Introduction
According to the American Cancer Society (ACS),
breast cancer (BC) is the most common malignancy and the second
most common cause of cancer-associated mortality, in the USA
(1). The ACS have estimated a total
of 268,670 newly diagnosed cases of invasive BC and 41,400
BC-associated mortalities among females in the USA in 2018
(1). Current treatments, including
surgery, endocrine therapy, chemotherapy and radiation, have
greatly improved the survival of females diagnosed with BC
(2); however, a cure remains to be
identified. Understanding the underlying mechanisms that contribute
to the progression of this disease is important for developing
novel targets.
The Ephrin (Eph) family is the largest family of
tyrosine kinase receptors (TKRs). Eph proteins are subdivided into
two categories, A and B, according to their sequence homology and
affinity for corresponding transmembrane ephrin ligands (3,4). EphB
TKRs are considered as candidates for novel anticancer therapies
due to their participation in both physiological and pathological
processes (5–7). It has been reported that metastatic BC
cell motility may be dynamically guided by the crosstalk between
epidermal growth factor-mediated chemotaxis and contact inhibition
of locomotion, mediated in part by EphB receptors (8). This suggests that EphB receptors may
serve a role in the recurrence of human BC (8). Other preclinical and laboratory studies
have also revealed the function of EphB TKRs in tumor growth,
invasion, metastasis and angiogenesis (9), including in BC (10). In the human genome there are five
distinct EphB receptors, which aberrantly bind three
membrane-anchored ephrin-B ligands (11–13).
This aberrant interaction between ligands and receptors results in
pleiotropic functions and bidirectional signaling, which makes the
role of EphB receptors in cancer complex (9). Additionally, the activities of EphB
receptors in cancer remain controversial, with evidence supporting
both tumor-promoting and tumor-inhibiting functions (9). Nevertheless, EphB receptors are
promising candidates for novel therapeutic targets in cancer.
The present study aimed to resolve this controversy
by comprehensively investigating the prognostic roles of EphB
receptors in BC, including EphB1, EphB2, EphB3, EphB4 and EphB6,
using a large population-based database. The Kaplan-Meier plotter
(KM plotter) database (14–18) was used to calculate the relapse-free
survival (RFS) from a total of 3,554 patients with BC and the
mRNA-level data of EphB receptors were downloaded. Relapse-free
survival was defined as the time from diagnosis to the first
relapse or death as a result of any cause. The analyses performed
revealed significant associations between EphB receptors and human
BC progression, which, to the best of our knowledge, have not been
previously investigated in BC.
Materials and methods
An online database was used to determine the
association between EphB mRNA expression and RFS. At present, the
database contains data regarding lung (14), ovarian (19), gastric and breast malignancies
(18). The database was first set up
from the Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/). The gene expression
data and survival information of 3,554 patients with BC, with 20
years of follow-up, were obtained for the current study, the
dataset of which was originally from Affymetrix HG-U133A (GPL96)
and HG-U133 Plus 2.0 (GPL570) microarrays that have 22,277 probe
sets in common (18). Briefly,
survival information and mRNA levels of five individual EphB
receptors, including EphB1, EphB2, EphB3, EphB4 and EphB6, were
downloaded from the KM plotter database (http://kmplot.com/analysis/index.php?p=service&cancer=breast,
2018 edition) to provide KM plots. All patients with RFS available
were included. The JetSet best probe sets were selected. The hazard
ratio (HR), number-at-risk and 95% confidence intervals (CI) were
displayed on the plot. All percentiles were computed and the
highest performing threshold was selected as a cut-off.
Specifically, patients were split based on the ‘auto select best
cut-off’, and all possible cut-off values between the lower and
upper quartiles were computed, and the best performing threshold
was used as a cut-off. A log-rank test was used to calculate
P-values. P<0.05 was considered to indicate statistically
significance.
To further evaluate the association between EphB
gene expression and tumor relapse in patients with BC, the
expression of selected genes was determined in patients stratified
by estrogen receptor (ER), progesterone receptor (PgR), human
epidermal growth factor receptor 2 (HER2), lymph node status,
pathological grade and molecular subtype. In addition, the cut-off
was set by the best performing threshold after all percentiles were
computed.
Results
Associations between mRNA expression
levels of EphB receptors and RFS
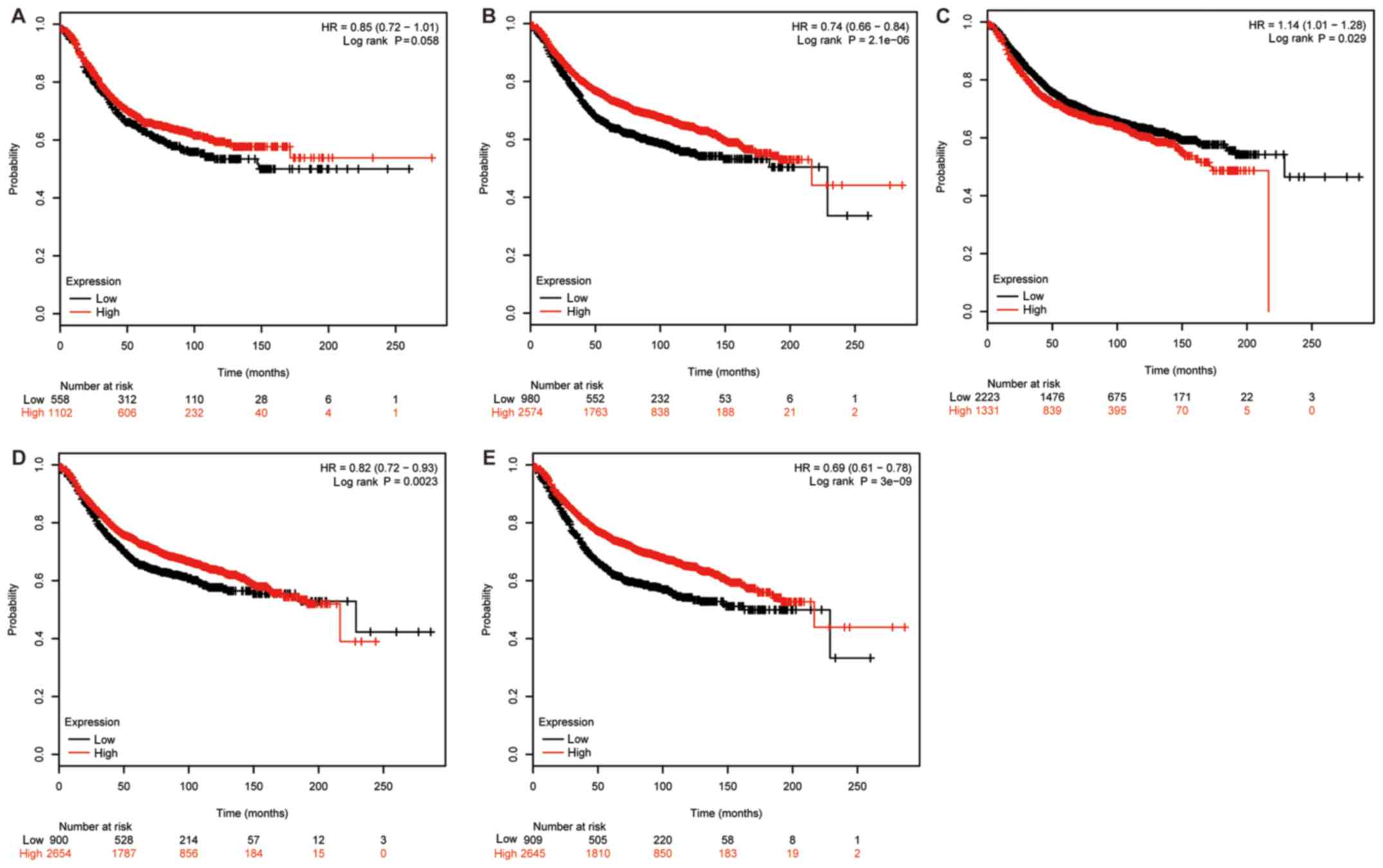
Firstly, the impact of EphB1 mRNA expression on the
prognosis of 1,660 patients with BC was evaluated using the KM
plotter database. A survival curve was generated using the
Affymetrix ID: 230425_at EPHB1 (Fig.
1A). A significant association was not identified between the
RFS and high mRNA expression of EphB1 for all patients with BC
followed up for 20 years (HR, 0.85; CI, 0.72–1.01; P=0.058).
Subsequently, the impact of EphB2 mRNA expression on the prognosis
of 3,554 patients was analyzed. High mRNA expression of EphB2 was
associated with improved RFS (HR, 0.74; CI, 0.66–0.84; P=2.1×10-6;
Affymetrix ID: 209588_at EPHB2; Fig.
1B). The prognostic effect of EphB3 mRNA expression in 3,554
patients with BC (Affymetrix ID: 204600_at EPHB3) is presented in
Fig. 1C. In contrast to EphB2, high
mRNA expression of EphB3 was associated with worse RFS. The
prognostic effect of EphB4 mRNA expression was then analyzed. High
expression of EphB4 mRNA was positively associated with RFS in
3,554 patients with BC (Affymetrix ID: 202894_at EPHB4; Fig. 1D). Finally, the impact of EphB6 mRNA
expression on the prognosis of 3,554 patients with BC was
investigated. High EphB6 mRNA expression was positively associated
with RFS (Affymetrix ID: 204718_at EPHB6; Fig. 1E).
Association between EphB receptors
with the clinicopathological features of patients with BC
The present study further determined the association
between EphB receptors with clinicopathological features, including
ER status (Table I), PgR status
(Table II), HER2 status (Table III), lymph node status (Table IV) and pathological grade (Table V) of patients with BC. It should be
noted that information on ER, PgR, HER2, lymph node status and
pathological grade was not available for all 3,554 patients.
 | Table I.Association of EphB receptors with ER
status in patients with breast cancer. |
Table I.
Association of EphB receptors with ER
status in patients with breast cancer.
| EphB receptor | ER status | Cases (n) | HR (95% CI) | P-value |
|---|
| B1 | Positive | 695 | 0.67
(0.45–0.99) | 0.044a |
|
| Negative | 313 | 0.56
(0.36–0.86) | 0.008a |
| B2 | Positive | 1,802 | 1.16
(0.97–1.39) | 0.110 |
|
| Negative | 671 | 0.78
(0.60–1.02) | 0.070 |
| B3 | Positive | 1,802 | 1.31
(1.09–1.56) | 0.003a |
|
| Negative | 671 | 0.76
(0.59–0.98) | 0.035a |
| B4 | Positive | 1,802 | 0.92
(0.77–1.10) | 0.360 |
|
| Negative | 671 | 1.21
(0.93–1.57) | 0.160 |
| B6 | Positive | 1,802 | 0.85
(0.71–1.02) | 0.085 |
|
| Negative | 671 | 0.72
(0.55–0.93) | 0.012a |
 | Table II.Association of EphB receptors with
PgR status in patients with breast cancer. |
Table II.
Association of EphB receptors with
PgR status in patients with breast cancer.
| EphB receptor | PgR status | Cases (n) | HR (95% CI) | P-value |
|---|
| B1 | Positive | 489 | 0.84
(0.56–1.25) | 0.380 |
|
| Negative | 372 | 0.60
(0.40–0.91) | 0.014a |
| B2 | Positive | 525 | 1.31
(0.86–1.98) | 0.200 |
|
| Negative | 483 | 1.66
(1.22–2.26) | 0.001a |
| B3 | Positive | 525 | 0.80
(0.54–1.20) | 0.280 |
|
| Negative | 483 | 1.38
(0.98–1.94) | 0.067 |
| B4 | Positive | 525 | 1.41
(0.98–2.03) | 0.059 |
|
| Negative | 483 | 1.51
(1.10–2.08) | 0.010a |
| B6 | Positive | 525 | 1.41
(0.94–2.10) | 0.093 |
|
| Negative | 483 | 1.21
(0.88–1.66) | 0.250 |
 | Table III.Association of EphB receptors with
HER2 status in patients with breast cancer. |
Table III.
Association of EphB receptors with
HER2 status in patients with breast cancer.
| EphB receptor | HER2 status | Cases (n) | HR (95% CI) | P-value |
|---|
| B1 | Positive | 150 | 0.59
(0.30–1.17) | 0.130 |
|
| Negative | 635 | 1.27
(0.94–1.71) | 0.110 |
| B2 | Positive | 168 | 2.03
(1.09–3.77) | 0.023a |
|
| Negative | 756 | 1.52
(1.15–2.02) | 0.003a |
| B3 | Positive | 168 | 1.43
(0.83–2.45) | 0.190 |
|
| Negative | 756 | 1.40
(1.05–1.86) | 0.019a |
| B4 | Positive | 168 | 1.95
(1.12–3.42) | 0.017a |
|
| Negative | 756 | 1.29
(0.99–1.68) | 0.057 |
| B6 | Positive | 168 | 2.19
(1.07–4.47) | 0.028a |
|
| Negative | 756 | 1.33
(1.00–1.77) | 0.052 |
 | Table IV.Association of EphB receptors with
lymph node status in patients with breast cancer. |
Table IV.
Association of EphB receptors with
lymph node status in patients with breast cancer.
| EphB receptor | Lymph node
status | Cases (n) | HR (95% CI) | P-value |
|---|
| B1 | Positive | 665 | 1.40
(1.02–1.94) | 0.039a |
|
| Negative | 451 | 0.73
(0.43–1.24) | 0.250 |
| B2 | Positive | 945 | 1.34
(1.07–1.67) | 0.011a |
|
| Negative | 1,813 | 1.16
(0.95–1.43) | 0.150 |
| B3 | Positive | 945 | 1.39
(1.11–1.73) | 0.004a |
|
| Negative | 1,813 | 1.36
(1.13–1.65) | 0.001a |
| B4 | Positive | 945 | 1.33
(1.06–1.67) | 0.013a |
|
| Negative | 1,813 | 1.21
(1.01–1.43) | 0.034a |
| B6 | Positive | 945 | 1.32
(1.05–1.65) | 0.016a |
|
| Negative | 1,813 | 0.85
(0.69–1.05) | 0.130 |
 | Table V.Association of EphB receptors with
pathological grades of patients with breast cancer. |
Table V.
Association of EphB receptors with
pathological grades of patients with breast cancer.
| EphB receptors | Grade | Cases (n) | HR (95% CI) | P-value |
|---|
| B1 | I | 97 | 0.31
(0.09–1.05) | 0.046a |
|
| II | 187 | 1.40
(0.78–2.49) | 0.250 |
|
| III | 391 | 0.73
(0.48–1.11) | 0.140 |
| B2 | I | 308 | 1.70
(0.98–2.95) | 0.057 |
|
| II | 724 | 1.29
(0.95–1.76) | 0.110 |
|
| III | 723 | 1.29
(1.00–1.65) | 0.046a |
| B3 | I | 308 | 0.72
(0.42–1.24) | 0.230 |
|
| II | 724 | 1.39
(1.06–1.82) | 0.015a |
|
| III | 723 | 1.22
(0.94–1.57) | 0.130 |
| B4 | I | 308 | 1.99
(0.99–3.99) | 0.048a |
|
| II | 724 | 1.16
(0.9–1.51) | 0.260 |
|
| III | 723 | 1.31
(1.02–1.68) | 0.034a |
| B6 | I | 308 | 0.69
(0.4–1.18) | 0.170 |
|
| II | 724 | 0.77
(0.59–0.99) | 0.044a |
|
| III | 723 | 0.85
(0.65–1.11) | 0.240 |
EphB1 expression data and ER status were available
for a total of 1,008 patients, and both ER status and EphB2, B3, B4
or B6 expression data were available for 2,473 patients. For
ER-positive patients, it was demonstrated that EphB1 was positively
associated with RFS (HR, 0.67; CI, 0.45–0.99, P=0.044); however,
EphB3 was negatively associated with RFS (HR, 1.31; CI, 1.09–1.56;
P=0.0029). By contrast, in ER-negative subgroups, EphB1 (HR, 0.56;
CI, 0.36–0.86; P=0.0081), EphB3 (HR, 0.76; CI, 0.59–0.98; P=0.035)
and EphB6 (HR, 0.72; CI, 0.55–0.93; P=0.012) were identified to be
associated with improved RFS.
As demonstrated in Table
II, both EphB1 expression and PgR status data were available
for 861 patients, and PgR status and EphB2, EphB3, EphB4 or EphB6
expression data were available for 1,008 patients. Only EphB1 was
associated with improved RFS in PgR-negative patients (HR, 0.6; CI,
0.4–0.91; P=0.014). However, EphB2 (HR, 1.66; CI, 1.22–2.26;
P=0.0012) and EphB4 (HR, 1.51; CI, 1.1–2.08; P=0.0098) expression
were revealed to be associated with worse RFS in PgR-negative
subgroups.
As presented in Table
III, both EphB1 expression and HER2 status data were available
for 785 patients, and HER2 status and EphB2, EphB3, EphB4 or EphB6
expression data were available for 924 patients. In HER2-positive
patients, EphB2 (HR, 2.03; CI, 1.09–3.77; P=0.023), EphB4 (HR,
1.95; CI, 1.12–3.42; P=0.017) and EphB6 (HR, 2.19; CI, 1.07–4.47;
P=0.028) were negatively associated with RFS. In HER2-negative
patients with BC, EphB2 (HR, 1.52; CI, 1.15–2.02; P=0.0033) and
EphB3 (HR, 1.4; CI, 1.05–1.86; P=0.019) were identified to be
associated with worse RFS.
As demonstrated in Table
IV, both EphB1 expression and lymph node status data were
available for 1,116 patients, and lymph node status and EphB2,
EphB3, EphB4 or EphB6 expression data were available for 2,758
patients. EphB1, EphB2, EphB3, EphB4 and EphB6 were all negatively
associated with RFS in lymph-node-positive patients with BC. EphB3
(HR, 1.36; CI, 1.13–1.65; P=0.0014) and EphB4 (HR, 1.21; CI,
1.01–1.43; P=0.034) were revealed to be associated with worse RFS
in lymph-node negative patients.
As presented in Table
V, both EphB1 expression and pathological grade data were
available for 675 patients, and pathological grade and EphB2,
EphB3, EphB4 or EphB6 expression data were available for 1,755
patients. EphB1 was demonstrated to be positively associated with
RFS in patients with grade I BC (HR, 0.31; CI, 0.09–1.05; P=0.046).
EphB2 was identified to be negatively associated with RFS in
patients with grade III BC (HR, 1.29; CI, 1–1.65; P=0.046). EphB3
was revealed to be associated with worse RFS in patients with grade
II BC (HR, 1.39; CI, 1.06–1.82; P=0.015). EphB4 was identified to
be associated with worse RFS in patients with both grade I BC (HR,
1.99; CI, 0.99–3.99; P=0.048) and grade III BC (HR, 1.31; CI,
1.02–1.68; P=0.034). EphB6 was revealed to be negatively associated
with RFS in patients with grade II BC (HR, 0.77; CI, 0.59–0.99;
P=0.044).
Associations of EphB receptors with
molecular subtypes of BC
BC can be classified into four distinct molecular
subtypes, including basal-like, luminal-A, luminal-B and
HER2-positive. As presented in Table
VI, EphB1 was revealed to be associated with improved RFS in
patients with luminal-B (HR, 0.57; CI, 0.38–0.86; P=0.0072) and
HER2-positive BC (HR, 0.58; CI, 0.35–0.95; P=0.029). EphB2 and
EphB6 were identified to be positively associated with RFS in each
molecular subgroup. EphB3 was revealed to be associated with
improved RFS in patients with basal-like BC (HR, 0.74; CI,
0.57–0.97; P=0.025); however, it was associated with worse RFS in
patients with HER2-positive BC (HR, 2.0; CI, 1.21–3.28; P=0.0055).
EphB4 was identified to be positively associated with RFS in
patients with luminal-A BC (HR, 0.64; CI, 0.53–0.77;
P=1.9×10-6).
 | Table VI.Associations of EphB receptors with
molecular subtypes of patients with breast cancer. |
Table VI.
Associations of EphB receptors with
molecular subtypes of patients with breast cancer.
| EphB receptor | Molecular
subtype | Cases (n) | HR (95% CI) | P-value |
|---|
| B1 | Basal | 339 | 0.75
(0.52–1.08) | 0.120 |
|
| Luminal A | 783 | 0.81
(0.62–1.05) | 0.110 |
|
| Luminal B | 389 | 0.57
(0.38–0.86) | 0.007a |
|
| HER2+ | 149 | 0.58
(0.35–0.95) | 0.029a |
| B2 | Basal | 580 | 0.69
(0.53–0.89) | 0.005a |
|
| Luminal A | 1,764 | 0.70
(0.59–0.84) |
<0.001a |
|
| Luminal B | 1,002 | 0.71
(0.56–0.89) | 0.003a |
|
| HER2+ | 208 | 0.5
(0.33–0.78) | 0.002a |
| B3 | Basal | 580 | 0.74
(0.57–0.97) | 0.025a |
|
| Luminal A | 1,764 | 0.89
(0.73–1.08) | 0.230 |
|
| Luminal B | 1,002 | 1.13
(0.92–1.39) | 0.240 |
|
| HER2+ | 208 | 2.00
(1.21–3.28) | 0.006a |
| B4 | Basal | 580 | 0.86
(0.66–1.12) | 0.260 |
|
| Luminal A | 1,764 | 0.64
(0.53–0.77) |
<0.001a |
|
| Luminal B | 1,002 | 0.85
(0.69–1.03) | 0.100 |
|
| HER2+ | 208 | 1.36
(0.85–2.18) | 0.190 |
| B6 | Basal | 580 | 0.70
(0.52–0.93) | 0.015a |
|
| Luminal A | 1,764 | 0.61
(0.51–0.72) |
<0.001a |
|
| Luminal B | 1,002 | 0.62
(0.48–0.80) |
<0.001a |
|
| HER2+ | 208 | 0.50
(0.30–0.84) | 0.008a |
Discussion
The KM plotter database was generated from the GEO
database. The expression data of 22,277 genes were initially
available for 1,809 patients with BC (18). Gene expression data and survival
information have since been validated and updated for 3,554
patients with BC. Therefore, the KM plotter can be used to analyze
the prognostic effect of individual genes with clinical outcomes,
including overall survival (OS) and RFS (http://kmplot.com/analysis/index.php?p=service&cancer=breast).
As certain patients lacked OS data, the current study focused on
RFS. The present study comprehensively and specifically analyzed
the associations between the expression levels of EphB receptors,
including EphB1, EphB2, EphB3, EphB4 and EphB6, and RFS in all
patients with BC, as well as in subgroups according to
clinicopathological features, which, to the best of our knowledge,
has not previously been reported. EphA receptors were not discussed
in the present study and may be investigated in future studies.
EphB receptors predominantly function independently on class B
ephrin ligands in BC, therefore, the current study only analyzed
and discussed EphB receptors (6,9).
A limited number of studies have investigated EphB1
in patients with BC. A BC-risk, genome-wide association study
suggested an association between carcinogenesis and
germline-somatic rs3732568 in EphB1 (20). The current study revealed that EphB1
was not associated with RFS. In subgroup analysis, EphB1 was
positively associated with RFS in patients with PgR-negative and
grade I BC. Previous studies have demonstrated that active EphB1
can activate the mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase (ERK), c-Jun and
integrin signaling pathways (21–23).
EphB2 has more widely been investigated in previous
studies. It has been demonstrated that EphB2 can regulate
apoptosis, autophagy and invasion in human BC cells (24,25). One
previous study examined the expression of EphB2 protein by
immunohistochemistry. This study revealed that associations with
clinical outcomes were the opposite for membranous and cytoplasmic
EphB2. Membranous EphB2 protein was associated with improved BC
prognosis, whereas cytoplasmic EphB2 protein predicted a worse BC
prognosis (26). EphB2 has also been
validated to exhibit a novel transforming growth factor-β (TGF-β)
targeting TGF-β3-mediated invasion and migration in BC (27). The present study identified that high
mRNA expression of EphB2 predicted a longer RFS in all patients
with BC, whereas it was associated with worse RFS in PgR-negative
and grade III subgroups. Similar to EphB1, EphB2 can also stimulate
the MAPK/ERK pathway, which leads to improved responsiveness to
EphB stimulation in a positive feedback loop (28). However, in a different context, EphB2
can inhibit the oncogenic ERK signaling pathway, which in turn
suppresses its activation by ephrins (29).
To the best of our knowledge, the role of EphB3 in
BC is largely unknown. The current study revealed that high mRNA
expression of EphB3 was associated with a longer RFS in all
patients with BC. In subgroup analysis, EphB3 was identified to
indicate a shorter RFS in patients with ER-positive, HER2-negative
and grade II BC, but an improved RFS for patients with ER-negative
BC. It has been demonstrated that EphB3 receptor can inhibit cell
adhesion and migration in a kinase-dependent/independent manner
(30). However, in malignant
lymphocytes it exhibits the opposite effect by activating the Akt
pathway and suppressing the apoptosis pathway (31).
EphB4 is involved in regulating mammary gland
development. EphB4 protein overexpression has been revealed to
induce delayed development of the mammary gland during puberty and
pregnancy (32,33). In addition, EphB4 knockdown has been
demonstrated to inhibit the survival, migration and invasion of BC
cells (34). Furthermore, EphB4 has
been identified to promote erythropoietin-induced tumor growth in
human BC (35). In one small-sample
study, patients with HER2-positive BC with EphB4 and EphB2
overexpression were associated with a shorter survival time
(36). However, to the best of our
knowledge, high expression of EphB4 alone remains to be identified
as an independent prognostic factor. On the contrary, another study
revealed negative associations between EphB4 and clinical outcomes
by investigating protein expression in breast tissue microarrays
(37). Notably, dual functions of
this receptor in tumor promotion and inhibition have been reported
on the basis of its ligand presence or absence (9,38).
Previous studies have been based on small samples. In the present
large analysis it was identified that EphB4 mRNA levels could
predict improved RFS in all patients with BC, while it was
associated with worse RFS in PgR-negative, HER2-positive, grade I
and grade III subgroups, which supports the dual function of EphB4
(10,34,39).
EphB4 activation leads to cell proliferation and enhanced migration
via the phosphoinositide 3-kinase (PI3K)/Akt pathway (40). However, in a mouse xenograft model,
EphB4 was demonstrated to inhibit cell growth via the Abl-Crk
pathway (10).
EphB6 is an uncommon Eph receptor and lacks
catalytic capacity due to its kinase domain changes (41). In an experiment conducted in
vitro, reduced EphB6 receptor expression resulted in increased
metastatic activity in BC (41).
Other studies have demonstrated that EphB6 transcriptional
silencing and its consequent effects on the Wnt pathway may
contribute to tumor progression in triple-negative BC (42). Additionally, EphB6 receptors with
kinase deficiency have been demonstrated to initiate signal
transduction from the cell surface to the nucleus, allowing for the
expression of a variety of genes alterations that are involved in
tumor progression via the PI3K/Akt/mammalian target of rapamycin
pathways (43). Furthermore, the
interaction of EphB6 with a number of proteins can lead to
proteomic profile changes in EphB6-overexpressed MDA-MB-231 cells
(44). Significantly positive
associations have been revealed between EphB6 and OS in BC tissue
microarrays (37). In the present
study, high expression of EphB6 mRNA predicted a longer RFS in all
patients with BC. In the subgroup analysis, EphB6 was associated
with improved RFS in patients with ER-negative and grade II BC, but
was associated with worse RFS in patients with HER2-positive BC,
which indicates a dual function of EphB6.
Expression of EphB2 mRNA has been demonstrated to
predict poor survival in lymph-node-positive BC (45). Notably, all EphB receptors were
associated with worse RFS in patients with lymph-node-positive BC
in the present study.
In addition, we attempted to investigate the
prognostic value of the expression of EphB receptors in another
independent METABRIC dataset through the cBioPortal for Cancer
Genomics (cbioportal.org) (46,47). The
genomic profiles of 2,509 patients with BC were available in the
dataset, with data of somatic mutations, copy number alterations
and gene expression. However, the detailed clinicopathological
features or the best performing thresholds could not be determined
using METABRIC. Therefore, important results could have been missed
using the cBioPortal; the data have not been presented in the
current study.
The functions of ephrin receptors in cancer are
paradoxical and complex (48). The
ephrin system is essentially present in all types of cancer cell
(48). Cancer cellular phenotypes
may partly be attributed to a combinatorial expression or
interactions of ephrin receptors among the same class, as well as
two different classes. For example, EphB6, a receptor with
kinase-deficiency, can interact with EphB2 or EphA2 in mammalian
cells (49). EphB4 can promote
cancer progression independent of EphB6, suggesting that the
balance may determine tumorigenesis in the EphB4-EphB6 system
(41). Decreased ephrin expression
levels have also been associated with tumor progression (50). Consistent with the dichotomy,
evidence has demonstrated that ephrin receptors and ephrins exhibit
both tumor-promoting and -suppressing activities. For example, in
the present study high mRNA expression of EphB3 predicted shorter
RFS in ER positive patients but longer RFS in ER-negative patients.
Additionally, EphB6 indicated longer RFS in patients with
ER-negative BC but a shorter RFS in patients with HER2-positive BC,
which suggests the multifaceted roles of these receptors. The
underlying mechanisms responsible for these divergent functions
have previously been investigated (7,50,51).
The results of the current study are limited for two
reasons. First, the database lacks information on
clinicopathological features in certain patients, which may lead to
statistical bias. Second, multivariate analysis cannot be used in
the database to correct the associations between different
clinicopathological features. Nevertheless, the comprehensive data
suggest that EphB receptors are prognostic factors and traceable
targets for BC, which may improve understanding regarding the
complexity and heterogeneity of BC molecular biology. In addition,
the current results may assist the development of tools to more
accurately predict prognosis and design customized therapies. ELISA
can be used as a rapid and sensitive method to detect EphB4 as
diagnostic and therapeutic biomarker in BC (45). EphB6 deficiency may be treated by
small molecule inhibitors in a synthetic lethality approach
(52). Biological products targeting
EphB receptors, including antibodies, peptides and recombinant
proteins, to reduce the progression of several types of cancer,
such as BC, are in the preclinical stages of investigation in
animal models. Additionally, at present, certain biopharmaceutical
agents are undergoing phase I or phase II clinical trials (e.g.,
NCT01642342, NCT03552796, NCT02495896) (53–55).
Acknowledgements
The authors wish to thank Dr Xiaosong Chen
(Comprehensive Breast Health Center, Ruijin Hospital, Shanghai
Jiaotong University School of Medicine, Shanghai, P.R. China), Dr
Jiayi Wu (Comprehensive Breast Health Center, Ruijin Hospital,
Shanghai Jiaotong University School of Medicine, Shanghai, P.R.
China), Dr Yan Mao Comprehensive Breast Health Center, Ruijin
Hospital, Shanghai Jiaotong University School of Medicine,
Shanghai, P.R. China), Dr Mingzhu Li (Department of Breast
Oncology, The First Hospital of Quanzhou Affiliated to Fujian
Medical University, Quanzhou, Fujian, P.R. China), Dr Qinglan Wang
(Department of Breast Oncology, The First Hospital of Quanzhou
Affiliated to Fujian Medical University, Quanzhou, Fujian, P.R.
China) and Dr Chengye Hong (Department of Breast Oncology, The
First Hospital of Quanzhou Affiliated to Fujian Medical University,
Quanzhou, Fujian, P.R. China) for their kind suggestions regarding
this manuscript. In addition, the authors wish to thank Professor
Meiyu Geng (Division of Anti-tumor Pharmacology, State Key
Laboratory of Drug Research, Shanghai Institute of Materia Medica,
Chinese Academy of Sciences, Shanghai, P.R. China), Professor Min
Huang (Division of Anti-tumor Pharmacology, State Key Laboratory of
Drug Research, Shanghai Institute of Materia Medica, Chinese
Academy of Sciences, Shanghai, P.R. China), Professor Hongchun Liu
(Division of Anti-tumor Pharmacology, State Key Laboratory of Drug
Research, Shanghai Institute of Materia Medica, Chinese Academy of
Sciences, Shanghai, P.R. China), Professor Wangmin Qiao (Shenzhen
Huada Gene Technology Co., Ltd, Shenzhen, P.R. China) and Professor
Ziguan Zhang (Xiamen Zhixin Biotechnology Co., Ltd, Xiamen, P.R.
China) for their technical help.
Funding
Not applicable.
Availability of data and materials
The datasets used during the current study are
available from Kaplan-Meier Plotter (http://kmplot.com).
Authors' contributions
XM, OH and XZ conceived and designed the study. XZ
and OH performed the statistical analysis. MJ, ZX and DC analyzed
the data. XZ and XM wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EphB
|
ephrin B
|
|
HR
|
hazard ratio
|
|
BC
|
breast cancer
|
|
KM plotter
|
Kaplan-Meier plotter
|
|
RFS
|
relapse-free survival
|
|
TKR
|
tyrosine kinase receptor
|
|
CI
|
confidence interval
|
|
ER
|
estrogen receptor
|
|
PgR
|
progesterone receptor
|
|
HER2
|
human epidermal receptor 2
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gonzalez-Conchas GA, Rodriguez-Romo L,
Hernandez-Barajas D, Gonzalez-Guerrero JF, Rodriguez-Fernandez IA,
Verdines-Perez A, Templeton AJ, Ocana A, Seruga B, Tannock IF, et
al: Epidermal growth factor receptor overexpression and outcomes in
early breast cancer: A systematic review and a meta-analysis.
Cancer Treat Rev. 62:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pasquale EB: Eph-ephrin bidirectional
signaling in physiology and disease. Cell. 133:38–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Himanen JP, Saha N and Nikolov DB:
Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell
Biol. 19:534–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brantley-Sieders DM: Clinical relevance of
Ephs and ephrins in cancer: Lessons from breast, colorectal, and
lung cancer profiling. Semin Cell Dev Biol. 23:102–108. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaenel P, Mosimann M and Andres AC: The
multifaceted roles of Eph/ephrin signaling in breast cancer. Cell
Adh Migr. 6:138–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Genander M and Frisén J: Ephrins and Eph
receptors in stem cells and cancer. Curr Opin Cell Biol.
22:611–616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin B, Yin T, Wu YI, Inoue T and Levchenko
A: Interplay between chemotaxis and contact inhibition of
locomotion determines exploratory cell migration. Nat Commun.
6:66192015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pasquale EB: Eph receptors and ephrins in
cancer: Bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noren NK, Foos G, Hauser CA and Pasquale
EB: The EphB4 receptor suppresses breast cancer cell tumorigenicity
through an Abl-Crk pathway. Nat Cell Biol. 8:815–825. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Janes PW, Griesshaber B, Atapattu L,
Nievergall E, Hii LL, Mensinga A, Chheang C, Day BW, Boyd AW,
Bastiaens PI, et al: Eph receptor function is modulated by
heterooligomerization of A and B type Eph receptors. J Cell Biol.
195:1033–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arvanitis D and Davy A: Eph/ephrin
signaling: Networks. Genes Dev. 22:416–429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cortina C, Palomo-Ponce S, Iglesias M,
Fernández-Masip JL, Vivancos A, Whissell G, Humà M, Peiró N,
Gallego L, Jonkheer S, et al: EphB-ephrin-B interactions suppress
colorectal cancer progression by compartmentalizing tumor cells.
Nat Genet. 39:1376–1383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pénzváltó Z, Lánczky A, Lénárt J,
Meggyesházi N, Krenács T, Szoboszlai N, Denkert C, Pete I and
Győrffy B: MEK1 is associated with carboplatin resistance and is a
prognostic biomarker in epithelial ovarian cancer. BMC Cancer.
14:8372014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Győrffy B, Bottai G, Lehmann-Che J, Kéri
G, Orfi L, Iwamoto T, Desmedt C, Bianchini G, Turner NC, de Thè H,
et al: TP53 mutation-correlated genes predict the risk of tumor
relapse and identify MPS1 as a potential therapeutic kinase in
TP53-mutated breast cancers. Mol Oncol. 8:508–519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pongor L, Kormos M, Hatzis C, Pusztai L,
Szabó A and Győrffy B: A genome-wide approach to link genotype to
clinical outcome by utilizing next generation sequencing and gene
chip data of 6,697 breast cancer patients. Genome Med. 7:1042015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Györffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gyorffy B, Lánczky A and Szállási Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonifaci N, Górski B, Masojć B,
Wokołorczyk D, Jakubowska A, Dębniak T, Berenguer A, Serra Musach
J, Brunet J, Dopazo J, et al: Exploring the link between germline
and somatic genetic alterations in breast carcinogenesis. PLoS One.
5:e140782010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Becker E, Huynh-Do U, Holland S, Pawson T,
Daniel TO and Skolnik EY: Nck-interacting Ste20 kinase couples Eph
receptors to c-Jun N-terminal kinase and integrin activation. Mol
Cell Biol. 20:1537–1545. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stein E, Huynh-Do U, Lane AA, Cerretti DP
and Daniel TO: Nck recruitment to Eph receptor, EphB1/ELK, couples
ligand activation to c-Jun kinase. J Biol Chem. 273:1303–1308.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vindis C, Cerretti DP, Daniel TO and
Huynh-Do U: EphB1 recruits c-Src and p52Shc to activate MAPK/ERK
and promote chemotaxis. J Cell Biol. 162:661–671. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chukkapalli S, Amessou M, Dilly AK, Dekhil
H, Zhao J, Liu Q, Bejna A, Thomas RD, Bandyopadhyay S, Bismar TA,
et al: Role of the EphB2 receptor in autophagy, apoptosis and
invasion in human breast cancer cells. Exp Cell Res. 320:233–246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Genander M, Halford MM, Xu NJ, Eriksson M,
Yu Z, Qiu Z, Martling A, Greicius G, Thakar S, Catchpole T, et al:
Dissociation of EphB2 signaling pathways mediating progenitor cell
proliferation and tumor suppression. Cell. 139:679–692. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Husa AM, Magić Ž, Larsson M, Fornander T
and Pérez-Tenorio G: EPH/ephrin profile and EPHB2 expression
predicts patient survival in breast cancer. Oncotarget.
7:21362–21380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lam S, Wiercinska E, Teunisse AF, Lodder
K, ten Dijke P and Jochemsen AG: Wild-type p53 inhibits
pro-invasive properties of TGF-β3 in breast cancer, in part through
regulation of EPHB2, a new TGF-β target gene. Breast Cancer Res
Treat. 148:7–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poliakov A, Cotrina ML, Pasini A and
Wilkinson DG: Regulation of EphB2 activation and cell repulsion by
feedback control of the MAPK pathway. J Cell Biol. 183:933–947.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dail M, Richter M, Godement P and Pasquale
EB: Eph receptors inactivate R-Ras through different mechanisms to
achieve cell repulsion. J Cell Sci. 119:1244–1254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miao H, Strebhardt K, Pasquale EB, Shen
TL, Guan JL and Wang B: Inhibition of integrin-mediated cell
adhesion but not directional cell migration requires catalytic
activity of EphB3 receptor tyrosine kinase. Role of Rho family
small GTPases. J Biol Chem. 280:923–932. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maddigan A, Truitt L, Arsenault R,
Freywald T, Allonby O, Dean J, Narendran A, Xiang J, Weng A, Napper
S and Freywald A: EphB receptors trigger Akt activation and
suppress Fas receptor-induced apoptosis in malignant T lymphocytes.
J Immunol. 187:5983–5994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nikolova Z, Djonov V, Zuercher G, Andres
AC and Ziemiecki A: Cell-type specific and estrogen dependent
expression of the receptor tyrosine kinase EphB4 and its ligand
ephrin-B2 during mammary gland morphogenesis. J Cell Sci.
111:2741–2751. 1998.PubMed/NCBI
|
|
33
|
Munarini N, Jäger R, Abderhalden S,
Zuercher G, Rohrbach V, Loercher S, Pfanner-Meyer B, Andres AC and
Ziemiecki A: Altered mammary epithelial development, pattern
formation and involution in transgenic mice expressing the EphB4
receptor tyrosine kinase. J Cell Sci. 115:25–37. 2002.PubMed/NCBI
|
|
34
|
Rutkowski R, Mertens-Walker I, Lisle JE,
Herington AC and Stephenson SA: Evidence for a dual function of
EphB4 as tumor promoter and suppressor regulated by the absence or
presence of the ephrin-B2 ligand. Int J Cancer. 131:E614–E624.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pradeep S, Huang J, Mora EM, Nick AM, Cho
MS, Wu SY, Noh K, Pecot CV, Rupaimoole R, Stein MA, et al:
Erythropoietin stimulates tumor growth via EphB4. Cancer Cell.
28:610–622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Song C, Huang G, Sun S, Qiao J, Zhao
J, Zhao Z and Li M: The coexpression of EphB4 and EphrinB2 is
associated with poor prognosis in HER2-positive breast cancer. Onco
Targets Ther. 10:1735–1742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brantley-Sieders DM, Jiang A, Sarma K,
Badu-Nkansah A, Walter DL, Shyr Y and Chen J: Eph/ephrin profiling
in human breast cancer reveals significant associations between
expression level and clinical outcome. PLoS One. 6:e244262011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barneh F, Moshayedi M, Mirmohammadsadeghi
H, Haghjooy-Javanmard S, Sabzghabaee AM and Badri S: EphB4 tyrosine
kinase stimulation inhibits growth of MDA-MB-231 breast cancer
cells in a dose and time dependent manner. Dis Markers. 35:933–938.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Noren NK and Pasquale EB: Paradoxes of the
EphB4 receptor in cancer. Cancer Res. 67:3994–3997. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Steinle JJ, Meininger CJ, Forough R, Wu G,
Wu MH and Granger HJ: Eph B4 receptor signaling mediates
endothelial cell migration and proliferation via the
phosphatidylinositol 3-kinase pathway. J Biol Chem.
277:43830–43835. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Truitt L, Freywald T, DeCoteau J, Sharfe N
and Freywald A: The EphB6 receptor cooperates with c-Cbl to
regulate the behavior of breast cancer cells. Cancer Res.
70:1141–1153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhushan L, Tavitian N, Dey D, Tumur Z,
Parsa C and Kandpal RP: Modulation of liver-intestine cadherin
(Cadherin 17) expression, ERK phosphorylation and WNT signaling in
EPHB6 receptor-expressing MDA-MB-231 cells. Cancer Genomics
Proteomics. 11:239–249. 2014.PubMed/NCBI
|
|
43
|
Fox BP and Kandpal RP: EphB6 receptor
significantly alters invasiveness and other phenotypic
characteristics of human breast carcinoma cells. Oncogene.
28:1706–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kandpal RP: Tyrosine kinase-deficient
EphB6 receptor-dependent alterations in proteomic profiles of
invasive breast carcinoma cells as determined by difference gel
electrophoresis. Cancer Genomics Proteomics. 7:253–260.
2010.PubMed/NCBI
|
|
45
|
Moshayedi M, Barneh F, Haghjooy-Javanmard
S, Sadeghi HM, Eskandari N and Sabzghabaee AM: A rapid and
sensitive method for EphB4 identification as a diagnostic and
therapeutic biomarker in invasive breast cancer. J Cancer Res Ther.
12:188–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al: The genomic and transcriptomic architecture of 2,000 breast
tumours reveals novel subgroups. Nature. 486:346–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pereira B, Chin SF, Rueda OM, Vollan HK,
Provenzano E, Bardwell HA, Pugh M, Jones L, Russell R, Sammut S, et
al: The somatic mutation profiles of 2,433 breast cancers refines
their genomic and transcriptomic landscapes. Nat Commun.
7:114792016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kullander K and Klein R: Mechanisms and
functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol.
3:475–486. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fox BP and Kandpal RP: A paradigm shift in
EPH receptor interaction: Biological relevance of EPHB6 interaction
with EPHA2 and EPHB2 in breast carcinoma cell lines. Cancer
Genomics Proteomics. 8:185–193. 2011.PubMed/NCBI
|
|
50
|
Klein R: Bidirectional modulation of
synaptic functions by Eph/ephrin signaling. Nat Neurosci. 12:15–20.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Orsulic S and Kemler R: Expression of Eph
receptors and ephrins is differentially regulated by E-cadherin. J
Cell Sci. 113:1793–1802. 2000.PubMed/NCBI
|
|
52
|
Paul JM, Toosi B, Vizeacoumar FS,
Bhanumathy KK, Li Y, Gerger C, El Zawily A, Freywald T, Anderson
DH, Mousseau D, et al: Targeting synthetic lethality between the
SRC kinase and the EPHB6 receptor may benefit cancer treatment.
Oncotarget. 7:50027–50042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lodola A, Giorgio C, Incerti M, Zanotti I
and Tognolini M: Targeting Eph/ephrin system in cancer therapy. Eur
J Med Chem. 142:152–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chrencik JE, Brooun A, Recht MI, Nicola G,
Davis LK, Abagyan R, Widmer H, Pasquale EB and Kuhn P:
Three-dimensional structure of the EphB2 receptor in complex with
an antagonistic peptide reveals a novel mode of inhibition. J Biol
Chem. 282:36505–36513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Koolpe M, Burgess R, Dail M and Pasquale
EB: EphB receptor-binding peptides identified by phage display
enable design of an antagonist with ephrin-like affinity. J Biol
Chem. 280:17301–17311. 2005. View Article : Google Scholar : PubMed/NCBI
|