Introduction
Osteosarcoma (OS) is a type of bone cancer that can
spread easily to other tissues or organs in the body (1). OS is considered the most frequent
primary malignant bone tumor among children, adolescents and young
adults and is responsible for approximately 5% of cancer-associated
mortality worldwide (2). OS is an
aggressive type of cancer and accounts for approximately 50% of
bone sarcomas with metastasis (3).
Statistical data have indicated that OS is more prevalent in males
compared with females, and has an increasing incidence rate of 3.8
per 1,000,000 in males and 2.8 per 1,000,000 in females around the
world (4). Although there is
considerable progress in the improvement of therapeutic strategies
for OS, such as surgical resection, chemotherapy, and radiotherapy,
the outcomes and prognosis of patients with cancer remain poor
(5). The 5-year survival rate for
patients suffering from OS is <53.9% (6). Therefore, studies on novel functional
molecules, which have pivotal roles during tumor progression, are
important for OS treatment, as they may uncover potential
therapeutic targets to improve cancer prognosis in patients with
OS.
Accumulated evidence suggested that various genes
are involved in tumor initiation and development and that genetic
alterations are critical events during tumor progression in various
types of human cancer, including colorectal carcinoma and gastric
cancer (7,8). MicroRNAs (miRNAs), a class of small and
noncoding RNAs, have a crucial gene regulation function that acts
by binding to the 3′-untranslated regions (3′UTRs) of target genes
(9). In addition, miRNAs have been
reported to be involved in biological processes in both normal and
tumor cells, such as cell proliferation, differentiation, invasion,
migration, cell cycle and cell apoptosis (10–13).
Emerging studies have shown that aberrant miRNA expression has been
observed in tumor samples and that this expression exerts a
functional role in the progression of various types of cancer,
including retinoblastoma, gastric cancer and non-small cell lung
cancer (14–16). Therefore, this study considered that
the identification of functional miRNAs could provide novel
prognostic biomarkers and effective therapeutic targets for the
treatment of OS. MicroRNA-106b (miR-106b) is a member of the
miR-106b-25 cluster, which has been found to be highly expressed in
OS, according to Arabi et al (17). However, to the best of our knowledge,
the clinical significance and functional role of miR-106b in OS
have rarely been reported in previous studies.
The aim of the present study was to assess the
expression patterns of miR-106b in OS tissues and cells, as well as
its prognostic value for patients with OS. In addition, the effects
of miR-106 on OS cell proliferation, migration, and invasion were
also analyzed to further investigate its functional role during OS
progression. The results of this study may provide novel insight on
the prognosis and target therapy of OS regarding the role of
miR-106b.
Materials and methods
Patients and tissues collection
The experimental protocols of this study were
approved by the Ethics Committee of Ningbo No.2 Hospital (Ningbo,
China). All patients provided written informed consent prior to
surgery. A total of 134 patients, who were pathologically diagnosed
with OS and received surgical resection at Ningbo No. 2 Hospital
(Zhejiang, China) between January 2008 and July 2012, were
recruited for this study. The patients with OS included 95 males
and 39 females with a mean age of 19.95±13.17 years (range, 4–48
years). The patients met the following inclusion criteria:
(1) None of the patients had
received any anti-tumor therapy before the surgery; (2) the tumor tissues were pathologically
diagnosed as OS by two experienced pathologists and (3) patients had a complete record in terms
of demography and clinicopathological data. OS tissues and adjacent
normal tissues were collected from the patients during the
resection surgery and were immediately frozen in liquid nitrogen
for subsequent RNA extraction. The tumors were classified into TNM
stage I–II (n=61) and TNM stage III–IV (n=73) based on the
guidelines of the American Joint Committee on Cancer (18). All patients had complete electronic
medical records, and their demographic data and clinicopathological
characteristics are summarized in Table
I. A 5-year follow-up survey was conducted for the patients
after the surgery, and the survival information was recorded for
the survival analysis.
 | Table I.Association between miR-106b
expression and clinicopathological features of patients with
OS. |
Table I.
Association between miR-106b
expression and clinicopathological features of patients with
OS.
|
|
| miR-106b
expression |
|
|---|
|
|
|
|
|
|---|
| Features | Total no. n=134 | Low (n=64) | High (n=70) | P-value |
|---|
| Age (years) |
|
|
| 0.982 |
| ≤40 | 92 | 44 | 48 |
|
|
>40 | 42 | 20 | 22 |
|
| Sex |
|
|
| 0.811 |
|
Female | 39 | 18 | 21 |
|
| Male | 95 | 46 | 49 |
|
| Tumor size (cm) |
|
|
| 0.941 |
| ≤5 | 80 | 38 | 42 |
|
|
>5 | 54 | 26 | 28 |
|
| Differentiation |
|
|
| 0.151 |
|
Well/moderate | 73 | 39 | 34 |
|
|
Poor | 61 | 25 | 36 |
|
| Metastasis |
|
|
| 0.028a |
|
Negative | 58 | 34 | 24 |
|
|
Positive | 76 | 30 | 46 |
|
| TNM stage |
|
|
| 0.017a |
|
I–II | 61 | 36 | 25 |
|
|
III–IV | 73 | 28 | 45 |
|
Cell culture and transfection
Four OS cell lines, including MG63, U2OS, HOS, and
SaOS2, and one osteoblastic cell line, hFOB1.19, were purchased
from the Cell Bank of the Chinese Academy of Sciences. All the
cells were cultured in RPMI-1640 medium (HyClone; GE Healthcare
Life Sciences), supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. The expression of miR-106b was regulated in MG63
and HOS cells by cell transfection using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), since these cell
lines exhibited higher expression levels of miR-106b. The vectors
(50 nM) used in the transfection were as follows: miR-106b mimic,
mimic negative control (NC), miR-106b inhibitor and inhibitor NC.
All vectors were synthesized by Shanghai GenePharma Co., Ltd. with
the sequences as follows: miR-106b mimic
5′-UAAAGUGCUGACAGUGCAGAU-3′, miR-106b inhibitor
5′-AUCUGCACUGUCAGCACUUUA-3′, mimic NC: 5′-UUCUCCGAACGUGUCACGUTT-3′,
inhibitor NC: 5′-CAGUACUUUUGUGUAGUACAA-3′. After 48 h of cell
transfection, the cells were used for subsequent cell
experiments.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
The total RNA, including the miRNAs, was extracted
from the collected tissues and all cell lines used in the present
study with TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocols. The concentration
and quality of the RNA were evaluated using a NanoDrop 2000 (Thermo
Fisher Scientific, Inc.). Reverse transcription was performed to
synthesize cDNA using a PrimeScript RT Reagent kit (Takara Bio,
Inc.), according to the manufacturer's protocols. The reaction
condition was as follow: 42°C for 30 min, 85°C for 5 sec, storage
at 4°C.
To estimate the expression levels of miR-106b,
RT-qPCR was carried out using a 7300 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the SYBR green I
Master Mix kit (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. U6 was selected
as an internal control in the reactions. The thermocycling
conditions included initial denaturation at 95°C for 10 min,
denaturation at 95°C for 30 sec, 56°C for 30 sec, 72°C for 15 sec
for a total of 40 cycles. The sequences of primer were as follows:
miR-106b, forward: 5′-GGGGCTAAAGTGCTGACAGT-3′, reverse:
5′-GGAGCAGCAAGTACCCACAG-3′; U6, forward:
5′-GCTTCGGCAGCACATATACTAAAAT-3′, reverse:
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The relative expression value of
miR-106b was computed using the 2−ΔΔCq method (19) and was normalized to U6.
Cell proliferation analysis
To analyze the effect of miR-106b on cell
proliferation of MG63 and HOS cells, an MTT assay was performed.
The stably transfected OS cells were seeded into 96-well plates,
and the cell density was adjusted to 4×105 cells/well.
The cell culture plates were stored at 37°C with 5% CO2
for 72 h. During the incubation, the 10 µl MTT (5 mg/ml) was added
to the wells every 24 h, and the samples were cultured for another
4 h. After removing of media, the cells were treated with 150 µl
DMSO for 10 min. The absorbance was measured at a wavelength of 490
nm by a microplate reader (Thermo Fisher Scientific, Inc.) to
evaluate the ability of OS cell proliferation.
Cell migration and invasion
analysis
The abilities of cell migration and invasion of MG63
and HOS cells were examined by Transwell analysis using Transwell
chambers with 8 µm pore size (Corning, Inc.). The stably
transfected OS cells were seeded into the upper chambers
(4×105 cells/well), which were filled with serum-free
RPMI-1640 medium. The medium was supplemented with 10% FBS and was
added to the lower chambers. The chambers were all cultured at 37°C
in a humidified incubator with 5% CO2 for 48 h. After
incubation, the migratory cells in the lower chambers were fixed
with precooled 3.7% formaldehyde for 5 min at room temperature and
stained using 0.1% crystal violet for 15 min at room temperature.
The cell number was counted under a microscope (magnification,
×200) in 7 randomly-selected fields of view. For the invasion
analysis, the Transwell chambers used were coated with Matrigel
(Corning, Inc.).
Statistical analysis
The data are expressed as the mean ± standard
deviation. All the statistical analyses were performed using SPSS
18.0 software (SPSS Inc.) and GraphPad Prism 5.0 software (GraphPad
Software, Inc.). The expression values of miR-106b in the clinical
samples were checked by Kolmogorov-Smirnov test. The comparisons of
the groups were assessed using Student's t-test and one-way
analysis of variance followed by Tukey's multiple comparison test.
The association of miR-106b expression with clinicopathological
features was analyzed using the χ2 test. The survival
analysis was carried out using the Kaplan-Meier method and the
log-rank test. Cox regression analysis was used to confirm the
prognostic value of miR-106b in patients with OS. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-106b in OS tissues
and cells
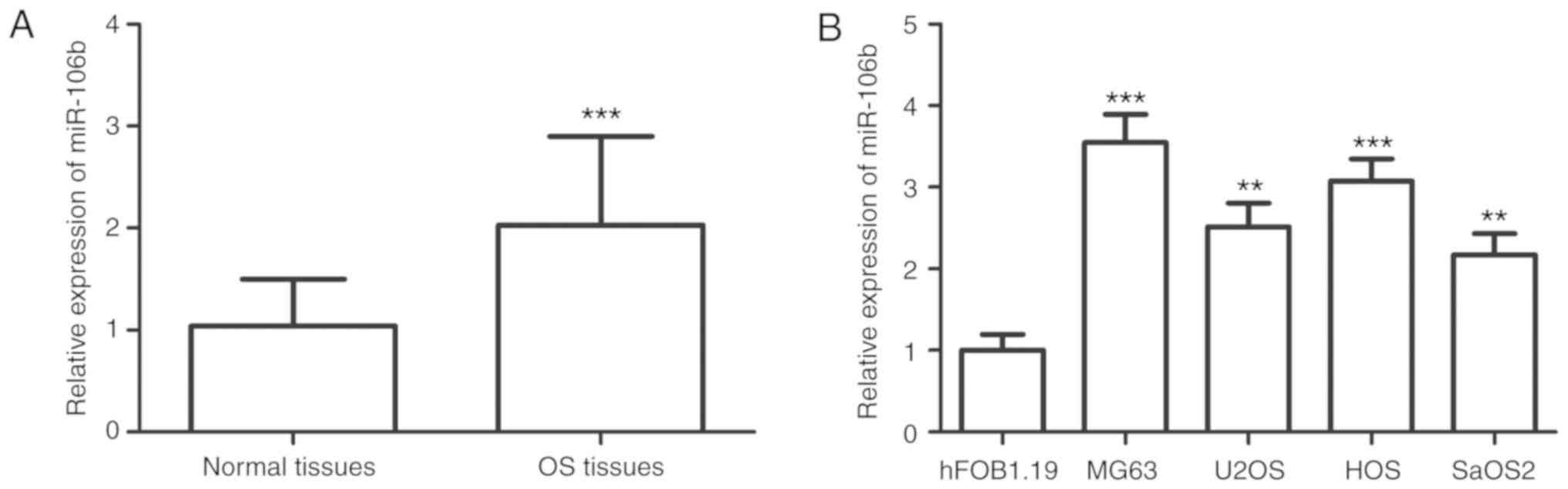
The expression values of miR-106b corresponded to a
Gaussian distribution, and were significantly upregulated in OS
tissues compared with that in the paired normal tissues
(P<0.001; Fig. 1A). Similar
results were observed in the OS cell lines, in which miR-106b
expression was higher in the four OS cell lines than the expression
in the normal cells (P<0.01; Fig.
1B).
Association between miR-106b
expression and the clinicopathological characteristics of patients
with OS
To investigate the potential role of miR-106b in OS
development, the association between miR-106b expression and the
clinicopathological data of patients with OS was assessed. The mean
miR-106b expression value (2.071) was used as a cut-off value to
classify miR-106b into low and high expression groups. The analysis
results presented in Table I
revealed that the expression of miR-106b was associated with
metastasis (P=0.028) and Tumor-Node-Metastasis (TNM) stage
(P=0.017). However, no association between miR-106b and other
clinicopathological parameters, including age, sex, tumor size, and
differentiation, was found in this analysis (P>0.05).
Prognostic value of miR-106b in
patients with OS
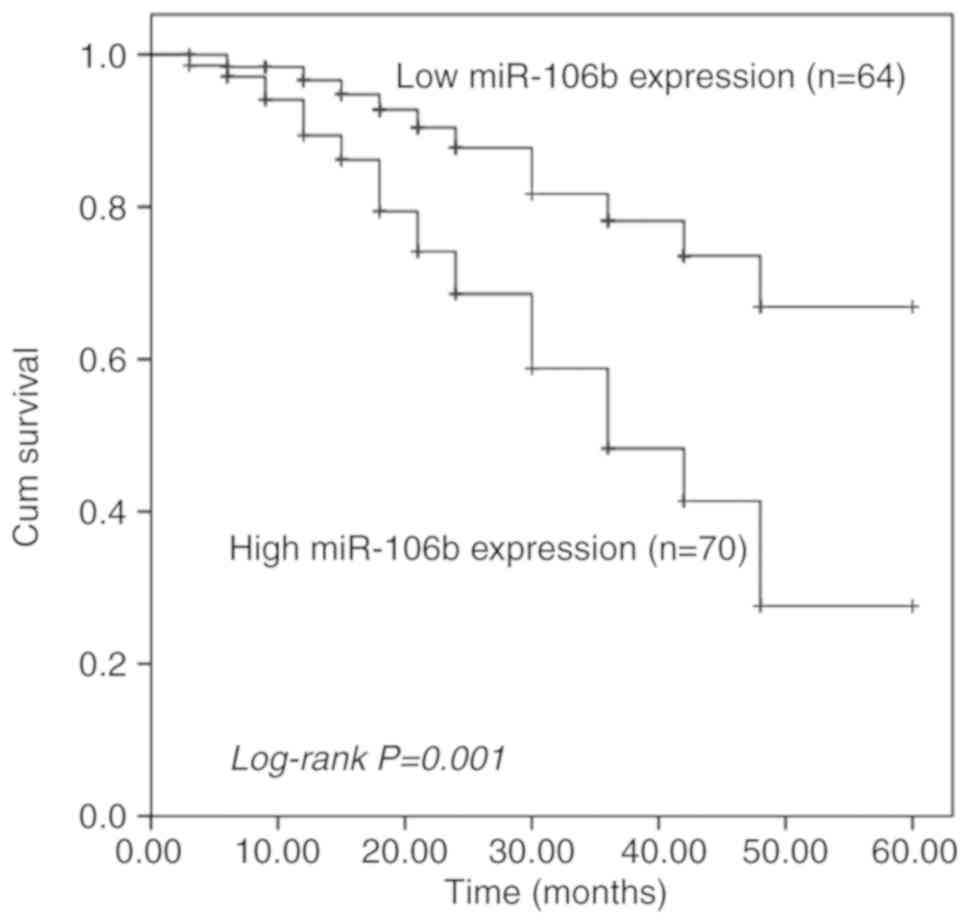
The focus of the present study was on the clinical
significance of miR-106b in OS prognosis. First, the survival
information collected from the 5-year follow-up survey was used to
perform a Kaplan-Meier survival analysis, and it was indicated that
patients with high miR-106b expression had shorter survival times
than those with low miR-106b expression (log-rank P=0.001; Fig. 2). Second, the expression of miR-106b
and other clinical parameters were included in the Cox regression
analysis. The multivariate Cox analysis indicated that miR-106b
expression [hazard ratio (HR)=2.769; 95% confidence interval
(CI)=1.369–5.599; P=0.005)] and metastasis (HR=2.235; 95%
CI=1.166–4.284; P=0.015) were two independent prognostic factors in
patients with OS (Table II).
 | Table II.Multivariate Cox regression analysis
for miR-106b in patients with OS. |
Table II.
Multivariate Cox regression analysis
for miR-106b in patients with OS.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value |
|---|
| miR-106b | 2.769 | 1.369–5.599 | 0.005b |
| Age | 1.070 | 0.564–2.030 | 0.835 |
| Sex | 1.080 | 0.575–2.030 | 0.810 |
| Tumor size | 1.304 | 0.727–2.341 | 0.373 |
|
Differentiation | 1.062 | 0.590–1.913 | 0.840 |
| Metastasis | 2.235 | 1.166–4.284 | 0.015a |
| TNM stage | 1.261 | 0.692–2.299 | 0.448 |
Effects of miR-106b on cell
proliferation of OS cells
Given the dysregulation of miR-106b in OS samples,
it was hypothesized that miR-106b may be involved in the tumor
progression of OS. Therefore, the effects of miR-106b expression on
OS cell biological behaviors were examined by regulating the
expression of miR-106b. This was done using an miR-106 mimic and an
miR-106 inhibitor. The OS cell lines MG63 and HOS were used for the
cell experiments, as they had a higher expression of miR-106b.
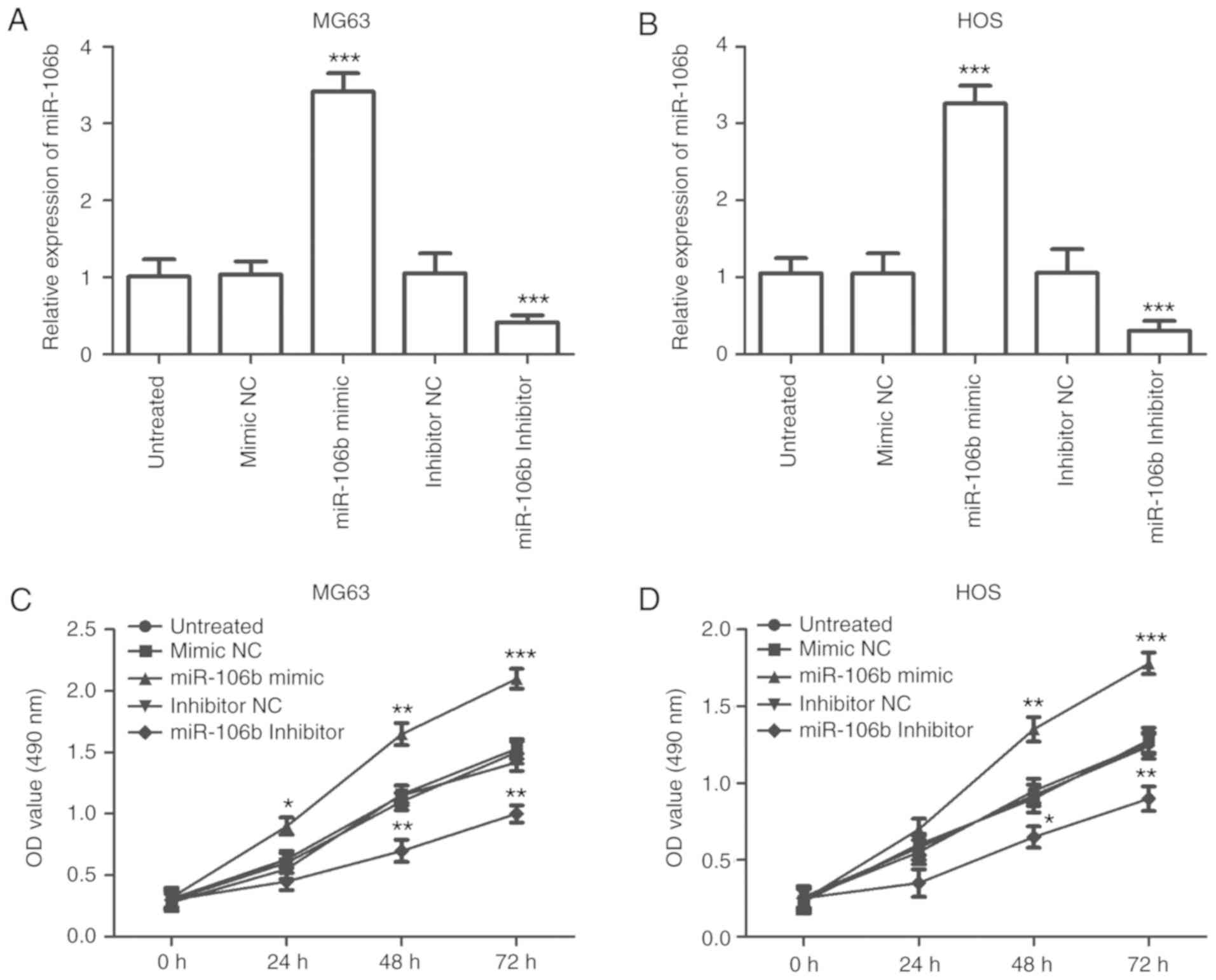
According to the RT-qPCR results, the expression of miR-106b was
elevated in the cells transfected with the miR-106b mimic
(P<0.001), and was decreased in the cells transfected with the
miR-106b inhibitor (P<0.001; Fig. 3A
and B). The results of the MTT assay demonstrated that cell
proliferation was promoted by the overexpression of miR-106b,
however it was inhibited by miR-106b reduction in both MG63 and HOS
cells (P<0.05; Fig. 3C and
D).
Effects of miR-106b on cell migration
and invasion of OS cells
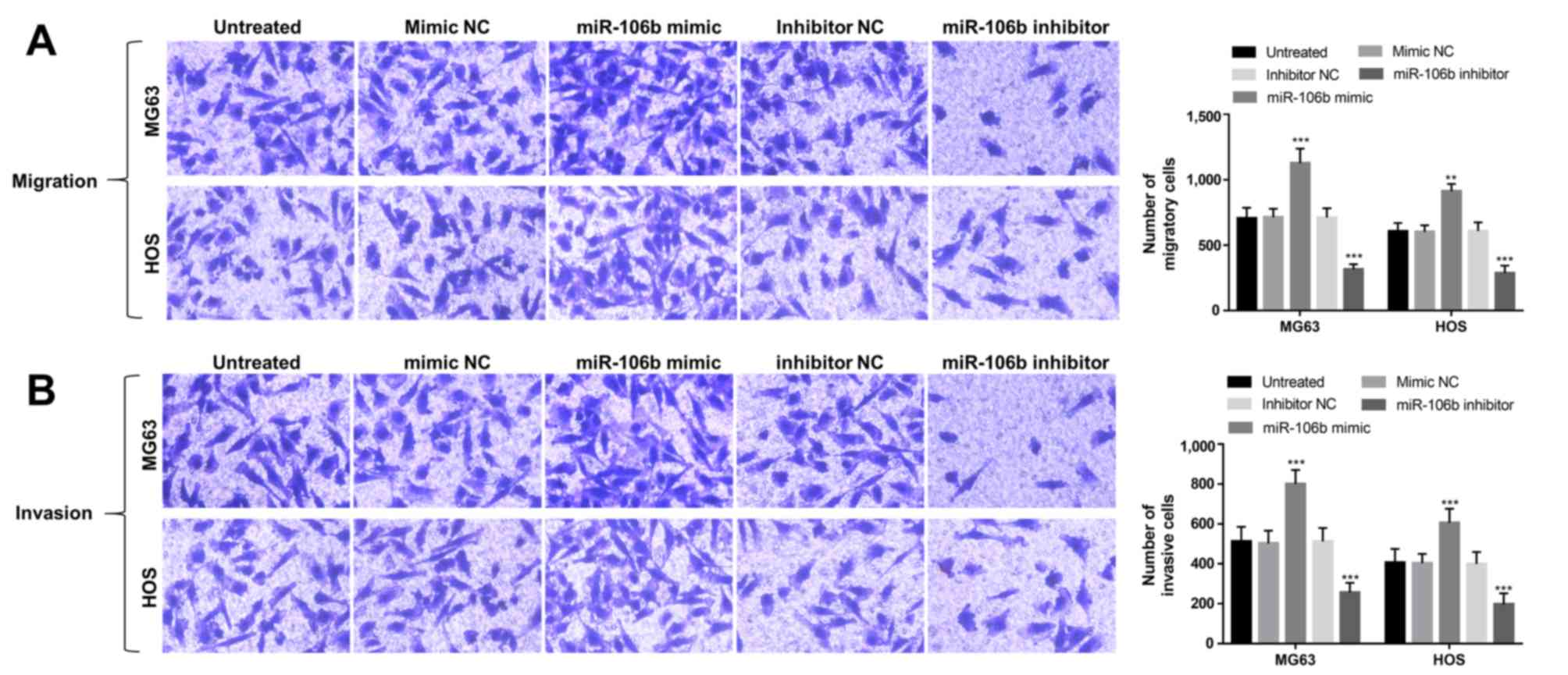
The abilities of cell migration and invasion in the
transfected OS cells were also analyzed using Transwell analysis.
As shown in Fig. 4A, this study
found that the upregulation of miR-106b could enhance cell
migration, whereas the downregulation of miR-106b could suppress
cell migration in MG63 and HOS cells (P<0.01). In addition, OS
cell invasion was also promoted by the overexpression of miR-106b,
but was inhibited by the knockdown of miR-106b (P<0.01; Fig. 4B).
Discussion
Emerging evidence has shown that miRNAs play pivotal
roles in the initiation and development of various types of human
cancer, and therefore, are applied in targeted therapy for these
malignant diseases (20,21). In addition, the clinical significance
of miRNAs has also attracted increasing attention for their
dramatic diagnostic and prognostic value in human cancers (22,23). OS,
as the most frequent primary malignancy among adolescents and
children, has become a serious health burden worldwide (24). Regardless of the advances in diverse
therapeutic strategies, including aggressive surgical resection and
adjuvant chemotherapy, the outcomes for patients diagnosed with OS
remain dismal. Therefore, the identification of the functional
miRNAs that are involved in OS progression, is urgently needed for
the improvement of OS treatment. Currently, some members of miRNAs
have been observed in OS samples with deregulated expression levels
and critical roles. A study scheduled by Zhang et al
(25) showed that miR-33a-5p was
downregulated in OS tissues and could suppress the cell growth of
OS cells. The increased expression of miR-148a detected in OS
tissues was shown to be involved in OS growth in vitro and
in vivo (26). Mao et
al (27) demonstrated that OS
cell migration and invasion could be suppressed by miR-195,
indicating that miR-195 served as a potential therapeutic target
for OS treatment. In addition, the clinical value of miRNAs has
also been assessed in the previous studies for patients with OS. Li
et al (28) have indicated
that the increased serum miR-17 was associated with the poor
overall survival of patients with OS and could play as an efficient
prognostic biomarker of OS. In the present study, the aberrant
expression of miR-106b was indicated in both OS tissues and cells.
Therefore, it was hypothesized that miR-106 may be involved in OS
progression with an important role.
miR-106 is a member of the miR-106b-25 cluster,
which has been described to play oncogenic roles in some types of
cancer, such as hepatocellular carcinoma (29) and myeloid leukemia (30). The dysregulation of miR-106b has been
observed in some cancers, and its expression variation trends are
diverse in different types of tumor. For example, the decreased
expression of miR-106b was detected in breast cancer, and the
miR-106b reduction could enhance breast cancer cell migration and
invasion (31). Furthermore, the
increased miR-106b expression was found in hepatocellular carcinoma
tissues compared with that in normal controls and may be involved
in tumor progression of this malignant disease (32). Xu et al (33) reported that the expression of
miR-106b was significantly increased in tumor tissues collected
from paediatric patients with OS, and was associated with cell
proliferation, migration and invasion of OS cell line U2OS. In this
study, the expression of miR-106b in a larger research cohort with
134 patients with OS was measured by RT-qPCR, and was found to be
upregulated compared with the adjacent normal tissues. Similarly,
the increased expression of miR-106b was also observed in four OS
cell lines compared to that in the normal cells. Therefore, this
study considered that miR-106b may be an oncogenic miRNA in OS. In
addition, the overexpression of miR-106b was associated with
positive metastasis and the advanced TNM stage of patients with OS,
indicating that miR-106b was involved in the tumor development of
OS.
Given the deregulated expression of miR-106b in OS,
the clinical significance of miR-106b in OS prognosis was further
analyzed. According to the Kaplan-Meier survival analysis, the
patients with OS with high miR-106b expression had a shorter
survival time compared with those with low miR-106b expression,
suggesting that the overexpression of miR-106b was associated with
poor overall survival time of patients with OS. Furthermore, the
results of multivariate Cox analysis implied that miR-106b was an
independent prognostic factor in patients with OS. Therefore, this
study suggests that the overexpression of miR-106b served as a
novel and non-invasive prognostic biomarker of OS.
To further explore the functional role of miR-106b
in OS progression, the effects of miR-106b expression on cell
proliferation, migration and invasion were examined in OS cells.
The results of the MTT assay revealed that OS cell proliferation
was promoted by the overexpression of miR-106b, however was
suppressed by miR-106b-knockdown. According to Transwell analysis,
it was found that the upregulation of miR-106b could enhance OS
cell migration and invasion, whereas the downregulation of miR-106b
could inhibit these functions. All the data above indicated that
the dysregulation of miR-106b may serve as a therapeutic target for
the treatment of OS. miR-106b has been demonstrated to promote the
cell proliferation, migration, and invasion of medulloblastoma
cells by directly targeting PTEN (34). Tumor cell proliferation, migration,
and invasion were also found to be enhanced by the overexpression
of miR-106b in esophageal squamous cell carcinoma, as these
tumor-promoting effects were exerted via the downregulation of
Smad7 (35). Although this
data also indicated that miR-106b may promote tumor progression in
OS, the molecular mechanisms underlying the role of miR-106b in OS
remain elusive and need to be examined in further studies.
In conclusion, the data in this study revealed that
the upregulated expression of miR-106b serves as a candidate
prognostic biomarker in patients with OS, and this overexpression
of miR-106b promotes OS cell proliferation, migration, and
invasion, suggesting that miR-106b may be a potential therapeutic
target in OS treatment. This study provides a novel prognostic
biomarker for patients with OS, and the strategies to downregulate
miR-106b may be effective therapeutic methods in OS treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KX and BW were responsible for the overall design of
the study, clinical research, clinical data analyses and manuscript
writing. WX and SZ performed the cell experiments and analyzed the
associated data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Ningbo No. 2 Hospital (Ningbo, China). Written informed consent was
obtained from each patient.
Patient consent for publication
Patients provided written informed consent for the
publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McTiernan A, Jinks RC, Sydes MR, Uscinska
B, Hook JM, van Glabbeke M, Bramwell V, Lewis IJ, Taminiau AH,
Nooij MA, et al: Presence of chemotherapy-induced toxicity predicts
improved survival in patients with localised extremity osteosarcoma
treated with doxorubicin and cisplatin: A report from the European
Osteosarcoma Intergroup. Eur J Cancer. 48:703–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li XP, Cao GW, Sun Q, Yang C, Yan B, Zhang
MY, Fu YF and Yang LM: Cancer incidence and patient survival rates
among the residents in the Pudong New Area of Shanghai between 2002
and 2006. Chin J Cancer. 32:512–519. 2013.PubMed/NCBI
|
|
6
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng Y, Dong YW, Song YN, Xiao JH, Guo XY,
Jiang WL and Lu LG: MicroRNA-449a is a potential predictor of
colitis-associated colorectal cancer progression. Oncol Rep.
40:1684–1694. 2018.PubMed/NCBI
|
|
8
|
Peng X, Zha L, Chen A and Wang Z: HOXA5 is
a tumor suppressor gene that is decreased in gastric cancer. Oncol
Rep. 40:1317–1329. 2018.PubMed/NCBI
|
|
9
|
Zhou B, Li Z, Yang H and He N:
Extracellular miRNAs: Origin, function and biomarkers in hepatic
diseases. J Biomed Nanotechnol. 10:2865–2890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Z, Tang ZY, He Y, Liu LF, Li DJ and
Chen X: miRNA-205 is a candidate tumor suppressor that targets ZEB2
in renal cell carcinoma. Oncol Res Treat. 37:658–664. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao J, Xu J and Zhang R: MicroRNA-539
inhibits colorectal cancer progression by directly targeting SOX4.
Oncol Lett. 16:2693–2700. 2018.PubMed/NCBI
|
|
12
|
Chen K, Chen Y, Chen Z, Shi Y, He Z, Ding
B, Wang C and Yu L: miR-134 increases the antitumor effects of
cytarabine by targeting Mnks in acute myeloid leukemia cells. Onco
Targets Ther. 11:3141–3147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Li L, Liu Z, Yuan Q and Lu X:
Downregulation of MiR-431 expression associated with lymph node
metastasis and promotes cell invasion in papillary thyroid
carcinoma. Cancer Biomark. 22:727–732. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Cao B, Zhao Y, Liang H and Hao F:
Upregulated miR-221/222 promotes cell proliferation and invasion
and is associated with invasive features in retinoblastoma. Cancer
Biomark. 22:621–629. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu N, Yang H and Wang H: miR-598 acts as
a tumor suppressor in human gastric cancer by targeting IGF-1R.
Onco Targets Ther. 11:2911–2923. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu W, Wan X, Mu Z, Li F, Wang L, Zhao J
and Huang X: MiR-1256 suppresses proliferation and migration of
non-small cell lung cancer via regulating TCTN1. Oncol Lett.
16:1708–1714. 2018.PubMed/NCBI
|
|
17
|
Arabi L, Gsponer JR, Smida J, Nathrath M,
Perrina V, Jundt G, Ruiz C, Quagliata L and Baumhoer D:
Upregulation of the miR-17-92 cluster and its two paraloga in
osteosarcoma-reasons and consequences. Genes Cancer. 5:56–63.
2014.PubMed/NCBI
|
|
18
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual. 7th. New York:
Springer; 2010
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiang J, Wu Y, Li DS, Wang ZY, Shen Q, Sun
TQ, Guan Q and Wang YJ: miR-584 suppresses invasion and cell
migration of thyroid carcinoma by regulating the target oncogene
ROCK1. Oncol Res Treat. 38:436–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L and Li S: miR-205-5p inhibits cell
migration and invasion in prostatic carcinoma by targeting ZEB1.
Oncol Lett. 16:1715–1721. 2018.PubMed/NCBI
|
|
22
|
Deng Y and Chen Y: Increased expression of
miR-29a and its prognostic significance in patients with
cholangiocarcinoma. Oncol Res Treat. 40:128–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dan B, Luo J, Li K and Chen S: Prognostic
value of miR-375 for survival outcomes in various cancers: A
systematic review and meta-analysis. Oncol Res Treat. 41:47–50.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
25
|
Zhang J, Wang D, Xiong J, Chen L and Huang
J: MicroRNA-33a-5p suppresses growth of osteosarcoma cells and is
downregulated in human osteosarcoma. Oncol Lett. 10:2135–2141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Wang Y, Xu T, Li C, Wu J, He Q,
Wang G, Ding C, Liu K, Tang H and Ji F: Increased expression of
microRNA-148a in osteosarcoma promotes cancer cell growth by
targeting PTEN. Oncol Lett. 12:3208–3214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao JH, Zhou RP, Peng AF, Liu ZL, Huang
SH, Long XH and Shu Y: microRNA-195 suppresses osteosarcoma cell
invasion and migration in vitro by targeting FASN. Oncol
Lett. 4:1125–1129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Gao Y, Wang Y, Wang K, Dai ZP, Xu D,
Liu W, Li ZL, Zhang ZD, Yang SH and Yang C: Serum microRNA-17
functions as a prognostic biomarker in osteosarcoma. Oncol Lett.
12:4905–4910. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan W, Li Y, Lim SG and Tan TM:
miR-106b-25/miR-17-92 clusters: Polycistrons with oncogenic roles
in hepatocellular carcinoma. World J Gastroenterol. 20:5962–5972.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Verboon LJ, Obulkasim A, de Rooij JD,
Katsman-Kuipers JE, Sonneveld E, Baruchel A, Trka J, Reinhardt D,
Pieters R, Cloos J, et al: MicroRNA-106b~25 cluster is upregulated
in relapsed MLL-rearranged pediatric acute myeloid leukemia.
Oncotarget. 7:48412–48422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ni X, Xia T, Zhao Y, Zhou W, Wu N, Liu X,
Ding Q, Zha X, Sha J and Wang S: Downregulation of miR-106b induced
breast cancer cell invasion and motility in association with
overexpression of matrix metalloproteinase 2. Cancer Sci.
105:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yen CS, Su ZR, Lee YP, Liu IT and Yen CJ:
miR-106b promotes cancer progression in hepatitis B
virus-associated hepatocellular carcinoma. World J Gastroenterol.
22:5183–5192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu M, Zhang YY, Wang HF and Yang GS: The
expression and function of miRNA-106 in pediatric osteosarcoma. Eur
Rev Med Pharmacol Sci. 21:715–722. 2017.PubMed/NCBI
|
|
34
|
Li KK, Xia T, Ma FM, Zhang R, Mao Y, Wang
Y, Zhou L, Lau KM and Ng HK: miR-106b is overexpressed in
medulloblastomas and interacts directly with PTEN. Neuropathol Appl
Neurobiol. 41:145–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dai F, Liu T, Zheng S, Liu Q, Yang C, Zhou
J, Chen Y, Sheyhidin I and Lu X: MiR-106b promotes migration and
invasion through enhancing EMT via downregulation of Smad 7 in
Kazakh's esophageal squamous cell carcinoma. Tumour Biol.
37:14595–14604. 2016. View Article : Google Scholar : PubMed/NCBI
|