Introduction
Comprising approximately 6% of all intracranial
tumors, vestibular schwannomas, also known as acoustic neuromas,
are a common disease found in the region of the cerebellopontine
angle (1). Although many data have
been gathered, numerous phenomena encountered in this disease are
not yet understood. Neurosurgeons have established that hearing
loss, deafness and tinnitus are common clinical presentations for
this disease; however, larger case studies are required to identify
the most common and significant clinical features of the disease.
Furthermore, controversy remains as to the clinical features that
predict the development of tumors. It is not clear how the various
symptoms, duration and sequence of occurrence correlate with the
size and extension of the tumor or with the actual objective
cranial nerve damage.
Currently, more and more research is focusing on
conservative management aimed at preventing the further growth of
lesions (2). A ‘watch-wait-rescan’
strategy appears to be justified in numerous patients. However,
there is a potential burden of worry for individuals with an
acoustic neuroma managed with a watch-wait-rescan strategy when
they are aware that their tumor may progress at any time. Thus,
distinguishing individual patients whose tumors will progress and
pose a threat from those whose tumors are likely to remain dormant
or even regress is central to the current management of these
patients. Increasingly, evidence of tumor growth has become the
defining criterion for intervention. Consequently, an analysis of
these epidemiological aspects of alarm features is necessary before
formulating an optimal diagnostic and therapeutic protocol.
In recent years, a number of studies concerning
vestibular schwannomas have focused on cranial nerve preservation
rather than description of clinical presentation. The 1,009 cases
treated in Shanghai Huashan Hospital between 1999 and 2009 offer an
opportunity to detail the clinical features and management of these
tumors. In this study, we focus on the clinical features of
intracranial vestibular schwannomas, and evaluate the symptom and
signs as well as their correlation with tumor extension.
Patients and methods
Patient population
A retrospective review of medical records from 1999
to 2009 identified 1,009 patients with intracranial vestibular
schwannomas. Tumor removal was performed using a sub-occipital
approach for all cases and the received histology results were
verified. The study was approved by the ethics committee of the
Institution of Neurology, Fudan University, Shanghai, China.
Written informed consent was obtained from the patient’s
family.
Clinical manifestation
In this study, we focused on the clinical evaluation
that was based in part on records of patients’ complaints and
neurological examinations in the following areas: i) general state
of health; ii) involvement of the cranial nerves; iii) involvement
of the cerebellum and the cerebrum; and iv) duration and sequence
of symptoms.
The objective investigation for all patients
preoperatively included bone window computed tomography (CT),
contrast-enhanced magnetic resonance imaging (MRI) and brainstem
electric response audiometry, which were performed before surgery
and 1 to 2 weeks postoperatively in each case.
Tumor size and classification
Tumor sizes were measured in the axial plane,
considering intra- and extrameatal tumor extension. The data were
recorded for each operative protocol. Tumors larger than 30×20 mm
were defined as large; small tumors measured less than 30×20 mm.
Tumor extension classes were described as follows: T1, purely
intrameatal; T2, intra-extrameatal; T3, filling the
cerebellopontine cistern or reaching the brain stem; T4 compressing
the brain stem or severely dislocating the brain stem and
compressing the fourth ventricle.
Statistical analysis
Categorical data were compared by the χ2
test with continuity correction if appropriate. Continuous
variables are expressed as the mean ± SD, and were compared using
the Student’s t-test. The diagnostic values of symptoms including
sensitivity, specificity, positive predictive value (PPV), negative
predictive value (NPV), positive likelihood ratio (PLR), negative
likelihood ratio (NLR) and their 95% CIs were calculated and
compared between classes T3 and T4. Two-tailed P-values <0.05
were considered to indicate a statistically significant difference.
Statistical analysis was performed with the Stata software package
(version 10.0).
Results
General patient data
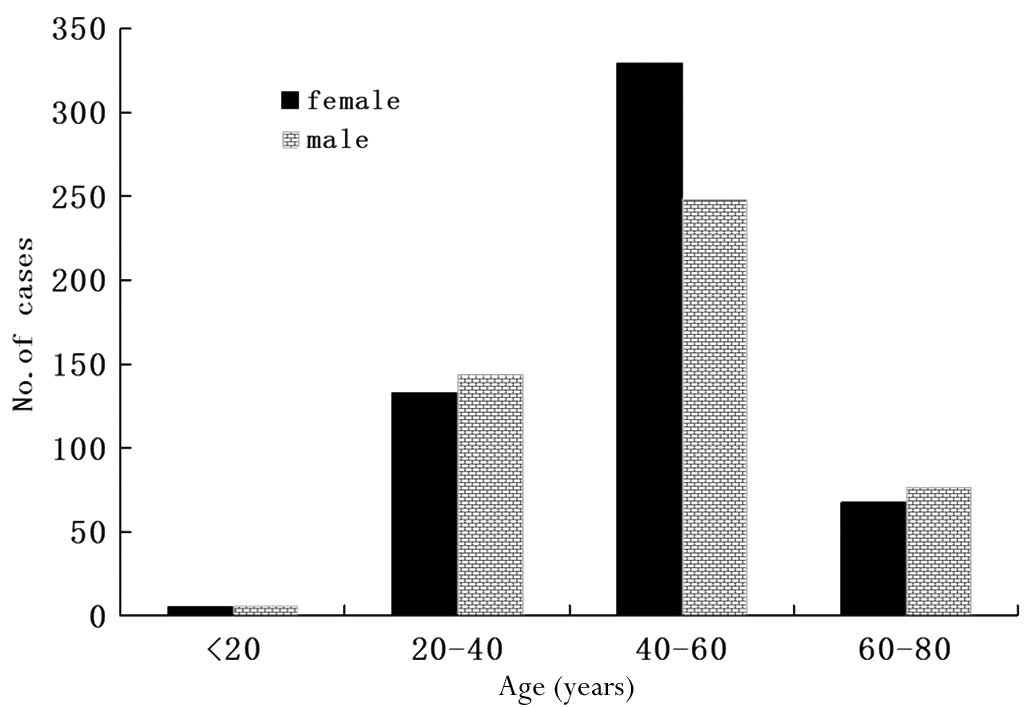
In this group of medical cases, the male to female
ratio was 475:534 and the age range was 12–80 years (median,
47.6±12.2). Most of the patients were 40–60 years of age,
accounting for 57.2% of the total patients. There were 11 patients
younger than 20 years of age, making up only 1.1% of cases. There
were 277 (27.5%) and 144 (14.2%) patients belonging to the 20–40
and 60–80 age groups, respectively. The age and gender distribution
revealed that middle-aged females between 40 and 60 years of age
were a high-risk population in this disease, accounting for 61.6%
of total female patients; this figure was 32.7% higher than that
observed for male patients in the same age group (Fig. 1).
Tumor extension analysis revealed that 42% of tumors
belonged to class T3 and 58% to class T4. A total of 53% of tumors
were located on the right and 47% on the left. Tumor length and
width were significantly different between these classes, with a
mean length and width of 30×25 mm in class T3 and 44×36 mm in class
T4.
Symptoms and signs
Among the 1,009 patients, the most frequent clinical
symptoms were hearing loss (85.8%), facial paresthesia (48.9%),
instability of gait (44.6%), tinnitus (40.1%), deafness (26.3%) and
facial paralysis (21.1%). During preoperative physical examination,
absent corneal reflex was the most common sign, observed in 15.2%
of the 1,009 patients. However, Romberg sign and abduction disorder
were both uncommon, observed in <2% of patients (Table I).
 | Table IMajor preoperative symptoms and signs
of 1,009 cases of intracranial vestibular schwannomas. |
Table I
Major preoperative symptoms and signs
of 1,009 cases of intracranial vestibular schwannomas.
| Symptoms and
signs | Total (%) | T3 (%) | T4 (%) | P-value |
|---|
| Headache | 259 (25.7) | 107 (25.2) | 152 (26.0) | 0.789 |
| Hypacusis | 866 (85.8) | 357 (84.2) | 509 (87.0) | 0.206 |
| Hearing loss | 265 (26.3) | 108 (25.5) | 157 (26.8) | 0.627 |
| Tinnitus | 405 (40.1) | 177 (41.7) | 228 (39.0) | 0.375 |
| Vertigo | 160 (15.9) | 55 (13.0) | 105 (17.9) | 0.033 |
| Instability of
gait | 450 (44.6) | 203 (47.9) | 247 (42.2) | 0.074 |
| Facial paralysis | 213 (21.1) | 77 (18.8) | 137 (23.3) | 0.035 |
| Facial
paresthesia | 493 (48.9) | 200 (47.2) | 293 (50.1) | 0.360 |
| Ear pain | 4 (0.4) | 1 (0.2) | 3 (0.5) | 0.854 |
| Bucking | 99 (9.8) | 39 (9.2) | 60 (10.3) | 0.577 |
| Hoarseness | 18 (1.8) | 9 (2.1) | 9 (1.5) | 0.489 |
| Visual disorder | 82 (8.1) | 35 (8.3) | 47 (8.0) | 0.899 |
| Movement
disorder | 41 (4.1) | 19 (4.5) | 22 (3.8) | 0.567 |
| Nausea and
vomiting | 57 (5.6) | 23 (5.4) | 34 (5.8) | 0.792 |
| Romberg sign | 20 (2.0) | 9 (2.1) | 11 (1.9) | 0.785 |
| Nystagmus | 52 (5.2) | 22 (5.2) | 30 (5.1) | 0.966 |
| Absent corneal
reflex | 153 (15.2) | 78 (18.4) | 75 (12.8) | 0.015 |
| Mastication
disorder | 26 (2.6) | 14 (3.3) | 12 (2.1) | 0.216 |
| Abduction
disorder | 17 (1.7) | 6 (1.4) | 11 (1.9) | 0.571 |
The most frequent cranial nerve disturbance was
related to the cochlear nerve (92.6%) and trigeminal nerve (53.5%).
There were only 17 (1.7%) cases involving the abducent nerve, which
became the minimal impact nerve by tumors (Table II).
 | Table IIPreoperative cranial nerve
disturbance of 1,009 cases of intracranial vestibular
schwannomas. |
Table II
Preoperative cranial nerve
disturbance of 1,009 cases of intracranial vestibular
schwannomas.
| Nerve | Symptom/sign | Total (%) | T3 (%) | T4 (%) | P-value |
|---|
| V | Trigeminal nerve
disturbance | 540 (53.5) | 222 (52.4) | 318 (54.4) | 0.529 |
| VI | Abduction
disorder | 17 (1.7) | 6 (1.4) | 11 (1.9) | 0.571 |
| VII | Facial nerve
disturbance | 230 (22.8) | 67 (14.9) | 163 (27.8) | 0.035 |
| VIII | Hearing
deficits | 934 (92.6) | 393 (92.7) | 541 (92.5) | 0.900 |
| VIII | Vestibular
disturbance | 209 (20.7) | 76 (17.9) | 133 (22.7) | 0.063 |
| IX–XII | Caudal cranial
nerve deficits | 153 (15.2) | 67 (15.8) | 86 (14.7) | 0.630 |
Cochlear nerve symptoms accounted for the most
frequent complaints (Table II). A
total of 648 patients noticed some degree of hearing deficit and
265 patients experienced deafness. Tinnitus occurred at an
incidence of 43.7 and 38.1% in hypacusis and deaf patients,
respectively. The incidence of facial paralysis and instability of
gait were significantly different between the two groups
(P<0.05).
Difference between class T3 and T4
The average ages of T3 and T4 patients were 47.9 and
47.3 years of age, respectively (P>0.05). There were 209 female
patients in T3, accounting for 49.3% of this class. However, the
female incidence in class T4 was much higher, making up 55.6% of
this stage (P<0.05). Twenty-one (5.0%) patients in class T3
suffered tumor hemorrhage compared with 16 (2.7%) patients in T4;
this difference was not significant (P>0.05). A total of 143
(33.7%) cystic tumors were observed in class T3 and 171 (29.2%)
were observed in T4 (P>0.05).
There were 55 (13.0%) patients who suffered from
vertigo in T3 compared with 105 (17.9%) in T4. The incidence of
facial paralysis showed a correlation with tumor extension;
patients with larger tumors had higher rates of facial paralysis.
The incidence was 18.8% in T3 and 23.3% in T4. We therefore
concluded that the ratio of gender, vertigo, facial paralysis and
absent corneal reflex were significantly different between class T3
and T4 (P<0.05; Table I).
As mentioned previously, the most frequent cranial
nerve disturbance was related to the cochlear nerve (92.6%) and
trigeminal nerve (53.5%). Facial nerve disturbance was more severe
in class T3 than T4. There were 67 (14.9%) patients in T3 and 163
(27.8%) in T4 for facial nerve disturbance (P<0.05; Table II).
Next, we also used diagnostic test parameters,
including sensitivity, specificity, PPV, NPV, PLR, NLR and their
95% CIs, to identify the differences between T3 and T4 (Table III). However, none of the clinical
symptoms had a PLR >10 for T4 prediction.
 | Table IIIDiagnostic accuracy of clinical
features in predicting the tumor progress. |
Table III
Diagnostic accuracy of clinical
features in predicting the tumor progress.
| Clinical
feature | Sensitivity (95%
CI) | Specificity (95%
CI) | PPV (95% CI) | NPV (95% CI) | PLR (95% CI) | NLR (95% CI) |
|---|
| Headache | 25.2
(21.3–29.6) | 74.0
(70.3–77.4) | 41.3
(35.5–47.4) | 57.7
(54.2–61.2) | 0.97
(0.79–1.20) | 1.01
(0.94–1.09) |
| Hypacusis | 84.2
(80.4–87.4) | 13.0
(10.5–16.0) | 41.2
(38.0–44.5) | 53.2
(45.0–61.1) | 0.97
(0.92–1.02) | 1.01
(0.94–1.09) |
| Deafness | 25.5
(21.6–30.0) | 73.2
(69.4–76.6) | 40.8
(35.0–46.8) | 57.5
(54.0–61.0) | 0.95
(0.77–1.17) | 1.02
(0.95–1.10) |
| Tinnitus | 41.7
(37.2–46.5) | 61.0
(57.0–64.9) | 43.7
(39.0–48.6) | 59.1
(55.1–63.0) | 1.07
(0.92–1.25) | 0.95
(0.86–1.06) |
| Vertigo | 13.0
(10.1–16.5) | 82.1
(78.7–85.0) | 34.4
(27.5–42.0) | 56.5
(53.2–59.8) | 0.72
(0.54–0.98) | 1.06
(1.01–1.12) |
| Instability of
gait | 47.9
(43.2–52.6) | 57.8
(53.7–61.7) | 45.1
(40.6–49.7) | 60.5
(56.4–64.4) | 1.13
(0.99–1.30) | 0.90
(0.80–1.01) |
| Facial
paralysis | 24.3
(20.5–28.6) | 81.2
(77.8–84.2) | 48.4
(41.7–55.0) | 59.7
(56.2–63.0) | 1.29
(1.02–1.64) | 0.93
(0.87–1.00) |
| Facial
paresthesia | 47.2
(42.5–51.9) | 49.9
(45.9–54.0) | 40.6
(36.3–45.0) | 56.6
(52.3–60.8) | 0.94
(0.83–1.07) | 1.06
(0.94–1.20) |
| Ear pain | 0.2 (0.1–1.3) | 99.5
(98.5–99.8) | 25.0
(4.6–70.0) | 57.9
(54.8–60.9) | 0.46
(0.05–4.41) | 1.00
(0.99–1.01) |
| Bucking | 9.2 (6.8 12.3) | 89.7 (87.0
92.0) | 39.4
(30.3–49.2) | 57.7
(54.5–60.9) | 0.90
(0.61–1.32) | 1.01
(0.97–1.05) |
| Hoarseness | 2.1 (1.1–4.0) | 98.5
(97.1–99.2) | 50.0
(29.0–71.0) | 58.1
(55.0–61.2) | 1.38
(0.55–3.45) | 0.99
(0.98–1.01) |
| Visual
disorder | 8.3 (6.0–11.3) | 92.0
(89.5–93.9) | 42.7
(32.5–53.5) | 58.0
(54.8–61.2) | 1.03
(0.68–1.56) | 1.00
(0.96–1.04) |
| Movement
disorder | 4.5 (2.9–6.9) | 96.2
(94.4–97.5) | 46.3
(32.1–61.3) | 58.2
(55.0–61.2) | 1.19
(0.65–2.17) | 0.99
(0.97–1.02) |
| Absent corneal
reflex | 18.4
(15.0–22.4) | 87.2
(84.2–89.7) | 51.0 (43.1
58.8) | 59.6
(56.3–62.8) | 1.43 (1.07
1.92) | 0.94 (0.89
0.99) |
| Mastication
disorder | 3.3 (2.0–5.5) | 97.9
(96.5–98.8) | 53.8
(35.5–71.2) | 58.3
(55.2–61.3) | 1.61
(0.75–3.45) | 0.99
(0.97–1.01) |
| Abduction
disorder | 1.4 (0.7–3.1) | 98.1
(96.7–99.0) | 35.3
(17.3–58.7) | 57.9
(54.8–60.9) | 0.75
(0.28–2.02) | 1.00
(0.99–1.02) |
| Nausea and
vomiting | 5.4 (3.6–8.0) | 94.2
(92.0–95.8) | 40.4
(28.6–53.3) | 57.9
(54.7–61.0) | 0.93
(0.56–1.56) | 1.00
(0.97–1.04) |
| Romberg sign | 2.1 (1.1–4.0) | 98.1
(96.7–99.0) | 45.0
(25.8–65.8) | 58.0
(54.9–61.1) | 1.13
(0.47–2.70) | 1.00
(0.98–1.02) |
| Nystagmus | 5.2 (3.5–7.7) | 94.9
(92.8–96.4) | 42.3
(29.9–55.8) | 58.0
(54.8–61.1) | 1.01
(0.59–1.73) | 1.00
(0.97–1.03) |
Discussion
General findings
Intracranial vestibular schwannomas comprise
approximately 85% of tumors in the region of the cerebellopontine
angle. Approximately 10 tumors are diagnosed per million
individuals each year (1). The
tumors may cause dysfunction of the cochlear nerve, including
unilateral hypacusis, hearing loss, tinnitus and vertigo. Diplopia
may occur due to abducens palsy. If the trigeminal nerve is
involved, facial paresthesia, facial pain, absent corneal reflex
and mastication disorder may occur. Facial paralysis or spasms are
uncommon. Large lesions may lead to dysphonia, dysarthria and
dysphagia due to involvement of the IX and X cranial nerve.
Impaired coordination and upper motor neuron signs in the limbs are
symptoms of cerebellar and/or brain stem compression. Certain
patients experience pain localized in the ear/mastoid region and
occasionally non-localized headache (3,4–9). Among
our 1,009 patients, the most frequent clinical symptoms were
hearing loss (85.8%), facial paresthesia (48.9%), instability of
gait (44.6%), tinnitus (40.1%), deafness (26.3%) and facial
paralysis (21.1%). During preoperative physical examination, absent
corneal reflex was the most common sign, observed in 15.2% of the
patients. However, Romberg sign and abduction disorder were both
uncommon, appearing in less than 2% of patients (Table I). The most frequent cranial nerve
disturbance was related to the cochlear nerve (92.6%) and
trigeminal nerve (53.5%).
Both conservative management and post-operative
follow up are needed to observe the tumor extension (10–12)
since certain vestibular schwannomas present with rapid growth that
may lead to serious morbidity and even mortality if left untreated.
In the present study, we attempted to find the clinical alarm
features for predicting tumor progress. This may guide clinical
conservative management of vestibular schwannomas and become the
first step for evaluating the tumor extension, which must be
followed by CT and MRI.
Factors associated with tumor
extension
Age
Matthies and Samii analyzed 1,000 vestibular
schwannomas and found an inverse correlation between patients’ ages
and the degree of tumor extension (younger age correlates with
larger tumors) (13). However, a
meta-analysis of 10 studies and 620 patients did not reveal age to
be a predictive factor for tumor growth (14). In another meta-analysis of 555
patients in 13 studies, no statistically significant difference was
found with regard to the mean age and mean initial size between
growth and non-growth groups (15).
In a systematic review of 1,340 patients, no correlation was found
between the proportion of tumors that grew and patient age at
diagnosis or follow-up duration (16). In our group, the average ages of T3
and T4 patients were 47.9 and 47.3 years (P>0.05) The data did
not reveal a correlation between age and tumor extension.
Similarly, Rosenberg concluded from a large retrospective study
that there was no significant correlation between tumor size and
patient age (17).
Gender.
Ogawa et al found tumor extension to be
correlated with gender (18). On
the basis of this analysis, female patients had larger tumors at
presentation and their tumors grew faster. Beenstock found that
probability of stability was independent of gender (11). However, Stangerup et al
analyzed 552 patients and found no correlation between gender and
tumor growth (19). Our analysis of
age and gender distribution revealed that middle-aged females
ranging from 40–60 years of age were most at risk, accounting for
61.6% of total female patients and 32.7% higher than for male
patients in the same age group. There were 209 female patients in
class T3, accounting for 49.3% of this class. However, the female
incidence in T4 was much higher, making up 55.6% of this class. The
present study supports the finding of Ogawa et al that
female patients had larger tumors and are possibly a high-risk
population for vestibular schwannomas.
Size.
In a review of 119 patients, Fucci et al
found that tumors larger than 20 mm at presentation are more likely
to exhibit rapid growth than those smaller than 20 mm (20). Walsh et al, in an 11-year
retrospective study, showed that small tumor confined to the
canaliculus did not exhibit significant growth compared with tumors
in the cerebellopontine angle cistern (21). In our study of 1,009 cases, the
tumor length and width were significantly different among classes
T3 and T4, with a mean length and width of 30×25 mm in T3 and 44×36
mm in T4.
Symptoms and signs predicting tumor
progress
Vertigo
In this study, we found the incidence of vertigo was
significant higher in class T3 than in class T4 (P<0.05) The
specificity for vertigo was 82.1% in the diagnostic assessment
(Table III). Similarly, Artz et
al analyzed 234 vestibular schwannoma patients and found
vertigo was a predictor for the growth of sporadic tumors (22). Theoretically, vertigo may occur due
to vestibular nerve dysfunction as well as brain stem compression.
Tumors with a diameter larger than 20 mm in the cerebellopontine
angle are likely to reach the brain stem, which obviously adds to
the symptoms of vertigo. In a recent study, the theory hypothesizes
that endolymph draining too rapidly from the cochlear duct (pars
inferior) causes attacks of vertigo. The endolymph overfills the
endolymphatic sinus and overflows into the utricle (pars superior),
stretching the cristae of the semicircular canals and causing the
attacks of vertigo (23). We
speculate that the tumor extension will affect the vestibular nerve
function in vessels and lymphatic backflow which cause vertigo. A
compensatory mechanism influenced by vision and the contralateral
vestibular apparatus possibly contributes to delaying the onset of
vertigo.
Facial paralysis.
Another phenomenon of these 1,009 cases was that the
incidence of facial paralysis showed a correlation with tumor
extension; patients with larger tumors had higher rates of facial
paralysis. The incidence was 18.8% in class T3 and 23.3% in class
T4 (P<0.05).
Notably, facial paralysis at admittance was a result
of two factors; firstly, a large number of patients had undergone
previous partial tumor resection elsewhere, and secondly, a high
rate of large tumors had brain stem compression and therefore
caused elongation of the facial nerve. Samii and Matthies analyzed
1,000 patients in 1997 (24). Of
the patients with preoperative facial nerve paresis, 1% were
assigned to class T1, 10% to T2, 23% to T3 and 55% to T4. This
study demonstrated that this symptom is largely dependent on a
patient’s ability to differentiate and perceive such slight
changes. A systematic search for taste disturbances should be
included in history taking; in case of doubt or suspicion,
electrogustometry is capable of detecting disorders early.
Notably, although the facial nerve may be thinly
stretched by the tumor, facial nerve weakness is not a prominent
sign in many cases preoperatively (25,26).
When facial nerve function is compromised it usually has an
insidious onset over several months and is not a sudden event.
Hearing loss.
It remains unclear whether hearing loss predicts
tumor extension. Artz et al analyzed 234 vestibular
schwannomas and concluded that the predictor factors for growth are
vertigo, no sudden onset of hearing loss and short duration of
hearing loss (22).
Tschudi et al found that patients with
progressive hearing loss as a first symptom had a significantly
lower tumor growth than those presenting with tinnitus, sudden
hearing loss and vertigo (27).
Other studies, however, revealed no significant differences in this
symptom between patients with growing tumors and non-growing tumors
(28,29).
In our research, hearing loss was one of the most
frequent symptoms in vestibular schwannomas. Cochlear nerve
symptoms accounted for the most frequent complaints (Table II). There were 648 patients who
noticed some degree of hearing deficit and 265 patients experienced
deafness. Although the proportion of patients suffering hearing
loss was similar in classes T3 and T4, the rates of vestibular
disturbances most often occur as some unsteadiness while walking
and facial disturbances, such as facial paralysis. These factors
are predictors of tumor extension, as mentioned previously, and
their incidence was significantly higher in deaf than in hearing
patients (P<0.05; Table IV).
This evidence supports the hypothesis that hearing loss may be a
predictive factor of tumor extension.
 | Table IVParameters and correlation with
preoperative hearing function. |
Table IV
Parameters and correlation with
preoperative hearing function.
| Parameter | Hypacusis | Hearing loss | P-value |
|---|
| Mean age
(years) | 47.8 | 48.8 | 0.235 |
| Tumor diameter
(mm) | 3.12 | 3.15 | 0.701 |
| Headache (%) | 166 (25.6) | 69 (26.0) | 0.895 |
| Trigeminal nerve
disturbance (%) | 354 (54.6) | 142 (53.6) | 0.774 |
| Tinnitus (%) | 283 (43.7) | 101 (38.1) | 0.122 |
| Vertigo (%) | 107 (16.5) | 37 (14.0) | 0.337 |
| Instability of gait
(%) | 281 (43.4) | 139 (52.5) | 0.012 |
| Facial paralysis
(%) | 117 (18.1) | 79 (29.8) | <0.001 |
| Movement disorder
(%) | 22 (3.4) | 11 (4.2) | 0.579 |
| Romberg sign
(%) | 12 (1.9) | 8 (3.0) | 0.274 |
Conclusions and future
considerations
Hearing deficit, facial paresthesia, ataxia and
tinnitus are the most frequent and significant symptoms of huge
vestibular schwannomas. The cochlear, trigeminal and facial nerves
are those most commonly affected by large tumors. Gender and tumor
size are associated with tumor extension. Although the predictive
value was limited, the symptoms of vertigo, facial paralysis and
hearing loss may be alarm features predicting tumor growth. The
symptoms and signs are the first step in determining tumor
extension. Further methods, including CT and MRI, are required to
evaluate tumor progress.
Acknowledgements
The authors thank the surgeons and
pathologists at the Department of Neurosurgery in Huashan Hospital.
Professor Ying Mao provided valuable advice for this study. This
study was presented in part at the 14th European Congress of
Neurosurgery (Rome, Italy; October 9–14, 2011) (30).
References
|
1
|
Evans DG, Moran A, King A, Saeed S,
Gurusinghe N and Ramsden R: Incidence of vestibular schwannoma and
neurofibromatosis 2 in the north west of england over a 10-year
period: higher incidence than previously thought. Otol Neurotol.
26:93–97. 2005.PubMed/NCBI
|
|
2
|
Nikolopoulos TP, Fortnum H, O’Donoghue G
and Baguley D: Acoustic neuroma growth: a systematic review of the
evidence. Otol Neurotol. 31:478–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou LF, Chen XC and Shi YQ: Modern
Neurosurgery. Fudan Press; Shanghai: 2001
|
|
4
|
Samii M, Gerganov V and Samii A: Improved
preservation of hearing and facial nerve function in vestibular
schwannoma surgery via the retrosigmoid approach in a series of 200
patients. J Neurosurg. 105:527–535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goksu N, Bayazit Y and Kemaloglu Y:
Endoscopy of the posterior fossa and endoscopic dissection of
acoustic neuroma. Neurosurg Focus. 6:e151999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tringali S, Bertholon P, Chelikh L,
Jacquet C, Prades JM and Martin C: Hearing preservation after
modified translabyrinthine approach performed to remove a
vestibular schwannoma. Ann Otol Rhinol Laryngol. 113:152–155. 2004.
View Article : Google Scholar
|
|
7
|
Sanna M, Khrais T, Russo A, Piccirillo E
and Augurio A: Hearing preservation surgery in vestibular
schwannoma: the hidden truth. Ann Otol Rhinol Laryngol.
113:156–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chee GH, Nedzelski JM and Rowed D:
Acoustic neuroma surgery: the results of long-term hearing
preservation. Otol Neurotol. 24:672–676. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brackmann DE, Owens RM, Friedman RA, et
al: Prognostic factors for hearing preservation in vestibular
schwannoma surgery. Am J Otol. 21:417–424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lassaletta L, Fontes L, Melcon E, Sarria
MJ and Gavilan J: Hearing preservation with the retrosigmoid
approach for vestibular schwannoma: Myth or reality? Otolaryngol
Head Neck Surg. 129:397–401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beenstock M: Predicting the stability and
growth of acoustic neuromas. Otol Neurotol. 23:542–549. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamakami I, Oka N and Yamaura A:
Intraoperative monitoring of cochlear nerve compound action
potential in cerebellopontine angle tumour removal. J Clin
Neurosci. 10:567–570. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matthies C and Samii M: Management of 1000
vestibular schwannomas (acoustic neuromas): clinical presentation.
Neurosurgery. 40:1–9, (discussion 9–10), 1997.
|
|
14
|
Smouha EE, Yoo M, Mohr K and Davis RP:
Conservative management of acoustic neuroma: a meta-analysis and
proposed treatment algorithm. Laryngoscope. 115:450–454. 2005.
View Article : Google Scholar
|
|
15
|
Selesnick SH and Johnson G: Radiologic
surveillance of acoustic neuromas. Am J Otol. 19:846–849.
1998.PubMed/NCBI
|
|
16
|
Yoshimoto Y: Systematic review of the
natural history of vestibular schwannoma. J Neurosurg. 103:59–63.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosenberg SI: Natural history of acoustic
neuromas. Laryngoscope. 110:497–508. 2000.
|
|
18
|
Ogawa K, Kanzaki J, Ogawa S, Yamamoto M,
Ikeda S and Shiobara R: The growth rate of acoustic neuromas. Acta
Otolaryngol Suppl. 487:157–163. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stangerup SE, Caye-Thomasen P, Tos M and
Thomsen J: The natural history of vestibular schwannoma. Otol
Neurotol. 27:547–552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fucci MJ, Buchman CA, Brackmann DE and
Berliner KI: Acoustic tumor growth: Implications for treatment
choices. Am J Otol. 20:495–499. 1999.PubMed/NCBI
|
|
21
|
Walsh RM, Bath AP, Bance ML, Keller A,
Tator CH and Rutka JA: The role of conservative management of
vestibular schwannomas. Clin Otolaryngol Allied Sci. 25:28–39.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Artz JC, Timmer FC, Mulder JJ, Cremers CW
and Graamans K: Predictors of future growth of sporadic vestibular
schwannomas obtained by history and radiologic assessment of the
tumor. Eur Arch Otorhinolaryngol. 266:641–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gibson WP: Hypothetical mechanism for
vertigo in Meniere’s disease. Otolaryngol Clin North Am.
43:1019–1027. 2010.
|
|
24
|
Samii M and Matthies C: Management of 1000
vestibular schwannomas (acoustic neuromas): the facial nerve -
preservation and restitution of function. Neurosurgery. 40:684–694,
(discussion 694–685), 1997.
|
|
25
|
Arts HA, Telian SA, El-Kashlan H and
Thompson BG: Hearing preservation and facial nerve outcomes in
vestibular schwannoma surgery: results using the middle cranial
fossa approach. Otol Neurotol. 27:234–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin TP: Conservative management of
acoustic neuroma: a meta-analysis and proposed treatment algorithm.
Laryngoscope. 115:17042005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tschudi DC, Linder TE and Fisch U:
Conservative management of unilateral acoustic neuromas. Am J Otol.
21:722–728. 2000.PubMed/NCBI
|
|
28
|
Flint D, Fagan P and Panarese A:
Conservative management of sporadic unilateral acoustic neuromas. J
Laryngol Otol. 119:424–428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Herwadker A, Vokurka EA, Evans DG, Ramsden
RT and Jackson A: Size and growth rate of sporadic vestibular
schwannoma: predictive value of information available at
presentation. Otol Neurotol. 26:86–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang X, Xu J and Zhong P: The clinical
feature of intracranial vestibular schwannomas-A retrospective
review of 1009 vestibular schwannomas in single hospital. Acta
Neurochirurgica. 153:1833–1905. 2011.
|