 76,277.022 | <0.001 |
| Wedge
resection | 75 | 84 | 27 | 200 | 105 |
|
|
|
Lobectomy | 59 | 156 | 18 | 102 | 24 |
|
|
|
Pneumonectomy | 8 | 9 | 1 | 12 | 3 |
|
|
| Surgery of the
metastases |
| No | 5,695 | 3,385 | 1,612 | 5,610 | 9,516 | 92,989.834 | P<0.001 |
|
Surgical procedure to other
regional sites | 25 | 16 | 6 | 27 | 26 |
|
|
|
Surgical procedure to distant
lymph node(s) | 24 | 26 | 3 | 20 | 51 |
|
|
|
Surgical procedure to distant
site | 241 | 827 | 21 | 56 | 519 |
|
|
|
Combination of all the
above | 4 | 1 | 0 | 4 | 10 |
|
|
| Radiation
therapy |
| No | 2,755 | 775 | 1,351 | 4,346 | 4,303 | 41,417.869 | P<0.001 |
| Beam
radiation | 3,226 | 3,476 | 289 | 1,362 | 5,801 |
|
|
|
Radioactive implants | 3 | 3 | 0 | 4 | 3 |
|
|
|
Radioisotopes | 2 | 0 | 1 | 1 | 6 |
|
|
|
Combination of 2 or | 3 | 1 | 1 | 4 | 9 |
|
|
Survival outcomes
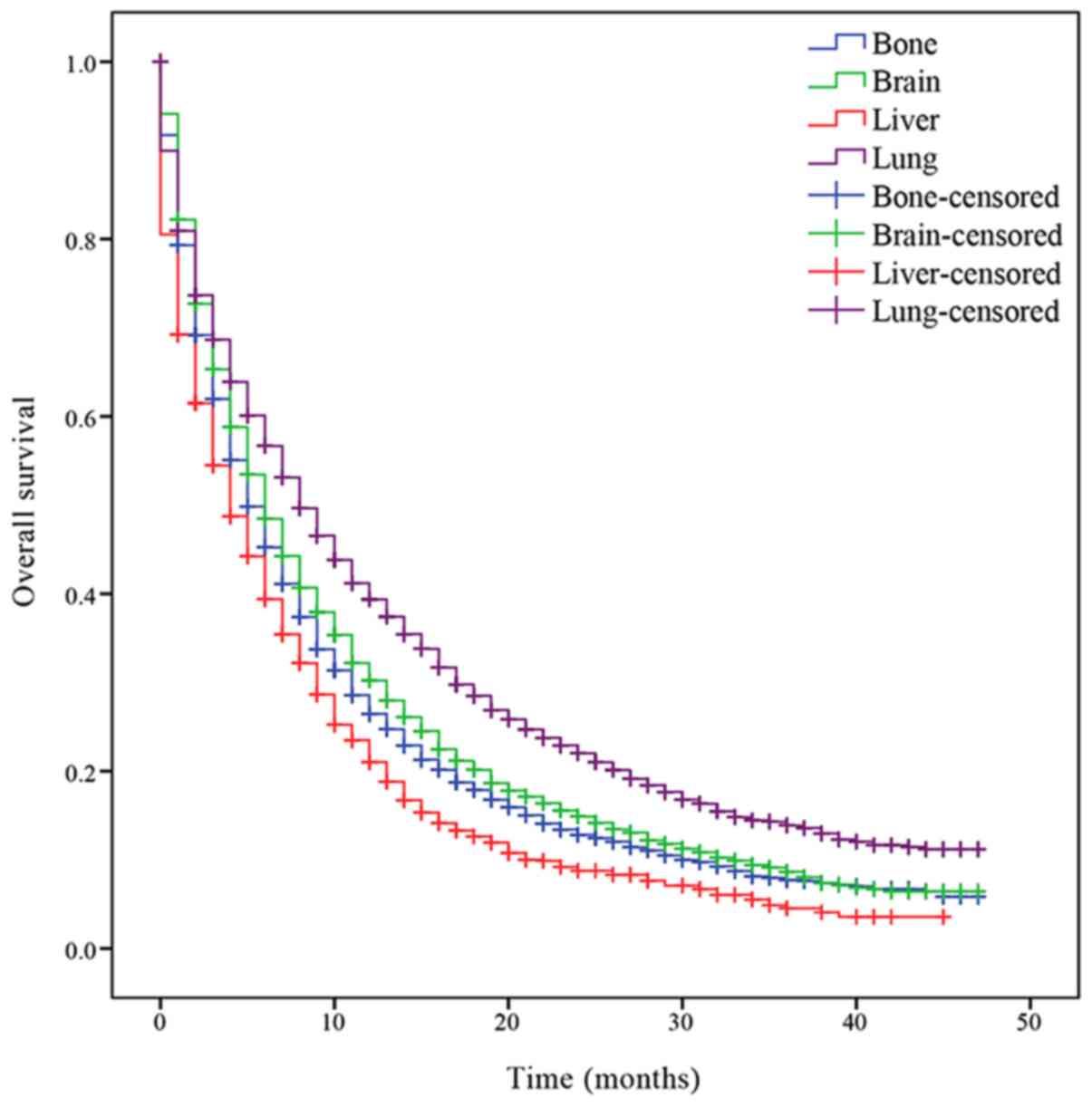
Survival analysis was performed to determine the OS
of patients with the different isolated metastatic lesions. The
median OS of patients with NSCLC with bone, brain, liver and lung
metastases was 5, 6, 4 and 8 months, respectively. Patients with
lung metastasis had an increased prognosis compared with the other
patients (P<0.001; Fig. 1), while
patients with liver metastasis had a decreased prognosis
(P<0.001; Fig. 1). Significant
differences of median OS time between patients with different organ
metastasis were indicated.
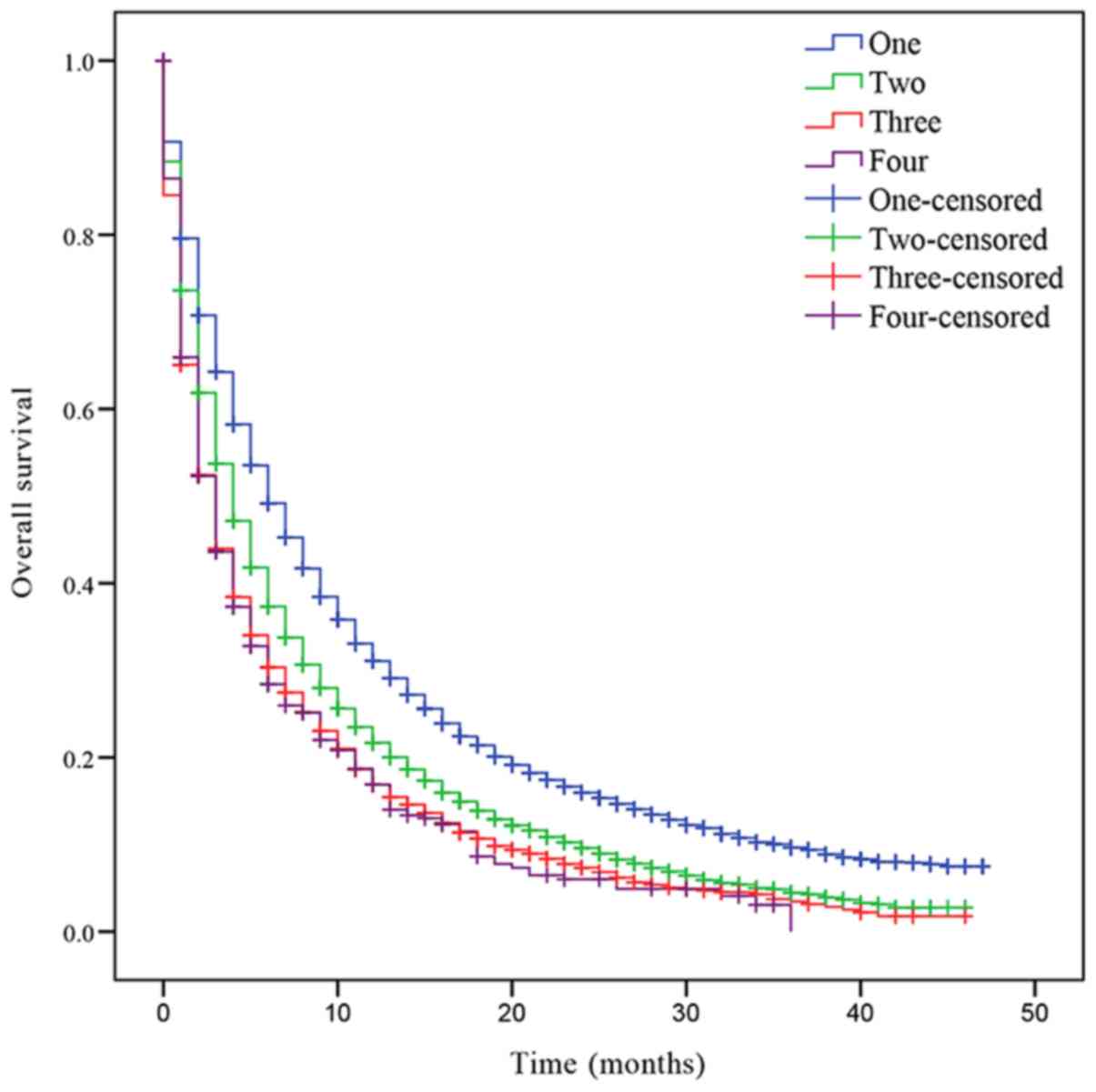
In addition, the OS was also assessed based on the
number of metastatic lesions. The median OS of patients with NSCLC
with one, two, three or four metastatic sites was 6, 4, 3 and 3
months, respectively. Patients with one metastatic lesion had a
significant increased prognosis compared with patients with >1
metastatic lesion (Fig. 2). Patients
with two metastases had a significantly improved prognosis compared
with patients with three and four metastases (two sites vs. three
sites, P<0.001; two sites vs. four sites, P<0.001; Fig. 2). However, there was no statistically
significant difference in OS between patients with three and four
metastatic lesions (P=0.721; Fig.
2).
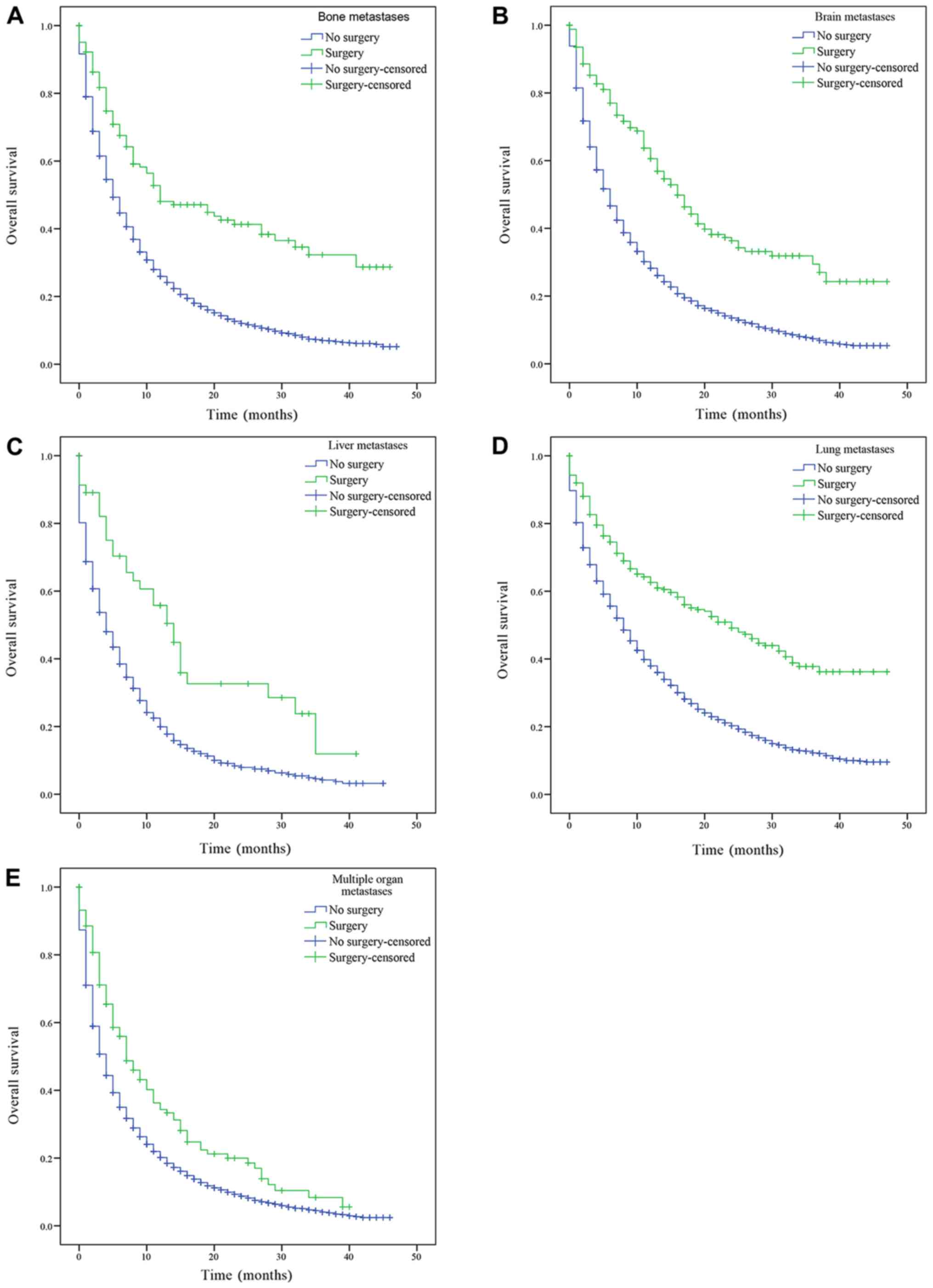
The patients with only bone, brain, liver, lung
metastasis and patients with multiple metastases were divided into
groups according to whether the primary or metastatic lesions were
treated by surgery, and their OS was subsequently estimated.
Patients with bone, brain, liver, lung metastasis and multiple
metastatic lesions had a significantly increased prognosis
following surgery on the primary lesions compared with patients who
had not received surgery (bone, brain, liver, lung metastasis and
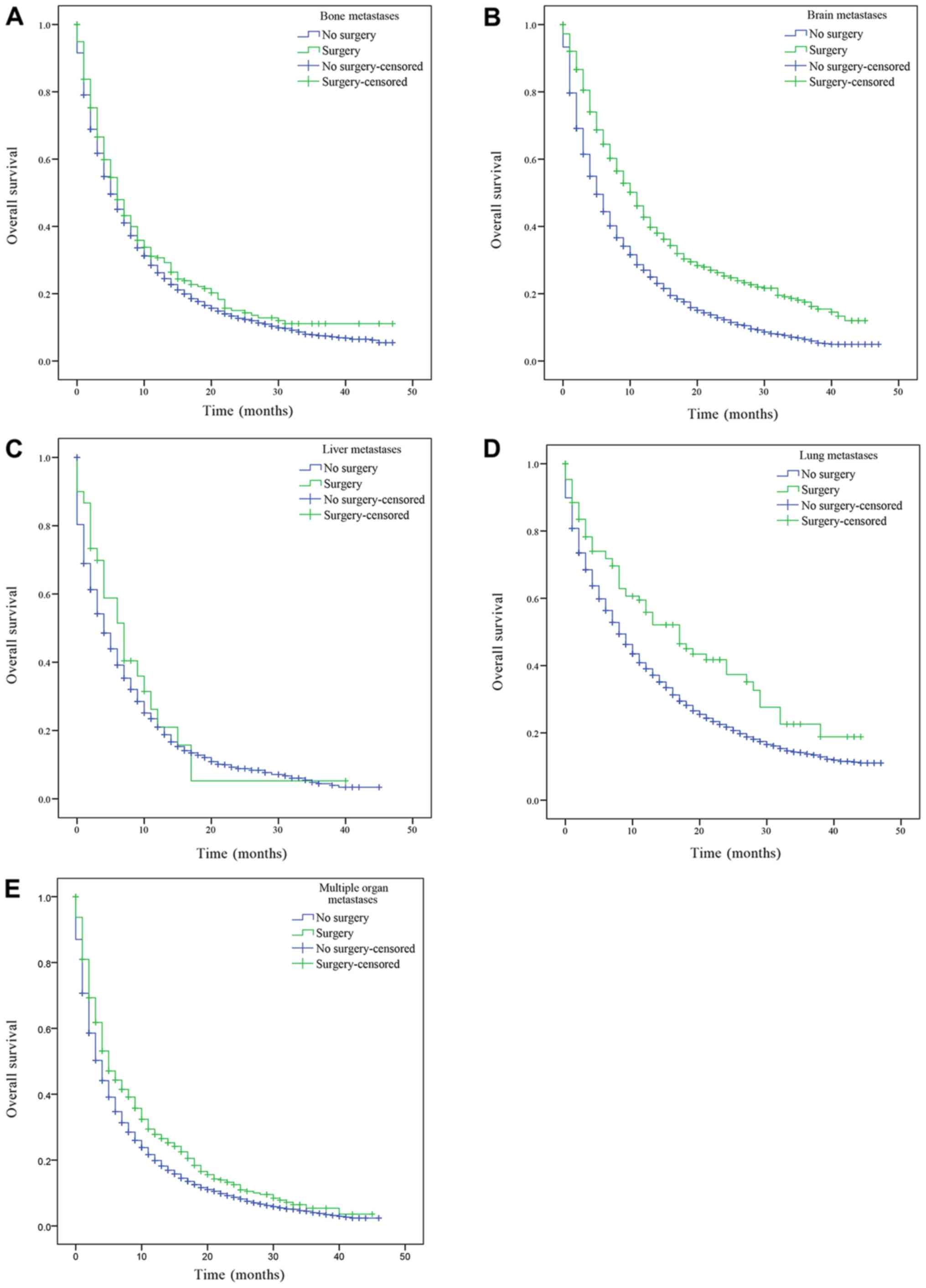
multiple organ metastases, P<0.001; Fig. 3). Similarly, patients with only bone,
brain, liver, lung metastasis and multiple metastatic lesions who
received surgery on distant lesions had an improved OS compared
with patients who had not received surgery (bone metastasis,
P=0.043; lung metastasis, P=0.001; brain and multiple organ
metastases, P<0.001; Fig. 4).
There was a statistically significant difference in OS between
patients who had underwent surgery and those who had not received
surgery. However, there was no statistically significant difference
in the median OS of patients with liver metastasis who had received
surgery compared with patients who had not (P=0.388; Fig. 4C).
Multivariate Cox proportional hazard models were
used to determine the prognostic factors of patients with NSCLC
with single and multiple organ metastases. The analysis in patients
with single organ metastases revealed that patients with the
following characteristics: i) Age (≤60); ii) sex (female); iii)
tumor type (adenocarcinoma); iv) marital status (married); v)
ethnicity (Asian); vi) N0 stage; vii) received surgery
of the primary tumor (wedge resection and lobectomy) and metastatic
lesion [distant tissue(s) or organ(s)]; and viii) received beam
radiation therapy had improved OS and CSS compared with other
patients (Table II). Using bone
metastasis as a reference, patients with brain and liver metastases
had a decreased OS (brain, HR, 1.162, 95% CI, 1.106–1.220; liver,
HR, 1.081, 95% CI, 1.015–1.151), while patients with lung
metastases had an improved OS (HR, 0.636; 95% CI, 0.607–0.667). The
analysis in overall metastatic patient cohort revealed that
patients with the following characteristics: i) Age (≤60); ii) sex
(female); iii) tumor type (adenocarcinoma); iv) marital status
(married); v) ethnicity (Asian); vi) N0 stage; vii) one
metastatic lesion; viii) received surgery of the primary (wedge
resection and lobectomy) and metastatic lesion (distant tissue(s)
or organ(s)); and ix) received beam radiation therapy had improved
OS and CSS (Table III). Using
single metastatic organ as a reference, patients with two, three
and four metastases had a decreased OS (two metastases, HR, 1.336,
95% CI, 1.294–1.379; three metastases, HR, 1.649, 95% CI,
1.571–1.732; four metastases, HR, 1.787, 95% CI, 1.613–1.980) and
CSS (two metastases, HR, 1.322, 95% CI, 1.275–1.372; three
metastases, HR, 1.628, 95% CI, 1.541–1.721; four metastases, HR,
1.805, 95% CI, 1.613–2.019).
 | Table II.Multivariate Cox regression analysis
for OS and CSS in patients with a single metastatic site. |
Table II.
Multivariate Cox regression analysis
for OS and CSS in patients with a single metastatic site.
|
| OS | CSS |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age at
diagnosis |
|
≤60 | 1.00
(reference) |
| 1.00
(reference) |
|
|
>60 | 1.017
(1.015–1.019) | <0.001 | 1.017
(1.015–1.019) | <0.001 |
| Sex |
|
Female | 1.00
(reference) |
| 1.00
(reference) |
|
|
Male | 1.291
(1.244–1.338) | <0.001 | 1.275
(1.222–1.330) | <0.001 |
| Tumor type |
|
Squamous cell | 1.00
(reference) |
| 1.00
(reference) |
|
|
carcinomas |
|
Adenocarcinoma | 0.930
(0.918–0.942) | <0.001 | 0.921
(0.907–0.935) | <0.001 |
| Marital status |
|
Unmarried | 1.00
(reference) |
| 1.00
(reference) |
|
|
Married | 0.841
(0.812–0.872) | <0.001 | 0.831
(0.797–0.866) | <0.001 |
| Ethnicity |
|
Caucasian | 1.00
(reference) |
| 1.00
(reference) |
|
|
African-American | 1.000
(0.949–1.053) | 0.991 | 1.022
(0.963–1.085) | 0.471 |
|
Asian | 0.674
(0.625–0.728) | <0.001 | 0.669
(0.614–0.730) | <0.001 |
|
Australoid | 0.717
(0.457–1.125) | 0.148 | 0.709
(0.427–1.178) | 0.185 |
| T stage |
| 0 | 1.00
(reference) |
| 1.00
(reference) |
|
| 1 | 0.781
(0.654–0.932) | 0.006 | 0.845
(0.689–1.035) | 0.104 |
| 2 | 0.888
(0.746–1.057) | 0.181 | 0.954
(0.781–1.165) | 0.642 |
| 3 | 1.008
(0.847–1.200) | 0.931 | 1.090
(0.893–1.332) | 0.396 |
| 4 | 1.042
(0.861–1.219) | 0.787 | 1.119
(0.917–1.366) | 0.268 |
| N stage |
| 0 | 1.00
(reference) |
| 1.00
(reference) |
|
| 1 | 1.134
(1.059–1.214) | <0.001 | 1.114
(1.029–1.207) | 0.008 |
| 2 | 1.276
(1.222–1.332) | <0.001 | 1.237
(1.176–1.302) | <0.001 |
| 3 | 1.340
(1.271–1.413) | <0.001 | 1.314
(1.235–1.398) | <0.001 |
| Surgery of the
primary |
| No | 1.00
(reference) |
| 1.00
(reference) |
|
| Wedge
resection | 0.718
(0.630–0.819) | <0.001 | 0.687
(0.586–0.805) | <0.001 |
|
Lobectomy | 0.361
(0.305–0.428) | <0.001 | 0.346
(0.281–0.426) | <0.001 |
|
Pneumonectomy | 0.791
(0.525–1.191) | 0.262 | 0.840
(0.535–1.319) | 0.449 |
| Surgery of the
metastases |
| No | 1.00
(reference) |
| 1.00
(reference) |
|
|
Surgical procedure to other
regional sites | 0.843
(0.634–1.120) | 0.237 | 0.786
(0.572–1.082) | 0.140 |
|
Surgical procedure to distant
lymph node(s) | 0.806
(0.605–1.075) | 0.142 | 0.796
(0.571–1.112) | 0.181 |
|
Surgical procedure to distant
site | 0.778
(0.720–0.841) | <0.001 | 0.768
(0.704–0.838) | <0.001 |
|
Combination of all the
above | 1.402
(0.629–3.126) | 0.409 | 1.419
(0.636–3.166) | 0.393 |
| Radiation
therapy |
| No | 1.00
(reference) |
| 1.00
(reference) |
|
| Beam
radiation | 0.808
(0.776–0.841) | <0.001 | 0.823
(0.786–0.863) | <0.001 |
|
Radioactive implants | 1.616
(0.769–3.396) | 0.205 | 1.315
(0.423–4.803) | 0.636 |
|
Radioisotopes | 1.317
(0.494–3.512) | 0.582 | 1.264
(0.407–3.923) | 0.685 |
|
Combination of 2 or 3
above | 0.958
(0.430–2.135) | 0.916 | 0.987
(0.410–2.377) | 0.976 |
| Metastatic
site |
| Bone
only | 1.00
(reference) |
| 1.00
(reference) |
|
| Brain
only | 1.162
(1.106–1.220) | <0.001 | 1.131
(1.070–1.197) | <0.001 |
| Liver
only | 1.081
(1.015–1.151) | 0.016 | 1.075
(0.999–1.157) | 0.053 |
| Lung
only | 0.636
(0.607–0.667) | <0.001 | 0.635
(0.600–0.671) | <0.001 |
 | Table III.Multivariate Cox regression analysis
for OS and CSS in overall metastatic patient cohort. |
Table III.
Multivariate Cox regression analysis
for OS and CSS in overall metastatic patient cohort.
|
| OS | CSS |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age at
diagnosis |
|
≤60 | 1.00
(reference) |
| 1.00
(reference) |
|
|
>60 | 1.269
(1.229–1.310) | <0.001 | 1.275
(1.231–1.321) | <0.001 |
| Sex |
|
Female | 1.00
(reference) |
| 1.00
(reference) |
|
|
Male | 1.287
(1.251–1.324) | <0.001 | 1.281
(1.240–1.323) | <0.001 |
| Tumor types |
|
Squamous cell carcinomas | 1.00
(reference) |
| 1.00
(reference) |
|
|
Adenocarcinoma | 0.930
(0.920–0.940) | <0.001 | 0.920
(0.909–0.931) | <0.001 |
| Marital status |
|
Unmarried | 1.00
(reference) |
| 1.00
(reference) |
|
|
Married | 0.834
(0.811–0.857) | <0.001 | 0.823
(0.797–0.849) | <0.001 |
| Ethnicity |
|
Caucasian | 1.00
(reference) |
| 1.00
(reference) |
|
|
African-American | 0.964
(0.825–1.004) | 0.074 | 0.981
(0.937–1.028) | 0.421 |
|
Asian | 0.658
(0.622–0.696) | <0.001 | 0.655
(0.616–0.697) | <0.001 |
|
Australoid | 0.745
(0.544–1.021) | 0.067 | 0.721
(0.507–1.026) | 0.069 |
| T stage |
| 0 | 1.00
(reference) |
| 1.00
(reference) |
|
| 1 | 0.756
(0.649–0.882) | <0.001 | 0.831
(0.697–0.990) | 0.039 |
| 2 | 0.850
(0.732–0.988) | 0.034 | 0.928
(0.781–1.102) | 0.393 |
| 3 | 0.874
(0.753–1.016) | 0.079 | 0.964
(0.812–1.145) | 0.675 |
| 4 | 0.842
(0.725–0.978) | 0.024 | 0.933
(0.787–1.107) | 0.430 |
| N stage |
| 0 | 1.00
(reference) |
| 1.00
(reference) |
|
| 1 | 1.129
(1.069–1.193) | <0.001 | 1.122
(1.052–1.196) | <0.001 |
| 2 | 1.231
(1.188–1.274) | <0.001 | 1.190
(1.143–1.240) | <0.001 |
| 3 | 1.194
(1.145–1.245) | <0.001 | 1.175
(1.120–1.234) | <0.001 |
| Surgery of the
primary |
| No | 1.00
(reference) |
| 1.00
(reference) |
|
| Wedge
resection | 0.670
(0.598–0.750) | <0.001 | 0.670
(0.587–0.765) | <0.001 |
|
Lobectomy | 0.362
(0.308–0.424) | <0.001 | 0.349
(0.287–0.425) | <0.001 |
|
Pneumonectomy | 0.720
(0.486–1.066) | 0.101 | 0.774
(0.504–1.188) | 0.242 |
| Surgery of the
metastases |
| No | 1.00
(reference) |
| 1.00
(reference) |
|
|
Surgical procedure to other
regional sites | 0.857
(0.676–1.086) | 0.202 | 0.836
(0.638–1.096) | 0.194 |
|
Surgical procedure to distant
lymph node(s) | 0.883
(0.713–1.093) | 0.252 | 0.875
(0.686–1.118) | 0.286 |
|
Surgical procedure to distant
site | 0.852
(0.802–0.906) | <0.001 | 0.829
(0.775–0.888) | <0.001 |
|
Combination of all the
above | 1.021
(0.604–1.726) | 0.938 | 0.984
(0.570–1.697) | 0.952 |
| Radiation
therapy |
| No | 1.00
(reference) |
| 1.00
(reference) |
|
| Beam
radiation | 0.881
(0.856–0.906) | <0.001 | 0.892
(0.864–0.921) | <0.001 |
|
Radioactive implants | 1.498
(0.805–2.786) | 0.202 | 1.302
(0.585–2.901) | 0.518 |
|
Radioisotopes | 0.646
(0.308–1.335) | 0.248 | 0.613
(0.255–1.475) | 0.275 |
|
Combination of 2 or 3
above | 0.821
(0.454–1.484) | 0.513 | 1.051
(0.565–1.958) | 0.874 |
| Number of
metastatic sites |
|
Single | 1.00
(reference) |
| 1.00
(reference) |
|
|
Double | 1.336
(1.294–1.379) | <0.001 | 1.322
(1.275–1.372) | <0.001 |
|
Triple | 1.649
(1.571–1.732) | <0.001 | 1.628
(1.541–1.721) | <0.001 |
|
Four | 1.787
(1.613–1.980) | <0.001 | 1.805
(1.613–2.019) | <0.001 |
Discussion
Improvements in lung cancer diagnosis and treatment
have increased the 5-year survival rate and median survival time of
patients with stage IV NSCLC (15).
In recent years, an increased understanding of the genetic changes
involved in lung cancer has led to molecular targeted therapy
(6). Additionally, surgical
techniques are also rapidly evolving (16). The emergence of radiofrequency
ablation (17) and endoscopy
(18) has contributed to the
improved safety of surgical procedures. Individualized treatment
plans may reduce the occurrence of adverse events and improve the
quality of life of the patients (19).
The current study involved a retrospective analysis
of a large population of patients with stage IV NSCLC selected from
the SEER program. Compared with patients with other organ
metastases, patients with lung metastasis had the longest OS, and
patients with single organ metastasis had an increased OS compared
with patients with multiple organ metastases. This suggested that
the type and number of metastatic organs may affect the prognosis
of patients with stage IV NSCLC. This is similar to previously
published studies investigating bladder and colorectal cancer
(20,21). Furthermore, the current study
established that surgical resection of the primary and metastatic
organs may significantly improve the prognosis of patients with
stage IV NSCLC.
The current study demonstrated that patients with
only lung metastases had the best prognosis, patients with only
brain metastases had a slightly improved prognosis compared with
patients with only bone metastases, while those with liver
metastases had the worst prognosis. Patients with only one
metastasis had an improved prognosis compared with patients with
multiple metastases. Previous studies have revealed similar
results; patients with NSCLC and SCLC with liver metastasis and
multiple metastases have the worst prognosis (22–25).
Similar results were obtained using the AJCC staging system, where
the number of metastatic organs had an effect on the prognosis of
patients (26). Notably, the effect
of the number of metastases is not same for different types of
cancer, the OS of patients with pancreatic cancer is not affected
by either single or multiple organ metastases (27). Pancreatic cancer is characterized by
rapid growth, abundant pancreatic blood and lymphatic vessels, and
incomplete pancreatic capsule. Therefore, the time of distant
metastasis is relatively early, so whether there is distant
metastasis or not, has little impact on OS (28).
Multivariate Cox regression analysis revealed that
the prognosis of patients who underwent surgery for primary and
metastatic lesions was better compared with patients who did not
undergo the above. Surgical treatment remains the main approach
used for the treatment of the majority of malignant tumors
(16). Previously published studies
revealed that certain patients with advanced NSCLC with unilateral
contralateral lung metastasis, single brain, bone or adrenal
metastasis may be treated surgically (29–31). For
patients with NSCLC with isolated metastases and resectable
pulmonary lesions, resection of the metastatic organs may also be
considered. However, how isolated liver metastases should be
removed remains unclear (32–34).
Previous studies demonstrated that surgery serves an important role
in the treatment of liver metastasis of neuroendocrine carcinoma
and colorectal cancer, but not in lung cancer (32,35).
With advances in liver resection and the continuous improvement of
surgical safety, previous case reports described surgical resection
of liver metastatic carcinoma with satisfactory results (33,34).
The benefit of surgical treatment on the prognosis
of patients with advanced lung cancer remains controversial
(29–32). A previous study based on SEER program
analysis suggested that no further surgical treatment is
recommended for patients with advanced lung cancer (36). However, additional studies do not
concur with this recommendation (37). Patients with stage IV NSCLC who
received pneumonectomy and thoracic wall enlargement resection had
an improved quality of life and 5-year survival (38). However, this is contrary to what was
observed in the current study. Results from a previous study
suggested that the long-term survival rate of patients is related
to the degree of tumor infiltration into the chest wall, and thus
the scope of resection should be determined according to the degree
of infiltration (39). Using the
SEER program, previous studies have revealed that the size of the
lung cancer lesions should guide the choice of surgical
intervention and expanding the scope of surgical resection will not
improve prognosis (40). A previous
study revealed that lymph node dissection for distant metastatic
lesions is necessary to improve the prognosis (41). Taken together, the results from the
aforementioned studies suggest that it is important to identify
specific patients who may benefit from surgical procedures.
Radiotherapy is widely used for patients with
advanced lung cancer (42). A
previous study revealed that surgery following radiotherapy may be
beneficial to patients (43). The
most commonly employed method of radiotherapy is beam radiation
(44), which was consistent with the
results obtained in the current study. However, previous studies
reported that radioactive implants and radioisotopes may offer
promising results for patients with advanced NSCLC (45,46).
Multivariate Cox regression analysis revealed that
age, sex, marital status, ethnicity, N stage and tumor type
affected the prognosis of patients with NSCLC in the current study.
Patients >60 years had an improved prognosis compared with other
patients. Toffart et al (47)
revealed that patients with NSCLC >63 years had significantly
decreased OS compared with other younger patients using a
multivariate cox analysis (HR=1.63; 95% CI: 1.013–2.63; P=0.04).
The current study demonstrated that the prognosis of female
patients was improved compared with male patient. This may be
attributed to different hormone and corresponding receptor
expression levels (48). In terms of
marital status, previous large epidemiological studies revealed
that marriage benefits patients with less aggressive cancer
(49,50), which is consistent with the results
obtained in the current study. The effect of ethnicity on the
prognosis of patients with NSCLC patients remains controversial
(51–54). A previous study revealed that
African-Americans with lung cancer had a decreased 5-year survival
rate compared with Caucasians (51).
Similar survival rates for African-Americans and Caucasians have
been reported for patients with lung cancer (52,53).
Tannenbaum et al (54)
reported that Asian patients with NSCLC had significantly increased
survival rates compared with Caucasian patients, which is
consistent with the results obtained in the current study.
The results obtained in the current study suggested
that there was no statistical difference in the prognosis of
patients with different T stages. This is not in accordance with
the AJCC staging system. However, the patients selected in the
current study all had stage IV NSCLC, according to the 7 Edition of
the AJCC staging system with only four sites of metastases
identified, and may not conform with the principles of the staging
system, due to a limited representative sample. The current study
revealed that the N stage influenced the prognosis of patients with
stage IV NSCLC. This was consistent with a previous study which
suggested that lymph node metastasis is an adverse prognostic
factor for the surgical treatment of patients with advanced NSCLC
(55). The aforementioned study
recommended that patients with N0 stage should be
eligible for surgical treatment and that surgery for patients with
extensive lymph node metastases may not be beneficial (55). There are few studies investigating
the prognosis of patients with stage IV NSCLC with adenocarcinoma
and squamous carcinoma (56,57). A retrospective study of 148 Chinese
patients with NSCLC revealed that non-lung adenocarcinoma was a
prognostic risk factor in patients with NSCLC (57), consistent with the results obtained
in the current study.
The present study had certain limitations. Firstly,
due to the retrospective nature of the study, confounding factors,
such as smoking history and age, were not easily excluded.
Secondly, the specific chemotherapy regimens and radiation doses
were not detailed in the SEER program, and these may have had an
impact on the prognosis of the patients (58). Thirdly, the SEER program did not
include data on whether the patients were treated with tyrosine
kinase inhibitors, due to EGFR mutations being more prevalent in
non-smoking, female, Asian patients (59). The OS would be affected if they were
treated with tyrosine kinase inhibitors (6), influencing our conclusions. Finally,
more distal metastases, such as in the adrenal gland and
gastrointestinal tract, cannot be included without relevant data,
and at the same time, the sequence of metastatic lesions cannot be
determined. The results obtained in the current study require
further examination by future well-designed studies to validate
this study's results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Surveillance, Epidemiology, and
End Results (SEER) repository (https://seer.cancer.gov/).
Authors' contributions
YL designed the study, work that led to the
submission, acquired data and played an important role in
interpreting the results. XF and XW analyzed the data and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The SEER database does not include any human or
demographic identifying information, and the data used for analysis
were de-identified. Therefore, ethics approval and formal informed
consent to participate was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noone AM, Howlader N, Krapcho M, Miller D,
Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, et al:
SEER Cancer Statistics Review, 1975–2015, National Cancer
Institute. Bethesda, MD: 2018, https://seer.cancer.gov/archive/csr/1975_2015/September
10–2018
|
|
4
|
Duan L, Pang HL, Chen WJ, Shen WW, Cao PP,
Wang SM, Liu LL and Zhang HL: The role of GDF15 in bone metastasis
of lung adenocarcinoma cells. Oncol Rep. 41:2379–2388.
2019.PubMed/NCBI
|
|
5
|
Riihimäki M, Hemminki A, Fallah M, Thomsen
H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and
survival in lung cancer. Lung Cancer. 86:78–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Xie S and He B: Effect of EGFR
gene polymorphism on efficacy of chemotherapy combined with
targeted therapy for non-small cell lung cancer in Chinese
patients. Am J Cancer Res. 9:619–627. 2019.PubMed/NCBI
|
|
7
|
Sosa Iglesias V, Giuranno L, Dubois LJ,
Theys J and Vooijs M: Drug resistance in non-small cell lung
cancer: A potential for NOTCH Targeting? Front Oncol. 8:2672018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kogure Y: Cost effectiveness and health
economics of immune checkpoint inhibitors for non-small cell lung
cancer. Gan To Kagaku Ryoho. 45:781–784. 2018.(In Japanese).
PubMed/NCBI
|
|
9
|
Skinner KE, Fernandes AW, Walker MS
Pavilack M and VanderWalde A: Healthcare costs in patients with
advanced non-small cell lung cancer and disease progression during
targeted therapy: A real-world observational study. J Med Econ.
21:192–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furák J, Troján I, Szöke T, Agócs L,
Csekeö A, Kas J, Svastics E, Eller J and Tiszlavicz L: Lung cancer
and its operable brain metastasis: Survival rate and staging
problems. Ann Thorac Surg. 79:241–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanagiri T, Takenaka M, Oka S, Shigematsu
Y, Nagata Y, Shimokawa H, Uramoto H and Tanaka F: Results of a
surgical resection for patients with stage IV non-small-cell lung
cancer. Clin Lung Cancer. 13:220–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Congedo MT, Cesario A, Lococo F, De Waure
C, Apolone G, Meacci E, Cavuto S and Granone P: Surgery for
oligometastatic non-small cell lung cancer: Long-term results from
a single center experience. J Thorac Cardiovasc Surg. 144:444–452.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tieqin L, Hongxu L, Yu L, Shun X and
Chunlu Y: Influencing factors of survival rate on 44 cases of
postoperative stage IV non-small-celllung cancer. Chin J Ciinicians
(Eiectronic Ed). 7:605–608. 2013.
|
|
14
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. (7th).
Springer. (New York, NY). 2009.
|
|
15
|
Delea T, Langer C, McKiernan J, Liss M,
Edelsberg J, Brandman J, Sung J, Raut M and Oster G: The cost of
treatment of skeletal-related events in patients with bone
metastases from lung cancer. Oncology. 67:390–396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hofferberth SC, Grinstaff MW and Colson
YL: Nanotechnology applications in thoracic surgery. Eur J
Cardiothorac Surg. 50:6–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaughn C, Mychaskiw G II and Sewell P:
Massive hemorrhage during radiofrequency ablation of a pulmonary
neoplasm. Anesth Analg. 94:1149–1151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tutar N, Yurci A, Güneş I, Gülmez İ,
Gürsoy Ş, Önal Ö and Canöz Ö: The role of endobronchial and
endoscopic ultrasound guided fine needle aspiration for mediastinal
nodal staging of non-small-cell lung cancer. Tuberk Toraks.
66:85–92. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reck M and Rabe KF: Precision diagnosis
and treatment for advanced non-small-cell lung cancer. N Engl J
Med. 377:849–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong F, Shen Y, Gao F, Xu T, Wang X, Zhang
X, Zhong S, Zhang M, Chen S and Shen Z: Prognostic value of
site-specific metastases and therapeutic roles of surgery for
patients with metastatic bladder cancer: A population-based study.
Cancer Manag Res. 9:611–626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo D, Liu Q, Yu W, Ma Y, Zhu J, Lian P,
Cai S, Li Q and Li X: Prognostic value of distant metastasis sites
and surgery in stage IV colorectal cancer: A population-based
study. Int J Colorectal Dis. 33:1241–1249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tamura T, Kurishima K, Nakazawa K,
Kagohashi K, Ishikawa H, Satoh H and Hizawa N: Specific organ
metastases and survival in metastatic non-small-cell lung cancer.
Mol Clin Oncol. 3:217–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakazawa K, Kurishima K, Tamura T,
Kagohashi K, Ishikawa H, Satoh H and Hizawa N: Specific organ
metastases and survival in small cell lung cancer. Oncol Lett.
4:617–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Zhang Y, Sun X, Gusdon AM, Song N,
Chen L, Jiang G and Huang Y: The prognostic value of multiorgan
metastases in patients with non-small cell lung cancer and its
variants: A SEER-based study. J Cancer Res Clin Oncol.
144:1835–1842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gibson AJW, Li H, D'Silva A, Tudor RA,
Elegbede AA, Otsuka SM, Bebb DG and Cheung WY: Impact of number
versus location of metastases on survival in stage IV M1b non-small
cell lung cancer. Med Oncol. 35:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rami-Porta R, Bolejack V, Crowley J, Ball
D, Kim J, Lyons G, Rice T, Suzuki K, Thomas CF Jr, Travis WD, et
al: The IASLC lung cancer staging project: Proposals for the
revisions of the T descriptors in the forthcoming eighth edition of
the TNM classification for lung cancer. J Thorac Oncol.
10:990–1003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oweira H, Petrausch U, Helbling D, Schmidt
J, Mannhart M, Mehrabi A, Schöb O, Giryes A, Decker M and
Abdel-Rahman O: Prognostic value of site-specific metastases in
pancreatic adenocarcinoma: A Surveillance Epidemiology and End
Results database analysis. World J Gastroenterol. 23:1872–1880.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Y, Sun Y, Yu J, Ding C, Wang Z, Wang
C, Wang D, Wang C, Wang Z, Wang M, et al: China experts consensus
on the diagnosis and treatment of advanced stage primary lung
cancer (2016 version). Asia Pac J Clin Oncol. 13:87–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi Y, Sun Y, Yu J, Ding C, Ma Z, Wang Z,
Wang D, Wang Z, Wang M, Wang Y, et al: China experts consensus on
the diagnosis and treatment of brain metastases of lung cancer
(2017 version). Zhongguo Fei Ai Za Zhi. 20:1–13. 2017.(In Chinese).
PubMed/NCBI
|
|
31
|
Sun Y, Guan Z, Liao M, Yu X, Wang C, Wang
J, Niu X, Shi Y, Zhi X, Liu Y, et al: Expert consensus on the
diagnosis and treatment of bone metastasis in lung cancer (2014
version). Zhongguo Fei Ai Za Zhi. 17:57–72. 2014.(In Chinese).
PubMed/NCBI
|
|
32
|
Schmelzle M, Eisenberger CF, Am Esch JS
II, Matthaei H, Krausch M and Knoefel WT: Non-colorectal,
non-neuroendocrine, and non-sarcoma metastases of the liver:
Resection as a promising tool in the palliative management.
Langenbecks Arch Surg. 395:227–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ileana E, Greillier L, Moutardier V and
Barlesi F: Surgical resection of liver non-small cell lung cancer
metastasis: A dual weapon? Lung Cancer. 70:221–222. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di Carlo I, Grasso G, Patane' D, Russello
D and Latteri F: Liver metastases from lung cancer: Is surgical
resection justified? Ann Thorac Surg. 76:291–293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reddy SK, Barbas AS, Marroquin CE, Morse
MA, Kuo PC and Clary BM: Resection of noncolorectal
nonneuroendocrine liver metastases: A comparative analysis. J Am
Coll Surg. 204:372–382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abdel-Rahman O: Outcomes of surgery as
part of the management of metastatic Non-small-cell lung cancer: A
Surveillance, epidemiology and End results database analysis.
Cancer Invest. 36:238–245. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu K, Zheng D, Xu G, Du Z and Wu S: Local
thoracic therapy improve prognosis for stage IV non-small cell lung
cancer patients combined with chemotherapy: A Surveillance,
Epidemiology, and End results database analysis. PLoS One.
12:e01873502017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Setzer M, Robinson LA and Vrionis FD:
Management of locally advanced pancoast (superior sulcus) tumors
with spine involvement. Cancer Control. 21:158–167. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rui M, Pengqing Y and Chang C: Progress in
resection and reconstruction of chest wall in non-small lung
cancer. Chin J Clin Thorac Cardiovasc Surg. 22:685–690. 2015.
|
|
40
|
Dai C, Shen J, Ren Y, Zhong S, Zheng H, He
J, Xie D, Fei K, Liang W, Jiang G, et al: Choice of surgical
procedure for patients with Non-small-cell lung cancer ≤1 cm or
>1 to 2 cm among lobectomy, segmentectomy, and wedge resection:
A population-based study. J Clin Oncol. 34:3175–3182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ludwig MS, Goodman M, Miller DL and
Johnstone PA: Postoperative survival and the number of lymph nodes
sampled during resection of node-negative non-small cell lung
cancer. Chest. 128:1545–1550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang R, Li P, Li Q, Qiao Y, Xu T, Ruan P,
Song Q and Fu Z: Radiotherapy improves the survival of patients
with stage IV NSCLC: A propensity score matched analysis of the
SEER database. Cancer Med. 7:5015–5026. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Herskovic A, Chitti B, Christos P,
Wernicke AG and Parashar B: Addition of surgery after radiation
significantly improves survival in stage IIIB Non-small cell lung
cancer: A population-based analysis. World J Surg. 41:758–762.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Verma V, Rwigema JM, Adeberg S and Simone
CB II: Enrollment of elderly patients with locally advanced
non-small cell lung cancer in multi-institutional trials of proton
beam radiation therapy. Clin Lung Cancer. 18:441–443. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He M, Li S, Chen Y, Ouyang M, Chen P and
Zhang J: 131I-chTNT injection to relieve tracheal obstruction in
advanced NSCLC patient. Technol Health Care. 24 (Suppl
2):S513–S519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hendriks LE, Hermans BC, van den
Beuken-van Everdingen MH, Hochstenbag MM and Dingemans AM: Effect
of Bisphosphonates, Denosumab, and radioisotopes on bone pain and
quality of life in patients with non-small cell lung cancer and
bone metastases: A systematic review. J Thorac Oncol. 11:155–173.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Toffart AC, Duruisseaux M, Brichon PY,
Pirvu A, Villa J, Selek L, Guillem P, Dumas I, Ferrer L, Levra MG
and Moro-Sibilot D: Operation and chemotherapy: Prognostic factors
for lung cancer with one synchronous metastasis. Ann Thorac Surg.
105:957–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cheng TD, Darke AK, Redman MW, Zirpoli GR,
Davis W, Payne Ondracek R, Bshara W, Omilian AR, Kratzke R, Reid
ME, et al: Smoking, Sex, and Non-small cell lung cancer: Steroid
hormone receptors in tumor tissue (S0424). J Natl Cancer Inst.
110:734–742. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Merrill RM and Johnson E: Benefits of
marriage on relative and conditional relative cancer survival
differ between males and females in the USA. J Cancer Surviv.
11:578–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu Y, Ai Z and Xu G: Marital status and
survival in patients with non-small cell lung cancer: An analysis
of 70006 patients in the SEER database. Oncotarget.
8:103518–103534. 2017.PubMed/NCBI
|
|
51
|
Richards TB, Henley SJ, Puckett MC, Weir
HK, Huang B, Tucker TC and Allemani C: Lung cancer survival in the
United States by race and stage (2001–2009): Findings from the
CONCORD-2 study. Cancer. 123:5079–5099. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Williams CD, Salama JK, Moghanaki D, Karas
TZ and Kelley MJ: Impact of race on treatment and survival among
U.S. Veterans with early-stage lung cancer. J Thorac Oncol.
11:1672–1681. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Videtic GM, Reddy CA, Chao ST, Rice TW,
Adelstein DJ, Barnett GH, Mekhail TM, Vogelbaum MA and Suh JH:
Gender, race, and survival: A study in non-small-cell lung cancer
brain metastases patients utilizing the radiation therapy oncology
group recursive partitioning analysis classification. Int J Radiat
Oncol Biol Phys. 75:1141–1147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tannenbaum SL, Koru-Sengul T, Zhao W, Miao
F and Byrne MM: Survival disparities in non-small cell lung cancer
by race, ethnicity, and socioeconomic status. Cancer J. 20:237–245.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yoshinaga Y, Enatsu S, Iwasaki A and
Shirakusa T: Surgical treatment for primary non-small cell lung
cancer with synchronous brain metastases. Kyobu Geka. 59:41–45.
2016.(In Japanese).
|
|
56
|
Ramalingam S, Dinan MA and Crawford J:
Survival comparison in patients with Stage IV lung cancer in
Academic versus Community centers in the United States. J Thorac
Oncol. 13:1842–1850. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xiaolan J and Jia W: Prognostic factors
for bone metastasis of patients with non-small-cell lung cancer.
Shandong Med. 56:88–90. 2016.
|
|
58
|
Masters GA, Temin S, Azzoli CG, Giaccone
G, Baker S Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller
JH, et al: Systemic therapy for stage IV non-small-cell lung
cancer: American Society of Clinical Oncology Clinical Practice
Guideline Update. J Clin Oncol. 33:3488–3515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR,
Threapleton D, Yang ZY, Mao C and Tang JL: The prevalence of EGFR
mutation in patients with non-small cell lung cancer: A systematic
review and meta-analysis. Oncotarget. 7:78985–78993.
2016.PubMed/NCBI
|