Introduction
Small cell lung cancer (SCLC) accounts for ~15% of
all lung cancers in the USA (1).
The majority of patients typically present with symptoms associated
with central airway disease, such as cough and dyspnea, as well as
symptoms of widespread metastatic disease, such as weight loss,
debility, bone pain and neurologic compromise. Histological
analysis may be sufficient for the diagnosis of SCLC, which
presents as poorly differentiated tumor that is categorized as a
high-grade neuroendocrine carcinoma. The majority of SCLCs are
immunoreactive for keratin and thyroid transcription factor-1
(TTF-1). Furthermore, at least one marker of neuroendocrine
differentiation, such as neuron-specific enolase (NSE), neural cell
adhesion molecule (CD56) or synaptophysin (2), is observed in 75% of SCLC cases
(2,3). Despite its rapid doubling time and
early development of widespread metastases, SCLC is highly
sensitive to chemotherapy and radiotherapy (4). At present, the etoposide and cisplatin
regimen is the standard first-line chemotherapy treatment for
patients with SCLC. However, the majority of patients exhibit
recurrences and subsequently succumb to the disease (5,6).
Patients with SCLC are usually classified as either limited-or
extensive-stage. Limited-stage SCLC is defined as cancer within
only one lung and/or in the lymph nodes in the mediastinum. The
majority of patients with SCLC are extensive-stage at the time of
initial diagnosis, where the cancer has spread either to the other
side of the chest or to more distant locations in the body. The
median survival rates are 10.3 months and 5.6 months for patients
with limited- and extensive-stage disease, respectively (7).
Smoking is, undoubtedly, the primary cause of lung
cancer (8,9). Environmental and occupational
exposures are also potential risk factors, but the effects of these
suspected factors remain equivocal (10,11).
Mosquito coils are a major type of residential insecticide, and are
frequently burned indoors and outdoors in Asia. In China, mosquito
coils that contain pyrethroid insecticides, particularly
d-allethrin, use different concentrations of octachlorodipropyl
ether (S-2) as a synergist or an active ingredient (12). S-2 has been reported to exhibit
carcinogenicity following its degradation to the extremely potent
lung carcinogen, bis(chloromethyl) ether (BCME) (13). The carcinogenicity of BCME was first
demonstrated in 1968 with skin painting in mice and subcutaneous
injection in rats (14),
subsequently a number of additional animal experiments have been
performed. Van Duuren et al (14) stated that BCME produced a moderate
tumor response as an initiator and caused hyperplasia in the
treated area. Preceding the increase in DNA synthesis, an
inhibition of DNA synthesis immediately followed treatment with
BCME. The reduction in apparent DNA synthesis caused by the agent
may occur via a number of mechanisms (15), including: i) Interference with
strand separation and/or DNA polymerase activity due to the
presence of a covalently bound foreign molecule in DNA, ii)
reduction in the number of cells synthesizing DNA due to of cell
death or other toxic reactions, iii) breakdown of (labeled) DNA
following treatment, and iv) defective enzymes resulting in a
failure to utilize nucleoside precursors. Furthermore, BCME is a
unique alkylating agent due to the participation of the oxonium ion
in addition to the carbonium ion usually encountered with
alkylating agent carcinogens. The existence of this equilibrium
reaction and the resonance stabilization in the ionic species
explains the induction of lung cancer in animals and humans by this
carcinogen and its potential for resulting in the induction of
malignant tumors at distant sites (16). Exposure to BCME via inhalation has
been associated with the formation of lung tumors in rats and mice
(17–19). Furthermore, previous studies have
shown that BCME leads to mutations in bacteria, as well as
unscheduled DNA synthesis in cultured human cells (15,20).
In addition, epidemiological studies from various geographical
locations, including the USA (21),
Germany (22) and Japan (23) have found that occupational exposure
to BCME is associated with the development of lung cancer, in
particular SCLC (16,20). The present study describes three
cases of small cell lung cancer (SCLC) that were likely to have
arisen due to exposure to mosquito coils. Consent was obtained from
the families of the patients.
Case reports
Case one
A 39-year-old male never-smoker presented to the
Shanghai Pulmonary Hospital (Tongji University, School of Medicine,
Shanghai, China) on March 6, 2008, with a productive cough that had
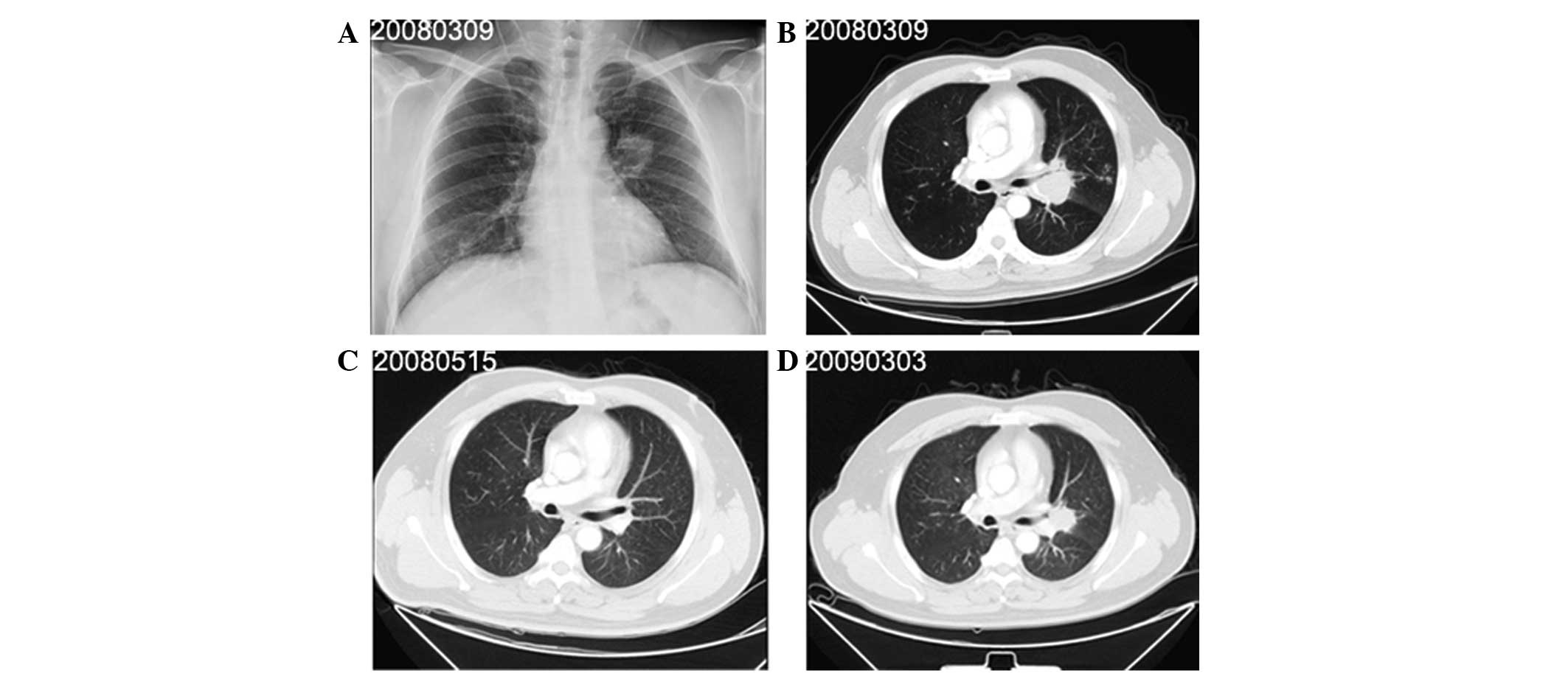
been apparent for one month. Radiography (Fig. 1A) and computed tomography (CT) of
the chest (Fig. 1B) revealed
enlarged lymph nodes and a mass measuring 4.8×3.4 cm in the upper
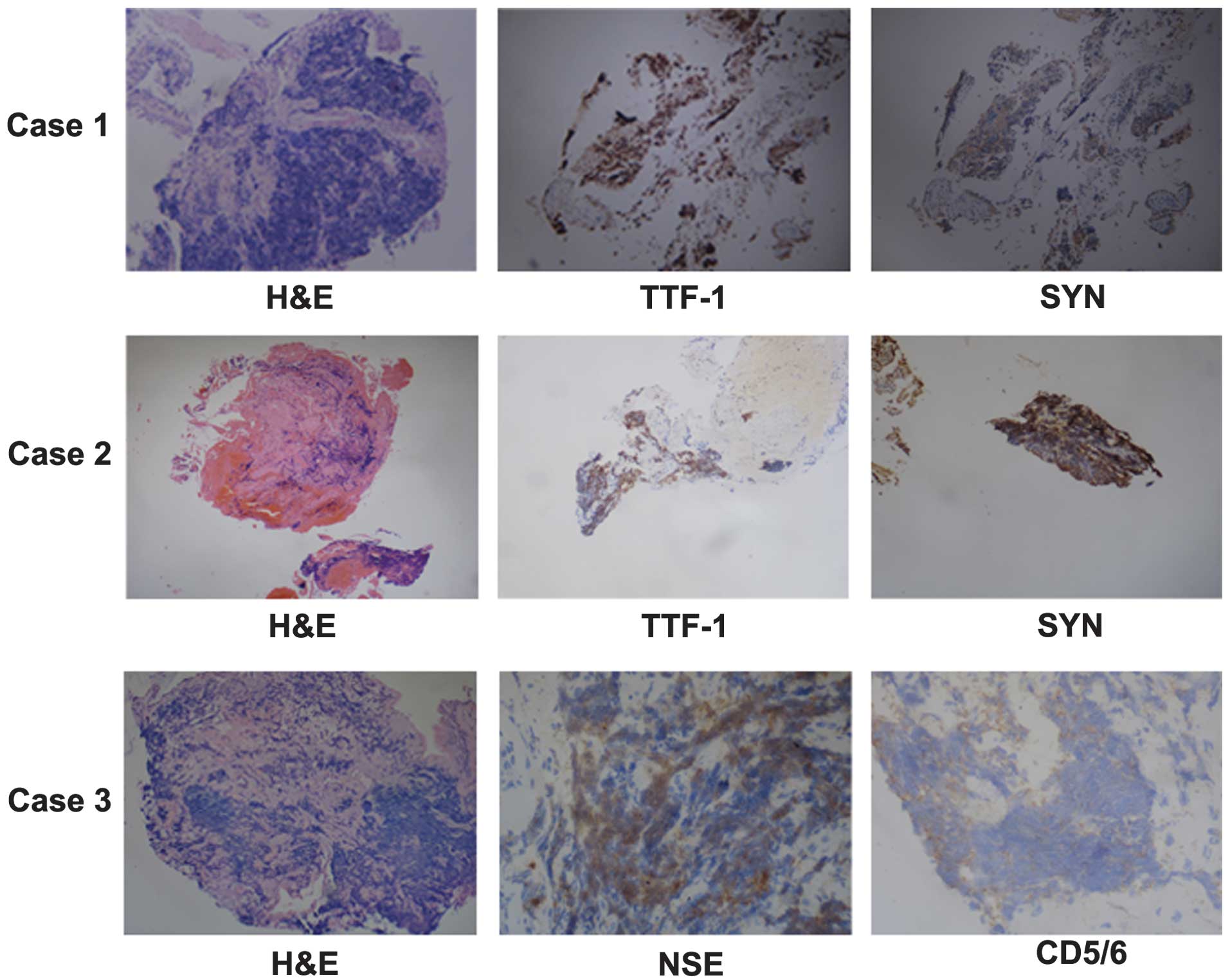
lobe of the left lung. Immunohistochemical analysis indicated that
the tumor was positive for thyroid transcription factor 1 (TTF-1)
and synaptophysin (SYN), but negative for cluster of
differentiation (CD)5 and 6 (Fig.
4). The patient was subsequently diagnosed with SCLC,
tumor-node-metastasis (TNM) stage T4N2M0 (IIIb). Following two
cycles of chemotherapy with 100 mg/m2 etoposide and 75
mg/m2 cisplatin on days one to three of three-weekly
cycles, the patient exhibited a complete response (CR) (Fig. 1C). The six cycles of chemotherapy
were completed on September 13, 2008. In March 2009, CT revealed
the presence of progressive disease (PD) (Fig. 1D) and second-line chemotherapy with
60 mg/m2 irinotecan on days one and eight of
three-weekly cycles, was subsequently initiated. Due to a poor
performance status, the patient proceeded to receive supportive
care, but succumbed to the disease on August 17, 2009.
Case two
A 41-year-old male presented to the Shanghai
Pulmonary Hospital on October 20, 2010, with a productive cough and
dyspnea. The patient had smoked 10 cigarettes per day for the past
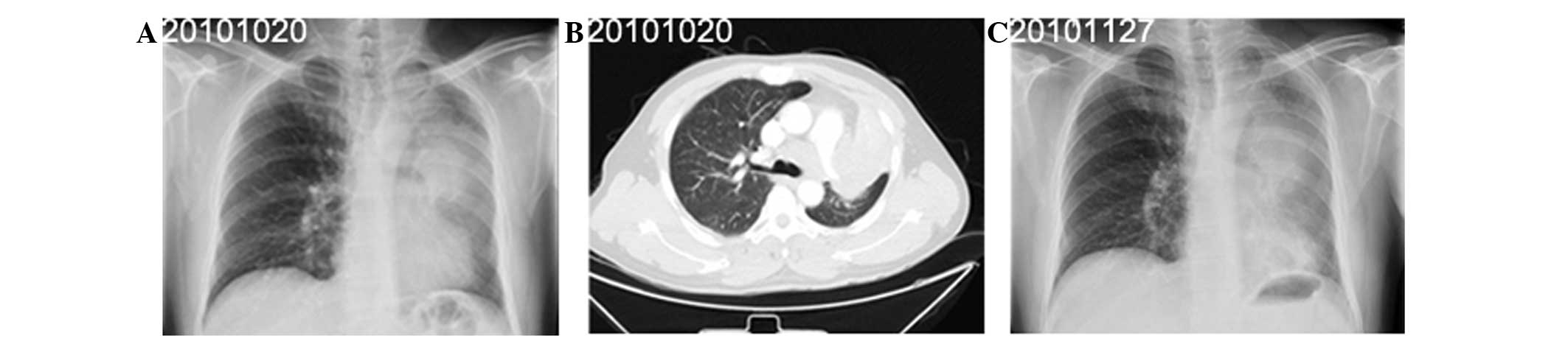
20 years. Radiography (Fig. 2A and
C) and CT (Fig. 2B) of the
chest revealed enlarged lymph nodes and a mass measuring 10.5×7.2
cm in the upper lobe of the left lung. Immunohistochemical analysis
indicated that the tumor was positive for TTF-1 and SYN, but
negative for CD5/6 (Fig. 4). The
patient was subsequently diagnosed with SCLC, stage T4N2M0 (IIIb).
Following two cycles of chemotherapy with 100 mg/m2
etoposide and 25 mg/m2 cisplatin on days one to three of
three-weekly cycles, the patient’s condition deteriorated, with
evidence of hemoptysis and thrombocytopenia. The patient succumbed
to the disease on January 25, 2011.
Case three
A 40-year-old male presented to the Shanghai
Pulmonary Hospital on September 27, 2012, with right-sided chest
pain, a productive cough and dyspnoea that had been apparent for
two weeks. The patient had smoked 15 cigarettes per day for the
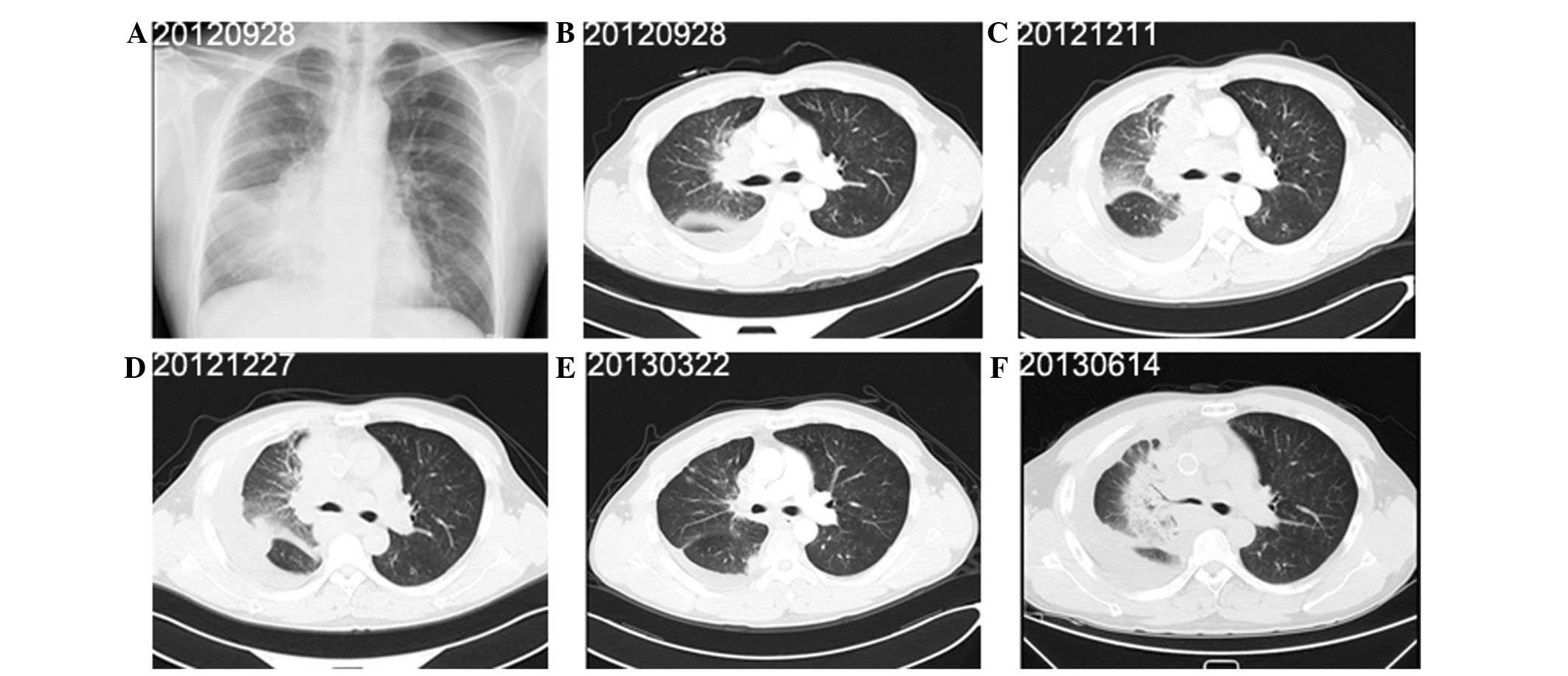
past 18 years. Radiography (Fig.
3A) and CT of the chest (Fig.
3B) revealed pleural effusion, enlarged lymph nodes and a mass
measuring 9.4×8.0 cm in the middle lobe of the right lung.
Immunohistochemical analysis indicated that the tumor was positive
for TTF-1, NSE, chromogranin A and Ki-67, but negative for SYN,
leukocyte common antigen, p63 and CD5/6 (Fig. 4). The patient was subsequently
diagnosed with SCLC, stage T4N3M1a (IV). Following two cycles of
chemotherapy with 100 mg/m2 etoposide and 25
mg/m2 cisplatin on days one to three of three-weekly
cycles, the tumor response was assessed as PD (Fig. 3C). Superior vena cava stenting and
25 Gy thoracic radiation therapy (2.5 Gy/fraction) were performed
in December, 2012 for two weeks. Four cycles of second-line
chemotherapy with 60 mg/m2 on days one and eight of
three-weekly cycles were also administered (Fig. 3E). On June 14, 2013, the tumor
response was evaluated as PD (Fig.
3F), at which time the patient’s performance status
deteriorated. The patient succumbed to the disease on July 02,
2013.
Discussion
In China, the burning of mosquito coils is a common
practice, particularly in summer. At present, a limited number of
toxicity studies have been published that describe the potential
carcinogenic effects associated with exposure to coil-containing
toxins. A previous case-control study (24) investigated whether exposure to
mosquito coil smoke was a risk factor for the development of lung
cancer. The results of the study revealed that mosquito coil smoke
exposure was more commonly observed in lung cancer patients than in
control subjects (38.1 vs. 17.8%; P<0.01), and that the risk of
lung cancer was significantly increased among those who frequently
burned mosquito coils compared with non-burners (adjusted odds
ratio=3.78; 95% CI, 1.55–6.90) (18). Another study demonstrated a positive
association between daily exposure to mosquito coils and lung
cancer among smokers in China (25). Therefore, exposure to mosquito coil
smoke may have a significant role in the pathogenesis of lung
cancer.
The key to recognizing cases of lung cancer that are
a result of occupational or environmental exposures is clinical
investigation and consideration of all possible causes for the
disease that are present. The histological type of
occupational-induced lung cancers is usually different to that of
lung cancers caused by another factor due to the difference in the
etiology of lung cancer. Between 2008 and 2012, three patients were
diagnosed with SCLC at the Shanghai Pulmonary Hospital. These
patients, whose cases have been reported in the present study, were
aged around 40 years old, which is younger than the general age of
patients with lung cancer. Notably, all patients had been employed
in the manufacture of mosquito coils in Xinghua, Jiangsu, for a
mean of 9.1 years. The mean overall survival time after
presentation was 10.7 months. The primary routes of occupational
exposure to mosquito coil toxins are inhalation and dermal contact.
Based upon the clinical and pathological findings, it was
hypothesized that occupational exposure to mosquito coils was a
major factor involved in the pathogenesis of the lung cancer.
Mosquito coils generally consist of an insecticide,
a binder, organic fillers capable of smoldering and additives, such
as synergists. It is reported that the majority of mosquito coils
used in China contain different concentrations of S-2 (26). Since mosquito coils that contain S-2
are unregistered and illegal for use in the United States (10), global studies regarding the effects
of S-2 are limited. Exposure to S-2 by inhalation results in
oxidative damage to the liver, spleen and lungs of mice. The lung,
however, is believed to be the main target organ (27). Since BCME can be produced from
impurities contained in S-2, or by the thermolytic degradation of
S-2, the use of S-2-containing mosquito coils has been established
to be a potential contributor to environmental exposure to BCME
(13). The risk of lung cancer
increases with increased exposure duration or cumulative exposure
(22). A previous study revealed
that for workers exposed to the highest doses, the relative risk of
developing lung cancer increased more than 10 fold (28). A further study revealed that the
mean time between first exposure and diagnosis was 13 years, and
that the average age of exposed individuals at diagnosis was 10.5
years lower than of non-exposed individuals (29).
Histological evaluation has indicated that exposure
to S-2 results primarily in small cell-type lung cancers,
particularly oat-cell carcinoma (29,30).
Based upon the histological evidence, it was hypothesized that the
inhalation of S-2 may have been the potential cause of SCLC in the
three patients included in the present study. However, the other
toxic products released following mosquito coil use have yet to be
adequately assessed, therefore, future controlled studies should be
conducted in order to evaluate their effects. Exposure is a
controllable factor, and workers therefore deserve preventive
actions in order to reduce exposure to toxins in the workplace.
Furthermore, the effects of daily use and exposure to mosquito
coils should be evaluated with respect to further health
implications.
References
|
1
|
Govindan R, Page N, Morgensztern D, et al:
Changing epidemiology of small-cell lung cancer in the United
States over the last 30 years: analysis of the surveillance,
epidemiologic, and end results database. J Clin Oncol.
24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Junker K, Wiethege T and Müller KM:
Pathology of small-cell lung cancer. J Cancer Res Clin Oncol.
126:361–368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guinee DG Jr, Fishback NF, Koss MN, et al:
The spectrum of immunohistochemical staining of small-cell lung
carcinoma in specimens fromtransbronchial and open-lung biopsies.
Am J Clin Pathol. 102:406–414. 1994.PubMed/NCBI
|
|
4
|
Elias AD: Small cell lung cancer:
state-of-the-art therapy in 1996. Chest. 112(4 Suppl): S251–S258.
1997. View Article : Google Scholar
|
|
5
|
Murray N and Turrisi AT III: A review of
first-line treatment for small-cell lung cancer. J Thorac Oncol.
1:270–278. 2006.
|
|
6
|
Hann CL and Rudin CM: Management of
small-cell lung cancer: incremental changes but hope for the
future. Oncology (Williston Park). 22:1486–1492. 2008.
|
|
7
|
Kuo YH, Lin ZZ, Yang YY, et al: Survival
of patients with small cell lung carcinoma in Taiwan. Oncology.
82:19–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gibbons DL, Byers LA and Kurie JM:
Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 12:3–13.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
White C: Research on smoking and lung
cancer: a landmark in the history of chronic disease epidemiology.
Yale J Biol Med. 63:29–46. 1990.PubMed/NCBI
|
|
10
|
Espina C, Porta M, Schüz J, et al:
Environmental and occupational interventions for primary prevention
of cancer: a cross-sectorial policy framework. Environ Health
Perspect. 121:420–426. 2013.PubMed/NCBI
|
|
11
|
Vinceti M, Bottecchi I, Fan A, et al: Are
environmental exposures to selenium, heavy metals, and pesticides
risk factors for amyotrophic lateral sclerosis? Rev Environ Health.
27:19–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krieger RI, Dinoff TM and Zhang X:
Octachlorodipropyl ether (s-2) mosquito coils are inadequately
studied for residential use in Asia and illegal in the United
States. Environ Health Perspect. 111:1439–1442. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pauluhn J: Overview of inhalation exposure
techniques: strengths and weaknesses. Exp Toxicol Pathol. 57(Suppl
1): 111–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Duuren BL, Sivak A, Goldschmidt BM, et
al: Carcinogenicity of halo-ethers. J Natl Cancer Inst. 43:481–486.
1969.PubMed/NCBI
|
|
15
|
Slaga TJ, Bowden GT, Shapas BG and
Boutwell RK: Macromolecular synthesis following a single
application of alkylating agents used as initiators of mouse skin
tumorigenesis. Cancer Res. 33:769–776. 1973.PubMed/NCBI
|
|
16
|
Van Duuren BL: Direct-acting alkylating
and acylating agents. DNA adduct formation, structure-activity, and
carcinogenesis. Ann NY Acad Sci. 534:620–634. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gargus JL, Reese WH Jr and Rutter HA:
Induction of lung aenomas in newborn mice by bis(chloromethyl)
ether. Toxicol Appl Pharmacol. 15:92–96. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laskin S, Kuschner M, Drew RT, Cappiello
VP and Nelson N: Tumors of the respiratory tract induced by
inhalation of bis(chloromethyl)ether. Arch Environ Health.
23:135–136. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toth B: A critical review of experiments
in chemical carcinogensis using newborn animals. Cancer Res.
28:727–738. 1968.PubMed/NCBI
|
|
20
|
National Toxicology Program.
Bis(chloromethyl) ether and technical-grade chloromethyl methyl
ether. Rep Carcinog. 12:71–73. 2011.PubMed/NCBI
|
|
21
|
Albert RE, Pasternack BS, Shore RE, et al:
Mortality patterns among workers exposed to chloromethyl ethers--a
preliminary report. Environ Health Perspect. 11:209–214.
1975.PubMed/NCBI
|
|
22
|
Figueroa WG, Raszkowski R and Weiss W:
Lung cancer in chloromethyl methyl ether workers. N Engl J Med.
288:1096–1097. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakabe H: Lung cancer due to exposure to
bis(chloromethyl)ether. Ind Health. 11:145–148. 1973. View Article : Google Scholar
|
|
24
|
Chen SC, Wong RH, Shiu LJ, Chiou MC and
Lee H: Exposure to mosquito coil smoke may be a risk factor for
lung cancer in Taiwan. J Epidemiol. 18:19–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang L, Lim WY, Eng P, Leong SS, Lim TK,
Ng AW, Tee A and Seow A: Lung cancer in Chinese women: evidence for
an interaction between tobacco smoking and exposure to inhalants in
the indoor environment. Environ Health Perspect. 118:1257–1260.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen ZM: Tri-dimension pollution chain in
tea ecosystem and its control. Scientia Agricultura Sinica.
40:948–958. 2007.
|
|
27
|
Zeng CH, Tang M and Xiong LL: Effect of
Octachlorodipropyl Ether Inhalation on Oxidative Damage in Mice. J
Environ Occup Med. 23:151–153. 2006.
|
|
28
|
Maher KV and DeFonso LR: Respiratory
cancer among chloromethyl ether workers. J Natl Cancer Inst.
78:839–843. 1987.PubMed/NCBI
|
|
29
|
Gowers DS, DeFonso LR, Schaffer P, et al:
Incidence of respiratory cancer among workers exposed to
chloromethyl-ethers. Am J Epidemiol. 137:31–42. 1993.PubMed/NCBI
|
|
30
|
Weiss W and Boucot KR: The respiratory
effects of chloromethyl methyl ether. JAMA. 234:1139–1142. 1975.
View Article : Google Scholar : PubMed/NCBI
|