Introduction
Various conditions of the jaws may present
multinucleated giant cells. These include central giant cell
lesions (CGCL), peripheral giant cell lesions (PGCL), brown tumor
of hyperparathyroidism (BTH), and cherubism. These lesions show
multinucleated osteoclast-like giant cells in a background of oval
to spindle-shaped mononuclear cells.
PGCL is a reactive lesion usually associated with
local irritating factors in gingiva (1). CGCL is an intra-osseous lesion of
unknown etiology that occurs mainly in the mandible of patients
ranging from 10 to 25 years (2).
Cherubism is an autosomal-dominant genetic disease that affects the
jaws (3). The disease is
characterized by bilateral expansion of the maxilla and/or
mandible, usually detected in early childhood, and shows
progressive growth until puberty (4). BTH is a non-neoplastic lesion
resulting from abnormal bone metabolism in hyperparathyroidism and
may be associated with the primary or secondary types of the
disease (5).
The gene mutated in cherubism has been mapped to
chromosome 4p16.3 (6). Missense
mutations were observed in the SH3BP2 gene mainly clustered
in exon 9, which encodes a proline-rich region of the protein
(7,8). Recently, a somatic mutation of this
gene was described in a case of CGCL (9).
NFATc1 plays a significant role in osteoclast
differentiation. This protein is considered to be the
osteoclastogenesis master transcription factor. NFATc1 is located
in cytoplasm and is activated after RANKL signaling in osteoclast
precursor cells (10). Downstream
stimulation by RANKL promotes the formation of a complex containing
a second messenger, SH3BP2, which results in the upregulation of
intracellular calcium (11).
Increased levels of calcium promote the displacement of NFATc1 to
the nucleus where it binds to its own promoter. This binding leads
to the autoamplification of NFATc1 and activation of
osteoclasteogenesis-specific genes. In lesions with the
SH3BP2 mutant, as in the case of cherubism, upregulation of
the calcium promotes a constant displacement of NFATc1 to the
nucleus and a subsequent increased osteoclast differentiation
(12).
Recently an increased transcription of the NFATc1 in
was found in giant cell lesions (13). The present study aimed to compare
the expression of NFATc1 in CGCL, PGCL, BTH and cherubism.
Materials and methods
A total of 14 formalin-fixed and paraffin-embedded
tissue samples of PGCL (n=5), CGCL (n=4), BTH (n=3) and cherubism
(n=2) were included in the present study. Table I shows the age, gender and tumor
location in each group.
 | Table IClinical data and nuclear
immunohistochemical expression of NFATc1. |
Table I
Clinical data and nuclear
immunohistochemical expression of NFATc1.
| Lesion | Age | Gender | Location | % positive giant
cells | % positive
mononuclear cells |
|---|
| CGCL |
| 1 | 19 | M | Mand. | 100.0 | 8.1 |
| 2 | 16 | F | Mand. | 93.8 | 4.7 |
| 3 | 15 | F | Max. | 96.3 | 16.7 |
| 4 | 12 | F | Max. | 91.0 | 9.0 |
| PGCL |
| 1 | 7 | F | Mand. | 93.0 | 2.4 |
| 2 | 11 | M | Max. | 98.2 | 8.9 |
| 3 | 37 | F | Mand. | 100.0 | 7.5 |
| 4 | 16 | M | Mand. | 94.6 | 5.4 |
| 5 | 22 | M | Max. | 78.0 | 3.3 |
| BTH |
| 1 | 43 | F | Mand. | 95.5 | 8 |
| 2 | 39 | M | Mand. | 90.8 | 6.9 |
| 3 | 53 | F | Max. | 99.2 | 15.3 |
| Cherubism |
| 1 | 15 | M | Mand. | 87.3 | 4.1 |
| 2 | 16 | M | Mand. | 93.0 | 8.9 |
The immunohistochemistry protocols used are
described elsewhere (13). Briefly,
paraffin-embedded tissue sections were incubated with NFATc1
antiserum (diluted 1:50, Clone 7A6, SantaCruz Biotechnology Inc.,
Santa Cruz, CA, USA). The immunohistochemical staining was
performed using a highly sensitive polymer-based system (EnVision,
Dako Corporation, Carpinteria, CA, USA) with the diaminobenzidine
substrate solution as chromogen (Sigma, St. Louis, MO, USA). The
sections were counterstained with hematoxylin.
Only sections containing sufficient tumor cells used
to assess the antibody reactivity were included in the
investigation. Two experienced pathologists made an independent
analysis of each case, regardless of staining intensity. Six
high-power fields (magnification, ×400) were examined and the
percentage of mononuclear and multinucleated positively stained
cells was obtained for each case. Only nuclear immunoexpression was
evaluated.
Results
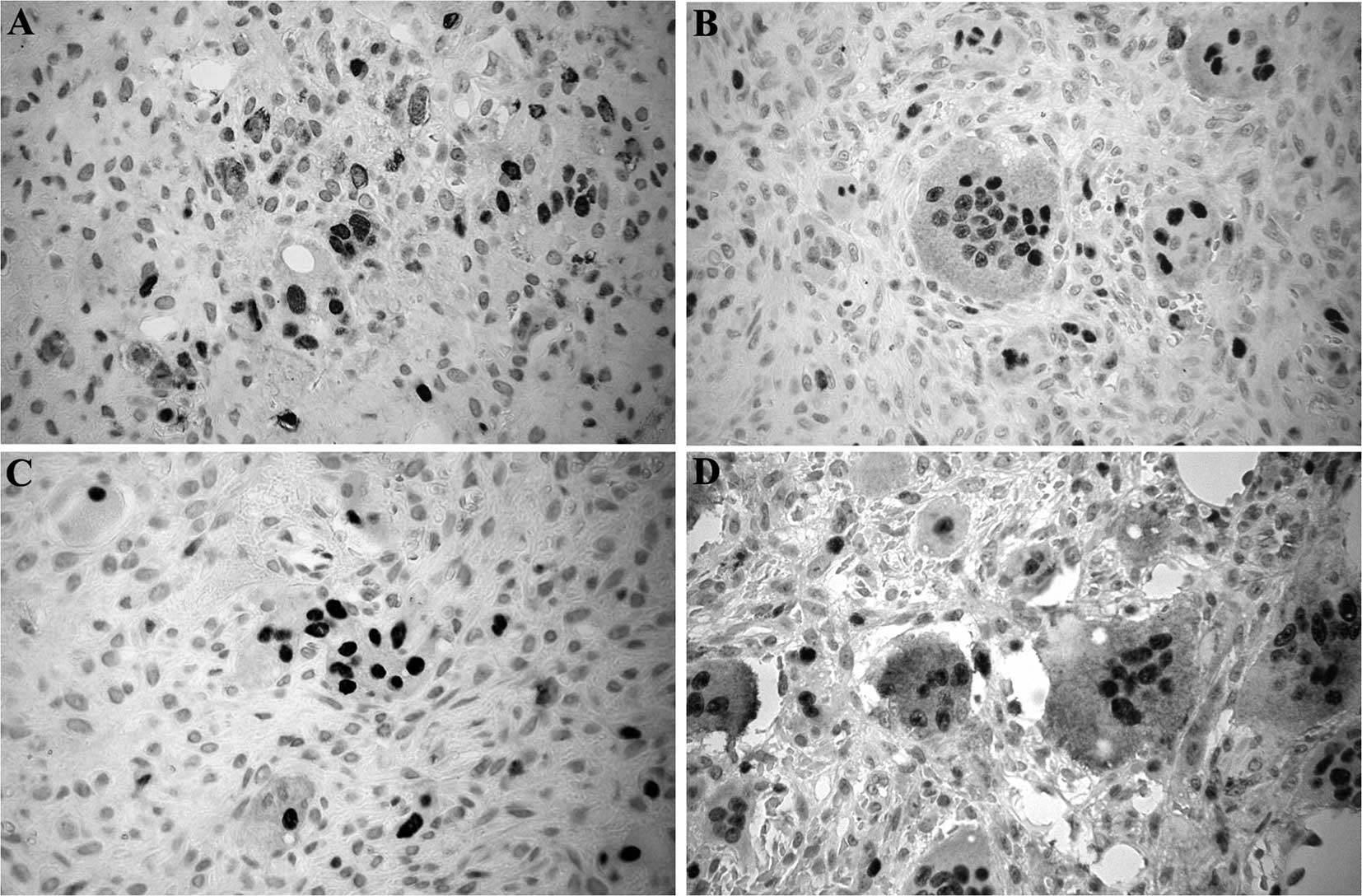
The imunohistochemical profile of NFATc1 in giant
cell lesions, BTH and cherubism are shown in Table I. It was found that most of the
giant cells in all of the cases were positive for nuclear NFATc1.
Less than 17% of the mononuclear ovoid and spindle-shaped cells
were immunopositive for this protein. Fig. 1 shows the immunostaining pattern of
NFATc1 in each lesion group. The immunostaining pattern was similar
in all lesions.
Discussion
Increased levels of NFATc1 in bone marrow cells play
an important role in osteoclastogenesis. Previous studies showed
that the giant cells found in PGCL, CGCL and cherubism exhibit
osteoclast-like characteristics (14). NFATc1 is a cytoplasmic protein
activated by calcineurin, Ca+/calmodulin-dependent
protein phosphatase. This activation increases the levels of
calcium, leading to NFATc1 translocation to the nucleus where it
binds to its own promoter. After RANKL signaling, a complex
containing a SH3BP2 second messenger is involved in a calcineurin
cascade that activates NFATc1, increasing osteoclastogenesis and
giant cell formation (11).
SH3BP2 is mutated in cherubism, and it was further
demonstrated that this gene is also mutated in CGCL (9).
Results of our previous study showed an increased
transcription of NFATc1 together with the positive immunostaining
of this protein mainly in the nucleus of the multinucleated giant
cell lesions of the jaws (13). In
the present study, the immunoexpression of NFATc1 was compared in
different lesions containing multinucleated giant cells. Most of
the giant cells in all of the lesions exhibited NFATc1-positive
nuclear staining. This finding supports the hypothesis that giant
cell accumulation in PGCL, CGCL, BTH and cherubism is mediated by
NFATc1. However, studies are required to examine whether NFATc1
modulation is an alternative molecular tool in the prevention or
regulation of the growth of these lesions.
Apart from the important role in osteoclastogenesis,
NFAT is crucial for thymocyte survival (15). This protein forms a complex with the
protein AP-1 to bind in specific DNA sites. Loss of NFAT:AP-1
complex activity in the nucleus promotes apoptosis of these cells
(16). Glucocorticoids impair this
complex formation and induce apoptosis of the immature thymocytes
(17). Corticosteroids have been
used successfully in the treatment of certain cases of giant cell
lesions of the jaws (18–20). A previous study showed that
glucocorticoids inhibit osteoclast precursor proliferation
(21). Findings of this study
showed that glucocorticoids disrupt osteoclasts in the cytoskeleton
and consequently affect the function of these cells, decreasing
bone resorption. Further studies are required to demonstrate
whether multinucleated giant cell formation is inhibited by
corticosteroids via modulation of the NFAT pathway.
Acknowledgements
This study was supported by grants from Conselho
Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and
Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG),
Brazil. Dr RS Gomez is a research fellow of CNPq.
References
|
1
|
Katsikeris N, Kakarantza-Angelopoulou E
and Angelopoulos AP: Peripheral giant cell granuloma.
Clinicopathologic study of 224 new cases and review of 956 reported
cases. Int J Oral Maxillofac Surg. 17:94–99. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Lange J, van den Akker HP and van den
Berg H: Central giant cell granuloma of the jaw: a review of the
literature with emphasis on therapy options. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 104:603–615. 2007.PubMed/NCBI
|
|
3
|
Von Wowern N: Cherubism: a 36-year
long-term follow-up of 2 generations in different families and
review of the literature. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 90:765–772. 2000.PubMed/NCBI
|
|
4
|
Hyckel P, Berndt A, Schleier P, Clement
JH, Beensen V, Peters H and Kosmehl: Cherubism - new hypotheses on
pathogenesis and therapeutic consequences. J Craniomaxillofac Surg.
33:61–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pecovnik Balon B and Kavalar R: Brown
tumor in association with secondary hyperparathyroidism. A case
report and review of the literature. Am J Nephrol. 18:460–463.
1998.PubMed/NCBI
|
|
6
|
Bell SM, Shaw M, Jou YS, Myers RM and
Knowles MA: Identification and characterization of the human
homologue of SH3BP2, an SH3 binding domain protein within a common
region of deletion at 4p16.3 involved in bladder cancer. Genomics.
44:163–170. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueki Y, Tiziani V, Santanna C, et al:
Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause
cherubism. Nat Genet. 28:125–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Lange J, van Maarle MC, van den Akker
HP and Redeker EJ: A new mutation in the SH3BP2 gene showing
reduced penetrance in a family affected with cherubism. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 103:378–381.
2007.PubMed/NCBI
|
|
9
|
Carvalho VM, Perdigao PF, Amaral FR, De
Souza PE, De Marco L and Gomez RS: Novel mutations in the SH3BP2
gene associated with sporadic central giant cell lesions and
cherubism. Oral Dis. 15:106–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lietman SA, Yin L and Levine MA: SH3BP2 is
an activator of NFAT activity and osteoclastogenesis. Biochem
Biophys Res Commun. 371:644–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Novack DV and Faccio R: Jawing about TNF:
new hope for cherubism. Cell. 128:15–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amaral FR, Brito JA, Perdigao PF, et al:
NFATc1 and TNF alpha expression in giant cell lesions of the jaws.
J Oral Pathol Med. 39:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itonaga I, Hussein I, Kudo O, Sabokbar A,
Watt-Smith S, Ferguson D and Athanasou NA: Cellular mechanisms of
osteoclast formation and lacunar resorption in giant cell granuloma
of the jaw. J Oral Pathol Med. 32:224–231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adachi S, Amasaki Y, Miyatake S, Arai N
and Iwata M: Successive expression and activation of NFAT family
members during thymocyte differentiation. J Biol Chem.
275:14708–14716. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wisniewska M, Stanczyk M,
Grzelakowska-Sztabert B and Kaminska B: Nuclear factor of activated
T cells (NFAT) is a possible target for dexamethasone in thymocyte
apoptosis. Cell Biol Int. 21:127–132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wisniewska M, Pyrzynska B and Kaminska B:
Impaired AP-1 dimers and NFAT complex formation in immature
thymocytes during in vivo glucocorticoid-induced apoptosis. Cell
Biol Int. 28:773–780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdo EN, Alves LC, Rodrigues AS, Mesquita
RA and Gomez RS: Treatment of a central giant cell granuloma with
intralesional corticosteroid. Br J Oral Maxillofac Surg. 43:74–76.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carlos R and Sedano HO: Intralesional
corticosteroids as an alternative treatment for central giant cell
granuloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
93:161–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adornato MC and Paticoff KA: Intralesional
corticosteroid injection for treatment of central giant-cell
granuloma. J Am Dent Assoc. 132:186–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HJ, Zhao H, Kitaura H, et al:
Glucocorticoids and the osteoclast. Ann N Y Acad Sci. 1116:335–339.
2007. View Article : Google Scholar
|