Introduction
Globally, colorectal cancer (CRC) is one of the most
common types of cancer (1). At the
early stage of CRC (i.e., stages I and II), CRC is amenable to
surgery and curative treatment, with a 5-year survival rate >60%
for patients with CRC. However, >50% of patients present with
advanced disease (at or beyond stage III) and a high incidence of
distant metastasis (2). In these
patients, the 5-year survival rate drops to 10% (2). During metastatic progression, cancer
cells can detach from the primary tumor site, passing through the
circulation system to form metastatic tumors in distant organs
(3). The epithelial-mesenchymal
transition (EMT) is a process that contributes to the early stages
of cancer metastasis, in which polarized epithelial cells lose
their cell polarity and cell-cell adhesion to acquire a mesenchymal
phenotype (4). The EMT process
allows cancer cells to gain migratory and invasive properties that
promote cancer metastasis (4). Since
metastasis is considered as the leading cause of failure in cancer
treatment, the development of novel pharmaceuticals for the
prevention and treatment of cancer metastasis is critical.
The transforming growth factor (TGF)-β1 pathway is
essential to the EMT in cancer cells. In this process, TGF-β1
initiates downstream signaling, by the dimerized TGF-β1 ligand
binding to the type III TGF-β1 receptor, which then presents the
ligand to the type II TGF-β1 receptor, which leads to the
phosphorylation of the type I TGF-β1 receptor (TβR-I) (5,6). In
canonical TGF-β1 signaling, activated TβR-I phosphorylates the
intracellular proteins Smad2/3, which in turn bind to Smad4, before
translocating into the nucleus where they initiate transcriptional
changes of target genes, including of the zinc finger E-box-binding
homeobox (ZEB) family (7,8). ZEB members act as suppressors of these
transcriptional factors, downregulating epithelial markers, such as
E-cadherin, while upregulating mesenchymal markers, such as
N-cadherin, which contributes to the development of cancer
metastasis (9). In addition, TGF-β1
can also signal through the non-canonical pathway, which includes
focal adhesion kinase (FAK). TGF-β1 has been shown to induce the
Src-dependent phosphorylation of FAK, and its consequent activation
is required for the upregulation of mesenchymal and invasiveness
markers and the delocalization of E-cadherin, which promotes
metastasis of cancer cells (10).
microRNAs (miRNAs) represent a class of non-coding
RNAs, that are 21–24 nucleotides long, that suppress target gene
expression in a sequence specific manner (11). The dysregulation of several miRNAs
has been identified to induce EMT and promote colorectal metastasis
associated with poorer survival (12). The miR-200 family consists of five
members, including miR-200a/b/c, miR-141 and miR-429, and has been
shown to be master regulators of EMT, promoting cell dissemination
from the primary tumor and subsequent metastasis (13). Indeed, loss of miR-200a/b/c
expression has been shown to promote cellular metastasis in several
cancers by inhibiting the ZEB transcription factor family and is
correlated with poorer survival in patients with CRC (14–16).
Notably, at the miRNA level, DNA methylation of miR-200a/b/c is a
key mechanism in the negative regulation of its expression, which
has been reported to be mediated by TGF-β1 signaling (17). Thus, the TGF-β1/ZEB/miR-200a/b/c
signaling network (a positive correlation between TGF-β1 and ZEB,
negative correlations between TGF-β1 and miR-200a/b/c and between
miR-200a/b/c and ZEB) supports the maintenance of the mesenchymal
phenotype required for metastasis (18).
Worldwide, agents used in Traditional Chinese
Medicine (TCM) have received increasing interest in recent years
for the treatment of various cancers, due to relatively low
toxicity and few side effects (19).
There is an urgent need to identify naturally occurring agents for
effective anticancer treatments. Ursolic acid (UA) is present in
many herbs and plants used in TCM, including Hedyotic diffusa,
Scutellaria barbata, Spica prunellae and Patrinia
scabiosaefolia, and possesses excellent anticancer properties
against various types of cancers, including CRC (20–23).
Increasing evidence indicates that UA has several biological
properties, such as anti-inflammatory, antiviral, antioxidant,
cytotoxic, anticancer, and antidiabetic (24–30). Our
previous studies have shown that UA induced CRC cell apoptosis and
suppressed cell proliferation and CRC angiogenesis via multiple
signaling pathways (28,29). In the present study, the effect of UA
on CRC cell migration and invasion in vitro was further
evaluated and the potential molecular mechanisms of its action were
elucidated.
Materials and methods
Material and reagents
UA was purchased from Sigma-Aldrich (Merck KGaA).
RPMI-1640 medium, PBS and penicillin-streptomycin were purchased
from Hyclone (GE Healthcare Life Sciences). Fetal bovine serum
(FBS) and trypsin-EDTA were obtained from Gibco (Thermo Fisher
Scientific. Inc.). 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) was purchased from Beijing
Solarbio Science & Technology Co., Ltd. miR-200a/b/c and U6
primers were synthesized by Takara Biotechnology Co., Ltd. RNAiso
for small RNA kit, Mir-X™ miRNA First-Strand Synthesis kit and TB
Green™ Premix Ex Taq II kit were purchased from Takara
Biotechnology Co., Ltd. N-cadherin (cat. no. ab18203) and
E-cadherin (cat. no. ab1416) antibodies were purchased from Abcam.
TGF-β1 (cat. no. 3711), phosphorylated (p-) Smad2/3 (cat. no.
8828), Smad2/3 (cat. no. 8685), p-FAK (cat. no. 3284), FAK (cat.
no. 71433), ZEB1 (cat. no. 3396) and horseradish peroxidase
(HRP)-conjugated secondary antibodies (anti-rabbit IgG; cat. no.
7074; and anti-mouse IgG; cat. no. 7076) were purchased from Cell
Signaling Technology, Inc. The β-actin antibody (cat. no.
66009-1-Ig) was purchased from ProteinTech Group, Inc. The
Transwell chambers were obtained from Corning Life Sciences and the
BD BioCoat Matrigel Invasion Chamber was purchased from BD
Bioscience. All the other chemicals, unless otherwise stated, were
obtained from Sigma-Aldrich (Merck KGaA).
Cell culture
Human colon cancer HCT116 and HCT-8 cell lines were
obtained from the Nanjing KeyGen Biotech. Co. Ltd. Cells were
cultured in RPMI-1640 medium, supplemented with 10% FBS, 100 U/ml
penicillin and 100 mg/ml streptomycin in a 37°C humidified
incubator supplemented with 5% CO2.
MTT assay
UA was dissolved in DMSO and diluted with culture
medium to the desired concentrations. The final concentration of
DMSO in the culture medium was <0.1% throughout the study.
HCT116 and HCT-8 cells were plated into 96-well plates at a
1×104 cells/well and treated with 0, 10, 20, 40 µM UA
for 24 h and 48 h. Treatment with DMSO was included as the vehicle
control. Following treatment, 100 µl of 0.5 mg/ml MTT solution was
added in each well at 37°C for 4 h. The MTT formazan precipitate
was dissolved in 100 µl of DMSO. Subsequently, the resulting
absorbance of the purple formazan product was determined at 570 nm
with a ELX800 microplate reader (BioTek Instruments, Inc.). The
cell viability was determined using the formula: Cell viability
(%)=sample optical density (OD)/control OD ×100.
Transwell assay
To evaluate the cell migration and invasion,
Transwell assays were conducted using Transwell cell culture
chambers with 8 µm pore filters (Corning Life Sciences). After
treatment with 0, 10, 20, 40 µM of UA for 24 h, HCT116 and HCT-8
cells were harvested and resuspended in serum-free RPMI-1640
without UA. Then, ~5×104 cells that survived after the
indicated concentrations of UA treatment for 24 h were seeded into
the upper chambers. The lower chambers were filled with RPMI-1640
media containing 10% FBS as a chemoattractant. Cells were allowed
to migrate towards the complete medium for 12 h in the migration
assay, the non-migrating cells in the upper chamber were wiped and
the migrated cells were stained with crystal violet for 15 min at
room temperature. For quantification, the average number of
migrated cells per field was assessed by counting three random
fields under a phase contrast microscope (Leica Microsystems GmbH)
at a magnification of ×200. The cell invasion assay was similar to
the migration assay, except that the upper chambers were coated
with Matrigel matrix (BD Biosciences).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Following treatment with the indicated
concentrations of UA for 24 h, total small RNA was extracted using
RNAiso for small RNA kit. Total small RNA was reverse transcribed
with an Mir-X™ miRNA First-Strand Synthesis kit following the
manufacturer's protocol. The primers for miR-200a (cat. no.
DHM0178), miR-200b (cat. no. DHM0179), miR-200c (cat. no. DHM0180)
and U6 (cat. no. D356-03) were obtained from Takara Biotechnology
Co., Ltd. The obtained cDNA was used to determine the levels of
miR-200a, miR-200b and miR-200c using TB Green™ Premix Ex Taq II in
an ABI 7500 Fast PCR system, according to the manufacturer's
instructions. The PCR conditions were as follows: Pre-denaturation
at 95°C for 2 min, then 45 cycles of 95°C for 3 sec and 60°C for 30
sec. U6 was used as an internal control. Relative quantification
was performed using the 2−ΔΔCq method (31). Each PCR amplification was carried out
in triplicate.
Western blot analysis
After treatment with the indicated concentrations of
UA for 24 h, total protein was extracted with cell lysis buffer
(Pierce; Thermo Fisher Scientific, Inc.) containing protease and
phosphatase inhibitors. The concentration of total protein was
detected using a bicinchoninic acid assay. A total of 50 µg protein
was separated on 10% SDS-PAGE gels and transferred onto
nitrocellulose membranes (EMD Millipore Corporation). The membranes
were blocked with 0.5% BSA for 2 h at room temperature and then
probed with primary antibodies against TGF-β1 (1:1,000), p-Smad2/3
(1:1,000), Smad2/3 (1:1,000), p-FAK (1:1,000), FAK (1:1,000),
N-cadherin (1:1,000), E-cadherin (1:2,000), ZEB1 (1:1,000) and
β-actin (1:5,000) overnight at 4°C, followed by 1 h incubation with
the anti-rabbit IgG HRP-conjugated secondary antibodies (1:2,000)
or anti-mouse IgG HRP-conjugated secondary antibodies (1:2,000) at
room temperature. Subsequently, using TBS/Tween-20 to wash the
membranes, the immunoreactive bands were visualized via Image Lab
software (version 3.0; Bio-Rad Laboratories, Inc.) using enhanced
chemiluminescence (Yuheng Biotechnology Co., Ltd.).
Statistical analysis
All data were expressed as mean ± standard
deviation. Data were analyzed using SPSS software (version 16.0;
SPSS Inc.). Statistical analysis was performed using one-way
analysis of variance and least significant difference post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
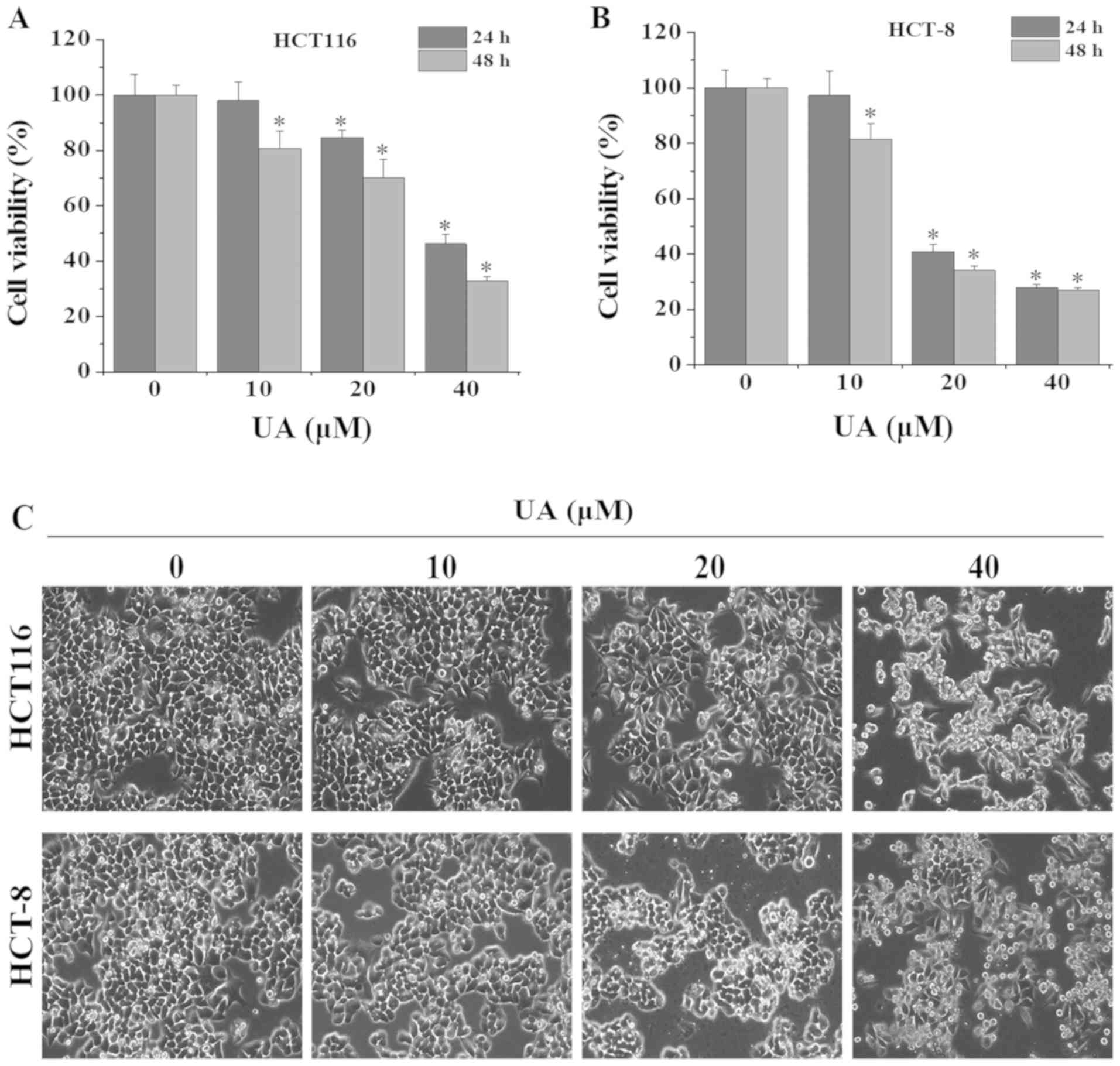
UA inhibits the growth of HCT116 and
HCT-8 cells
To evaluate the inhibitory effect of UA on the
growth of HCT116 and HCT-8 cells, MTT assays were performed. UA
treatment significantly inhibited cell viability in both a dose-
and time-dependent manner (Fig. 1A and
B). After 24 h of treatment, the half maximal inhibitory
concentration (IC50) values of UA for HCT116 and HCT-8
cells were calculated to be 37.2 and 25.2 µM, respectively.
Similarly, after 48 h of treatment, the IC50 values of
UA were determined to be 28.0 and 19.4 µM for HCT116 and HCT-8
cells, respectively. Cells exhibiting condensed and fragmented
nuclei are considered as growth inhibited. Phase-contrast
microscopy was used to examine the effect of UA on HCT116 and HCT-8
cell morphology. Untreated control cells were observed in a
confluent monolayer, healthy and attached to the culture plate,
whereas UA treatment significantly decreased the confluence of
these two cell lines and resulted in condensed, fragmented and
detached cells, in a dose-dependent manner (Fig. 1C). Taken together, these results
indicated that UA significantly inhibited the growth of HCT116 and
HCT-8 cells.
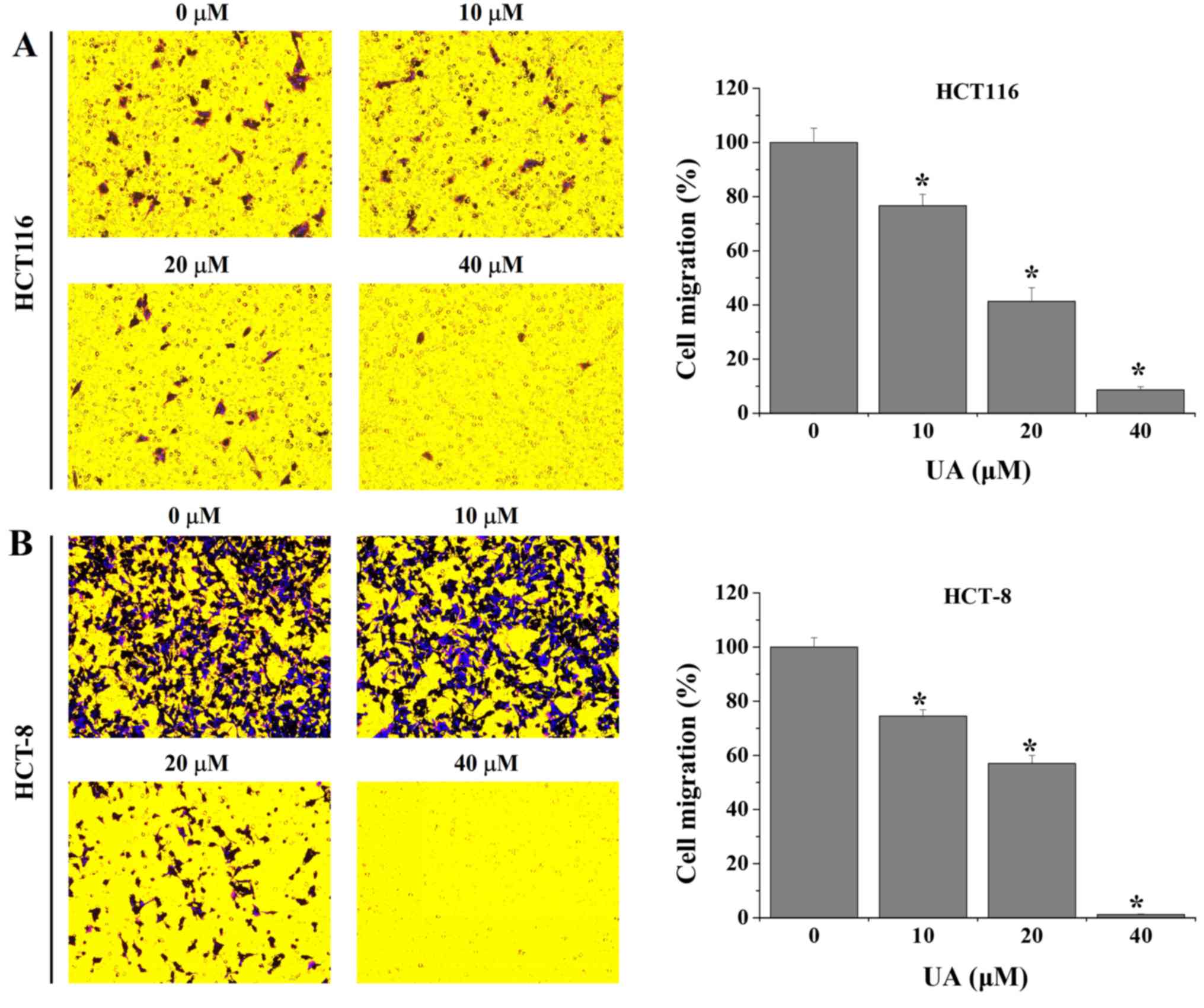
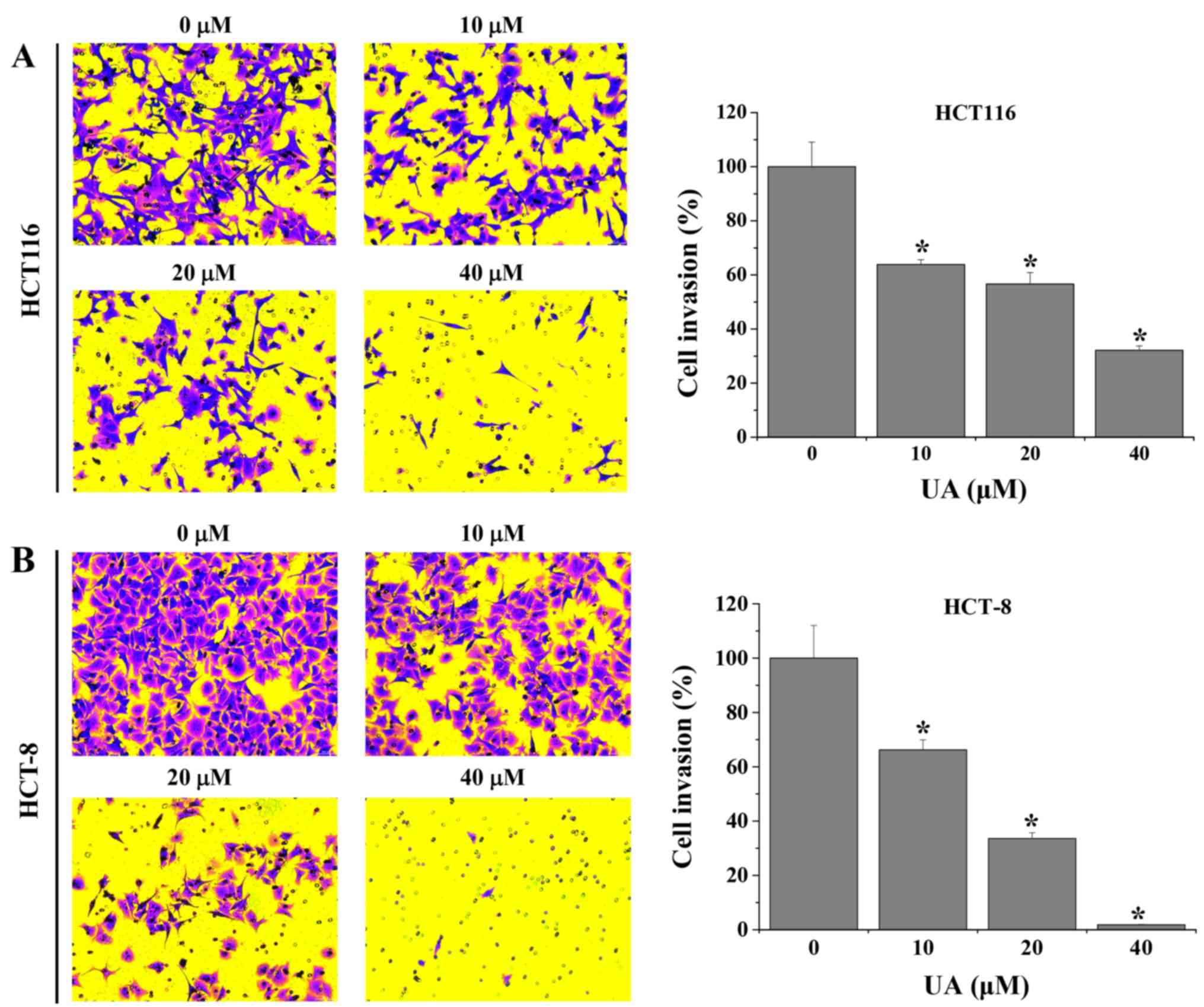
UA inhibits migration and invasion in
HCT116 and HCT-8 cells
Transwell assays were performed to evaluate the
effect of UA on the migration of HCT116 and HCT-8 cells. As shown
in Fig. 2, the results demonstrated
that treatment with 10–40 µM UA significantly decreased the
migratory rate of HCT116 and HCT-8 cells by 23.3±4.2–91.3±1.2% and
25.5±2.4–98.8±0.2%, respectively, when compared with untreated
cells. Furthermore, UA was demonstrated to inhibit the invasive
abilities of HCT116 and HCT-8 cells. The results revealed that the
invasion rate of HCT116 and HCT-8 cells following UA treatment was
36.2±1.8–67.9±1.6% and 33.8±3.7–98.2±0.2%, respectively, compared
with that in the untreated cells (Fig.
3). Taken together, these results suggest that UA exhibited an
inhibitory effect on the migration and invasion properties of
HCT116 and HCT-8 cells in a dose-dependent manner.
UA inhibits TGF-β1/Smad and TGF-β1/FAK
signaling pathways and regulates EMT-related proteins in HCT116 and
HCT-8 cells
EMT has been shown to be associated with the
metastasis of tumor cells (32).
TGF-β1 signaling pathways, including the canonical TGF-β1/Smad
pathway and the non-canonical TGF-β1/FAK signaling pathway, can
trigger EMT (10,33). To better understand whether UA
inhibited TGF-β1 signaling pathways, the expression of several
pivotal mediators of TGF-β1/Smad and TGF-β1/FAK signaling pathways
was assessed in HCT116 and HCT-8 cells. As shown in Fig. 4, western blot analysis revealed that
UA treatment (0, 10, 20, and 40 µM) dose-dependently decreased the
expression levels of TGF-β1, and the expression levels of the
TGF-β1 target gene ZEB1. Activation of Smad2/3 and FAK is mediated
by their phosphorylation, and UA treatment also significantly
reduced the phosphorylation levels of both Smad2/3 and FAK
(Fig. 4). Inhibition of the
TGF-β1/Smad and TGF-β1/FAK signaling pathways by UA led to a
decrease in the expression of the mesenchymal marker N-cadherin
compared with that in the control cells (Fig. 4). However, no difference was observed
between the control cells and UA-treated cells regarding the
protein expression levels of the epithelial marker E-cadherin
(Fig. 4). These results indicated
that the antitumor properties of UA may be mediated by the
inhibition of the TGF-β1 signaling pathways.
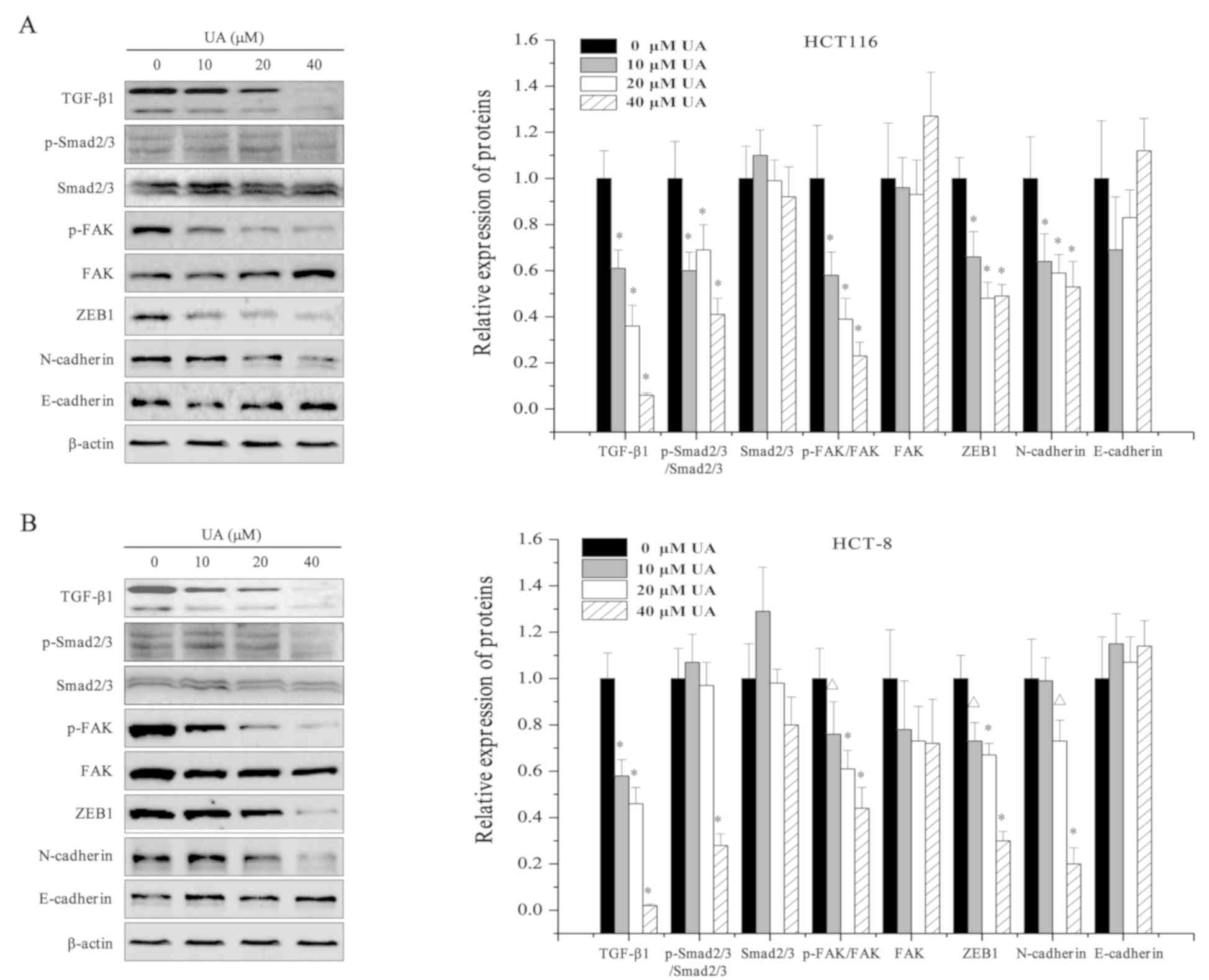 | Figure 4.Effect of UA on the expression of
TGF-β1 pathway-associated proteins in HCT116 and HCT-8 cells. (A)
The protein expression levels of TGF-β1, p-Smad2/3, Smad2/3, p-FAK,
FAK, ZEB1, N-cadherin and E-cadherin in HCT116 and (B) HCT-8 cells
were determined using western blot analysis following treatment
with 0, 10, 20, 40 µM UA for 24 h. β-actin was used as the internal
control. Images are representative of three independent
experiments. Relative densitometric analysis is shown.
ΔP<0.05 and *P<0.01 vs. untreated control cells.
UA, ursolic acid; TGF-β1, transforming growth factor β1; p-,
phosphorylated; FAK, focal adhesion kinase; ZEB, zinc finger
E-box-binding homeobox. |
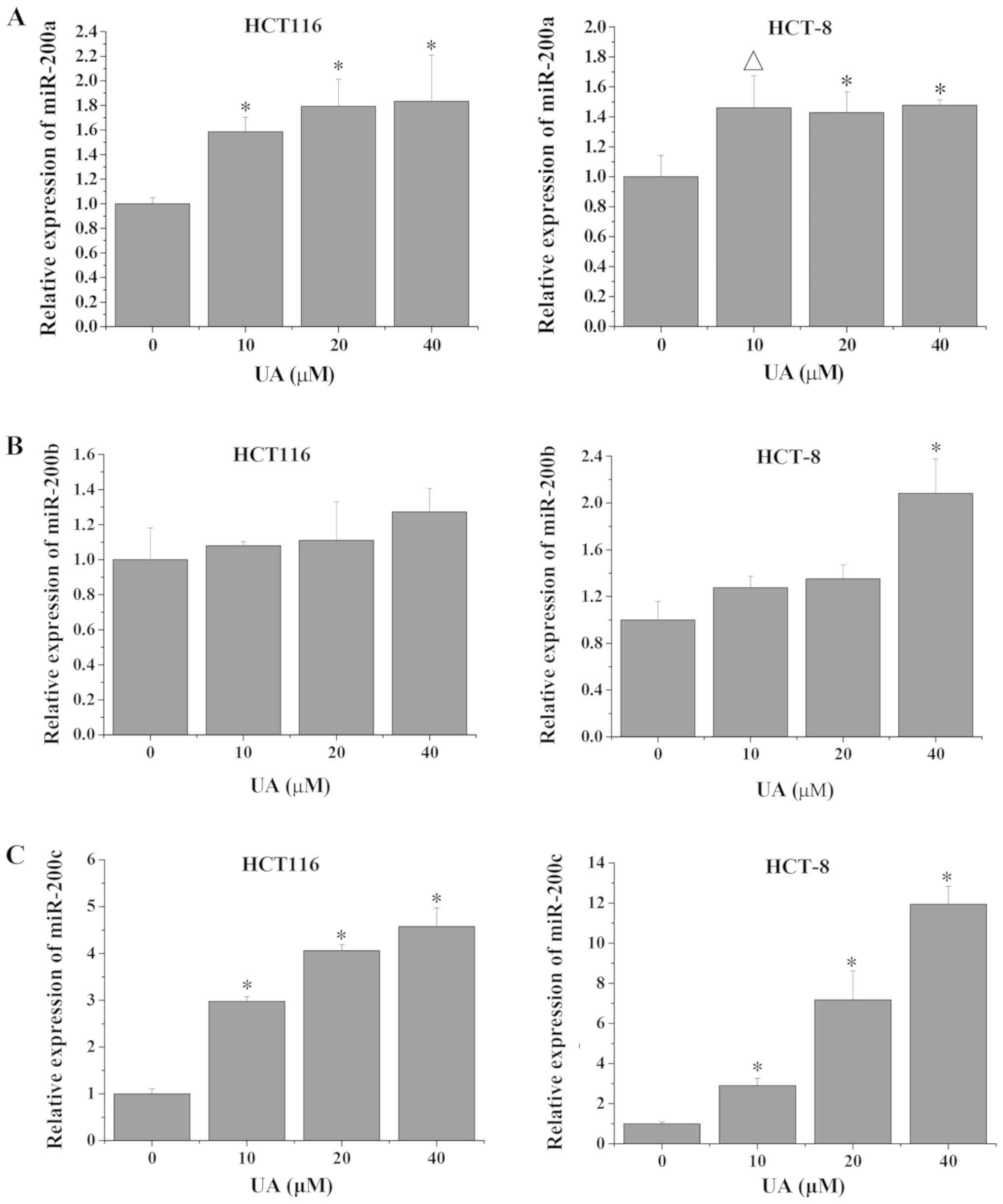
UA regulates the expression of
miR-200a/b/c in HCT116 and HCT-8 cells
miR-200a/b/c maintains the epithelial phenotype and
inhibits cell metastasis via downregulation of its target gene ZEB1
(34). In this process, TGF-β1
signaling increases the DNA methylation of miR-200a/b/c, thereby
negatively regulating its expression (17). Thus, to further investigate whether
UA inhibited colorectal cell invasion via regulation of the
TGF-β1/ZEB1/miR-200a/b/c feedback loop, the expression levels of
miR-200a/b/c were determined via RT-qPCR analysis. As shown in
Fig. 5, the expression levels of
miR-200a and miR-200c in HCT116 and HCT-8 cells were significantly
increased following UA treatment compared with that in the
untreated control cells. While the expression levels of miR-200b
did not significantly change after UA treatment in HCT116 cells
compared with the untreated control, HCT-8 cells exhibited a
significant increase in miR-200b levels following treatment with 40
µM UA (Fig. 5). These findings are
consistent with the observation that UA inhibited the TGF-β1
pathway and the expression of ZEB1 (Fig.
4). As shown in Fig. 5, the
expression levels of miR-200c exhibited the highest increase
following UA treatment. Therefore, the present results suggested
that UA may inhibit cell growth and invasion in HCT116 and HCT-8
cells via regulation of the TGF-β1/ZEB1/miR-200c feedback loop.
Discussion
UA is a natural triterpene acid present in various
plants, fruits, flowers and berries used in TCM (35). It mediates several pharmacological
processes and can be used as a preventive and therapeutic medicine
against multiple chronic diseases, including cancer, metabolic
syndrome, cardiovascular diseases, brain disease, liver disease,
and sarcopenia (36). In particular,
several studies have revealed the antitumor effects of UA in
gastric (37), prostate (38), breast (39), lung (40), liver (41), and osteosarcoma cancer (42). It was observed that UA inhibited CRC
cell growth while having no adverse effects on the weight of mice
in vivo (29). In addition,
UA significantly promotes CRC cell apoptosis and suppresses cell
proliferation via the regulation of numerous CRC-related signaling
pathways, including STAT3, ERK, JNK, and p38 (29). Of note, UA also inhibits CRC
angiogenesis through the suppression of several pivotal mediators,
such as vascular endothelial growth factor-A and basic fibroblast
growth factor. The inhibition of multiple signaling pathways,
including those related to hedgehog, STAT3, AKT and ribosomal
protein S6 kinase β-1, may be the potential mechanisms by which UA
may represent a promising compound against tumor angiogenesis
(28). Furthermore, UA has
anti-inflammatory effects on a dextran sodium sulfate-mediated
colitis model, whereas it has no effect on normal intestinal
epithelial cells (data not shown). In the present study, the effect
of UA on the metastatic potential of CRC cells was investigated.
The inhibitory effect of UA on cell viability was confirmed in the
human colon cancer cell lines HCT116 and HCT-8 and these results
are consistent with previous results in HT-29 cells (29). In addition, UA treatment
significantly and dose-dependently inhibited the migration and
invasion of HCT116 and HCT-8 cells in Transwell assays, suggesting
that UA may strong suppressive effects on cancer progression.
Metastasis is considered the predominant cause of
malignant cancer progression (43).
Similar to angiogenesis, the process of metastasis is complex and
involves complicated interactions between the tumor and the stroma
(44). Indeed, multiple signaling
pathways are involved in metastasis, including the integrin
pathway, the TGF-β pathway, the chemokine pathway, and the
dependence receptor pathway (45).
These signaling pathways regulate multiple mesenchymal and
invasiveness markers, as well as epithelial markers. A recent study
has reported that UA inhibited the invasive phenotype of human
gastric cancer cells by decreasing the expression of matrix
metalloproteinase-2 (37). The
synergism between UA and metformin has been shown to significantly
inhibit the invasion and migration of breast cancer cells via
modulation of the 5′-AMP activated protein kinase/TOR signaling
pathways (46). Furthermore, UA
attenuates EMT in non-small cell lung carcinoma by targeting
integrin αVβ5/matrix metalloproteinase signaling (47). Aspirin combined with UA exhibits
anti-metastatic ability via influencing both EMT and epidermal
growth factor receptor-mediated pathways (48). In the present study, the focus was on
the TGF-β1 pathways, including canonical TGF-β1/Smad and
non-canonical TGF-β1/FAK. The results demonstrated that UA
treatment significantly reduced the expression of several crucial
mediators of these TGF-β1 signaling pathways, including TGF-β1,
p-Smad2/3, p-FAK and ZEB1, leading to a decrease in N-cadherin
protein expression. A recent study has shown that ZEB1 is the
direct downstream target of miR-200a/b/c and is downregulated
following miR-200a/b/c activation (49). miR-200a/b/c, ZEB1 and TGF-β1 are
known to regulate tumor progression (50–53), and
increased the expression of miR-200a/b/c or the decrease in ZEB1 or
TGF-β1 could inhibit cancer cell EMT, which deactivates cellular
mobility and subsequently suppresses tumor metastasis (54–57).
Notably, the present study demonstrated that UA regulated the
expression levels of miR-200a/b/c, with miR-200c exhibiting the
highest upregulation in HCT116 and HCT-8 cells following UA
treatment.
In summary, UA inhibited the viability, migration
and invasion of CRC cells in vitro, by modulating the
TGF-β1/ZEB1/miR-200c signaling network. The present findings
elucidated the potential underlying mechanisms of UA, and suggested
that it may be an effective and promising therapeutic agent in the
treatment of CRC.
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the National
Natural Science Foundation of China (grant nos. 81704069 and
81774121), the Training of Young and Middle-aged Backbone Personnel
of Fujian Provincial Health and Family Planning Commission (grant
no. 2016-ZQN-67) and the Scientific Research Foundation of
Traditional Chinese Medicine of Fujian Provincial Health and Family
Planning Commission, China (grant no. 2017FJZYZY203).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
LZ and JML conceived and designed the experiments,
analyzed the data and drafted the manuscript. LZ, QYC and JXL
performed the cell experiments. QYC, JXL and YQC performed the
RT-qPCR and western blot experiments. TS and JP were involved in
analyzing the data and revised the manuscript critically for
important intellectual content. JML gave final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
UA
|
ursolic acid
|
|
TCM
|
Traditional Chinese Medicine
|
|
miR-200a/b/c
|
microRNA-200a/b/c
|
|
EMT
|
epithelial-mesenchymal transition
|
|
TGF-β1
|
transforming growth factor-β1
|
|
TβR-I
|
type I TGF-β1 receptor
|
|
ZEB
|
zinc finger E-box-binding homeobox
|
|
FAK
|
focal adhesion kinase
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics, 2012. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mcquade RM, Stojanovska V, Bornstein JC
and Nurgali K: Colorectal cancer chemotherapy: The evolution of
treatment and new approaches. Curr Med Chem. 24:1537–1557. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
López-Casillas F, Wrana JL and Massagué J:
Betaglycan presents ligand to the TGF beta signaling receptor.
Cell. 73:1435–1444. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franzén P, ten Dijke P, Ichijo H,
Yamashita H, Schulz P, Heldin CH and Miyazono K: Cloning of a TGF
beta type I receptor that forms a heteromeric complex with the TGF
beta type II receptor. Cell. 75:681–692. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Lebrun JJ and Vale W: Regulation
of transforming growth factor beta- and activin-induced
transcription by mammalian Mad proteins. Proc Natl Acad Sci USA.
93:12992–12997. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Feng X, Wu R and Derynck R:
Receptor-associated mad homologues synergize as effectors of the
TGF-beta response. Nature. 383:168–172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cicchini C, Laudadio I, Citarella F,
Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L and
Tripodi M: TGFbeta-induced EMT requires focal adhesion kinase (FAK)
signaling. Exp Cell Res. 314:143–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chitwood DH and Timmermans MC: Small RNAs
are on the move. Nature. 467:415–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Y, Bao Y and Yang W: Regulatory miRNAs
in colorectal carcinogenesis and metastasis. Int J Mol Sci.
18:E8902017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knezevic J, Pfefferle AD, Petrovic I,
Greene SB, Perou CM and Rosen JM: Expression of miR-200c in
claudin-low breast cancer alters stem cell functionality, enhances
chemosensitivity and reduces metastatic potential. Oncogene.
34:5997–6006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Wang Y, Shan B, Han J, Zhu H, Lv
Y, Fan X, Sang M, Liu XD and Liu W: The downregulation of
miR-200c/141 promotes ZEB1/2 expression and gastric cancer
progression. Med Oncol. 32:4282015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu WR, Sun H, Zhang R, Yu XH, Shi XD, Zhu
MS, Zeng H, Yan LX, Xu LB and Liu C: Methylation-associated
silencing of miR-200b facilitates human hepatocellular carcinoma
progression by directly targetingBMI1. Oncotarget. 7:18684–18693.
2016.PubMed/NCBI
|
|
16
|
Kumar S, Nag A and Mandal CC: A
comprehensive review on miR-200c, a promising cancer biomarker with
therapeutic potential. Curr Drug Targets. 16:1381–1403. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gregory PA, Bracken CP, Smith E, Bert AG,
Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, et al:
An autocrine TGF-beta/ZEB/miR-200 signaling network regulates
establishment and maintenance of epithelial-mesenchymal transition.
Mol Biol Cell. 22:1686–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong M, Jiang L, Zhou Y, Qiu W, Fang L,
Tan R, Wen P and Yang J: The miR-200 family regulates
TGF-β1-induced renal tubular epithelial to mesenchymal transition
through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J
Physiol Renal Physiol. 302:F369–F379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsiao WL and Liu L: The role of
traditional Chinese herbal medicines in cancer therapy-from tcm
theory to mechanistic insights. Planta Med. 76:1118–1131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin J, Wei L, Xu W, Hong Z, Liu X and Peng
J: Effect of Hedyotis Diffusa Willd extract on tumor angiogenesis.
Mol Med Rep. 4:1283–1288. 2011.PubMed/NCBI
|
|
21
|
Lin W, Zheng L, Zhuang Q, Zhao J, Cao Z,
Zeng J, Lin S, Xu W and Peng J: Spica prunellaepromotes cancer cell
apoptosis, inhibits cell proliferation and tumor angiogenesis in a
mouse model of colorectal cancer via suppression of stat3 pathway.
BMC Complement Altern Med. 13:1442013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng J, Chen Y, Lin J, Zhuang Q, Xu W,
Hong Z and Sferra TJ: Patrinia scabiosaefolia extract suppresses
proliferation and promotes apoptosis by inhibiting the STAT3
pathway in human multiple myeloma cells. Mol Med Rep. 4:313–318.
2011.PubMed/NCBI
|
|
23
|
Zhang L, Fang Y, Feng JY, Cai QY, Wei LH,
Lin S and Peng J: Chloroform fraction of Scutellaria barbata D. Don
inhibits the growth of colorectal cancer cells by activating
miR-34a. Oncol Rep. 37:3695–3701. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Checker R, Sandur SK, Sharma D, Patwardhan
RS, Jayakumar S, Kohli V, Sethi G, Aggarwal BB and Sainis KB:
Potent anti-inflammatory activity of ursolic acid, a triterpenoid
antioxidant, is mediated through suppression of NF-κB, AP-1 and
NF-AT. PLoS One. 7:e313182012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong L, Li S, Liao Q, Zhang Y, Sun R, Zhu
X, Zhang Q, Wang J, Wu X, Fang X and Zhu Y: Oleanolic acid and
ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B
activity. Antiviral Res. 98:44–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liobikas J, Majiene D, Trumbeckaite S,
Kursvietiene L, Masteikova R, Kopustinskiene DM, Savickas A and
Bernatoniene J: Uncoupling and antioxidant effects of ursolic acid
in isolated rat heart mitochondria. J Nat Prod. 74:1640–1644. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma CM, Cai SQ, Cui JR, Wang RQ, Tu PF,
Hattori M and Daneshtalab M: The cytotoxic activity of ursolic acid
derivatives. Eur J Med Chem. 40:582–589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin J, Chen Y, Wei L, Hong Z, Sferra TJ
and Peng J: Ursolic acid inhibits colorectal cancer angiogenesis
through suppression of multiple signaling pathways. Int J Oncol.
43:1666–1674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin J, Chen Y, Wei L, Shen A, Sferra TJ,
Hong Z and Peng J: Ursolic acid promotes colorectal cancer cell
apoptosis and inhibits cell proliferation via modulation of
multiple signaling pathways. Int J Oncol. 43:1235–1243. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao YN, Zhang XX and Jiang H: A review on
anti-diabetic effects of ursolic acid. Clin J Chin Med. 8:142–145.
2016.(In Chinese).
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pang L, Li Q, Wei C, Zou H, Li S, Cao W,
He J, Zhou Y, Ju X, Lan J, et al: TGF-β1/Smad signaling pathway
regulates epithelial-to-mesenchymal transition in esophageal
squamous cell carcinoma: In vitro and clinical analyses of cell
lines and nomadic Kazakh patients from northwest Xinjiang, China.
Plos One. 9:e1123002014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jäger S, Trojan H, Kopp T, Laszczyk MN and
Scheffler A: Pentacyclic triterpene distribution in various
plants-rich sources for a new group of multi-potent plant extracts.
Molecules. 14:2016–2031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seo DY, Lee SR, Heo JW, No MH, Rhee BD, Ko
KS, Kwak HB and Han J: Ursolic acid in health and disease. Korean J
Physiol Pharmacol. 22:235–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim ES and Moon A: Ursolic acid inhibits
the invasive phenotype of SNU-484 human gastric cancer cells. Oncol
Lett. 9:897–902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng Y, Lin ZM, Ge N, Zhang DL, Huang J
and Kong F: Ursolic acid induces apoptosis of prostate cancer cells
via the PI3K/Akt/mTOR pathway. Am J Chin Med. 43:1471–1486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wen JH, Wei XH, Sheng XY, Zhou DQ, Peng
HW, Lu YN and Zhou J: Effect of ursolic acid on breast cancer
resistance protein-mediated transport of rosuvastatin in vivo and
vitro. Chin Med Sci J. 30:218–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang CY, Lin CY, Tsai CW and Yin MC:
Inhibition of cell proliferation, invasion and migration by ursolic
acid in human lung cancer cell lines. Toxicol In Vitro.
25:1274–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan SL, Huang CY, Wu ST and Yin MC:
Oleanolic acid and ursolic acid induce apoptosis in four human
liver cancer cell lines. Toxicol In Vitro. 24:842–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu CC, Cheng CH, Lee YH, Chang IL, Chen
HY, Hsieh CP and Chueh PJ: Ursolic acid triggers apoptosis in human
osteosarcoma cells via caspase activation and the ERK1/2 MAPK
pathway. J Agric Food Chem. 64:4220–4226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yokota J: Tumor progression and
metastasis. Carcinogenesis. 21:497–503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited-the role of tumor-stroma interactions in
metastasis to different organs. Int J Cancer. 128:2527–2535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Robert J: Biology of cancer metastasis.
Bull Cancer. 100:333–342. 2013.(In French). PubMed/NCBI
|
|
46
|
Zheng G, Shen Z, Xu A, Jiang K, Wu P, Yang
X, Chen X and Shao J: Synergistic chemopreventive and therapeutic
effects of co-drug ua-met: Implication in tumor metastasis. J Agric
Food Chem. 65:10973–10983. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ruan JS, Zhou H, Yang L, Wang L, Jiang ZS,
Sun H and Wang SM: Ursolic acid attenuates TGF-β1 induced
epithelial-mesenchymal transition in NSCLC by targeting integrin
αVβ5/MMPs signaling. Oncol Res. 7:593–600. 2017.
|
|
48
|
Tang Q, Liu Y, Li T, Yang X, Zheng G, Chen
H, Jia L and Shao J: A novel co-drug of aspirin and ursolic acid
interrupts adhesion, invasion and migration of cancer cells to
vascular endothelium via regulating EMT and EGFR-mediated signaling
pathways: Multiple targets for cancer metastasis prevention and
treatment. Oncotarget. 7:73114–73129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Burk U, Schubert J, Wellner U, Schmalhofer
O, Vincan E, Spaderna S and Brabletz T: A reciprocal repression
between ZEB1 and members of the miR-200 family promotes EMT and
invasion in cancer cells. EMBO Rep. 9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Zeng C, Tu M, Jiang W, Dai Z, Hu Y,
Deng Z and Xiao W: MicroRNA-200b acts as a tumor suppressor in
osteosarcoma via targeting ZEB1. Onco Targets Ther. 9:3101–3111.
2016.PubMed/NCBI
|
|
51
|
Tang H, Deng M, Tang Y and Xie X, Guo J,
Kong Y, Ye F, Su Q and Xie X: miR-200b and miR-200c as prognostic
factors and mediators of gastric cancer cell progression. Clin
Cancer Res. 19:5602–5612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang HF, Xu LY and Li EM: A family of
pleiotropically acting microRNAs in cancer progression, miR-200:
Potential cancer therapeutic targets. Curr Pharm Des. 20:1896–1903.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hasegawa Y, Takanashi S, Kanehira Y,
Tsushima T, Imai T and Okumura K: Transforming growth factor-beta1
level correlates with angiogenesis, tumor progression, and
prognosis in patients with nonsmall cell lung carcinoma. Cancer.
91:964–971. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bracken CP, Gregory PA, Kolesnikoff N,
Bert AG, Wang J, Shannon MF and Goodall GJ: A double-negative
feedback loop between ZEB1-SIP1 and the microRNA-200 family
regulates epithelial-mesenchymal transition. Cancer Res.
68:7846–7854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen D, Wang J, Zhang Y, Chen J, Yang C,
Cao W, Zhang H, Liu Y and Dou J: Effect of down-regulated
transcriptional repressor ZEB1 on the epithelial-mesenchymal
transition of ovarian cancer cells. Int J Gynecol Cancer.
23:1357–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen D, Zhang Y, Wang J, Chen J, Yang C,
Cai K, Wang X, Shi F and Dou J: MicroRNA-200c overexpression
inhibits tumorigenicity and metastasis of CD117+ CD44+ ovarian
cancer stem cells by regulating epithelial-mesenchymal transition.
J Ovarian Res. 6:502013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alb M, Sie C, Adam C, Chen S, Becker JC
and Schrama D: Cellular and cytokine-dependent immunosuppressive
mechanisms of grm1-transgenic murine melanoma. Cancer Immunol
Immunother. 61:2239–2249. 2012. View Article : Google Scholar : PubMed/NCBI
|