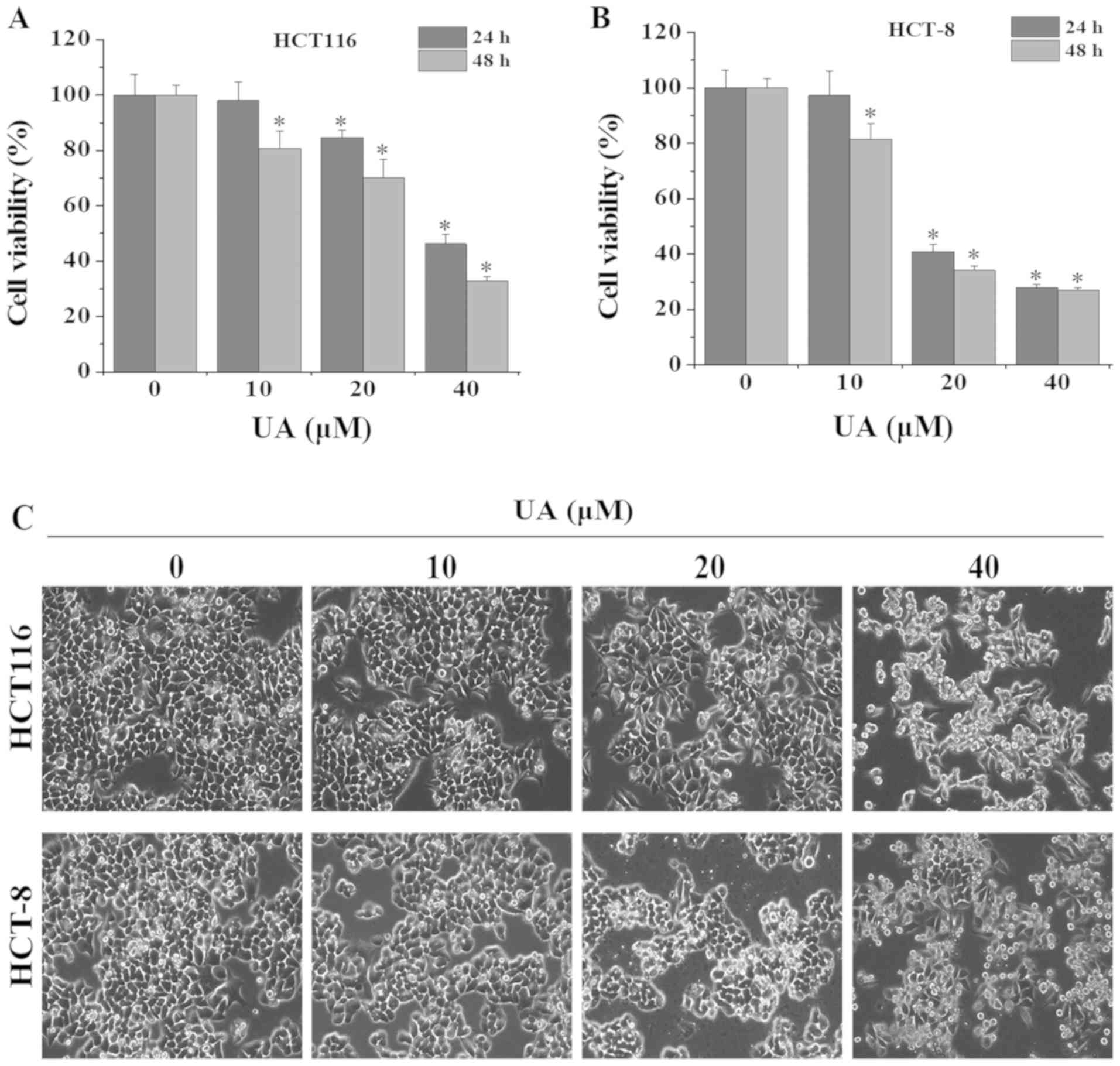
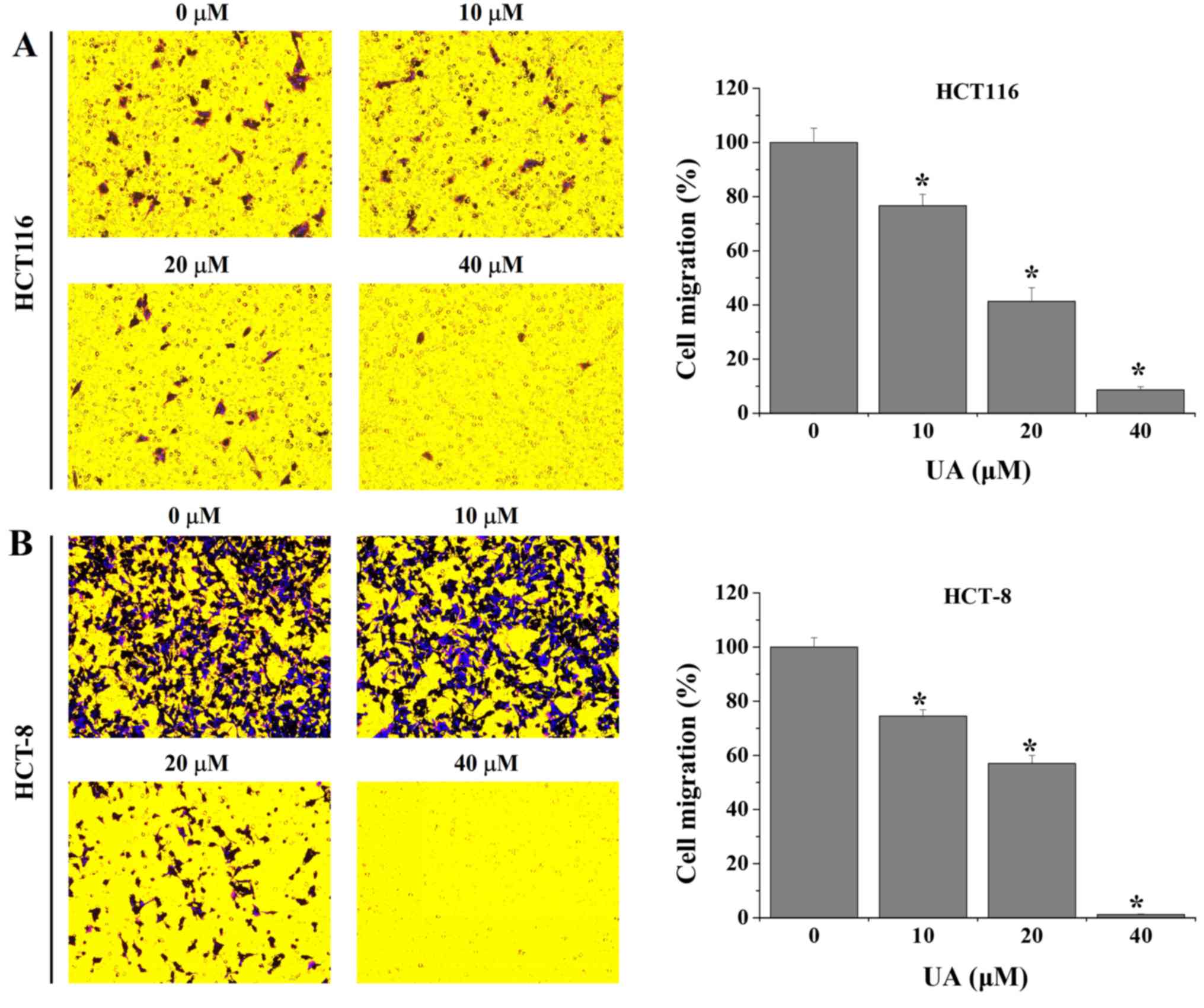
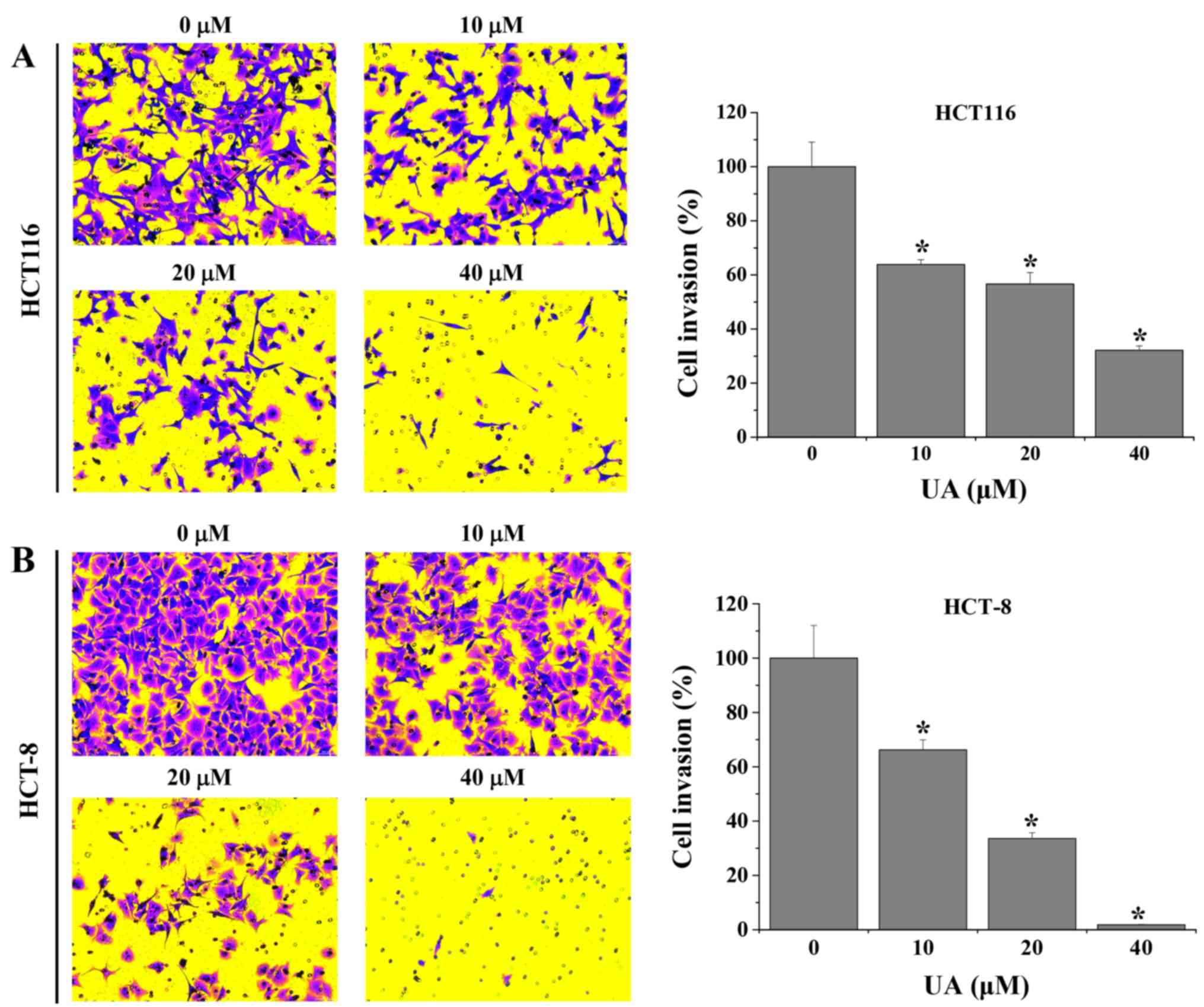
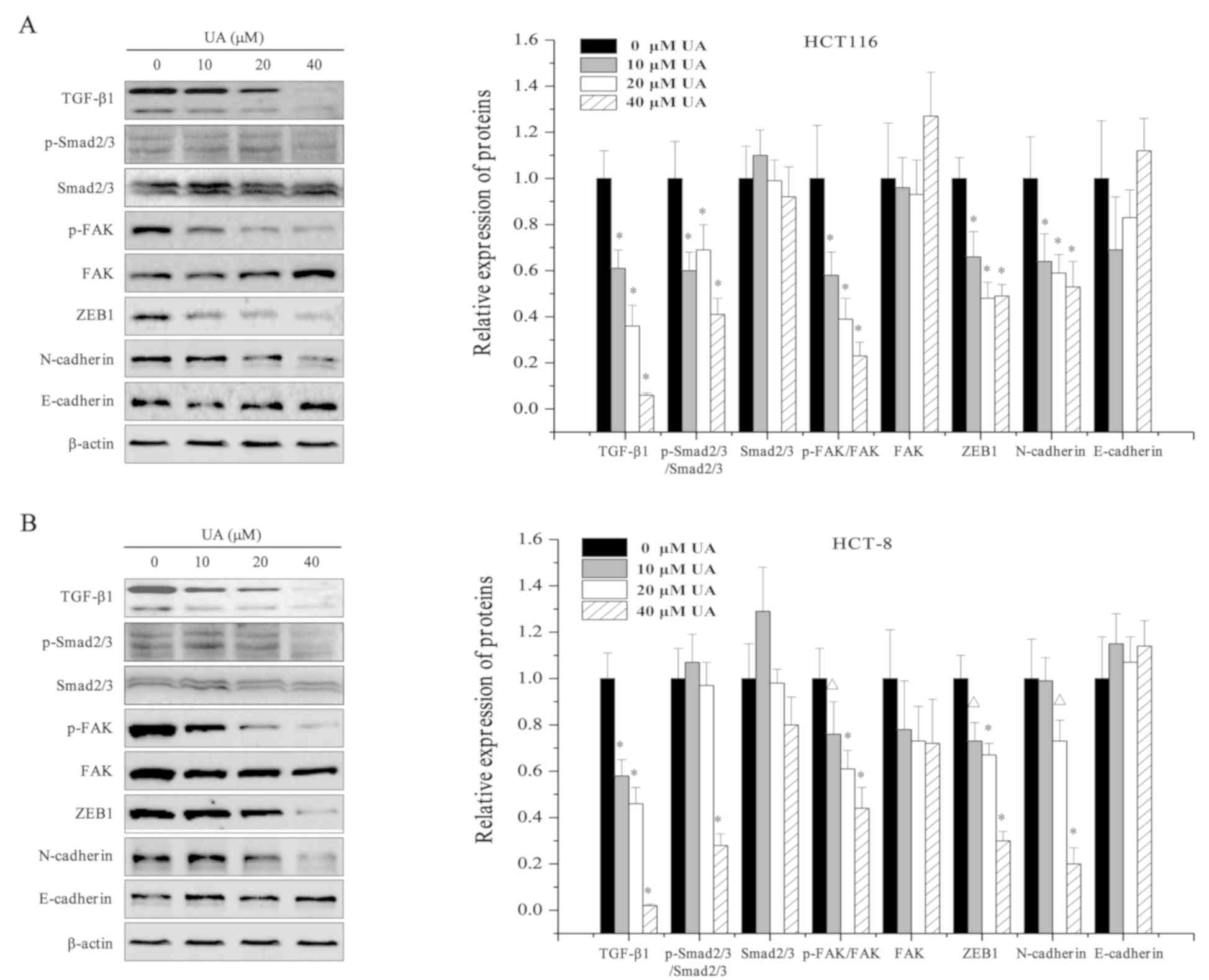
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics, 2012. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mcquade RM, Stojanovska V, Bornstein JC
and Nurgali K: Colorectal cancer chemotherapy: The evolution of
treatment and new approaches. Curr Med Chem. 24:1537–1557. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
López-Casillas F, Wrana JL and Massagué J:
Betaglycan presents ligand to the TGF beta signaling receptor.
Cell. 73:1435–1444. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franzén P, ten Dijke P, Ichijo H,
Yamashita H, Schulz P, Heldin CH and Miyazono K: Cloning of a TGF
beta type I receptor that forms a heteromeric complex with the TGF
beta type II receptor. Cell. 75:681–692. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Lebrun JJ and Vale W: Regulation
of transforming growth factor beta- and activin-induced
transcription by mammalian Mad proteins. Proc Natl Acad Sci USA.
93:12992–12997. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Feng X, Wu R and Derynck R:
Receptor-associated mad homologues synergize as effectors of the
TGF-beta response. Nature. 383:168–172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cicchini C, Laudadio I, Citarella F,
Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L and
Tripodi M: TGFbeta-induced EMT requires focal adhesion kinase (FAK)
signaling. Exp Cell Res. 314:143–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chitwood DH and Timmermans MC: Small RNAs
are on the move. Nature. 467:415–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Y, Bao Y and Yang W: Regulatory miRNAs
in colorectal carcinogenesis and metastasis. Int J Mol Sci.
18:E8902017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knezevic J, Pfefferle AD, Petrovic I,
Greene SB, Perou CM and Rosen JM: Expression of miR-200c in
claudin-low breast cancer alters stem cell functionality, enhances
chemosensitivity and reduces metastatic potential. Oncogene.
34:5997–6006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Wang Y, Shan B, Han J, Zhu H, Lv
Y, Fan X, Sang M, Liu XD and Liu W: The downregulation of
miR-200c/141 promotes ZEB1/2 expression and gastric cancer
progression. Med Oncol. 32:4282015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu WR, Sun H, Zhang R, Yu XH, Shi XD, Zhu
MS, Zeng H, Yan LX, Xu LB and Liu C: Methylation-associated
silencing of miR-200b facilitates human hepatocellular carcinoma
progression by directly targetingBMI1. Oncotarget. 7:18684–18693.
2016.PubMed/NCBI
|
|
16
|
Kumar S, Nag A and Mandal CC: A
comprehensive review on miR-200c, a promising cancer biomarker with
therapeutic potential. Curr Drug Targets. 16:1381–1403. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gregory PA, Bracken CP, Smith E, Bert AG,
Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, et al:
An autocrine TGF-beta/ZEB/miR-200 signaling network regulates
establishment and maintenance of epithelial-mesenchymal transition.
Mol Biol Cell. 22:1686–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong M, Jiang L, Zhou Y, Qiu W, Fang L,
Tan R, Wen P and Yang J: The miR-200 family regulates
TGF-β1-induced renal tubular epithelial to mesenchymal transition
through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J
Physiol Renal Physiol. 302:F369–F379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsiao WL and Liu L: The role of
traditional Chinese herbal medicines in cancer therapy-from tcm
theory to mechanistic insights. Planta Med. 76:1118–1131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin J, Wei L, Xu W, Hong Z, Liu X and Peng
J: Effect of Hedyotis Diffusa Willd extract on tumor angiogenesis.
Mol Med Rep. 4:1283–1288. 2011.PubMed/NCBI
|
|
21
|
Lin W, Zheng L, Zhuang Q, Zhao J, Cao Z,
Zeng J, Lin S, Xu W and Peng J: Spica prunellaepromotes cancer cell
apoptosis, inhibits cell proliferation and tumor angiogenesis in a
mouse model of colorectal cancer via suppression of stat3 pathway.
BMC Complement Altern Med. 13:1442013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng J, Chen Y, Lin J, Zhuang Q, Xu W,
Hong Z and Sferra TJ: Patrinia scabiosaefolia extract suppresses
proliferation and promotes apoptosis by inhibiting the STAT3
pathway in human multiple myeloma cells. Mol Med Rep. 4:313–318.
2011.PubMed/NCBI
|
|
23
|
Zhang L, Fang Y, Feng JY, Cai QY, Wei LH,
Lin S and Peng J: Chloroform fraction of Scutellaria barbata D. Don
inhibits the growth of colorectal cancer cells by activating
miR-34a. Oncol Rep. 37:3695–3701. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Checker R, Sandur SK, Sharma D, Patwardhan
RS, Jayakumar S, Kohli V, Sethi G, Aggarwal BB and Sainis KB:
Potent anti-inflammatory activity of ursolic acid, a triterpenoid
antioxidant, is mediated through suppression of NF-κB, AP-1 and
NF-AT. PLoS One. 7:e313182012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong L, Li S, Liao Q, Zhang Y, Sun R, Zhu
X, Zhang Q, Wang J, Wu X, Fang X and Zhu Y: Oleanolic acid and
ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B
activity. Antiviral Res. 98:44–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liobikas J, Majiene D, Trumbeckaite S,
Kursvietiene L, Masteikova R, Kopustinskiene DM, Savickas A and
Bernatoniene J: Uncoupling and antioxidant effects of ursolic acid
in isolated rat heart mitochondria. J Nat Prod. 74:1640–1644. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma CM, Cai SQ, Cui JR, Wang RQ, Tu PF,
Hattori M and Daneshtalab M: The cytotoxic activity of ursolic acid
derivatives. Eur J Med Chem. 40:582–589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin J, Chen Y, Wei L, Hong Z, Sferra TJ
and Peng J: Ursolic acid inhibits colorectal cancer angiogenesis
through suppression of multiple signaling pathways. Int J Oncol.
43:1666–1674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin J, Chen Y, Wei L, Shen A, Sferra TJ,
Hong Z and Peng J: Ursolic acid promotes colorectal cancer cell
apoptosis and inhibits cell proliferation via modulation of
multiple signaling pathways. Int J Oncol. 43:1235–1243. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao YN, Zhang XX and Jiang H: A review on
anti-diabetic effects of ursolic acid. Clin J Chin Med. 8:142–145.
2016.(In Chinese).
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pang L, Li Q, Wei C, Zou H, Li S, Cao W,
He J, Zhou Y, Ju X, Lan J, et al: TGF-β1/Smad signaling pathway
regulates epithelial-to-mesenchymal transition in esophageal
squamous cell carcinoma: In vitro and clinical analyses of cell
lines and nomadic Kazakh patients from northwest Xinjiang, China.
Plos One. 9:e1123002014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jäger S, Trojan H, Kopp T, Laszczyk MN and
Scheffler A: Pentacyclic triterpene distribution in various
plants-rich sources for a new group of multi-potent plant extracts.
Molecules. 14:2016–2031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seo DY, Lee SR, Heo JW, No MH, Rhee BD, Ko
KS, Kwak HB and Han J: Ursolic acid in health and disease. Korean J
Physiol Pharmacol. 22:235–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim ES and Moon A: Ursolic acid inhibits
the invasive phenotype of SNU-484 human gastric cancer cells. Oncol
Lett. 9:897–902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng Y, Lin ZM, Ge N, Zhang DL, Huang J
and Kong F: Ursolic acid induces apoptosis of prostate cancer cells
via the PI3K/Akt/mTOR pathway. Am J Chin Med. 43:1471–1486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wen JH, Wei XH, Sheng XY, Zhou DQ, Peng
HW, Lu YN and Zhou J: Effect of ursolic acid on breast cancer
resistance protein-mediated transport of rosuvastatin in vivo and
vitro. Chin Med Sci J. 30:218–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang CY, Lin CY, Tsai CW and Yin MC:
Inhibition of cell proliferation, invasion and migration by ursolic
acid in human lung cancer cell lines. Toxicol In Vitro.
25:1274–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan SL, Huang CY, Wu ST and Yin MC:
Oleanolic acid and ursolic acid induce apoptosis in four human
liver cancer cell lines. Toxicol In Vitro. 24:842–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu CC, Cheng CH, Lee YH, Chang IL, Chen
HY, Hsieh CP and Chueh PJ: Ursolic acid triggers apoptosis in human
osteosarcoma cells via caspase activation and the ERK1/2 MAPK
pathway. J Agric Food Chem. 64:4220–4226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yokota J: Tumor progression and
metastasis. Carcinogenesis. 21:497–503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited-the role of tumor-stroma interactions in
metastasis to different organs. Int J Cancer. 128:2527–2535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Robert J: Biology of cancer metastasis.
Bull Cancer. 100:333–342. 2013.(In French). PubMed/NCBI
|
|
46
|
Zheng G, Shen Z, Xu A, Jiang K, Wu P, Yang
X, Chen X and Shao J: Synergistic chemopreventive and therapeutic
effects of co-drug ua-met: Implication in tumor metastasis. J Agric
Food Chem. 65:10973–10983. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ruan JS, Zhou H, Yang L, Wang L, Jiang ZS,
Sun H and Wang SM: Ursolic acid attenuates TGF-β1 induced
epithelial-mesenchymal transition in NSCLC by targeting integrin
αVβ5/MMPs signaling. Oncol Res. 7:593–600. 2017.
|
|
48
|
Tang Q, Liu Y, Li T, Yang X, Zheng G, Chen
H, Jia L and Shao J: A novel co-drug of aspirin and ursolic acid
interrupts adhesion, invasion and migration of cancer cells to
vascular endothelium via regulating EMT and EGFR-mediated signaling
pathways: Multiple targets for cancer metastasis prevention and
treatment. Oncotarget. 7:73114–73129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Burk U, Schubert J, Wellner U, Schmalhofer
O, Vincan E, Spaderna S and Brabletz T: A reciprocal repression
between ZEB1 and members of the miR-200 family promotes EMT and
invasion in cancer cells. EMBO Rep. 9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Zeng C, Tu M, Jiang W, Dai Z, Hu Y,
Deng Z and Xiao W: MicroRNA-200b acts as a tumor suppressor in
osteosarcoma via targeting ZEB1. Onco Targets Ther. 9:3101–3111.
2016.PubMed/NCBI
|
|
51
|
Tang H, Deng M, Tang Y and Xie X, Guo J,
Kong Y, Ye F, Su Q and Xie X: miR-200b and miR-200c as prognostic
factors and mediators of gastric cancer cell progression. Clin
Cancer Res. 19:5602–5612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang HF, Xu LY and Li EM: A family of
pleiotropically acting microRNAs in cancer progression, miR-200:
Potential cancer therapeutic targets. Curr Pharm Des. 20:1896–1903.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hasegawa Y, Takanashi S, Kanehira Y,
Tsushima T, Imai T and Okumura K: Transforming growth factor-beta1
level correlates with angiogenesis, tumor progression, and
prognosis in patients with nonsmall cell lung carcinoma. Cancer.
91:964–971. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bracken CP, Gregory PA, Kolesnikoff N,
Bert AG, Wang J, Shannon MF and Goodall GJ: A double-negative
feedback loop between ZEB1-SIP1 and the microRNA-200 family
regulates epithelial-mesenchymal transition. Cancer Res.
68:7846–7854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen D, Wang J, Zhang Y, Chen J, Yang C,
Cao W, Zhang H, Liu Y and Dou J: Effect of down-regulated
transcriptional repressor ZEB1 on the epithelial-mesenchymal
transition of ovarian cancer cells. Int J Gynecol Cancer.
23:1357–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen D, Zhang Y, Wang J, Chen J, Yang C,
Cai K, Wang X, Shi F and Dou J: MicroRNA-200c overexpression
inhibits tumorigenicity and metastasis of CD117+ CD44+ ovarian
cancer stem cells by regulating epithelial-mesenchymal transition.
J Ovarian Res. 6:502013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alb M, Sie C, Adam C, Chen S, Becker JC
and Schrama D: Cellular and cytokine-dependent immunosuppressive
mechanisms of grm1-transgenic murine melanoma. Cancer Immunol
Immunother. 61:2239–2249. 2012. View Article : Google Scholar : PubMed/NCBI
|