Introduction
Breast cancer is the leading cause of cancer-related
mortality in females worldwide (1).
Primary breast cancer tumors may be removed or irradiated
relatively simply, but distant metastases are difficult to treat.
Tumor cell motility is the hallmark of invasion and an essential
step in metastasis. One important insight came from the discovery
that the increased motility and invasiveness of cancer cells is
reminiscent of the epithelial-mesenchymal transition (EMT) that
occurs during embryonic development (2). In this process, epithelial cells
acquire fibroblast-like properties such as the functional loss of
E-cadherin. Studies have shown that several transcription factors,
including the Snail/Slug family (3), δEF1/ZEB1 (4) and SIP1 (5) have been implicated in E-cadherin
repression. Snail, a zinc finger protein, is considered to be a
critical EMT regulator (6).
Although a direct link between Snail expression and tumor
metastasis has not yet been reported, a number of studies have
shown that the overexpression of Snail is correlated with tumor
invasion (7).
Studies have shown a correlation between Snail
expression and the degree of infiltration in breast carcinomas
(8). Snail is also an upstream
protein of metalloproteinase-2 (9),
which is a mesenchymal marker sufficient to trigger EMT in
vivo (10). Although a report
has shown that RhoB, a small GTPase involved in cytoskeletal actin
rearrangement, lies downstream of Slug (11), another member of the Snail
super-family, there is no evidence to support a link between Snail
and Rho GTPases.
Rho GTPases belong to the Ras superfamily of small
GTPases and are highly conserved throughout eukaryotes. These
proteins control the dynamics of the actin cytoskeleton and thus
represent key regulatory molecules that are active during cell
migration (12). RhoA has been
implicated in the formation of stress fibers and cell adhesion in
fibroblasts. A number of reports have shown that RhoA expression is
upregulated in a group of malignancies (13) and that the activity of RhoA is
correlated with lymph node metastasis in colorectal cancer
(14). The expression level of RhoA
is positively correlated with the progress of these carcinomas.
In the present study, Snail and RhoA were observed
to be overexpressed in breast cancer tissues compared with normal
breast tissues and the expression of Snail and RhoA was associated
with the differentiation grade and lymph node metastasis of breast
cancer, respectively. We hypothesized that Snail, as a
transcription factor, may promote breast cancer metastasis through
the regulation of RhoA expression and activity.
Materials and methods
Cell lines and antibodies
The breast cancer cell lines MDA-MB-231, MDA-MB-435S
and human mammary epithelial cells (HMEC; Invitrogen Life
Technologies Corporation, Carlsbad, CA, USA) were maintained in
Dulbecco’s minimum essential medium (DMEM) supplemented with 10%
FCS and 1% antibiotic/antimycotic solution. Polyclonal antibodies
against Snail, E-cadherin, MMP-2 and β-actin, and monoclonal
antibodies against fibronectin, RhoA, Rac1, Cdc42 and PECAM-1 were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Immunohistochemical staining
Conventional paraffin-embedded tissue sections
obtained from breast tumors and normal breast tissue were obtained
from surgical specimens resected at the Tongji Hospital of the
Huazhong Science and Technology University (Wuhan, China). The
avidin-biotin complex immunoperoxidase method was used to study the
levels of Snail and RhoA expression by immunostaining. The
frequency of each protein was scored as the percentage of positive
cells as follows: negative, <5%; weak, 5 to 25%; moderate, 25 to
50%; and strong, >50%.
Vectors and transfections
cDNA encoding the open reading frame of Snail was
amplified and cloned into the pIRES2-EGFP vector (Invitrogen,
Carlsbad, CA, USA) in the inverted direction to produce an
antisense-Snail cDNA construct (AsSn). Transient transfection for
GFP alone (mock, Invitrogen), GFP-fused forms of wild-type RhoA,
dominant-negative (DN) N19-RhoA and activated (Act) V14-RhoA
(kindly provided by Professor Richard Pestell, Albert Einstein
College of Medicine, New York, NY, USA) were also transfected into
MDA-MB-231 and MDA-MB-435S cells and were then selected using 800
μg/ml G418 for 12 days. Clones were picked and expanded for
an additional two months. Experiments with the transiently
transfected cells were performed 72 h after the transfections.
Real-time PCR
Total RNA was extracted according to the TRIzol
instructions (Invitrogen), then combined with an RNase-free DNase
kit (Promega, Madison, WI, USA) and reverse transcribed with random
primers. The resulting cDNA was used for PCR using SYBR-Green
master PCR mix (Toyobo, Osaka, Japan) in triplicate. PCR and data
collection were performed on a M×3000P™ Real-Time PCR system
(Stratagene, La Jolla, CA, USA).
Western blotting
The protein content of each lysate was determined by
a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Each lysate
(10 μg) was resolved on a 10 to 12% denaturing
polyacrylamide gel and transferred electrophoretically to a
nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ,
USA). After incubation with primary antibodies at 4°C overnight,
the immunoreactive proteins were localized with horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The reactants were
developed using an enhanced chemiluminescence kit (Amersham
Biosciences).
Reporter assays
The RhoA promoter sequence (−2,112 to +75 bp) was
cloned into the pGL3-Basic vector (Promega, Madison, WI, USA) to be
used as a reporter assay. The AsSn (or pIRES2-EGFP vector) and
fly-luciferase plasmids were cotransfected into MDA-MB-231 and
MDA-MB-435S cells using Lipofectamine 2000 (Invitrogen). After 48
h, firefly and Renilla luciferase activities were measured using
the Dual Luciferase Reporter Assay kit (Promega).
Cell mobility assay
For the wound-healing migration assay, ∼250,000
cells were seeded in six-well plates and grown to 100% confluence.
A 10-μl pipette tip was used to create a scratch down the
middle of each well. Representative images were photographed using
phase-contrast microscopy at the indicated times.
For the cell migration assay, 1×104 cells
were seeded on the top of Transwell membranes treated with Matrigel
matrix (BD Biosciences, Franklin Lakes, NJ, USA) following the
manufacturer’s instructions. After 10 h of incubation, filters were
fixed with 4% paraformaldehyde (15 min, 4°C); the number of cells
on the top surface of the filters was estimated by counting three
independent visual fields using a microscope. The average number of
cells in four replicate wells was determined for each cell line in
each of three independent experiments.
Cell proliferation assay
Cells (5×103) were plated in 96-well
plates and allowed to attach and grow in regular medium for 4 h. At
each time point (0, 12, 24, 36, 48, 60 and 72 h of culture), 10
μl MTT (5 mg/ml) was added to each well. The reaction was
stopped after 4 h of incubation by adding 150 μl dimethyl
sulfoxide (DMSO). The optical density (OD) value was obtained by
measuring the absorbance at a wavelength of 570 nm. The
proliferation assay was performed in triplicate and repeated three
times.
GTPase activity assays
The activities of Rac1, Cdc42 and RhoA were studied
as described previously (15).
Glutathione S-transferase (GST)-C21 was used to detect Act-RhoA.
GST-PAK-CD, a fusion protein that selectively binds to GTP-Rac1,
was used to active Rac1 and Cdc42 fusion proteins was generated as
described previously. Cells were lysed at 4°C in 300 μl
lysis buffer and then centrifuged; 15 μl supernatant was
kept as a total lysate control and the remaining volume was mixed
with fusion proteins in the presence of glutathione-agarose beads.
The mixtures were incubated for 16 h at 4°C, beads were pelleted
and washed and bound proteins were eluted in Laemmli
electrophoresis buffer. The proteins were resolved by SDS-PAGE on
12% polyacrylamide gels and transferred to polyvinylidene fluoride
membranes (Hybond-P, Amersham Pharmacia Biotech, Amersham, UK),
which were incubated with antibodies to RhoA, Rac1 or Cdc42
(diluted 1:1000).
In vivo tumorigenicity studies
Female athymic nude mice (BALB/c nu/nu) aged between
four and five weeks were obtained from the Shanghai Institute of
Medical Material (Shanghai, China). Parental and AsSn-transfected
MDA-MB-231 and MDA-MB-435s cells were harvested, washed,
resuspended in PBS and injected into the mammary fat pad of mice.
Tumor growth was measured three times each week. For survival
analysis, the mice were euthanized and sacrificed when they
appeared moribund. All experiments were performed at least twice,
and samples from the breast tumors, lungs, liver and lymph nodes
were obtained. Tissues for histological examination were fixed and
embedded in paraffin using standard methods.
Statistical analysis
Spearman’s rank tests were used to evaluate the
correlation between Snail or RhoA expression and clinical
pathological parameters. SPSS 16.0 software (SPSS, Chicago, IL,
USA) was used to carry out all statistical analyses. P<0.05 was
considered significant to indicate a statistically significant
difference.
Results
Increased expression levels of Snail and
RhoA were observed in human breast cancer tissues
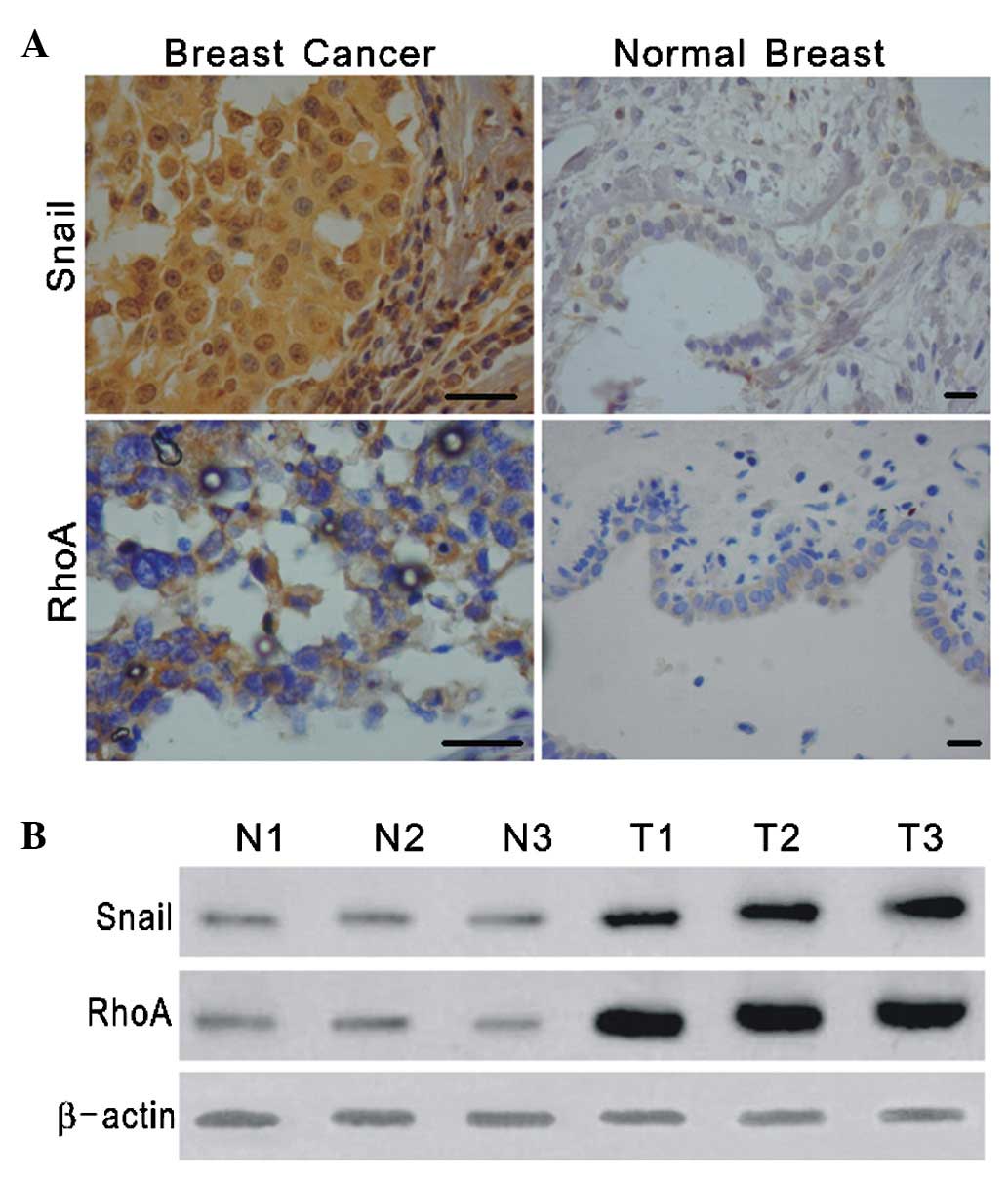
The expression and subcellular localization of Snail
and RhoA were studied in a set of specimens derived from 20 normal
breast tissues and 60 breast cancer tissues. In the normal breast
tissues examined, only faint nuclear staining for Snail and
cytoplasmic staining for RhoA were detected, whereas the breast
carcinomas exhibited strong staining for Snail and RhoA (Fig. 1A). To further investigate the
possible correlation of Snail and RhoA expression with the
progression of breast cancer, we evaluated the protein expression
of Snail and RhoA in normal breast and breast tumor, and the
clinicopathological characteristics in these specimens. It was
found that Snail and RhoA expression significantly higher in breast
cancer (Table I), and RhoA
expression was correlated with differentiation grades of breast
tumor (Table II). Western blotting
analysis for Snail and RhoA in breast cancer and adjacent normal
tissues obtained from 15 patients showed increased levels of Snail
and RhoA in the cancerous tissues (Fig.
1B). These results suggest that Snail and RhoA may be involved
in the progression of breast cancer.
 | Table I.Snail and RhoA expression in normal
breast and breast tumor. |
Table I.
Snail and RhoA expression in normal
breast and breast tumor.
| Factor | No. of cases | - | + | Snail staining
| RhoA staining | P-value |
|---|
| ++ | +++ | P-value | - | + | ++ | +++ |
|---|
| Normal breast | 20 | 8 | 11 | 1 | 0 | 0.000 | 10 | 9 | 1 | 0 | 0.000 |
| Breast tumor | 60 | 5 | 8 | 15 | 32 | | 3 | 7 | 30 | 20 | |
 | Table II.Clinicopathological association of
Snail and RhoA expression in patients with breast cancer. |
Table II.
Clinicopathological association of
Snail and RhoA expression in patients with breast cancer.
| No. of cases | Snail staining
| RhoA staining
|
|---|
| - | + | ++ | +++ | P-value | - | + | ++ | +++ | P-value |
|---|
|
Differentiation | | | | | | 0.848 | | | | | 0.020 |
| Well | 12 | 2 | 4 | 4 | 2 | | 1 | 5 | 4 | 0 | |
| Moderately | 18 | 2 | 3 | 5 | 8 | | 2 | 2 | 3 | 3 | |
| Poorly | 30 | 3 | 9 | 10 | 8 | | 2 | 3 | 18 | 7 | |
| TNM | | | | | | 0.079 | | | | | 0.169 |
| I+II | 24 | 3 | 8 | 7 | 6 | | 2 | 6 | 8 | 8 | |
| III+IV | 36 | 4 | 5 | 10 | 17 | | 2 | 4 | 13 | 17 | |
| Metastasis | | | | | | | | | | | |
| With | 40 | 5 | 6 | 11 | 18 | 0.164 | 2 | 5 | 15 | 18 | 0.235 |
| Without | 20 | 2 | 7 | 6 | 5 | | 2 | 5 | 6 | 7 | |
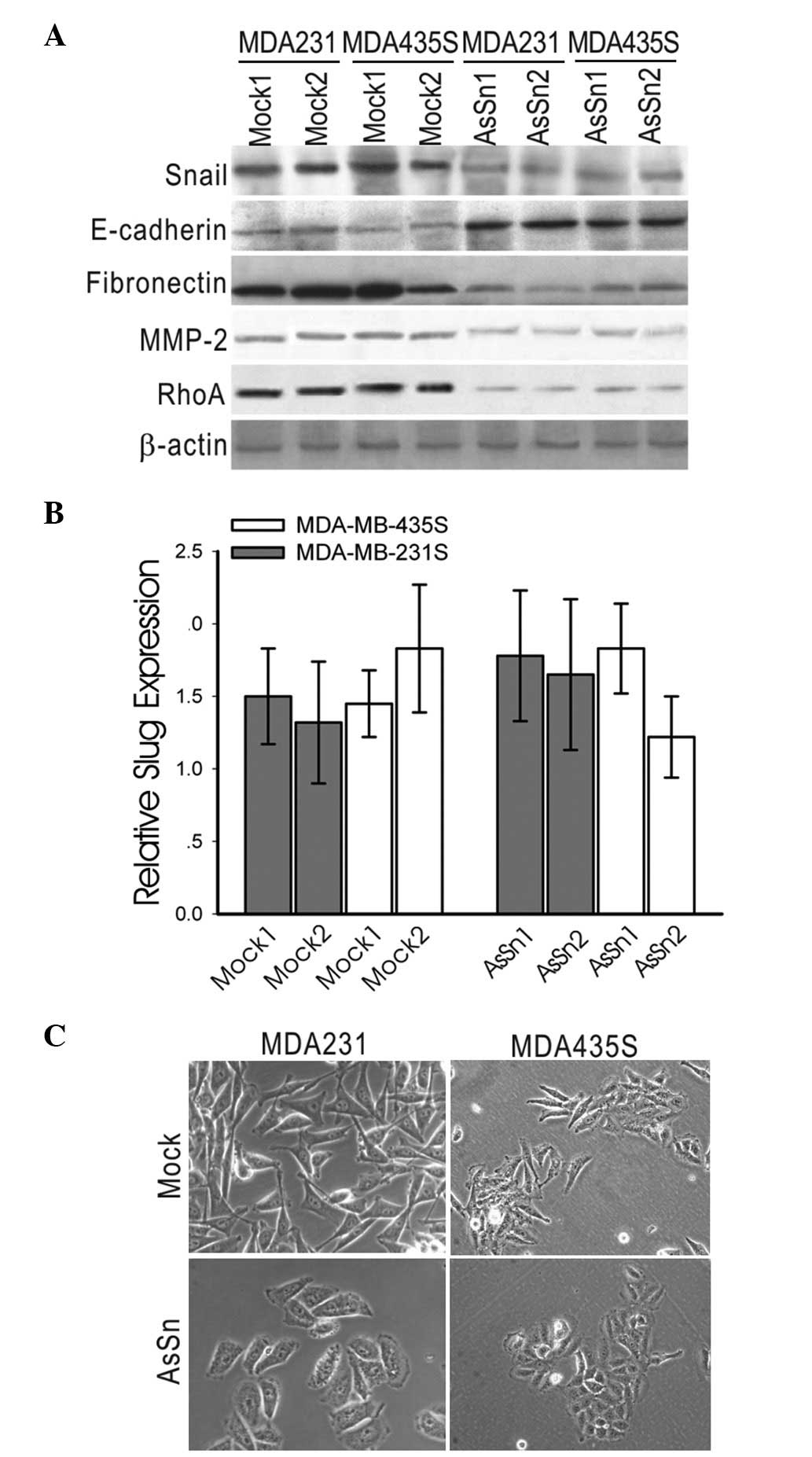
Gene expression involved in EM T was
altered in AsSn-transfected MDA-MB-231 and MDA-MB-435S cells
AsSn was stably introduced into the human breast
cancer cell lines MDA-MB-231 and MDA-MB-435S, which express
relatively high levels of Snail mRNA and exhibit the invasive
phenotype. A low level of Snail expression in AsSn MDA-MB-231
clones was demonstrated by western blotting with anti-Snail
antibody, while Snail expression was not affected in the
mock-transfected cells. A similar result was observed in the
MDA-MB-435S cell line (Fig. 2A),
demonstrating that the loss of Snail expression is achieved in
independent clones.
Whether the introduction of AsSn affected the
expression of EMT genes using western blotting analysis MDA-MB-231
was investigated. Analysis of several independent clones revealed
increased expression levels of E-cadherin and a significant
decrease in endogenous fibronectin and MMP-2 expression in AsSn
cells, whereas RhoA expression was reduced by ∼60% in AsSn cells
(Fig. 2A).
To exclude the possibility that the knockdown by the
anti-sense vector was not Snail-specific, the mRNA expression level
of Slug, a close homolog of Snail, was detected by real-time PCR.
No significant changes in the level of endogenous Slug mRNA were
detected in the AsSn- or mock-transfected clones (Fig. 2B).
Furthermore, the mock-transfected clones exhibited
spindle-shaped cells and fibroblastic morphology, whereas AsSn
clones typically displayed a cobblestone-like, epithelial
morphology (Fig. 2C).
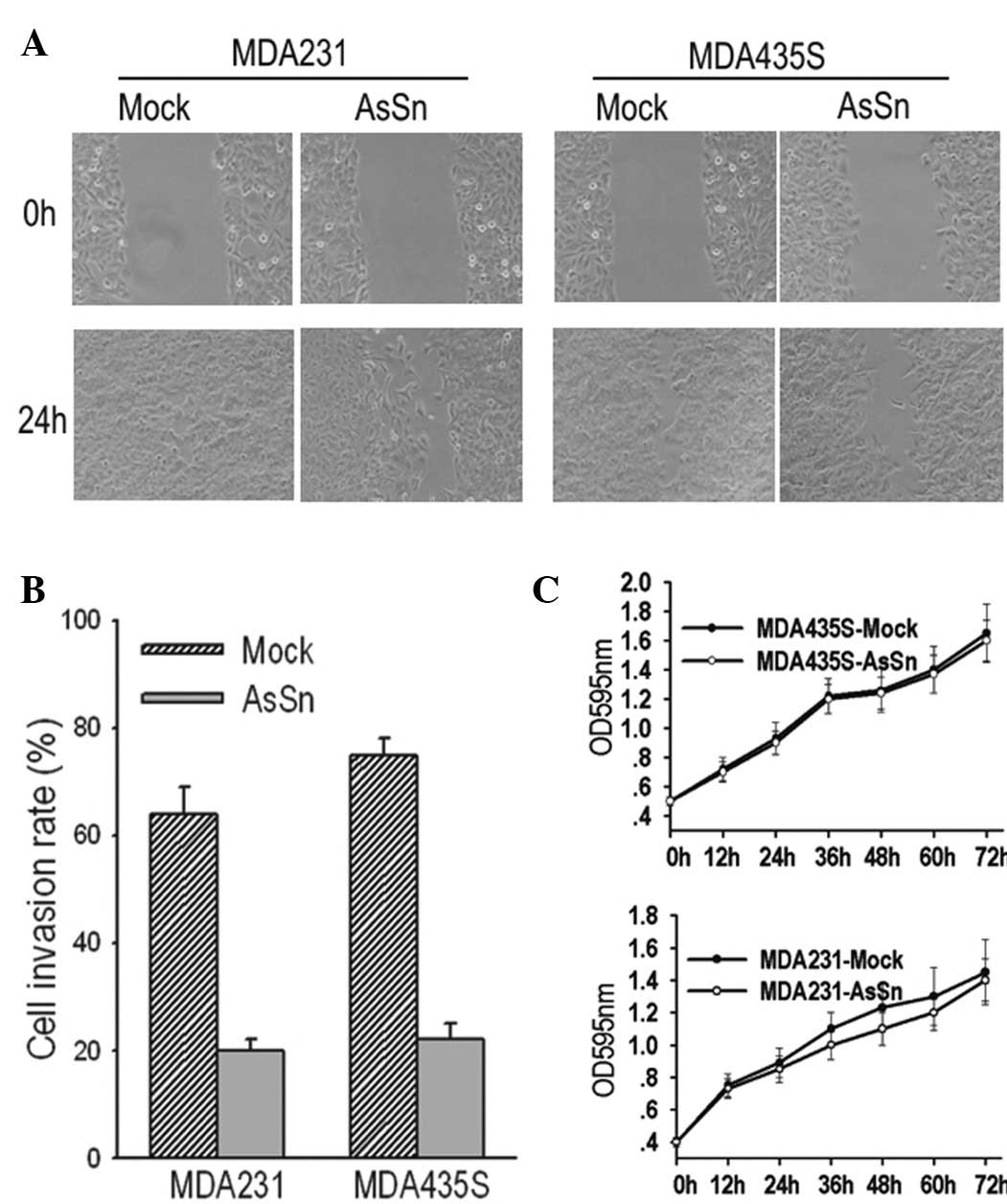
AsSn alters the motility of MDA-MB-231
and MDA-MB-435S transfected cells in vitro
It was observed that the downregulation of Snail in
breast tumor cells significantly reduced cell migration from the
edge of the wound 24 h after scratching (Fig. 3A). Similarly, when the cell invasion
potential was measured in a Matrigel-coated Transwell assay,
mock-transfected and AsSn cells were observed to invade the bottom
of the membrane. However, in the 10-h period, the number of
migrating mock-transfected cells was three- to four-fold greater
than that of the AsSn cells (Fig.
3B) and this difference persisted over a period of 24 h.
To exclude the possibility that differences in the
growth rates of the mock-transfected and AsSn cells affected the
interpretation of these results, cell growth was observed over 72 h
and curves were plotted. There was no significant difference
between the proliferation of the mock-transfected and AsSn cells
after 72 h, excluding such a possibility (Fig. 3C). This result indicates that AsSn
does not affect cell proliferation in vitro.
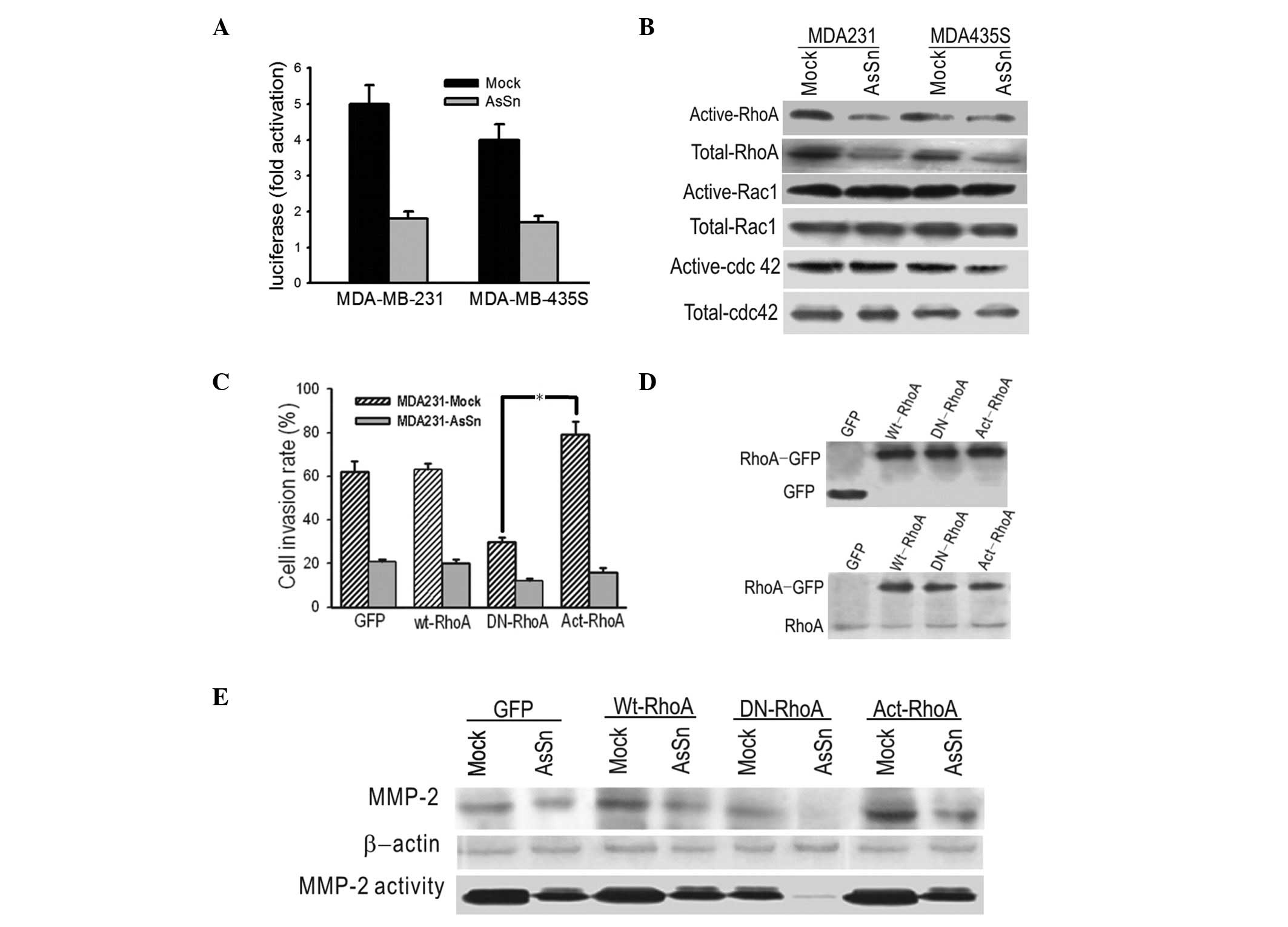
AsSn downregulates the expression and
alters the activity of RhoA in transfected cells, suggesting a role
in invasion in vitro
A reporter construct containing the RhoA promoter
was transfected into the same cells and which were analyzed for
promoter activity. The transient transfection results showed a two-
to three-fold repression of RhoA promoter activity by Snail
downregulation, suggesting that RhoA expression was under the
control of Snail (Fig. 4A).
To investigate whether AsSn was capable of
inactivating Rho GTPase and whether this inactivation was involved
in the decreased migration ability, GTPase assays were performed
using GST fusion proteins with binding domains that bind only
activated forms of these GTPases. As shown in Fig. 4B, there was a decrease in the
expression level of Act-RhoA in AsSn cells compared with
mock-transfected cells. By contrast, the levels of active Rac1 and
Cdc42 show no difference between the AsSn- and mock-transfected
cells.
To further analyze the involvement of RhoA
inactivation in invasion, GFP-fused wild-type, DN (N19-RhoA) or
active (V14-RhoA) RhoA vectors were transfected into the cells and
the invasion ability was measured in vitro. A significant
decrease in the invasion ability of AsSn cells was obtained with
transfectants expressing DN-RhoA, whereas overexpression of
Act-RhoA increased the invasion ability of the transfectants
compared with transfectants expressing GFP alone (Fig. 4C). Western blotting control
experiments using anti-GFP and anti-RhoA antibodies demonstrated
the expression of the GTPase forms in transfected cells (Fig. 4D). Whether there was an association
between RhoA and MMP-2 activation was then studied. As shown in
Fig. 4E, MMP-2 expression and
activity decreased in cells transfected with DN-RhoA and increased
in cells with Act-RhoA.
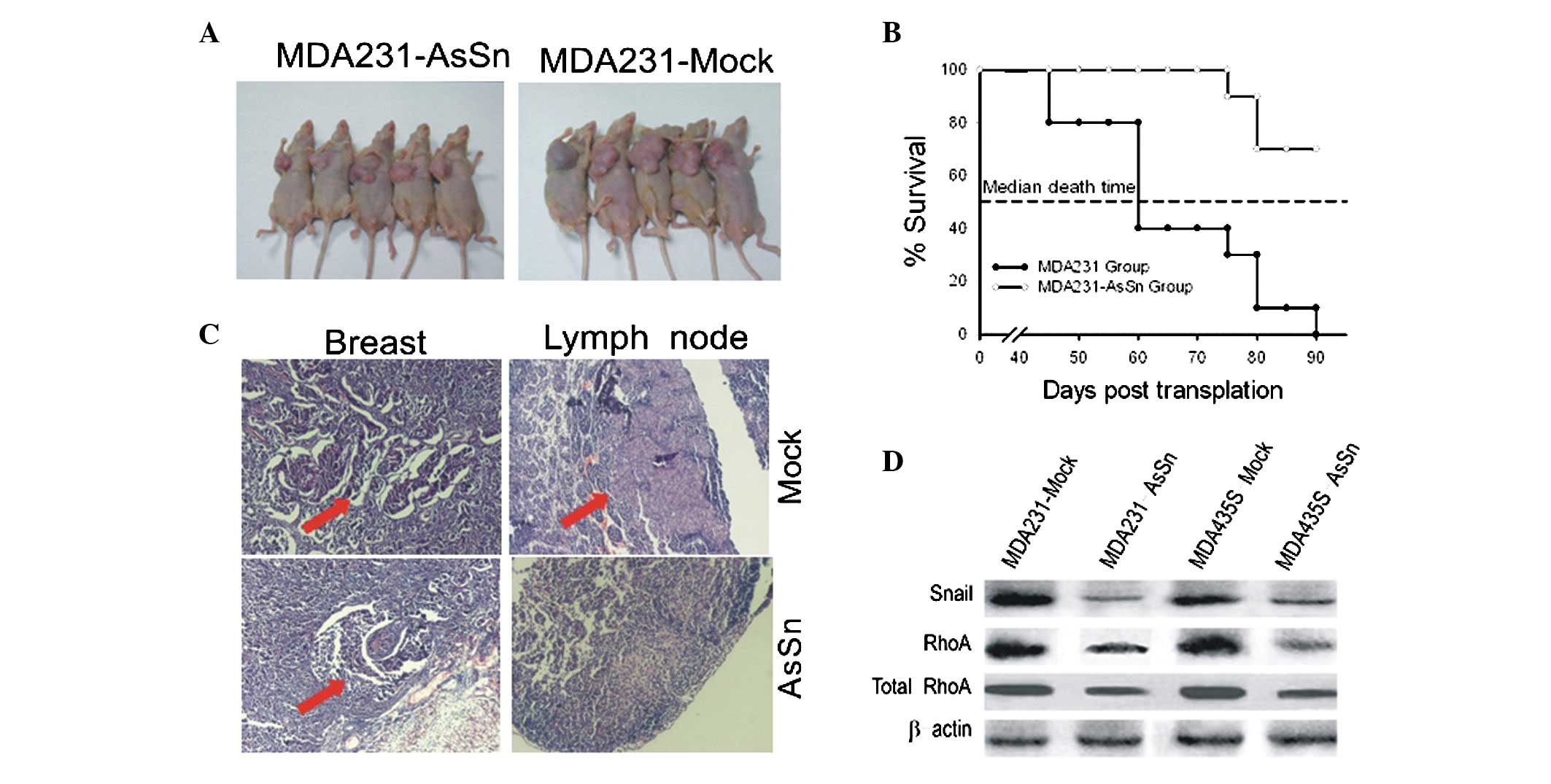
AsSn alters primary tumor growth and
lymph node metastasis in vivo
Mice that received an injection of mock-transfected
cells developed tumors of >1.5 cm in diameter (Fig. 5A) and their mean survival time was
60 days (Fig. 5B). However, mice
that received an injection with AsSn-transfected cells developed
tumors with a diameter of <0.5 cm (Fig. 5A) and their mean survival time was
>90 days (Fig. 5B).
Microscopically, no metastases were observed in the liver or lungs,
although they were detected in the lymph node (Fig. 5C). Lymph node metastases developed
in 80–90% of the mice injected with mock-transfected cells, whereas
the mice injected with AsSn cells developed lymph node metastases
in only 20% of cases. Similar results were observed with
MDA-MB-435s cells (data not shown).
Western blotting analysis showed decreased levels of
Snail and RhoA proteins in the tumors that received an injection of
AsSn cells (Fig. 5D), compared with
the control group that received an injection of mock-transfected
cells.
Discussion
In the present study, it was observed that Snail and
RhoA were coexpressed at significantly higher levels in breast
cancer tissues compared with normal tissues, according to
immunohistochemical analyses and western blotting. Analysis of the
clinicopathological characteristics (Table I) shows for the first time that
Snail and RhoA protein expression is associated with
differentiation grades and lymph node metastasis. We hypothesized
that Snail and RhoA may be involved in the progression of malignant
behavior in breast cancer and RhoA may act as a downstream target
of Snail. Previous studies have suggested that Snail expression is
correlated with the presence of lymph node metastases (16) and RhoA protein expression is
upregulated in breast cancer tissue (17), enhancing migration and invasion in
breast cancer cell lines (18). To
investigate this issue, the potential role of Snail in the invasion
of breast tumor cells was assessed at the molecular and cellular
level, with the in vitro and in vivo metastasis
properties of estrogen receptor (ER)-negative MDA-MB-231 and
MDA-MB-435S cell lines transfected with AsSn and DN-RhoA or
Act-RhoA vectors.
By inhibiting Snail expression, clear changes were
observed in the expression of several important components of the
EMT proteome in AsSn cells. The EMT proteome reflects a fundamental
change in the proteins gained or lost in the transition of tumor
epithelia to metastatic cells (19). Since RhoA is required for the
generation of the contractile force which leads to the rounding of
the cell body and is required for the regulation of microtubule
polymerization in cell mobility (20), RhoA may increase cell motility.
MMP-2, a candidate invasion gene, may also increase motility as it
is capable of degrading the extracellular matrix and components of
the basement membrane (21). In the
present study, the induced protein expression of E-cadherin and
decreased expression of RhoA, MMP-2 and fibronectin, a component of
the extracellular matrix, were observed in AsSn-transfected breast
cancer cells. Unchanged mRNA expression of Slug was also detected
in AsSn cells, excluding a possible off-target effect of AsSn.
These findings suggest that AsSn has an inhibiting effect on MMP-2,
fibronectin and RhoA.
Cell invasion is associated not only with the
ability to be motile, but also the ability to degrade the
extracellular matrix. The decreased invasion ability of AsSn cells
was coupled with the downregulated expression of RhoA and MMP-2,
suggesting that cell motility and extracellular matrix degradation
are likely to be functionally interdependent for cell invasion. We
suggest that Snail may modulate RhoA expression and trigger Rho
GTPase-dependent signaling, leading to the control of MMP-2
expression.
The metastasis and survival time after injection of
mock-transfected and AsSn MDA-MB-231 cells into BALB/C SCID mice
was further tested in vivo. At the end of the experiment, a
significant reduction in the volume of tumors induced by AsSn cells
and less lymph node metastasis was detected compared with
mock-transfected cells. The results indicate that the full
biological effect of blocking Snail must be markedly affected by
the tumor microenvironment. Furthermore, mice injected with AsSn
cells survived longer than those injected with mock-transfected
cells. Significantly decreased levels of RhoA protein were observed
in the tumors derived from mice injected with AsSn cells. These
details clarify the role of Snail in the regulation of RhoA, which
is correlated with tumor metastasis by affecting cell movement.
A previous study indicated that the use of RNA
interference may be an effective tool for blocking Snail function
(22). We have also reported a
strategy of combining antisense apoptosis-associated cDNA with an
oncolytic adenovirus (23) and it
appears that arming an oncolytic adenovirus with siRNA is also
reliable in cancer gene therapy (24). Future studies should apply these
methods progressively to Snail-targeted cancer gene therapy.
In conclusion, the present data support a novel role
for Snail in the progression of breast tumors and provide evidence
that this effect is mainly mediated through the regulation of RhoA
activity which is involved in cell movement and growth in
vivo. Based on these findings, Snail may be considered, in the
future, as a putative molecular target for antineoplastic
therapy.
Abbreviations:
|
AsSn
|
antisense-Snail cDNA construct;
|
|
DMEM
|
Dulbecco’s minimum essential
medium;
|
|
EMT
|
epithelial-mesenchymal transition;
|
|
HMEC
|
human mammary epithelial cells
|
Acknowledgements
The present study was supported by the
National Nature and Science Foundation of China (30700895,
30770913, 30500596 and 30528012), the National Basic Research
Program of China (973 Program, 2009CB521808), the Nature and
Science Foundation of Hubei Province (2011CBD542) and Fundamental
Research Funds for the Central Universities (HUST: 2012TS058).
References
|
1.
|
World Health Organization: Cancer: Fact
Sheet No 297. http://www.who.int/mediacentre/factsheets/fs297/en/.
Accessed August 12, 2012.
|
|
2.
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Jethwa P, Naqvi M, Hardy RG, et al:
Overexpression of Slug is associated with malignant progression of
esophageal adenocarcinoma. World J Gastroenterol. 14:1044–1052.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Xiong H, Hong J, Du W, et al: Roles of
STAT3 and ZEB1 in E-cadherin down-regulation and human colorectal
cancer epithelial-mesenchymal transition. J Biol Chem.
287:5813–5832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sakamoto K, Imanishi Y, Tomita T, et al:
Overexpression of SIP1 and downregulation of E-cadherin predict
delayed neck metastasis in stage I/II oral tongue squamous cell
carcinoma after partial glossectomy. Ann Surg Oncol. 19:612–619
|
|
6.
|
Baritaki S, Huerta-Yepez S, Sahakyan A, et
al: Mechanisms of nitric oxide-mediated inhibition of EMT in
cancer: inhibition of the metastasis-inducer Snail and induction of
the metastasis-suppressor RKIP. Cell Cycle. 9:4931–4940. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yin T, Wang C, Liu T, Zhao G, Zha Y and
Yang M: Expression of snail in pancreatic cancer promotes
metastasis and chemoresistance. J Surg Res. 141:196–203. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Côme C, Magnino F, Bibeau F, et al: Snail
and slug play distinct roles during breast carcinoma progression.
Clin Cancer Res. 12:5395–5402. 2006.PubMed/NCBI
|
|
9.
|
Jordà M, Olmeda D, Vinyals A, et al:
Upregulation of MMP-9 in MDCK epithelial cell line in response to
expression of the Snail transcription factor. J Cell Sci.
118:3371–3385. 2005.PubMed/NCBI
|
|
10.
|
Cheng CW, Wu PE, Yu JC, et al: Mechanisms
of inactivation of E-cadherin in breast carcinoma: modification of
the two-hit hypo- thesis of tumor suppressor gene. Oncogene.
20:3814–3823. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
del Barrio M and Nieto M: Overexpression
of Snail family members highlights their ability to promote chick
neural crest formation. Development. 1583–1593. 2002.PubMed/NCBI
|
|
12.
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar
|
|
13.
|
Gou L, Wang W, Tong A, et al: Proteomic
identification of RhoA as a potential biomarker for proliferation
and metastasis in hepatocellular carcinoma. J Mol Med (Berl).
89:817–827. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Takami Y, Higashi M, Kumagai S, et al: The
activity of RhoA is correlated with lymph node metastasis in human
colorectal cancer. Dig Dis Sci. 53:467–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sander EE, ten Klooster JP, van Delft S,
van der Kammen R and Collard JG: Rac downregulates Rho activity:
reciprocal balance between both GTPases determines cellular
morphology and migratory behavior. J Cell Biol. 147:1009–1022.
1999. View Article : Google Scholar
|
|
16.
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Simpson K, Dugan AS and Mercurio AM:
Functional analysis of the contribution of RhoA and RhoC GTPases to
invasive breast carcinoma. Cancer Res. 64:8694–8701. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Rosman DS, Phukan S, Huang CC and Pasche
B: TGFBR1*6A enhances the migration and invasion of MCF-7 breast
cancer cells through RhoA activation. Cancer Res. 68:1319–1328.
2008.
|
|
19.
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tsutsumi S, Gupta SK, Hogan V, Collard JG
and Raz A: Activation of small GTPase Rho is required for autocrine
motility factor signaling. Cancer Res. 62:4484–4490.
2002.PubMed/NCBI
|
|
21.
|
Tester AM, Waltham M, Oh SJ, et al:
Pro-matrix metalloproteinase-2 transfection increases orthotopic
primary growth and experimental metastasis of MDA-MB-231 human
breast cancer cells in nude mice. Cancer Res. 64:652–658. 2004.
View Article : Google Scholar
|
|
22.
|
Olmeda D, Jordá M, Peinado H, Fabra A and
Cano A: Snail silencing effectively suppresses tumour growth and
invasiveness. Oncogene. 26:1862–1874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhou J, Gao Q, Chen G, et al: Novel
oncolytic adenovirus selectively targets tumor-associated polo-like
kinase 1 and tumor cell viability. Clin Cancer Res. 11:8431–8440.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Carette JE, Overmeer RM, Schagen FH, et
al: Conditionally replicating adenoviruses expressing short hairpin
RNAs silence the expression of a target gene in cancer cells.
Cancer Research. 64:2663–2667. 2004. View Article : Google Scholar
|