Introduction
Defense mechanisms are effective only in the early
stages of cancer development. Throughout tumor growth, cancer cells
acquire various properties that allow them to reduce the body
defense mechanisms (1). Cancer cells
display low antigenic immunogenicity and rapid growth (2). Furthermore, cancer cells exhibit
immunosuppressive activity on the extracellular matrix. These
properties weaken the immune response during colorectal
carcinogenesis (2). In the field of
tumor microenvironment research, the role of macrophages, also
known as tumor-associated macrophages (TAM), is of particular
interest. It has been hypothesized that the macrophages population
in the tumor may not be homogeneous, and that certain macrophages
may undergo abnormal stimulation. These macrophages may transform
from antigen-presenting cells that stimulate the immune system to
fight cancer, into cells that secrete factors that increase cancer
cell proliferation and stimulate tumor angiogenesis (3). Subsequently, neutrophils serve a
fundamental role in the antimicrobial defense system of the body.
Neutrophils comprise granules that contain large amounts of free
radicals and serine proteases, including peroxidase and lysozyme
(4). It has been reported that minor
inflammatory activity is maintained in patients with colorectal
adenomas, which confirms the influence of inflammation on
intestinal cell proliferation (5).
In such cases, neutrophils undergoing apoptosis permanently
accumulate in the mucous membrane; they are therefore not cleared
by macrophages and release their intracellular granules, which can
cause tissue damage and loss of control over their regeneration
(6). Neutrophil granulocytes and
macrophages secrete tumor growth factors, including vascular
endothelial growth factor, hepatocyte growth factor, interleukin
(IL)-6, IL-8, matrix metalloproteinases and elastase, which can
contribute to the stimulation of the tumor microenvironment
(7,8). In addition, it has been demonstrated
that the presence of eosinophils in the tumor mass may be a
prognostic factor in patients with colorectal cancer (CRC)
(9). It is therefore crucial to
determine the cellular composition of the inflammatory mass,
including neutrophils, macrophages and eosinophils in the tissues
of patients with CRC, and to evaluate their association with other
clinicopathological parameters and disease-free survival (DFS).
Materials and methods
Patients
The present study included 144 patients diagnosed
with CRC (88 men and 56 women) who underwent surgery in the
Department of Oncological Surgery, Comprehensive Cancer Center
(Białystok, Poland) between April 2014 and December 2016. The mean
age of the patients was 67.5 years, including 34 patients <60
years-old and 110 patients >60 years-old. The majority of
patients presented similar symptoms, including abdominal pain,
anemia, rectal bleeding, constipation, diarrhea, vomiting and
anorexia. In the majority of cases, patients additionally received
treatment for hypertension, type II diabetes, osteoarthritis and
coronary heart disease. However, none of the patients had received
inflammatory therapy.
All patients underwent routine diagnostic tests,
including basic diagnostic laboratory tests (morphological tests
and lipid profiles), electrocardiography, spirometry, arterial
blood gasometric test, X-ray and computerized chest tomography. The
clinical stage of CRC was evaluated according to the
Tumor-Node-Metastasis (TNM) classification (10) and the Dukes staging systems (11). Prior to surgery, patients with tumors
identified in other sites received no inflammatory or
immunosuppressive therapy. The response to preoperative therapy was
estimated according to the Response Evaluation Criteria in Solid
Tumors (12).
The present study was performed in accordance with
the Declaration of Helsinki for Human Experimentation, and the
protocol was approved by The Bioethics Committee of the Medical
University of Bialystok (approval no. R-I-002/351/2016). Written
informed consent was obtained from all participants.
Histopathological examination of CRC
tumor tissue
Tissues obtained from surgery were fixed in 4%
buffered formalin for 24 to 72 h at room temperature. Small section
of tissue were embedded in paraffin. Sections (4 µm-thick) were cut
from paraffin blocks and stained with hematoxylin and eosin
(H&E) at room temperature for 4 min (cat. no. 468802128; POCH
S.A.; Avantor Performance Materials Poland, Gliwice, Poland)
according to the manufacturer's protocol. The slides were
deparaffinized in an oven at 60°C for 5 min. Subsequently, slides
were rehydrated in xylene (three washes, 10 min each) and graded
ethanol (100, 95, 85 and 75%, 1 min at each concentration). Routine
histopathological assessment was of the sections was performed by
two pathologists blinded to the clinical information. The type of
tumor growth, tumor size, histological type, percentage of mucinous
components, grade of malignancy, TNM and Dukes stages was
determined by the pathologists. Venous, lymphatic and perineural
invasion of cancer cells were also analyzed. Characteristic
features of lymph node invasion were examined, including the number
of resected and invaded lymph nodes, the presence of micro- and
macro-metastases, invasion of the pouch lymph node, presence of
distant metastases and the size of metastases. The presence, number
and size of the deposits of cancer cells were also assessed
(13).
The tumor stroma ratio was then determined, as
previously described by Huijbers et al (14). The analysis was performed in the most
invasive tumor area of each slide by using ×25 or ×50
magnification. The tumor and stromal tissues were identified and
subsequently viewed at ×100 magnification. According to above
criteria, tumor cells were present at all edges of the image field
whereas necrotic areas and mucinous components were excluded. Tumor
budding was analyzed as previously described by Morodomi et
al (15). The extent of the
inflammatory cell reaction in the invasive front of the tumor and
the center of the tumor mass was also observed and classified
according to the Klintrup-Makinen criteria (16). Inflammatory reaction in the invasive
margin and center of the tumor were scored on a 4-point scale: 0,
No increase in inflammatory cell infiltrate; 1, a mild or patchy
increase; 2, a prominent inflammatory reaction with some evidence
of cancer cell destruction; and 3, florid ‘cup-like’ inflammatory
infiltrate. The extent of necrosis and fibrosis in the central
tumor was evaluated according to Richards et al (17) and graded as follows: Absent, none;
focal, <10% of tumor area; moderate, 10–30%; or extensive,
>30%. Crohn's-like aggregates of lymphocytes (CLR) were
evaluated based on the Väyrynen criteria (18). CLR was defined as lymphoid structures
surrounding the primary tumors, excluding mucosa-associated
lymphoid tissue or pre-existing lymph nodes. Histological
categorization of fibrotic cancer stroma was performed based on the
criteria from Ueno et al (19). Fibrotic cancer stroma was classified
as follows: Mature, consist of mature collagen fibers and elongated
fibers with fibrocytes stratified into multiple layers;
intermediate, broad bands of collagen with brightly eosinophilic
hyalinization and keloid-like structures; and immature, consist of
randomly orientated keloid-like collagen bundles surrounded by
myxoid stroma.
Morphological examination of
inflammatory cells at the invasive front and in the center of CRC
tumor tissue
Tissue samples obtained from routine
histopathological diagnosis were stained with H&E, and used to
assess inflammatory cells in the invasive front and center of the
tumor with light microscopy under a high-power magnification (×400;
Leica DM6 B, KAWA.SKA, Sp. z o.o., Piaseczno, Poland). Analysis was
performed by two independent pathologists blinded to patients'
clinical information. Morphologically, neutrophils are
polymorphonuclear cells with segmented nuclei that present clumped
chromatin, eosinophilic cytoplasm and pink granules. Cells were
counted and quantified as a percentage of all examined cells.
Neutrophils were divided into three groups: 1, 2 and 3, whether
they corresponded to 0, <20 or >21% of all cells examined.
Macrophages are mononuclear cells that contain numerous lysosomal
acid phosphatases in their cytoplasm. Eosinophils are
double-segmented nuclear cells with acidophilic cytoplasm that
contains large Babès-Ernst granules (20). In the present study, macrophages and
eosinophils were evaluated as absent (lack of cells) or present
(>5 cells observed in the examined field).
Statistical analysis
Statistical analysis was performed using the
STATISTICA 10.0 program (Statsoft, Cracow, Poland). A Mann-Whitney
U-test was used to compare the groups. Correlations between the
parameters were calculated using the Spearman's correlation
coefficient test. P<0.05 was considered to indicate a
statistically significant difference. DFS time was calculated as
the duration between the date of the diagnosis and the date of
disease progression, including local or distant relapse. The DFS
rate was estimated using the Kaplan Meier estimator method and the
survival curves were compared using log-rank tests.
Results
Neutrophils, macrophages and
eosinophils in CRC tissue
Neutrophils in the invasive front of the CRC tumor
were absent in 48 cases (group 1), present in 77 cases (group 2)
and a further 19 cases (group 3; Table
I). In addition, neutrophils in the center of the tumor mass
were observed in 16 cases (group 2) and 40 cases (group 3; Table I). The percentage of neutrophils in
the invasive front were significantly higher compared with that in
the center of the tumor mass (P<0.01). Macrophages and
eosinophils were present in the invasive front and in the center of
the tumor mass in 65–72% cases (Table
II).
 | Table I.Evaluation of the neutrophils in the
stroma at the front and tumor center of colorectal cancer. |
Table I.
Evaluation of the neutrophils in the
stroma at the front and tumor center of colorectal cancer.
|
| Invasive front
(%) | Centre of main
tumor mass (%) |
|
|---|
| Type of cell |
|
| P-value |
|---|
| Group (percentage
of neutrophils) | 1 (0) | 2 (<20) | 3 (>21) | 1 (0) | 2 (<20) | 3 (>21) |
|
| Number of
cases | 48 (33.3) | 77 (53.4) | 19 (13.3) | 88 (61.1) | 16 (11.1) | 40 (27.8) | <0.010 |
 | Table II.Evaluation of the macrophages and
eosinophils in the stroma at the front and tumor center of
colorectal cancer. |
Table II.
Evaluation of the macrophages and
eosinophils in the stroma at the front and tumor center of
colorectal cancer.
|
| Invasive front | Center of main
tumor mass |
|
|---|
|
|
|
|
|
|---|
| Type of cell | Absent | Present | Absent | Present | P-value |
|---|
| Macrophages | 48 (33.3) | 96 (66.7) | 51 (35.4) | 93 (64.6) | 0.711 |
| Eosinophils | 40 (27.8) | 104 (72.2) | 45 (31.2) | 99 (68.8) | 0.998 |
Correlation analysis between each type
of inflammatory cell
According to the observations, neutrophils in the
invasive front of the CRC tumor positively correlated with
macrophages in the center of the tumor mass (R=0.239, P=0.004).
Furthermore, macrophages present in the invasive front were
negatively correlated with neutrophils, and positively correlated
with eosinophils in the deeper layer of the tumor mass (R=−0.170,
P=0.041; and R=0.318, P<0.010, respectively). Positive
correlations between eosinophils from the invasive front, and
macrophages and eosinophils in the center of the tumor mass were
observed (R=0.315, P<0.010; and R=0.353, P<0.010,
respectively). However, the presence of eosinophils was negatively
correlated with neutrophils in the main mass of CRC tumor
(R=−0.207, P=0.013). The results are presented in Table III.
 | Table III.Correlations of the cellular
composition of inflammatory infiltration in the stroma at the front
and tumor center of colorectal cancer. |
Table III.
Correlations of the cellular
composition of inflammatory infiltration in the stroma at the front
and tumor center of colorectal cancer.
|
| Neutrophils | Macrophages | Eosinophils |
|---|
|
|
|
|
|
|---|
| Centre of main mass
Invasive front | R | P-value | R | P-value | R | P-value |
|---|
| Neutrophils | −0.094 | 0.262 | 0.239 | 0.004 | 0.206 | 0.013 |
| Macrophages | −0.170 | 0.041 | 0.030 | 0.308 | 0.318 | <0.010 |
| Eosinophils | −0.207 | 0.013 | 0.315 | <0.010 | 0.353 | <0.010 |
Correlation between presence of
inflammatory cells and clinicopathological features of patients
with CRC
Macrophages localized in the center of the tumor
mass negatively correlated with histological type of adenocarcinoma
and the percentage of mucinous component (R=−0.171, P=0.040;
R=−0.221, P<0.010, respectively; Table IV). Macrophages were also associated
with tumor stroma percentage (R=0.220, P=0.011; Table IV). Patients with macrophages in the
center of the tumor mass were associated with venous invasion,
presence of deposits, and larger deposit size (R=−0.432;
P<0.010; R=−0.158, P<0.010; R=−0.203, P=0.015, respectively;
Table V).
 | Table IV.Correlation between a particular
population of inflammatory infiltrate located in the center of the
tumor and anatomic parameters in patients with colorectal
cancer. |
Table IV.
Correlation between a particular
population of inflammatory infiltrate located in the center of the
tumor and anatomic parameters in patients with colorectal
cancer.
| Variables | N | Neutrophils | Macrophages | Eosinophils |
|---|
| Age (years) |
|
|
|
|
|
<60 | 30 | NS | NS | NS |
|
≥60 | 114 |
|
|
|
| Sex |
|
|
|
|
|
Female | 56 | NS | NS | NS |
|
Male | 88 |
|
|
|
| Localization |
|
|
|
|
|
Right-side | 18 | NS | NS | −0.0186 |
|
Transverse | 13 |
|
| 0.031 |
|
Left-side | 12 |
|
|
|
|
Sigmoid | 25 |
|
|
|
|
Rectum | 76 |
|
|
|
| Tumor size
(cm) |
|
|
|
|
|
<2.5 | 23 | NS | NS | NS |
|
2.5–5.0 | 99 |
|
|
|
|
>5.0 | 22 |
|
|
|
| TNM stage |
|
|
|
|
| 1 | 36 | NS | NS | NS |
| 2 | 27 |
|
|
|
| 3 | 69 |
|
|
|
| 4 | 12 |
|
|
|
| Duke stage |
|
|
|
|
| A | 31 | NS | NS | NS |
| B | 33 |
|
|
|
| C | 69 |
|
|
|
| D | 11 |
|
|
|
| Adenocarcinoma
type |
|
|
|
|
| Partim
mucinous | 19 | NS | −0.221 | NS |
|
Adenocarcinoma | 125 |
| <0.010 |
|
| Malignancy
grade |
|
|
|
|
| 2 | 135 | NS | NS | NS |
| 3 | 9 |
|
|
|
| pT stage |
|
|
|
|
| 1 | 3 | NS | NS | NS |
| 2 | 52 |
|
|
|
| 3 | 86 |
|
|
|
| 4 | 3 |
|
|
|
| TSP (%) |
|
|
|
|
|
<50 | 73 | NS | 0.220 | NS |
|
>50 | 71 |
| 0.011 |
|
 | Table V.Correlation between a particular
population of inflammatory infiltrates located in the center of the
tumor and disease progression parameters in patients with
colorectal cancer. |
Table V.
Correlation between a particular
population of inflammatory infiltrates located in the center of the
tumor and disease progression parameters in patients with
colorectal cancer.
| Variables | N | Neutrophils | Macrophages | Eosinophils |
|---|
| Venous
invasion |
|
|
|
|
|
Absent | 123 |
| −0.432 |
|
|
Present | 21 | NS | <0.010 | NS |
| Lymphatic
invasion |
|
|
|
|
|
Absent | 118 | NS | NS | NS |
|
Present | 26 |
|
|
|
| Perineural
invasion |
|
|
|
|
|
Absent | 134 | NS | NS | NS |
|
Present | 10 |
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
Absent | 71 | NS | NS | NS |
|
Present | 73 |
|
|
|
| Distant
metastasis |
|
|
|
|
|
Absent | 129 | NS | NS | NS |
|
Present | 15 |
|
|
|
| Tumor deposits |
|
|
|
|
|
Absent | 122 | 0.354 | −0.220 | −0.158 |
|
Present | 22 | 0.000 | 0.008 | 0.000 |
| Size of tumor
deposits (mm) |
|
|
|
|
|
<2.5 | 12 | 0.170 | −0.203 | NS |
|
≥2.5 | 10 | 0.041 | 0.015 |
|
| K-M grade |
|
|
|
|
|
Low | 76 | NS | NS | NS |
|
High | 68 |
|
|
|
| Tumor budding |
|
|
|
|
|
Absence | 86 | NS | NS | NS |
|
Presence | 58 |
|
|
|
| Crohn's-like
aggregates of lymphocyte |
|
|
|
|
|
Absence | 112 | NS | NS | 0.236 |
|
Presence | 32 |
|
| <0.010 |
| Necrosis |
|
|
|
|
|
Absent | 42 | NS | NS | NS |
|
Focal | 51 |
|
|
|
|
Moderate | 34 |
|
|
|
|
Extensive | 17 |
|
|
|
| Fibrosis |
|
|
|
|
|
Absent | 9 | NS | NS | NS |
|
Focal | 67 |
|
|
|
|
Moderate | 38 |
|
|
|
|
Extensive | 30 |
|
|
|
| Maturation of
fibrotic stroma |
|
|
|
|
|
Immature | 10 | NS | NS | NS |
|
Intermediate | 84 |
|
|
|
|
Mature | 50 |
|
|
|
Neutrophils were positively correlated with the
presence of deposits of cancer cells deposits (R=0.354, P<0.010;
Table V). In addition, neutrophils
were positively correlated with deposits of >2.5 mm in size
(R=0.170, P=0.041, Table V).
The presence of eosinophils in the center of tumor
mass was negatively correlated with tumor localization and deposits
presence (R=−0.186, P=0.031, Table
V; and R=−0.220, P=0.008, Table
IV, respectively), but positively with Crohn's-like aggregates
of lymphocytes (R=0.236, P<0.010).
Correlation between DFS time and
inflammatory cells in the center of the tumor mass
The mean DFS time was 12.1 months for all patients.
Patients with macrophages present in the center of the tumor mass
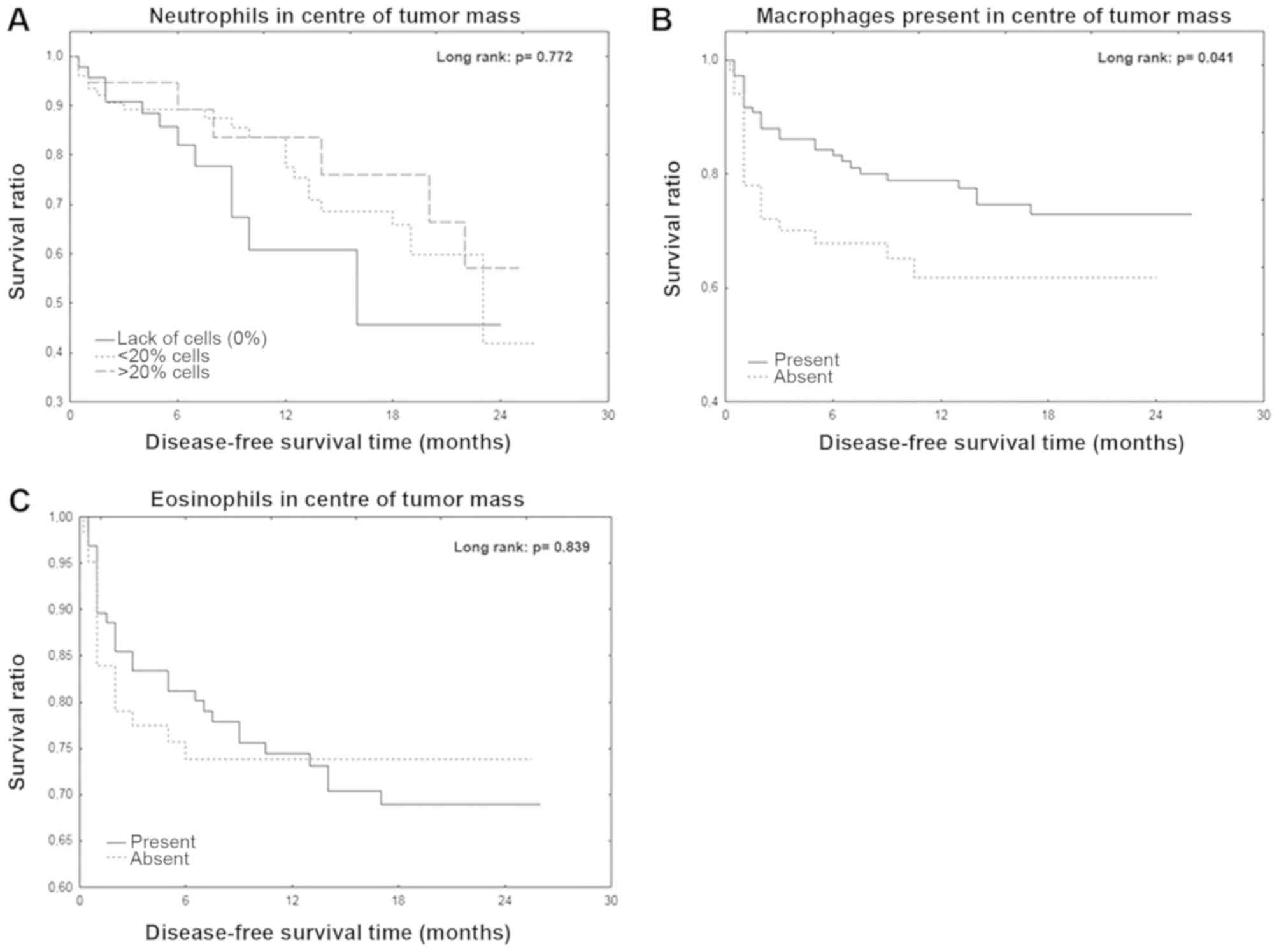
exhibited a significantly longer DFS time (P=0.041; Fig. 1A). However, the Kaplan-Meier curve
analyses of neutrophils and eosinophils in the center of tumor mass
were not statistically significant (Fig.
1B and C).
Discussion
Antigens located on cancer cells determine the
reactivity and quality of the immune response. Immune system cells
migrate to the location of a foreign entity and initiate the
inflammatory response. Neutrophil granulocytes represent the first
line of immunological defense, and activate or inhibit subsequent
populations of immunocompetent cells (21). These mechanisms confirm the
interdependencies between certain types of inflammatory cells,
including macrophages, neutrophils and eosinophils. The present
study demonstrated that neutrophils were mainly present in the
invasive front of the primary tumor (60% of cases) compared with in
the center of the tumor (30% of patients). Rao et al
(22) reported that intratumoral
neutrophil infiltration was observed in 45.4% of patients diagnosed
with CRC. However, Arelaki et al (23) observed that neutrophils gradually
move from the tumor mass to the invasive front. The present study
also reported that neutrophils were located in the center of the
primary tumor of patients with CRC was positively correlated with
the presence of tumor deposits and the size of these deposits.
Furthermore, the results from this study were consistent with the
observations from Rao et al (22) who reported that the increase in the
number of intratumoral neutrophils is associated with determinants
of disease progression, including TNM. However, Galdiero et
al (24) indicated that the
density of tumor-associated neutrophils dramatically decreased in
patients with stage IV CRC compared with patients with stages I–III
CRC. Neutrophils are the first inflammatory infiltration cells that
can activate further inflammatory reaction pathways, such as
lymphocyte activation. However, a long-lasting infiltration of
numerous neutrophils, particularly in the center of the tumor,
causes the degradation of the extracellular matrix, leading to the
secretion of metalloproteinases into the extracellular matrix,
resulting in its degradation and enabling the progression of cancer
development (25). The presence and
number of neutrophils in the primary tumor in patients with
colorectal cancer may depend on the ability of tumor cells to evade
the immune response (26).
Eosinophil granulocytes are involved in the
following step of the inflammatory reaction cascade, originally in
the allergic reaction, but also in the neoplastic changes (9). The present study demonstrated that
eosinophils were present in the invasive tumor front and in the
center of the tumor in ~70% of patients with CRC. A previous study
by Kiziltaş et al (27)
confirmed the presence of eosinophil infiltration in ~75% of
patients with CRC. In addition, Cho et al (28) observed a decrease in the number of
eosinophils in the tumor tissue of patients with CRC. Furthermore,
a previous study reported that a high percentage of eosinophils is
present in CRC tissue (29). The
present study also demonstrated that the percentage of eosinophils
in the center of the tumor mass was negatively correlated with the
presence of cancer cell deposits. Prizment et al (9) reported that a high eosinophil score in
the tumor is negatively correlated with age and tumor stage. In
addition, Harbaum et al (30)
demonstrated that increased peri- and intratumoral eosinophil
counts are significantly associated with T and N classification,
tumor differentiation and vascular invasion. The presence of
eosinophils in the tumor center in patients with CRC may therefore
influence the activation of the immune system due to their
association with other determinants of disease progression.
TAMs are macrophages originating from the
circulating monocytes subpopulation, which settle into the tumor
tissue stroma (31). TAMs divide
into one of two subpopulations, M1 and M2. M2 TAMs possess the
ability to secrete various types of growth factors that stimulate
tumor growth and neoangiogenesis (32). In addition, TAMs produce numerous
proteolytic enzymes, including metalloproteinases and cathepsins,
which are involved in extracellular matrix degradation that
subsequently conditions the tumor cell aggressiveness and viability
(33,34). However, some anti-cancer properties
of M1 TAMs have been demonstrated in previous studies (35,36). The
presence of both CD68-positive TAMs and VEGF were associated with
better prognosis in colon carcinoma (36). Due to their pro- and anti-tumor, the
present study investigated the occurrence of TAMs in the tumor
tissue of patients with CRC. The results demonstrated that such
macrophages were present in 65–72% of all cases, in the invasive
front and in center of the tumor. In addition, the present study
revealed that presence of macrophages was accompanied by tumors
primarily containing nonmucinous component. Colon tumors containing
mucus-producing cells typically have poor connective tissue stroma.
Compared with tumors with glandular cancer spurs, Nonmucinous
tumors are widely distributed in a rich stroma. In this case,
inflammatory cells have the possibility to accumulate and freely
migrate (37). The results from the
present study confirmed that the presence of macrophages in the
tumor center was associated with the tumor stroma percentage. In
addition, the results revealed that macrophages were present in
tumors that contained relatively more stroma compared with
cancerous tissue. A previous study reported that TAMs present
increased expression of tumor growth factor β and IL-6, which may
activate tumor angiogenesis (38).
However, Funada et al (39)
reported that low levels of macrophage infiltration at the invasive
margin were associated with a higher rate of vascular invasion and
lymph node metastasis. In addition, Zhou et al (40) demonstrated that a low TAMs density at
the invasive front is associated with a higher occurrence of
hepatic metastasis. According to Koelzer et al (41), strong infiltration of TAM type M2
predicts a lower tumor grade and reduces lymph node metastasis.
Ammendola et al (42)
confirmed that macrophages presence is correlated with angiogenesis
in patients with locally advanced CRC. Gulubova et al
(43) reported that a low number of
CD68-positive cells, including macrophages, is associated with
disease progression parameters, including the presence of
metastases in local lymph nodes, distant metastases, advanced tumor
stage, tumor cell invasion of blood and lymph vessels or perineural
invasion. This was consistent with the results from the present
study, which demonstrated that the presence of macrophages was
negatively correlated with the tumor disease progression factors,
including blood vessel involvement and the presence and size of
cancer cell deposits. The present study confirmed the crucial role
of macrophages in the immune response from the tumors of patients
with CRC. However, the exact subtype of macrophages involved
requires further immunophenotypic assessment.
The cellular composition of inflammatory infiltrate
may represent a potential prognostic marker for patients with CRC.
The present study demonstrated that the presence of macrophages in
the center of the primary tumor was associated with a significantly
longer DFS time. The beneficial effect of the presence of
macrophage infiltration on the DFS of patients with CRC was also
confirmed by Funada et al (39), Zhou et al (40), Gulubova et al (43) and Kinouchi et al (44). The present study investigated the
association between neutrophils and eosinophils located in the
tumor center with DFS. No significant differences were observed,
conversely with previous studies that indicated a beneficial effect
of neutrophils and eosinophils in the tumor center on CRC patients
DFS (9,44–46).
In conclusion, the results from the present study
confirmed the importance of assessing the cellular composition of
the inflammatory mass in the primary tumor of patients with CRC.
Notably, macrophages located in the stroma of the tumor center were
associated with a longer DFS in patients.
Acknowledgements
Not applicable.
Funding
The author(s) received funding support from Medical
University of Bialystok for this work.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KJ is responsible for the conception and design the
study, data collection, statistical analysis and writing the paper.
MK and LKK performed the histological examination. WK and WF
collected the data and reviewed the article. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki for Human Experimentation, and the
protocol was approved by The Bioethics Committee of the Medical
University of Bialystok (approval no. R-I-002/351/2016). Written
informed consent was obtained from all participants.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Testa U, Pelosi E and Castelli G:
Colorectal cancer: Genetic abnormalities, tumor progression, tumor
heterogeneity, clonal evolution and tumor-initiating cells. Med Sci
(Basel). 6:E312018.PubMed/NCBI
|
|
2
|
Merlano MC, Granetto C, Fea E, Ricci V and
Garrone O: Heterogeneity of colon cancer: From bench to bedside.
ESMO Open. 2:e0002182017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eljaszewicz A, Gackowska L, Kubiszewska I,
Jankowski M, Urbańska M, Wiese M, Helmin-Basa A, Michałkiewicz J
and Zegarski W: Aktywność makrofagów w rozwoju choroby
nowotworowej. Współczesna Onkologia. 1:1–6. 2010.(In Poland).
View Article : Google Scholar
|
|
4
|
Hong-Fang T, Yan-Hui P, Lei B and Wan-Xing
Z: Effects of granulocyte colony-stimulating factor on opsonin
receptor expression and neutrophil antibacterial activity in a
mouse model of severe acute pancreatitis. Postepy Hig Med Dosw
(Online). 71:352–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dubois RN: Role of inflammation and
inflammatory mediators in colorectal cancer. Trans Am Clin Climatol
Assoc. 125:358–372; discussion 372–373. 2014.PubMed/NCBI
|
|
6
|
Dunn GP, Old LJ and Schreiber RD: The
immunobiology of cancer immunosurveillance and immunoediting.
Immunity. 21:137–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCourt M, Wang JH, Sookhai S and Redmond
HP: Activated human neutrophils release hepatocyte growth
factor/scatter factor. Eur J Surg Oncol. 27:396–403. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shamamian P, Schwartz JD, Pocock BJ, Monea
S, Whiting D, Marcus SG and Mignatti P: Activation of progelatinase
A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: A
role for inflammatory cells in tumor invasion and angiogenesis. J
Cell Physiol. 189:197–206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prizment AE, Vierkant RA, Smyrk TC,
Tillmans LS, Lee JJ, Sriramarao P, Nelson HH, Lynch CF, Thibodeau
SN, Church TR, et al: Tumor eosinophil infiltration and improved
survival of colorectal cancer patients: Iowa women's health study.
Mod Pathol. 29:516–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
NCCN Clinical Practice Guidelines in
Oncology, . Colon Cancer. https://www.nccn.org.asp
|
|
11
|
Kyriakos M: The President cancer, the
Dukes classification, and confusion. Arch Pathol Lab Med.
109:1063–1066. 1985.PubMed/NCBI
|
|
12
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the United States, national cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin Q, Wei Y, Ren L, Zhong Y, Qin C, Zheng
P, Xu P, Zhu D, Ji M and Xu J: Tumor deposit is a poor prognostic
indicator in patients who underwent simultaneous resection for
synchronous colorectal liver metastases. Onco Targets Ther.
8:233–240. 2015.PubMed/NCBI
|
|
14
|
Huijbers A, Tollenaar RA, v Pelt GW,
Zeestraten EC, Dutton S, McConkey CC, Domingo E, Smit VT, Midgley
R, Warren BF, et al: The proportion of tumor-stroma as a strong
prognosticator for stage II and III colon cancer patients:
Validation in the VICTOR trial. Ann Oncol. 24:179–185. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morodomi T, Isomoto H, Shirouzu K,
Kakegawa K, Irie K and Morimatsu M: An index for estimating the
probability of lymph node metastasis in rectal cancers. Lymph node
metastasis and the histopathology of actively invasive regions of
cancer. Cancer. 63:539–543. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klintrup K, Mäkinen JM, Kauppila S, Väre
PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ and
Mäkinen MJ: Inflammation and prognosis in colorectal cancer. Eur J
Cancer. 41:2645–2654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richards CH, Flegg KM, Roxburgh CS, Going
JJ, Mohammed Z, Horgan PG and McMillan DC: The relationships
between cellular components of the peritumoural inflammatory
response, clinicopathological characteristics and survival in
patients with primary operable colorectal cancer. Br J Cancer.
106:2010–2015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Väyrynen JP, Sajanti SA, Klintrup K,
Mäkelä J, Herzig KH, Karttunen TJ, Tuomisto A and Mäkinen MJ:
Characteristics and significance of colorectal cancer associated
lymphoid reaction. Int J Cancer. 134:2126–2135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ueno H, Jones AM, Wilkinson KH, Jass JR
and Talbot IC: Histological categorization of fibrotic cancer
stroma in advanced rectal cancer. Gut. 53:581–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lichtman MA, Kipps TJ, Seligsohn U,
Kaushansky K and Prchal JT: Williams Hematology. (8th). McGraw-Hill
Companies. 875–999. 2010.
|
|
21
|
Nowak B: Patomechanizm rozwoju reakcji
zapalnej. Przegl Reumatol. R.1 nr 4; s.5. 2005.
|
|
22
|
Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng
YX, Cai MY and Xie D: Increased intratumoral neutrophil in
colorectal carcinomas correlates closely with malignant phenotype
and predicts patients' adverse prognosis. PLoS One. 7:e308062012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arelaki S, Arampatzioglou A, Kambas K,
Papagoras C, Miltiades P, Angelidou I, Mitsios A, Kotsianidis I,
Skendros P, Sivridis E, et al: Gradient infiltration of neutrophil
extracellular traps in colon cancer and evidence for their
involvement in tumour growth. PLoS One. 11:e01544842016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galdiero MR, Bianchi P, Grizzi F, Di Caro
G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S,
Polentarutti N, et al: Occurrence and significance of
tumor-associated neutrophils in patients with colorectal cancer.
Int J Cancer. 139:446–456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mizuno R, Kawada K, Itatani Y, Ogawa R,
Kiyasu Y and Sakai Y: The role of tumor-associated neutrophils in
colorectal cancer. Int J Mol Sci. 20:E5292019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mlecnik B, Tosolini M, Kirilovsky A,
Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman
WH, Pagès F and Galon J: Histopathologic-based prognostic factors
of colorectal cancers are associated with the state of the local
immune reaction. J Clin Oncol. 29:610–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kiziltaş S, Sezgin Ramadan S, Topuzoğlu A
and Küllü S: Does the severity of tissue eosinophilia of colonic
neoplasms reflect their malignancy potential? Turk J Gastroenterol.
19:239–244. 2008.PubMed/NCBI
|
|
28
|
Cho H, Lim SJ, Won KY, Bae GE, Kim GY, Min
JW and Noh BJ: Eosinophils in colorectal neoplasms associated with
expression of CCL11 and CCL24. J Pathol Transl Med. 50:45–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moezzi J, Gopalswamy N, Haas RJ Jr,
Markert RJ, Suryaprasad S and Bhutani MS: Stromal eosinophilia in
colonic epithelial neoplasms. Am J Gastroenterol. 95:520–523. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harbaum L, Pollheimer MJ, Kornprat P,
Lindtner RA, Bokemeyer C and Langner C: Peritumoral eosinophils
predict recurrence in colorectal cancer. Mod Pathol. 28:403–413.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mantovani A, Bottazzi B, Colotta F,
Sozzani S and Ruco L: The origin and function of tumor-associated
macrophages. Immunol Today. 13:265–270. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leek RD, Lewis CE, Whitehouse R, Greenall
M, Clarke J and Harris AL: Association of macrophage infiltration
with angiogenesis and prognosis in invasive breast carcinoma.
Cancer Res. 56:4625–4629. 1996.PubMed/NCBI
|
|
33
|
Mantovani A: Tumor-associated macrophages
in neoplastic progression: A paradigm for the in vivo function of
chemokines. Lab Invest. 71:5–16. 1994.PubMed/NCBI
|
|
34
|
Afik R, Zigmond E, Vugman M, Klepfish M,
Shimshoni E, Pasmanik-Chor M, Shenoy A, Bassat E, Halpern Z, Geiger
T, et al: Tumor macrophages are pivotal constructors of tumor
collagenous matrix. J Exp Med. 213:2315–2331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohtani H, Naito Y, Saito K and Nagura H:
Expression of costimulatory molecules B7-1 and B7-2 by macrophages
along invasive margin of colon cancer: A possible antitumor
immunity? Lab Invest. 77:231–241. 1997.PubMed/NCBI
|
|
36
|
Khorana AA, Ryan CK, Cox C, Eberly S and
Sahasrabudhe DM: Vascular endothelial growth factor, CD68, and
epidermal growth factor receptor expression and survival in
patients with Stage II and Stage III colon carcinoma: A role for
the host response in prognosis. Cancer. 97:960–968. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hamilton SR, Bosman FT, Boffetta P, et al:
Carcinoma of the colon and rectum In: WHO classification of tumours
of the digestive system. Bosman FT, Carneiro F, Hruban RH and
Theise ND: (4th). IARC. (Lyon). 134–146. 2010.
|
|
38
|
Popovic ZV, Sandhoff R, Sijmonsma TP,
Kaden S, Jennemann R, Kiss E, Tone E, Autschbach F, Platt N, Malle
E and Gröne HJ: Sulfated glycosphingolipid as mediator of
phagocytosis: SM4s enhances apoptotic cell clearance and modulates
macrophage activity. J Immunol. 179:6770–6782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Funada Y, Noguchi T, Kikuchi R, Takeno S,
Uchida Y and Gabbert HE: Prognostic significance of CD8+ T cell and
macrophage peritumoral infiltration in colorectal cancer. Oncol
Rep. 10:309–313. 2003.PubMed/NCBI
|
|
40
|
Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH,
Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, et al: The density of
macrophages in the invasive front is inversely correlated to liver
metastasis in colon cancer. J Transl Med. 8:132010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koelzer VH, Canonica K, Dawson H, Sokol L,
Karamitopoulou-Diamantis E, Lugli A and Zlobec I: Phenotyping of
tumor-associated macrophages in colorectal cancer: Impact on single
cell invasion (tumor budding) and clinicopathological outcome.
Oncoimmunology. 5:e11066772015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ammendola M, Patruno R, Sacco R, Marech I,
Sammarco G, Zuccalà V, Luposella M, Zizzo N, Gadaleta C, Porcelli
M, et al: Mast cells positive to tryptase and tumour-associated
macrophages correlate with angiogenesis in locally advanced
colorectal cancer patients undergone to surgery. Expert Opin Ther
Targets. 20:533–540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gulubova M, Ananiev J, Yovchev Y, Julianov
A, Karashmalakov A and Vlaykova T: The density of macrophages in
colorectal cancer is inversely correlated to TGF-β1 expression and
patients' survival. J Mol Histol. 44:679–692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kinouchi M, Miura K, Mizoi T, Ishida K,
Fujibuchi W, Sasaki H, Ohnuma S, Saito K, Katayose Y, Naitoh T, et
al: Infiltration of CD40-positive tumor-associated macrophages
indicates a favorable prognosis in colorectal cancer patients.
Hepatogastroenterology. 60:83–88. 2013.PubMed/NCBI
|
|
45
|
Fernández-Aceñero MJ, Galindo-Gallego M,
Sanz J and Aljama A: Prognostic influence of tumor-associated
eosinophilic infiltrate in colorectal carcinoma. Cancer.
88:1544–1548. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wikberg ML, Ling A, Li X, Öberg Å, Edin S
and Palmqvist R: Neutrophil infiltration is a favorable prognostic
factor in early stages of colon cancer. Hum Pathol. 68:193–202.
2017. View Article : Google Scholar : PubMed/NCBI
|