Introduction
Hepatocellular carcinoma (HCC) is the seventh most
common malignancy and the third leading cause of cancer-related
deaths worldwide (1). Despite
improvements in detection and clinical treatment strategies, the
5-year survival rate for HCC is still very low (2). The main cause of death in HCC patients
is tumor progression with invasion and metastasis. However, the
underlying mechanisms of HCC invasion and metastasis are still not
fully understood (3). Thus, the
discovery and subsequent development of novel agents to block HCC
invasion and metastasis are primary research objectives for
HCC.
Tumor metastasis occurs by a series of steps,
including cell invasion, degradation of basement membranes, and the
stromal extracellular matrix, ultimately leading to tumor cell
invasion and metastasis. Matrix metalloproteinases (MMPs) are a
family of related enzymes that degrade the extracellular matrix
(ECM) and activation of these enzymes allow tumor cells access to
the vasculature, migration, and invasion into target organs, and
the development of tumor metastasis (4). Among the previously reported human
MMPs, MMP-2 and MMP-9 play the most important role in tumor
invasion and metastasis because of their specificity for type IV
collagen which is the principal component of the basement membrane
(5,6). Angiogenesis plays an important role in
tumor metastasis from the initial stage of carcinogenesis to the
end stage of metastatic disease (7). The development of neovasculature in
the tumor provides essential functions for growth, invasion and
metastasis. VEGF is one of the isolated angiogenic peptides, and is
the most well studied angiogenic factor so far. Moreover, VEGF is
known to play a vital role in tumor-associated invasion (8,9).
The Notch signaling pathway includes Notch ligands,
receptors, negative and positive modifiers, and Notch target
transcription factors. As an important signaling pathway, Notch is
not only involved in cell development and fate determination, but
also plays an important role in tumor development (10,11).
The Notch signaling pathway is aberrantly activated in a variety of
human tumors, including T-cell acute lymphoblastic leukemia, lung,
colorectal, prostate, and breast carcinomas (12–15).
In contrast to its tumor-facilitative role, the Notch signaling
pathway has been identified in B-cell malignancies (16), neural crest tumors (17) and skin cancer (18). Therefore, the Notch signaling
pathway seems to function as an oncogene or a tumor suppressor,
depending on the tissue type. Pharmacologic manipulation of the
Notch signaling pathway is becoming a new strategy for human
tumors. γ-secretase inhibitors (GSI) can inhibit the proteolytic
processing of Notch receptors by γ-secretase, which is essential
for Notch activation (19), and are
being investigated clinically in T-cell leukemia and breast
cancer.
However, knowledge of the role of the Notch
signaling pathway in invasion of HCC is still limited. Therefore,
in this study, we investigated the role and mechanism of Notch
signaling pathway inhibition by DAPT. DAPT, a GSI, resulted in
inhibiting HCC cells invasion in vitro. Our results suggest
that the inhibition of Notch signaling pathway caused decreases of
MMP-2, MMP-9 and VEGF, thus resulting in the inhibition of HCC cell
invasion, mediated through the inactivation of extracellular
signal-regulated kinase (ERK) phosphorylation.
Materials and methods
Cell culture and reagents
The human liver non-tumor cell line (HL-7702) and
the HCC cell lines (HepG2, HuH-7, SMMC-7721 and MHCC97H) were
cultivated in DMEM medium supplemented with 10% fetal calf serum
(Sigma Chemicals Co., St. Louis, MO). The liver non-tumor cell and
HCC cells were seeded into 6-well cell culture plates at a density
of 1×105 cells/well. All experiments were carried out
using confluent cultures. To attain normoxic condition, cultures
were maintained at 37°C in a humidified incubator containing 20%
O2, 5% CO2, and 75% N2. Primary
antibodies for MMP-2, MMP-9 and VEGF were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA). Primary antibodies for the Notch1
intracellular domain (NICD) and ERK1/2 were purchased from Abcam
(Cambridge, UK). All secondary antibodies were obtained from Pierce
(Rockford, IL). MMP-2 small interfering RNA (siRNA), MMP-9 siRNA,
VEGF siRNA, and siRNA control were obtained from Santa Cruz
Biotechnology. Lipofectamine 2000 was purchased from Invitrogen
(Carlsbad, CA, USA). To suppress the Notch signaling pathway, DAPT
in DMSO was used at different doses. To inhibit ERK1/2, PD98059
(Calbiochem, San Diego, CA) in DMSO was used at 10 μmol/l. All
other chemicals and solutions were purchased from Sigma-Aldrich,
unless otherwise indicated.
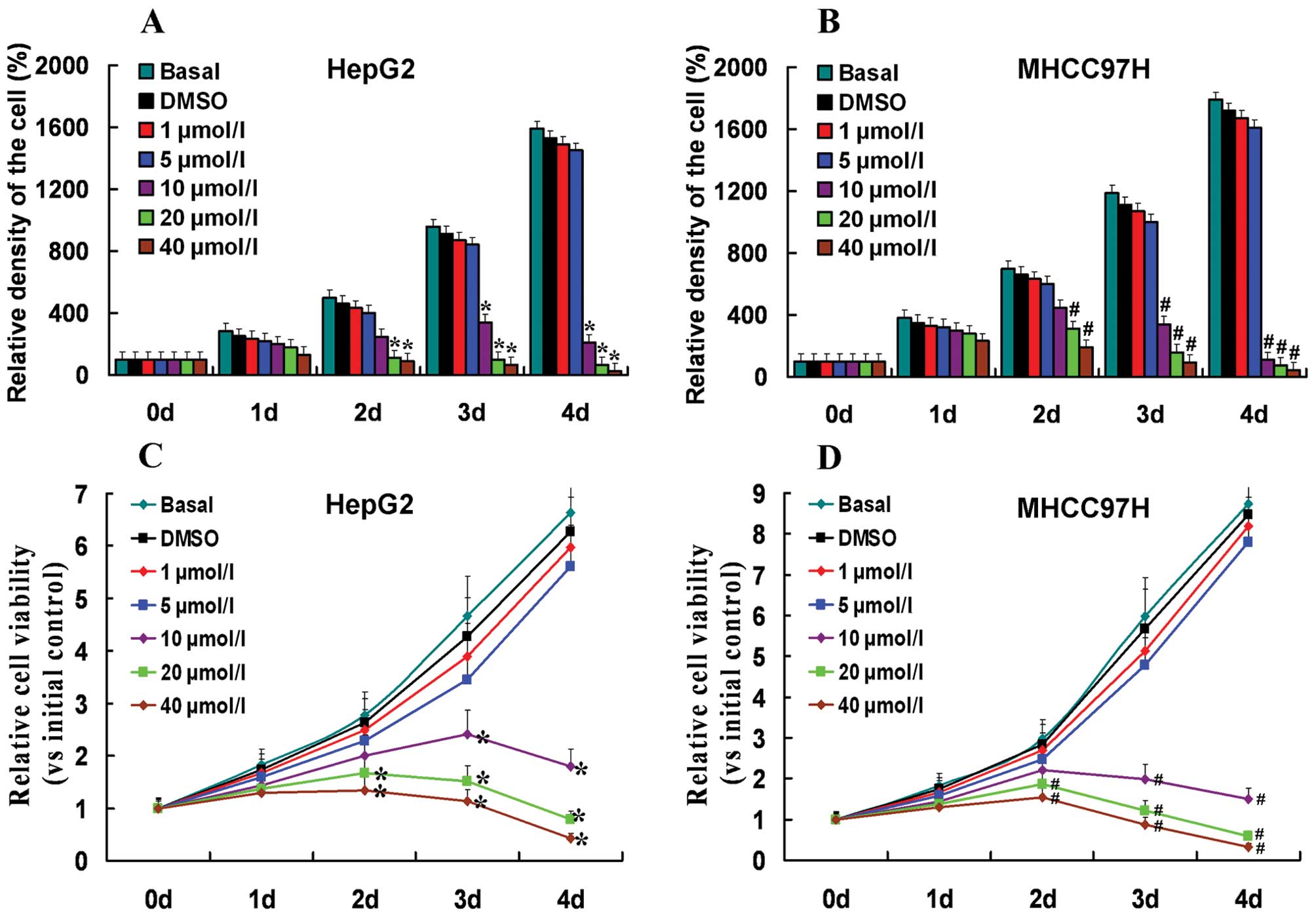
Growth curves and cell growth
HepG2 and MHCC97H cells treated with different doses
of DAPT were seeded onto 6-well cell culture plates at a density of
5×103 cells/well and were grown for up to 4 days. Each
day, we used a hemocytometer to determine the number of cells. We
used the relative density of the cells (vs. the density of the
primary cells at 100%) to establish the growth curve. Each
experiment included six replications and was repeated three times.
The data are summarized as means ± SDs.
MTT assay
The HepG2 and MHCC97H cells treated with different
doses of DAPT were seeded into 6-well cell culture plates at a
density of 1×104 cells/well and were grown for up to 4
days. Cell viability was assessed using the
3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay (Sigma Chemicals Co.) in accordance with the
manufacturer’s protocol. Each experiment included six replications
and was repeated three times. The data are summarized as means ±
SDs.
Small interfering RNA transfection
According to the protocol of Lipofectamine 2000, the
HepG2 and MHCC97H cells were transfected with MMP-2 siRNA, MMP-9
siRNA, VEGF siRNA and siRNA control respectively. Cells transfected
with siRNA were seeded into 6-well cell culture plates at a density
of 1×105 cells/well. The cells were allowed to grow
further for 24 h and were then harvested for further analysis.
Real-time reverse transcription-PCR
analysis for gene expression
Total RNA from different cells was isolated by
TRIzol (Invitrogen, Carlsbad, CA, USA) and purified using an RNeasy
Mini kit and RNase-free DNase Set (Qiagen, Valencia, CA) according
to the protocols of the manufacturer. Total RNA (1 μg) from each
sample was subjected to first-strand cDNA synthesis using TaqMan
reverse transcription reagent kit (Applied Biosystems, Foster City,
CA) in a total volume of 50 μl, including 6.25 units MultiScribe
reverse transcriptase and 25 pmol random hexamers. The reverse
transcription reaction was performed at 25°C for 10 min followed by
48°C for 30 min and 95°C for 5 min. The primers used in the PCR
reaction are as follows: Hes1, forward primer (5′-AGGCGGA
CATTCTGGAAATG-3′) and reverse primer (5′-TCGTTCA TGCACTCGCTGA-3′);
Hes5, forward primer (5′-ACCGCAT CAACAGCAGCATT-3′) and reverse
primer (5′-AGGCTTT GCTGTGCTTCAGGT-3′); Hey1, forward primer
(5′-AACTG TTGGTGGCCTGAATC-3′) and reverse primer (5′-GCGGT
AAATGCAGGCGTAT-3′); GAPDH, forward primer (5′-AA
ATCCCATCACCATCTTCC-3′) and reverse primer (5′-TCA
CACCCATGACGAACA-3′). The primers were checked by running a virtual
PCR and a primer concentration was optimized to avoid primer dimer
formation. Also, dissociation curves were checked in order to avoid
a non-specific amplification. Real-time PCR amplifications were
undertaken in a M×4000 Multiplex QPCR System (Stratagene, La Jolla,
CA) using 2X SYBR-Green PCR Master mix (Applied Biosystems). One
microliter of reverse transcription reaction was used for a total
volume of 25 μl quantitative PCR reactions. The thermal profile for
SYBR real-time PCR was 95°C for 10 min followed by 40 cycles of
95°C for 15 sec and 60°C for 1 min. Data were analyzed according to
the comparative Ct method and were normalized by GAPDH expression
in each sample.
Protein extraction and western
blotting
The cells were lysed in lysis buffer [50 mmol/l Tris
(pH 7.5), 100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% NP40, 0.5% Triton
X-100, 2.5 mmol/l sodium orthovanadate, 10 μl/ml protease inhibitor
cocktail, and 1 mmol/l PMSF] by incubating for 20 min at 4°C. The
protein concentration was determined using the Bio-Rad assay system
(Bio-Rad Laboratories, Hercules, CA). Total proteins were
fractionated using SDS-PAGE and were transferred onto
nitrocellulose membranes. The membranes were blocked with 5%
non-fat dried milk or bovine serum albumin in 1X TBS buffer
containing 0.1% Tween-20 and then were incubated with the
appropriate primary antibodies. Horseradish peroxidase-conjugated
anti-rabbit or anti-goat IgG was used as the secondary antibody,
and the protein bands were detected using the enhanced
chemiluminescence detection system (Amersham Pharmacia Biotech,
Amersham, UK). Quantification of western blots was performed using
laser densitometry, and relative protein expression was then
normalized to GAPDH levels in each sample. The results are
presented as the means of three independent experiments with error
bars representing SDs. For reprobing, membranes were incubated for
30 min at 50°C in a buffer containing 2% SDS, 62.5 mmol/l Tris (pH
6.7), and 100 mmol/l 2-mercaptoethanol, washed and incubated with
the desired primary antibody.
Invasion assays
The cell invasion capacity was analyzed by using
Matrigel-coated Transwell cell culture chambers (8 μm pore size)
(Millipore, Billerica, MA, USA). Briefly, treated cells
(5×104 cells/well) were serum-starved for 24 h and were
plated in the upper insert of a 24-well culture plates in
serum-free medium. Medium containing 10% serum as a chemoattractant
was added to the well. The cells were incubated under normoxic
conditions for 24 h. Non-invading cells were removed from the upper
surface by scrubbing with a cotton swab, after which the membrane
was fixed with 4% formaldehyde for 10 min at room temperature and
was stained with 0.5% crystal violet for 10 min. Finally, invasive
cells were counted at ×200 magnification from 10 different fields
of each filter. For treatment with DAPT and PD98059, the cells were
pretreated for 2–4 h, and the treatment continued during the
invasion experiment.
ELISA assay
Enzyme-linked immunosorbent assay (ELISA) technique
(Amersham Pharmacia Biotech) was used to quantify the activity of
individual MMP-2, MMP-9, VEGF and ERK1/2. The samples were thawed
on ice, and all reagents were equilibrated to room temperature.
Assays were carried out according to the manufacturer’s
instructions.
Statistical analysis
Each experiment was repeated at least three times.
All data were summarized and are presented as means ± SDs. The
differences among means were statistically analyzed using a t-test.
All statistical analyses were performed using the SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered as
statistically significant.
Results
Expression of Notch signaling pathway
increased in HCC cells
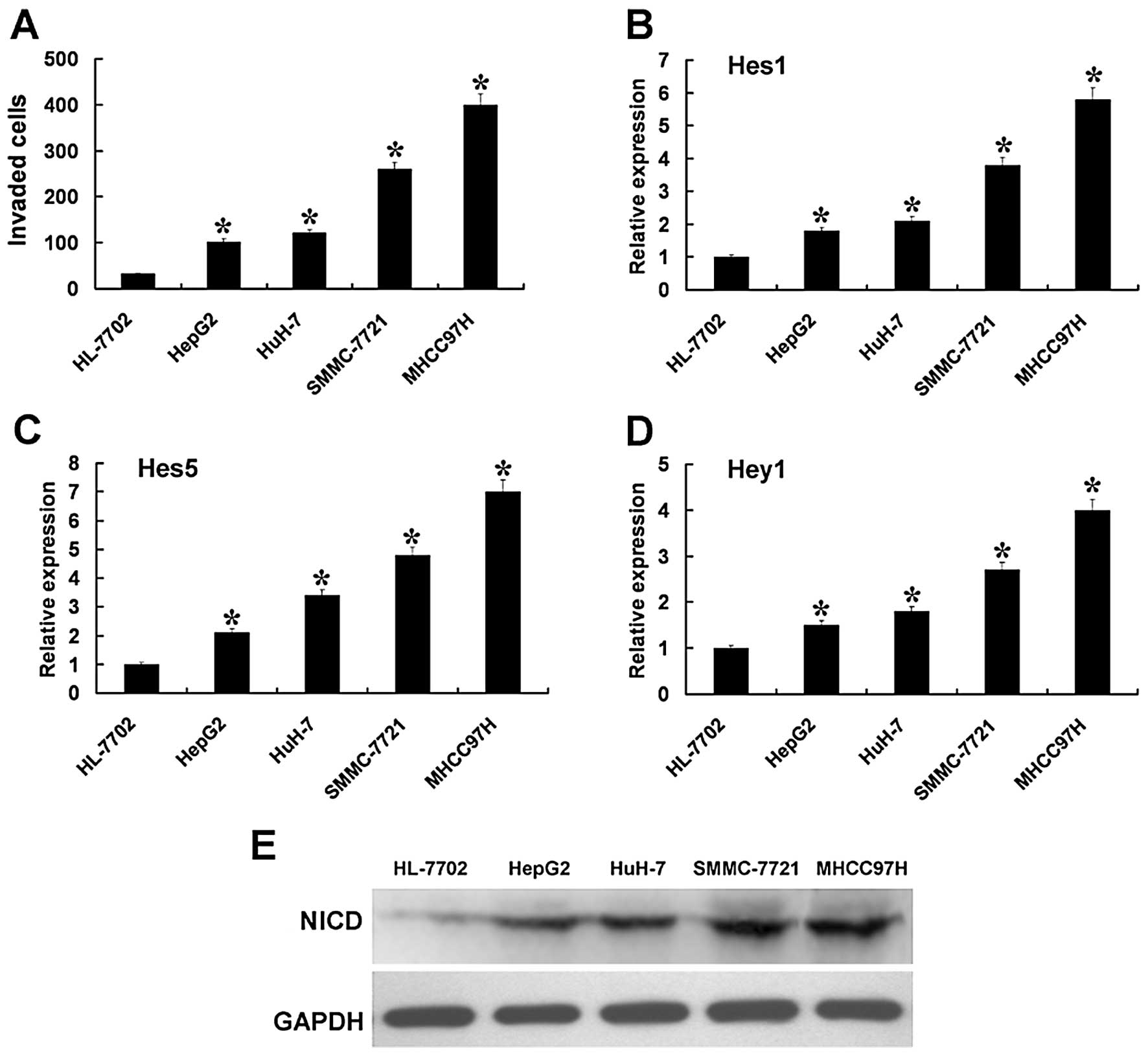
As illustrated in Fig.
1A, the HCC cell lines showed higher levels of penetration
through Transwell cell culture chambers, with Matrigel-coating vs.
the liver non-tumor cell. In HCC cell lines, the level of
penetration was the lowest in HepG2 cells and was the highest in
MHCC97H cells. These results demonstrated that HCC cells had higher
invasion capabilities than the liver non-tumor cells. In HCC cells,
HepG2 cells had the lowest invasion capability and the MHCC97H
cells had the highest invasion capability.
Next, we examined the expression of the Notch
signaling pathway in the liver non-tumor and HCC cells. RT-PCR
analysis showed that HCC cells had higher mRNA levels of the Notch
signaling pathway downstream target genes Hes1, Hes5, and Hey1
compared to the liver non-tumor cells (Fig. 1B-D). NICD also exhibited similar
increased tendencies regarding the protein levels (Fig. 1E). These results may illustrate that
the expression of the Notch signaling pathway is upregulated in HCC
cells. The expression of Hes1, Hes5, Hey1 and NICD were the lowest
in HepG2 cells and were the highest in MHCC97H cells. This
indicates that the upregulated levels of the Notch signaling
pathway may have a correlation with the invasion capability.
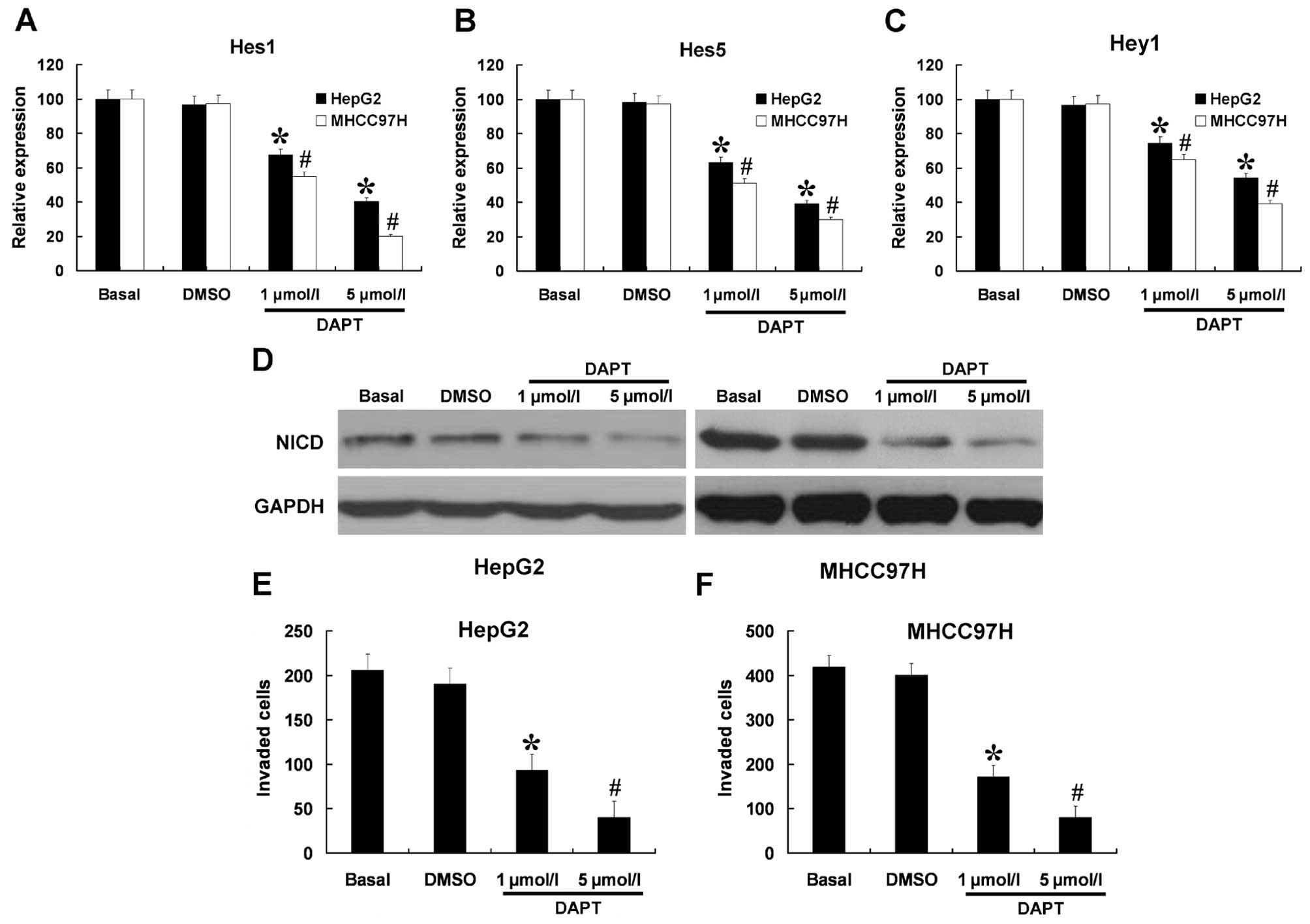
DAPT can efficiently downregulate the
Notch signaling pathway and inhibit invasion in HCC cells
Invasion capacity was the lowest in the HepG2 cell
and the highest in the MHCC97H cells. Therefore, we only used HepG2
and MHCC97H cells for the next experiment. We further investigated
the role of DAPT on the expression of the Notch signaling pathway
in HepG2 and MHCC97H cells. The cells were treated with DAPT at
different doses (1 and 5 μmol/l) and the cells not-treated or
treated with DMSO were used as controls. The mRNA expression levels
of Hes1, Hes5, and Hey1 were measured by RT-PCR and protein
expression of NICD was measured by western blotting. As shown in
Fig. 2A-D, HepG2 and MHCC97H cells
treated with DAPT reduced the mRNA expression levels of Hes1, Hes5,
Hey1, and the protein expression of NICD in a dose-dependent
manner. We next sought to determine whether DAPT affected the
invasion capabilities of HepG2 and MHCC97H cells. As shown in
Fig. 2E and F, the invasion
capabilities of HepG2 and MHCC97H cells were strongly inhibited by
DAPT in a dose-dependent manner. As shown in Fig. 3, the indicated concentrations of
DAPT (1 or 5 μmol/l) had no effect on the growth and viability of
HepG2 and MHCC97H cells. These results indicate that the inhibitory
effects of DAPT (1 or 5 μmol/l) on cell invasion were independent
of cellular cytotoxicity.
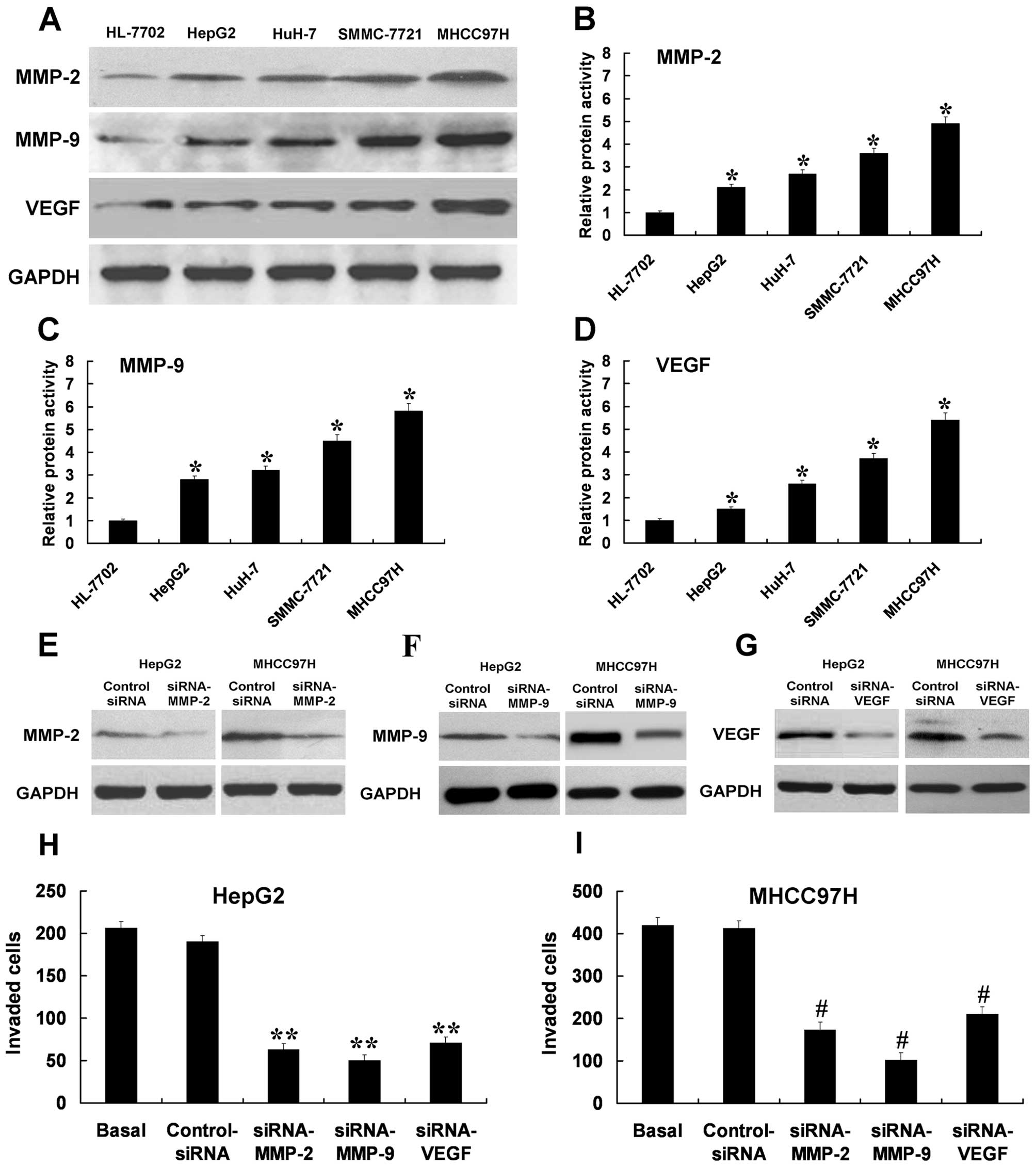
MMP-2, MMP-9 and VEGF may participate in
HCC cell invasion
MMP-2, MMP-9 and VEGF are associated with enhanced
invasion of tumor cells. With western blotting, the protein
expressions of MMP-2, MMP-9 and VEGF were upregulated in the HCC
cells compared with the liver non-tumor cells at the protein level
(Fig. 4A). The proteolytic
activities of MMP-2, MMP-9 and VEGF exhibited similar increased
tendencies in HCC cells (Fig.
4B-D). The protein expression levels and proteolytic activities
of MMP-2, MMP-9 and VEGF were the lowest in the HepG2 cells and the
highest in the MHCC97H cells. These results also showed upregulated
levels of MMP-2, MMP-9 and VEGF may correlate with invasion
capability. The HepG2 and MHCC97H cells were transfected with human
MMP-2 siRNA, MMP-9 siRNA, and VEGF siRNA. The cells non-transfected
and transfected with control siRNA were as control. siRNA can
efficiently downregulate the expression of MMP-2, MMP-9 and VEGF
(Fig. 4E-G). Cells transfected with
MMP-2 siRNA or MMP-9 siRNA or VEGF siRNA showed a lower level of
penetration through the membrane, compared with control
siRNA-transfected cells (Fig. 4H and
I). These results indicated that MMP-2, MMP-9 and VEGF may
participate in HCC cell invasion.
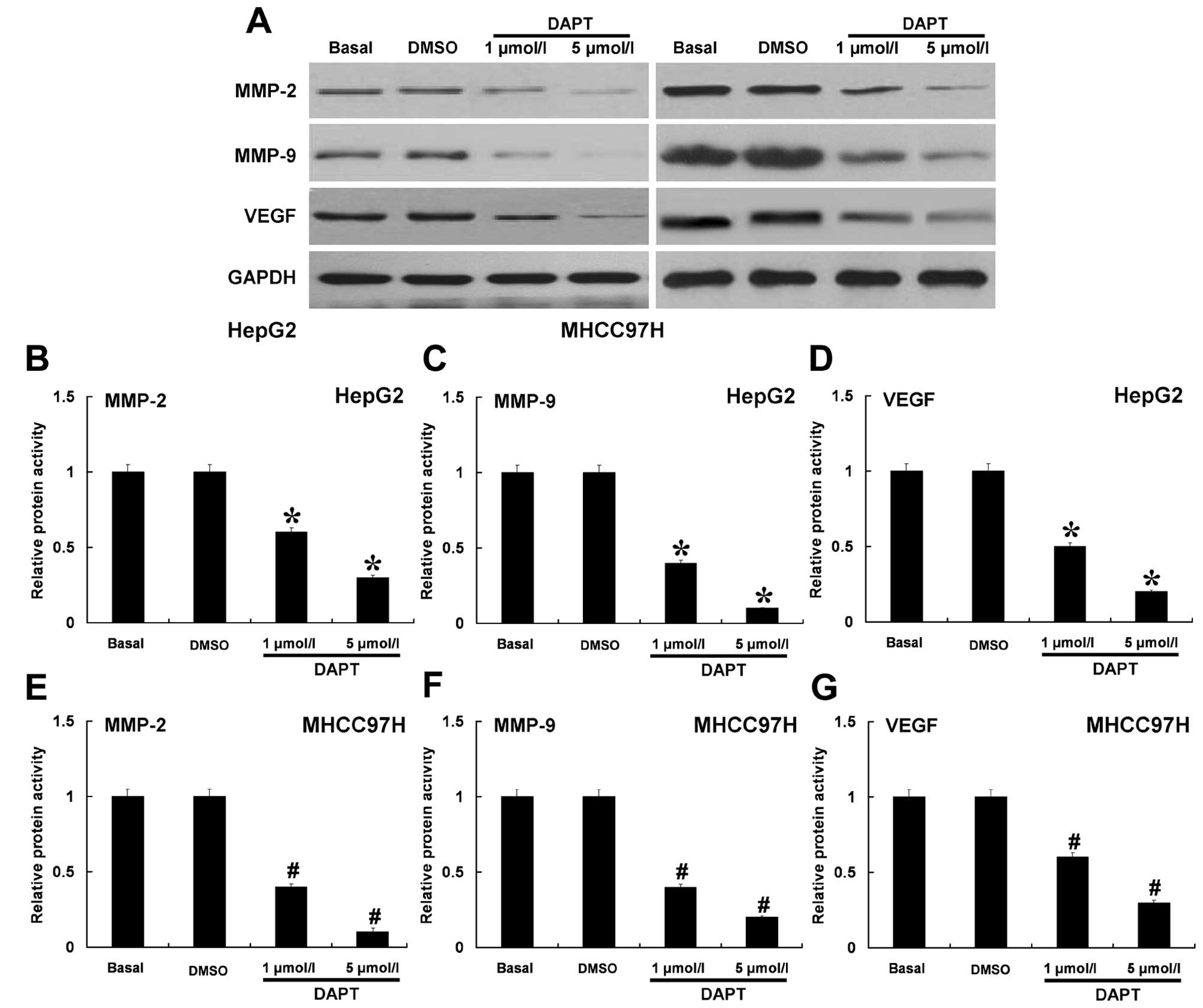
Inhibition of Notch signaling pathway by
DAPT decreased the protein expression and proteolytic activities of
MMP-2, MMP-9 or VEGF
We determined whether inhibition of Notch signaling
pathway by different doses of DAPT could have effects on MMP-2,
MMP-9 and VEGF. Western blotting and ELISA assay were used to
analyze the protein expression and proteolytic activity. DAPT was
able to effectively inhibit the protein expressions of MMP-2, MMP-9
and VEGF in a dose-dependent manner in HepG2 and MHCC97H cells
(Fig. 5A). Using ELISA assay
(Fig. 5B-G), we found that DAPT
also could effectively inhibit the proteolytic activities of MMP-2,
MMP-9 and VEGF. These results indicated that the Notch signaling
pathway may regulate MMP-2, MMP-9 and VEGF in HCC cells.
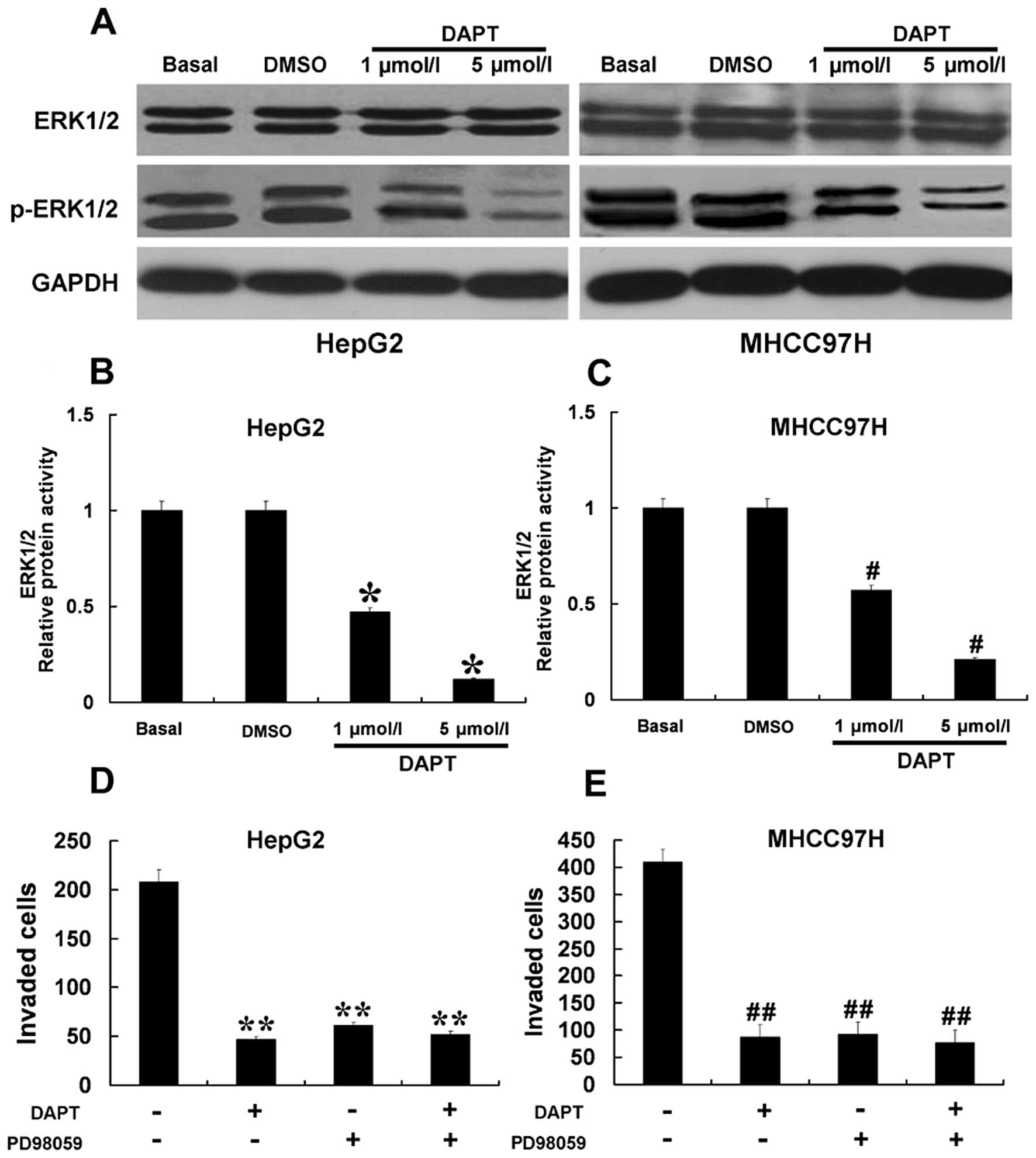
Downregulation of the Notch signaling
pathway by DAPT inhibited ERK1/2 activity resulting in inhibition
of the invasion of HCC cells
ERK1/2 is known to play a major role in signaling
pathways concerning invasion, and regulates the expression of MMPs
and VEGF. We investigated whether the antagonistic effects of DAPT
on the upregulation of MMP-2, MMP-9 and VEGF expression and
decreased invasion in HCC cells could be attributed to the
inhibition of ERK1/2. As shown in Fig.
6A, using western blot analysis, we found that increasing doses
of DAPT abolished ERK1/2 phosphorylation in a dose-dependent
manner. We further confirmed that DAPT inhibited ERK1/2 activity by
ELISA assay (Fig. 6B and C). The
results indicated that DAPT significantly inhibited the activity of
ERK1/2 in HCC cells. To further study the relationship between
ERK1/2 activity and the Notch signaling pathway in the control of
tumor invasion, HepG2 and MHCC97H cells were treated with 10 μmol/l
PD98059 and 5 μmol/l DAPT to block ERK1/2 activity and the Notch
signaling pathway, respectively (Fig.
6D and E). Treatment with PD98059 or DAPT alone reduced HepG2
and MHCC97H cell invasion. However, treatment with PD98059 in
combination with DAPT did not block these biological functions of
HCC cells to a greater extent than treatment with PD98059 or DAPT
alone. These results suggest that the Notch signaling pathway may
modulate HCC cell invasion through ERK1/2 regulating MMP-2, MMP-9
and VEGF.
Discussion
The high recurrence rate of intrahepatic and distant
metastasis is a major obstacle in improving the survival rate of
patients with HCC (20). If the
mechanisms regulating HCC invasion can be clearly defined, there
are likely to be key elements that can be exploited
therapeutically, reducing metastasis and improving survival. There
has been increasing evidence indicating that the Notch signaling
pathway increases the metastatic potential of tumor cells by
increasing processes such as invasion (21,22).
Our present studies investigated the role of the Notch signaling
pathway on HCC cell invasion. Our findings indicate that the
inhibition of the Notch signaling pathway decreased the protein
expression and proteolytic activities of MMP-2, MMP-9, and VEGF by
inhibiting ERK1/2 activity and suppressed HCC cell invasion. Taken
together, the data indicate that inhibition of the Notch signaling
pathway should be further evaluated in HCC invasion therapy.
Invasion and metastasis are the processes by which
tumor spreads from the place it first appears as a primary tumor to
distant locations in the body. This includes a series of sequential
steps, including tumor-induced angiogenesis, tumor invasion, and
the establishment of metastatic foci at the secondary site
involving various molecules (23,24).
The MMPs family proteins are the proteolytic enzymes in ECM that
contribute to tumor invasion, angiogenesis and metastasis (25). Among the previously reported human
MMPs, MMP-2 and MMP-9 have been implicated in invasion and
metastasis because of their role in the degradation of basement
membrane collagen (26,27). It was reported that MMP-2 and MMP-9
are correlated with an aggressive, invasive or metastatic tumor
phenotype (28,29). It is well known that the MMP
inhibitors block endothelial cell activities which are essential
for new vessel development leading to proliferation and invasion
(30). Therefore, MMP-2 and MMP-9
are considered therapeutic targets of anticancer drugs based on the
degrading action of both enzymes on gelatins which are major
components of the basement membrane. Another important molecule
involved in tumor cell invasion and metastasis is VEGF. The
expression of VEGF is commonly found to be upregulated in tumors
and there was a trend toward an association between the expression
of VEGF and distant metastasis. Investigations by other
laboratories have shown that VEGF promotes migration and invasion
of tumor cells (31,32). Here, we showed that the protein
expressions and proteolytic activities of MMP-2, MMP-9 and VEGF
were higher in HCC cells. The inhibition of MMP-2, MMP-9 and VEGF
by siRNA can also decrease the invasion capabilities of HCC cells.
These results indicate that MMP-2, MMP-9 and VEGF may participate
in HCC cell invasion.
The Notch signaling pathway is involved in the
carcinogenesis, progress, invasion and neurovascular formation of
many malignant tumors (22,33–35).
The Notch signaling pathway can regulate MMP-2, MMP-9 and VEGF
(22,36–40),
which are important in the processes of invasion and metastasis of
tumor. In the current study, the invasion capabilities of HepG2 and
MHCC97H cells treated with DAPT decreased. We showed that an
increase in the DAPT dose in response to Notch signaling pathway
inhibition resulted in suppression of MMP-2, MMP-9 and VEGF. These
results suggest that the inhibitory effect of DAPT on HCC cell
invasion can be partially attributable to the downregulation of
MMP-2, MMP-9 and VEGF. Some studies have shown that the Notch
signaling pathway regulates MMPs and VEGF partly due to activation
of the NF-γB pathway in cell invasion in pancreatic cancer cells
(22). However, the potential
mechanisms between Notch signaling pathway, MMPs and VEGF in HCC
invasion are poorly understood.
Extracellular signal-regulated kinase 1 and 2
(ERK1/2) belongs to the family of mitogen-activated protein kinases
(MAPKs) which play a major role in the signaling pathways
concerning scattering/motility, invasion, proliferation and
survival (41–43). ERK1/2 activation has also been
reported to regulate the expression of a variety of important genes
in some cellular responses, including metastasis related genes,
such as VEGF and MMP-2 and -9 (44–47).
Because ERK1/2 plays an important role in many cellular processes,
studies on the interaction of ERK1/2 activation with other cell
signal transduction pathways, including the Notch signaling
pathway, has received increased attention in recent years. The
Notch signaling pathway has also been reported to crosstalk with
the ERK1/2 pathway (48). In the
present study, we showed that the downregulation of the Notch
signaling pathway by DAPT reduced ERK1/2 activity and concomitantly
inhibited the protein expression and proteolytic activities of
MMP-2, MMP-9 and VEGF. We also found that the inhibited Notch
signaling pathway and/or ERK1/2 has the same role in suppressing
invasion of HCC cells. Thus, the downregulation of the Notch
signaling pathway results in lower ERK1/2 activity and its
downstream targets (MMP-2 and -9 and VEGF). Therefore, it is
possible that Notch signaling pathway induced HCC cell invasion is
partly due to the activation of the ERK1/2 pathway.
Taken together, our data showed that the Notch
signaling pathway inhibitor could suppress invasion of HCC cells
via ERK1/2 signaling pathways, resulting in the downregulation of
MMP-2, MMP-9 and VEGF. Inhibition of Notch signaling pathway could
be useful as a therapeutic target for inhibiting HCC invasion.
Further studies will elucidate the mechanism of the Notch signaling
pathway and ERK1/2 interaction.
Acknowledgements
We are grateful to Fuqin Zhang who provided
technical support. This study was supported by grants from the
National Natural Science Foundation of China (grants no. 30872480)
and the Major Program of the National Natural Science Foundation of
China (grants no. 81030010/H0318).
References
|
1
|
Yang JD, Nakamura I and Roberts LR: The
tumor microenvironment in hepatocellular carcinoma: current status
and therapeutic targets. Semin Cancer Biol. 21:35–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas MB and Zhu AX: Hepatocellular
carcinoma: the need for progress. J Clin Oncol. 23:2892–2899. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang DJ, Dong SS, Ma NF, et al:
Overexpression of eukaryotic initiation factor 5A2 enhances cell
motility and promotes tumor metastasis in hepatocellular carcinoma.
Hepatology. 51:1255–1263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Itoh Y and Nagase H: Matrix
metalloproteinases in cancer. Essays Biochem. 38:21–36.
2002.PubMed/NCBI
|
|
5
|
Zeng ZS, Cohen AM and Guillem JG: Loss of
basement membrane type IV collagen is associated with increased
expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during
human colorectal tumorigenesis. Carcinogenesis. 20:749–755. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Komatsu K, Nakanishi Y, Nemoto N, Hori T,
Sawada T and Kobayashi M: Expression and quantitative analysis of
matrix metalloproteinase-2 and -9 in human gliomas. Brain Tumor
Pathol. 21:105–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joo YE, Sohn YH, Lee WS, et al: Expression
of vascular endothelial growth factor and p53 in pancreatic
carcinomas. Korean J Intern Med. 17:153–159. 2002.PubMed/NCBI
|
|
9
|
Zeng H, Datta K, Neid M, Li J, Parangi S
and Mukhopadhyay D: Requirement of different signaling pathways
mediated by insulin-like growth factor-I receptor for
proliferation, invasion, and VPF/VEGF expression in a pancreatic
carcinoma cell line. Biochem Biophys Res Commun. 302:46–55. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miele L and Osborne B: Arbiter of
differentiation and death: Notch signaling meets apoptosis. J Cell
Physiol. 181:393–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allenspach EJ, Maillard I, Aster JC and
Pear WS: Notch signaling in cancer. Cancer Biol Ther. 1:466–476.
2002. View Article : Google Scholar
|
|
13
|
Nickoloff BJ, Osborne BA and Miele L:
Notch signaling as a therapeutic target in cancer: a new approach
to the development of cell fate modifying agents. Oncogene.
22:6598–6608. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leong KG and Karsan A: Recent insights
into the role of Notch signaling in tumorigenesis. Blood.
107:2223–2233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Radtke F and Raj K: The role of Notch in
tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer.
3:756–767. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zweidler-McKay PA, He Y, Xu L, et al:
Notch signaling is a potent inducer of growth arrest and apoptosis
in a wide range of B-cell malignancies. Blood. 106:3898–3906. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kunnimalaiyaan M and Chen H: Tumor
suppressor role of Notch-1 signaling in neuroendocrine tumors.
Oncologist. 12:535–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Proweller A, Tu L, Lepore JJ, et al:
Impaired notch signaling promotes de novo squamous cell carcinoma
formation. Cancer Res. 66:7438–7444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seiffert D, Bradley JD, Rominger CM, et
al: Presenilin-1 and -2 are molecular targets for gamma-secretase
inhibitors. J Biol Chem. 275:34086–34091. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tung-Ping Poon R, Fan ST and Wong J: Risk
factors, prevention, and management of postoperative recurrence
after resection of hepatocellular carcinoma. Ann Surg. 232:10–24.
2000.PubMed/NCBI
|
|
21
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Banerjee S, Li Y, Rahman KM, Zhang
Y and Sarkar FH: Down-regulation of notch-1 inhibits invasion by
inactivation of nuclear factor-kappaB, vascular endothelial growth
factor, and matrix metalloproteinase-9 in pancreatic cancer cells.
Cancer Res. 66:2778–2784. 2006. View Article : Google Scholar
|
|
23
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
24
|
Weiss L: Metastasis of cancer: a
conceptual history from antiquity to the 1990s. Cancer Metastasis
Rev. 19:193–383. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vihinen P and Kahari VM: Matrix
metalloproteinases in cancer: prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Curran S and Murray GI: Matrix
metalloproteinases: molecular aspects of their roles in tumour
invasion and metastasis. Eur J Cancer. 36:1621–1630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
John A and Tuszynski G: The role of matrix
metalloproteinases in tumor angiogenesis and tumor metastasis.
Pathol Oncol Res. 7:14–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cockett MI, Murphy G, Birch ML, et al:
Matrix metalloproteinases and metastatic cancer. Biochem Soc Symp.
63:295–313. 1998.PubMed/NCBI
|
|
29
|
Bianco FJ Jr, Gervasi DC, Tiguert R, et
al: Matrix metalloproteinase-9 expression in bladder washes from
bladder cancer patients predicts pathological stage and grade. Clin
Cancer Res. 4:3011–3016. 1998.PubMed/NCBI
|
|
30
|
Murphy AN, Unsworth EJ and
Stetler-Stevenson WG: Tissue inhibitor of metalloproteinases-2
inhibits bFGF-induced human microvascular endothelial cell
proliferation. J Cell Physiol. 157:351–358. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wey JS, Fan F, Gray MJ, et al: Vascular
endothelial growth factor receptor-1 promotes migration and
invasion in pancreatic carcinoma cell lines. Cancer. 104:427–438.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
33
|
Balint K, Xiao M, Pinnix CC, et al:
Activation of Notch1 signaling is required for
beta-catenin-mediated human primary melanoma progression. J Clin
Invest. 115:3166–3176. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jundt F, Anagnostopoulos I, Forster R,
Mathas S, Stein H and Dorken B: Activated Notch1 signaling promotes
tumor cell proliferation and survival in Hodgkin and anaplastic
large cell lymphoma. Blood. 99:3398–3403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Buchler P, Gazdhar A, Schubert M, et al:
The Notch signaling pathway is related to neurovascular progression
of pancreatic cancer. Ann Surg. 242:791–801. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu B, Wei J, Qian X, Lei D, Ma Q and Liu
Y: Notch1 signaling pathway participates in cancer invasion by
regulating MMPs in lingual squamous cell carcinoma. Oncol Rep.
27:547–552. 2012.PubMed/NCBI
|
|
37
|
Wang J, Fu L, Gu F and Ma Y: Notch1 is
involved in migration and invasion of human breast cancer cells.
Oncol Rep. 26:1295–1303. 2011.PubMed/NCBI
|
|
38
|
Delbosc S, Glorian M, Le Port AS, Bereziat
G, Andreani M and Limon I: The benefit of docosahexanoic acid on
the migration of vascular smooth muscle cells is partially
dependent on Notch regulation of MMP-2/-9. Am J Pathol.
172:1430–1440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiong HQ, Abbruzzese JL, Lin E, Wang L,
Zheng L and Xie K: NF-kappaB activity blockade impairs the
angiogenic potential of human pancreatic cancer cells. Int J
Cancer. 108:181–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Funahashi Y, Shawber CJ, Sharma A,
Kanamaru E, Choi YK and Kitajewski J: Notch modulates VEGF action
in endothelial cells by inducing Matrix Metalloprotease activity.
Vasc Cell. 3:22011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chan-Hui PY and Weaver R: Human
mitogen-activated protein kinase kinase kinase mediates the
stress-induced activation of mitogen-activated protein kinase
cascades. Biochem J. 336:599–609. 1998.PubMed/NCBI
|
|
42
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen PN, Hsieh YS, Chiou HL and Chu SC:
Silibinin inhibits cell invasion through inactivation of both
PI3K-Akt and MAPK signaling pathways. Chem Biol Interact.
156:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Arai K, Lee SR and Lo EH: Essential role
for ERK mitogen-activated protein kinase in matrix
metalloproteinase-9 regulation in rat cortical astrocytes. Glia.
43:254–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zeigler ME, Chi Y, Schmidt T and Varani J:
Role of ERK and JNK pathways in regulating cell motility and matrix
metalloproteinase 9 production in growth factor-stimulated human
epidermal keratinocytes. J Cell Physiol. 180:271–284. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Giuliani N, Lunghi P, Morandi F, et al:
Downmodulation of ERK protein kinase activity inhibits VEGF
secretion by human myeloma cells and myeloma-induced angiogenesis.
Leukemia. 18:628–635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Okajima E and Thorgeirsson UP: Different
regulation of vascular endothelial growth factor expression by the
ERK and p38 kinase pathways in v-ras, v-raf, and v-myc transformed
cells. Biochem Biophys Res Commun. 270:108–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Z, Li Y, Banerjee S and Sarkar FH:
Exploitation of the Notch signaling pathway as a novel target for
cancer therapy. Anticancer Res. 28:3621–3630. 2008.PubMed/NCBI
|