Introduction
Colorectal cancer (CRC) is the second and third most
commonly diagnosed cancer in females and males, respectively
(1). Approximately 25% of patients
with CRC have liver metastasis at initial diagnosis and an
additional 50% of patients develop metachronous liver metastasis.
Surgery is considered the only curative treatment option for
patients with resectable liver metastasis, and the five year
survival rate is approximately 35% (2).
It has been shown that chemokines play a role in
cancer progression and/or organ-specific metastasis (3–5).
Chemokine (C-X-C motif) ligand 16 (CXCL16) is a membrane-bound
chemokine that exists in a transmembrane form (TM-CXCL16) and a
soluble form (sCXCL16) cleaved by proteolytic enzymes, ADAM10 and
ADAM17 (6–11). CXCL16 is expressed in macrophages,
dendritic cells, monocytes, and B cells (6–8) as
well as in various cancer cell lines and tumor tissues (12–20).
CXCL16 has multiple biological functions. TM-CXCL16 can act as a
cell adhesion molecule for CXCR6-expressing cells and also as a
scavenger receptor for phosphatidylserine and oxidized lipoprotein
(7), whereas sCXCL16 is a
chemotactic factor for cells expressing its receptor CXCR6 such as
activated CD8 T cells, CD4 T cells, and natural killer T (NKT)
cells (21,22).
NKT cells are important regulatory immune cells
which express NK1.1 and TCR on their surface. Among mouse NKT
cells, type I NKT (iNKT) cells such as a Vα14+ NKT
express an invariant TCR containing the gene segment Vα14-Jα281
(23). Activated iNKT cells with
exogenous glycolipids presented via CD1d molecules are directly
cytotoxic to a number of tumor cell lines and play important roles
in antitumor and anti-metastatic responses (24–30).
Of note, localized iNKT cell infiltration into primary colorectal
cancer is associated with a good prognosis (31).
Mouse iNKT cells express high levels of CXCR6 and
CXCL16/CXCR6 regulate iNKT cell functions (6,22,31–35).
Recent studies have demonstrated that CXCL16 and its receptor CXCR6
are important in the homeostatic distribution of iNKT cells to the
liver (7,34,35).
In addition to regulating iNKT cell homing, CXCR6 and CXCL16 have
been shown to play a critical role in iNKT cell activation in
response to glycolipid antigens (7,35). As
CXCL16 is expressed as a transmembrane protein on the surface of
APCs (7), it is likely that CXCR6
and CXCL16 mediate costimulatory interactions between iNKT cells
and glycolipid-presenting cells that modulate activation and
effector functions (35).
Previously, we showed that primary tissues highly
expressing CXCL16 generated tumor-infiltrating lymphocytes (TIL)
and improved the prognosis of CRC patients (15). It has since been reported that
CXCL16/CXCR6 expression is involved in the metastasis of various
types of cancer (36,37). By contrast, the role of CXCL16 in
controlling the metastasis of CRC is unknown. In this study, we
investigated the involvement of CXCL16 and metastasis to the liver
by CRC cells in an experimental model.
Materials and methods
Cell culture
Colon 38 SL4 cells, a highly metastatic variant of
the mouse colon 38 colon cancer cell line, were used. This cell
line was maintained in a 1:1 mixture of Dulbecco’s modified Eagle’s
medium and Ham’s F-12 medium (DMEM/F12; Invitrogen, Carlsbad, CA,
USA) supplemented with 10% fetal calf serum (FCS), 2 mM
L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in a
humidified atmosphere of 95% air and 5% CO2 at 37°C.
Transfection of plasmid DNAs
Colon 38 SL4 cells were transfected by Nucleofector™
(Amaxa, Inc., Gaithersburg, MD, USA). Expression vectors (pcDNA
3.1(+) vector; Life Technologies Ltd., Japan) for mouse
membrane-bound CXCL16, pcDNA 3.1(+)-CXCL16 were used. DNA was
adjusted to 1 μg with empty vectors. After transfection,
CXCL16-positive colon 38 SL4 cells were selected by using the
antibiotic G148 (Invitrogen). CXCL16-stable expression cells
(SL4-CXCL16) and control cells (SL4-Mock) were maintained in
DMEM/F12 supplemented with 10% FBS and antibiotics.
Assays of cell growth
Cell growth was quantified using the cell
proliferation reagent WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt] (Dojindo, Kumamoto, Japan). SL4 cells were seeded
in 96-well microplates at 2×103 cells/well and incubated
24 h. The medium was then changed (90 μl), 10 μl of WST-8 solution
was added, and absorbance was measured at 450 nm.
Migration and adhesion assay
The migration assay was performed in Transwell cell
culture chambers (Corning Incorporated Life Sciences, Tewksbury,
MA, USA). Polyvinylpyrrolidone-free polycarbonate (PVFP) filters
(8.0 μm pore size; Nuclepore, Pleasanton, CA, USA) were pre-coated
with Matrigel (BD Biosciences) on the lower part. SL4-Mock or
SL4-CXCL16 cell suspension [1×105 cells/100 μl in
DMEM/F12 with 0.1% bovine serum albumin (BSA)] was added to the
upper compartment and incubated for 4, 8 and 12 h at 37°C. Cells
that invaded the lower surface were stained with hematoxylin and
eosin and counted under a microscope at ×400 magnification. The
adhesion assay was performed in 96-well plates. Triplicate wells
were precoated with Matrigel and after removing the Matrigel,
SL4-Mock or SL4-CXCL16 cells (1×104 cells/100 μl in
DMEM/F12) were incubated for 30 min at 37°C. Cells were aspirated
and fixed with 4% formaldehyde approximately 30 min. Adhered cells
were stained with hematoxylin for 20 min and counted under the
microscope in five predetermined fields at ×400 magnification.
Mice
C57BL/6 female and KSN/slc male 5-week-old nude mice
were purchased from Sankyo Lab Service (Hamamatsu, Japan). The
study was conducted in accordance with the standards established in
the guidelines for the Care and Use of Laboratory Animals of Toyama
University.
Experimental liver metastasis of colon 38
SL4 cells
Colon 38 SL4 cells were harvested and resuspended in
1 mM EDTA in phosphate-buffered saline (PBS). For experimental
liver metastasis, colon 38 SL4 cells were injected into the
intraportal vein. The mice were sacrificed 17 days later and the
livers were removed. The increase in tumor weight and the number of
tumor colonies in the liver were measured to evaluate tumor
metastasis.
CD8 T cell depletion model
Hybridomas producing a 53-6.72 CD8 T cell a specific
monoclonal antibody (ATCC, Manassas, VA, USA) were injected
intraperitoneally into KSN/slc mice. C57BL/6 female mice were
intraperitoneally injected with the ascites fluid of KSN/slc mice 3
days prior to and 3 and 6 days following the inoculation with
cancer cells. CD8 T cell depletion was verified by flow
cytometry.
NKT cell depletion model
di-palmitoyl-phosphatidylethanolamine
polyethyleneglycol (DPPE-PEG) which is used as a reagent of NKT
cell depletion (38), purchased
from NOF Corporation, was dissolved with saline and injected
intraperitoneally (250 μg/50 μl/mouse/day) until the mice were
sacrificed.
Real-time PCR
Total-RNA was extracted using an RNeasy Mini kit
(Qiagen, Valencia, CA, USA) according to the manufacturer’s
directions. First-strand cDNA was synthesized from an RNA template
(2 μg) using SuperScript II reverse transcriptase (Invitrogen). The
primer sequences are as follows: CD4, 5′-ACACACCTGTGCAAGAAGCA-3′
(forward) and 5′-GCT CTTGTTGGTTGGGAATC-3′ (reverse); CD8,
5′-CTCACCT GTGCACCCTACC-3′ (forward) and 5′-ATCCGGTCCCCTT CACTG-3′
(reverse); Vα14-Jα281, 5′-TGGGAGATACTCAG CAACTCTGG-3′ (forward) and
5′-CAGGTATGACAATCA GCTGAGTCC-3′ (reverse); CD3ɛ, 5′-AACACGTACTTGT
ACCTGAAAGCTC-3′ (forward) and 5′-GATGATTATGGC TACTGCTGTCA-3′
(reverse); β-actin, 5′-CTAAGGCCAACC GTGAAAAG-3′ (forward) and
5′-ACCAGAGGCATACAGG GACA-3′ (reverse). Real-time quantitative
RT-PCR was performed using a SYBR Premix Ex Taq (Takara Bio, Inc.,
Otsu, Japan) and Lightcycler nano system (Roche, Pleasanton, CA,
USA). All data were normalized to β-actin mRNA.
Lymphocyte isolation and flow
cytometry
To isolate lymphocytes from the liver, normal and
tumor tissues from the liver were dissected in 20% RPMI-1640
medium. Samples were then further homogenized through wire mesh and
mononuclear cells were isolated using a 30% Percoll gradient (GE
Healthcare, Little Chalfont, UK) 5 ml, 10X PBS 365 μl, 7%
NaHCO3 (Meylon) 65 μl, Heparin 50 μl, and RPMI-1640 10
ml/mouse. Red blood cells were lysed by lysis buffer
(NH4Cl solution: 155 mM NH4Cl, 100 mM
KHCO3 and 1 mM EDTA-2Na to 1 L with DW, Tris-HCl
solution: Trizma 20.6 g was dissolved in 900 ml DW, adjusted pH 7.6
with concentrated HCl, diluted to 1 L with DW mix 900 ml
NH4Cl solution and 100 ml Tris-HCl solution, autoclaved,
stored at 4°C). To isolate lymphocytes from the spleen, the Percoll
gradient was used. For flow cytometry, lymphocytes were first
pre-incubated with CD16/32 (2.4G2) mAb and then incubated with a
saturating amount of mAb. Antibodies to CD3ɛ (145-2C11), NK1.1
(PK136), and CD1d (RA3-6B2) were purchased from eBioscience (San
Diego, CA, USA). The samples were analyzed with the FACSCanto II
system (BD Biosciences).
Statistical analysis
Data were analyzed for statistical significance
using the Student’s t-test. P-values <0.05 were considered to
indicate statistically significant differences. The mean and SD
were calculated for all variables.
Results
Stable expression of mouse CXCL16 in
mouse colon cancer cells
We initially intended to obtain CXCL16
stable-expression colon 38 SL4 cells (SL4-CXCL16) by the antibiotic
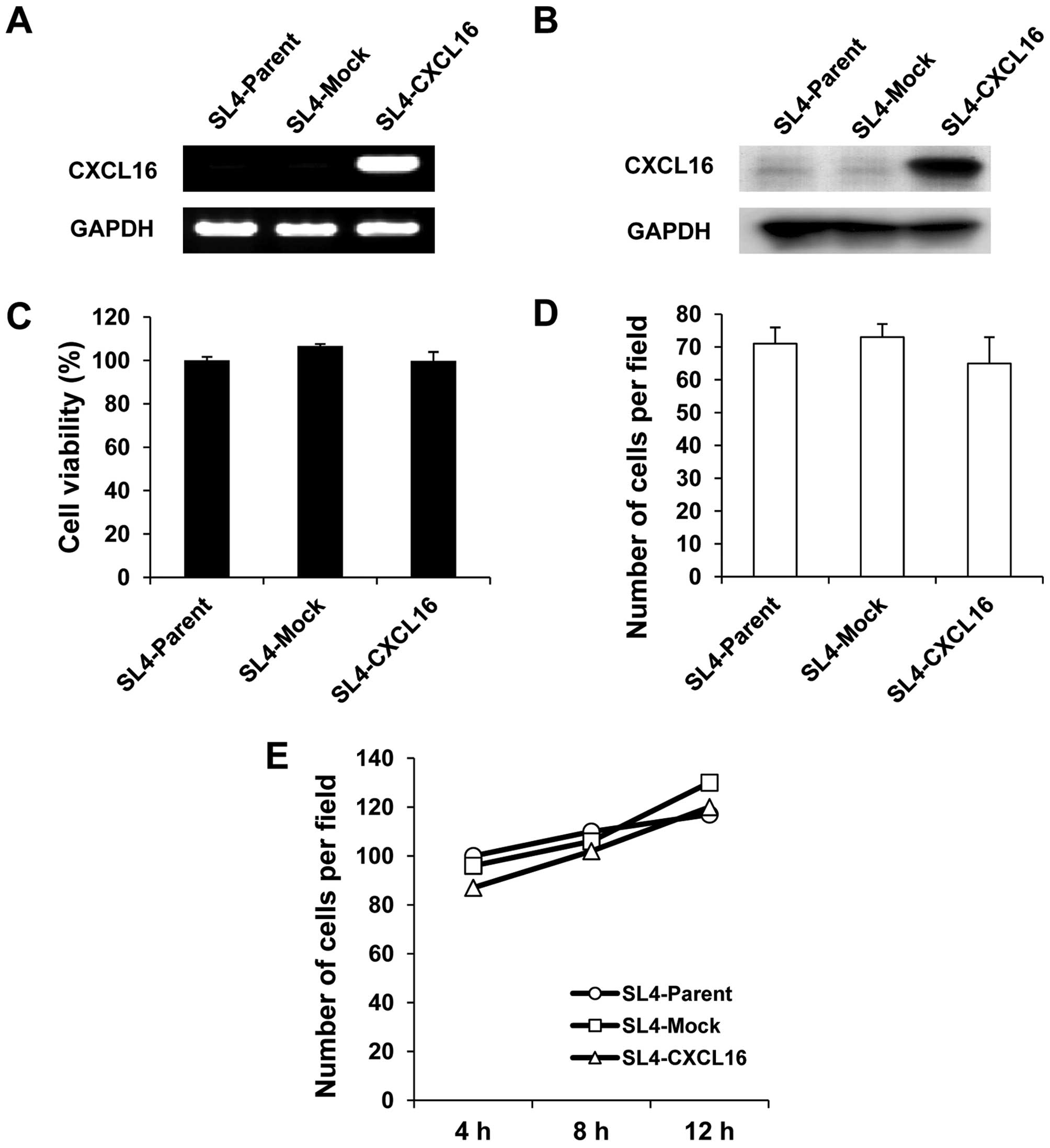
selection. Higher CXCL16 expression in SL4-CXCL16 than SL4-Mock
cells was confirmed by RT-PCR and western blotting (Fig. 1A and B). Gene introduction into
cells often causes various property changes, compared with control
cells. Compared with SL4-Mock cells, there was no significant
difference in cell growth (Fig.
1C), migration (Fig. 1D) and
adhesion (Fig. 1E) in SL4-CXCL16
cells.
Liver metastasis is inhibited by
CXCL16-expressing CRC cells
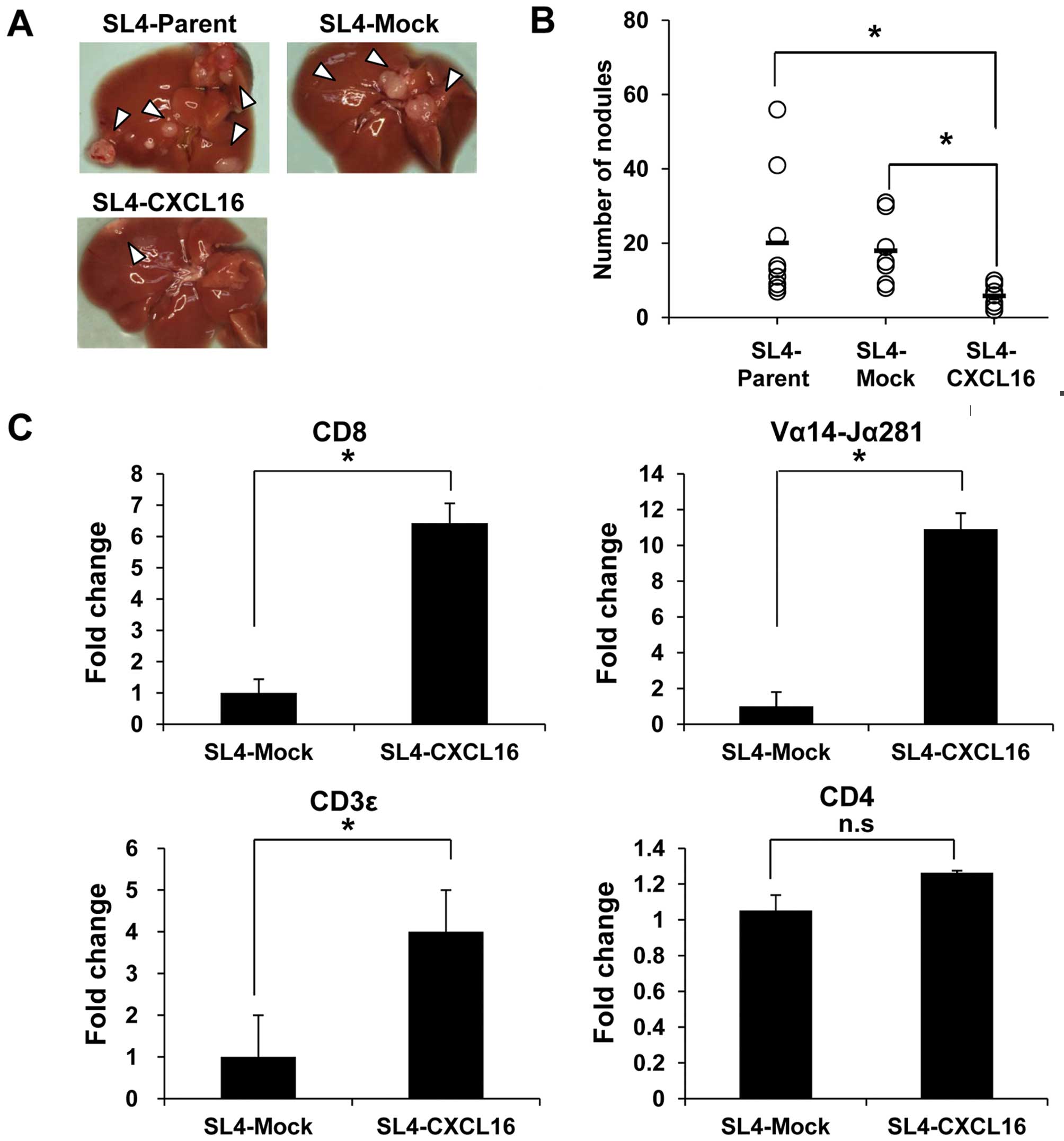
Colon 38 SL4 cells (Parent, Mock and CXCL16) were
injected into the intraportal vein to confirm the effect of CXCL16
expression on liver metastasis. Mice were sacrificed on Day 17 and
liver metastasis was evaluated by counting nodules. Metastasis into
the liver was significantly decreased by SL4-CXCL16 cells (Fig. 2A). Also, the number of nodules was
significantly decreased in CXCL16-expressing tumor tissue (Fig. 2B). Since CXCL16 acts as a
chemoattractant as well as a cell adhesion molecule for
CXCR6-expressing cells (21,22),
we conducted a real-time PCR analysis with tumor tissues to confirm
which immune cells were recruited to the liver by CXCL16. Among the
CXCR6-expressing cells, mRNA levels of CD8 T cell (CD8), NKT cell
(Vα14-Jα281) and T cell (CD3ɛ) markers, but not CD4 T cell marker
(CD4), were increased 6-, 10- and 4-fold compared with SL4-Mock
(Fig. 2C). These results suggested
that CXCL16 expression in metastatic tissues recruited
CXCR6-expressing cells and then inhibited metastasis to the
liver.
Inhibition of liver metastasis by CXCL16
expression is recovered in nude mice
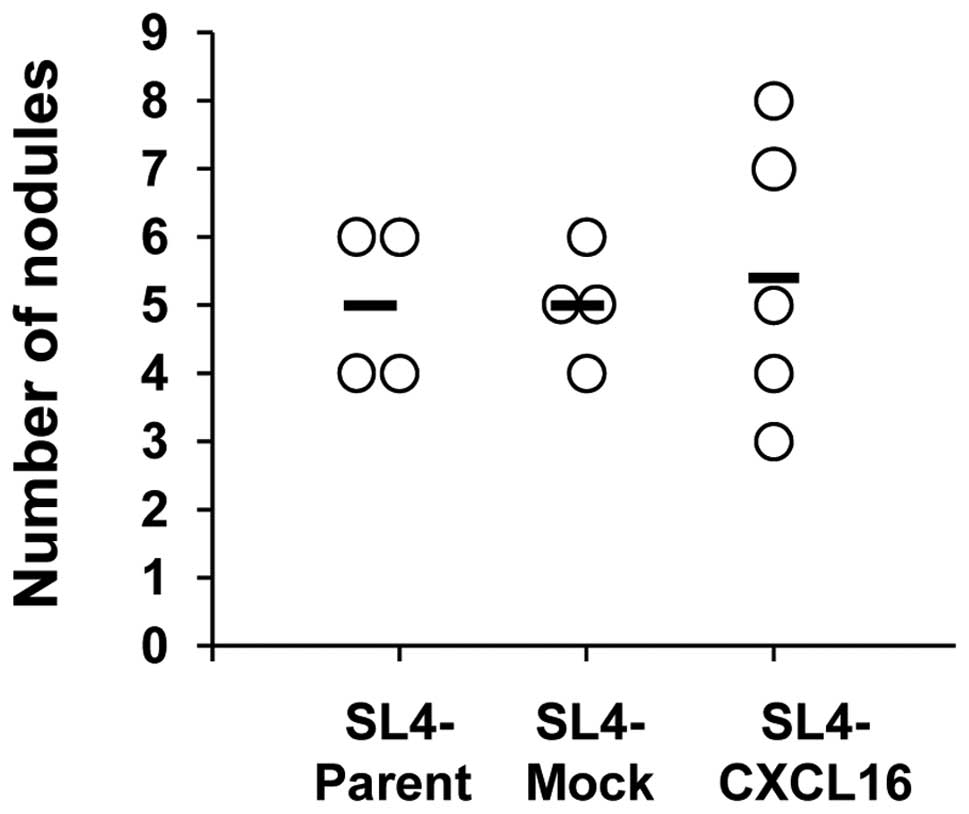
To confirm that CXCR6-expressing T cells are
involved in liver metastasis by CXCL16- expressing CRC cells, we
performed intraportal vein (i.p.v.) injections of SL4 cells
(7.5×104 cells/200 μl PBS/mouse) into nude mice (T
cell-deficient mice). Metastasis to the liver did not differ
significantly in the SL4-CXCL16 group (Fig. 3). Inhibition of metastasis to the
liver by CXCL16 expression was not observed in T cell-deficient
mice. These results indicate that T cells mediated the inhibition
of liver metastasis by CXCL16.
CD8 T cells are not related to inhibition
of liver metastasis by CXCL16
Activated CD8 lymphocytes were recruited to inflamed
liver in a mouse model and human patients (39,40).
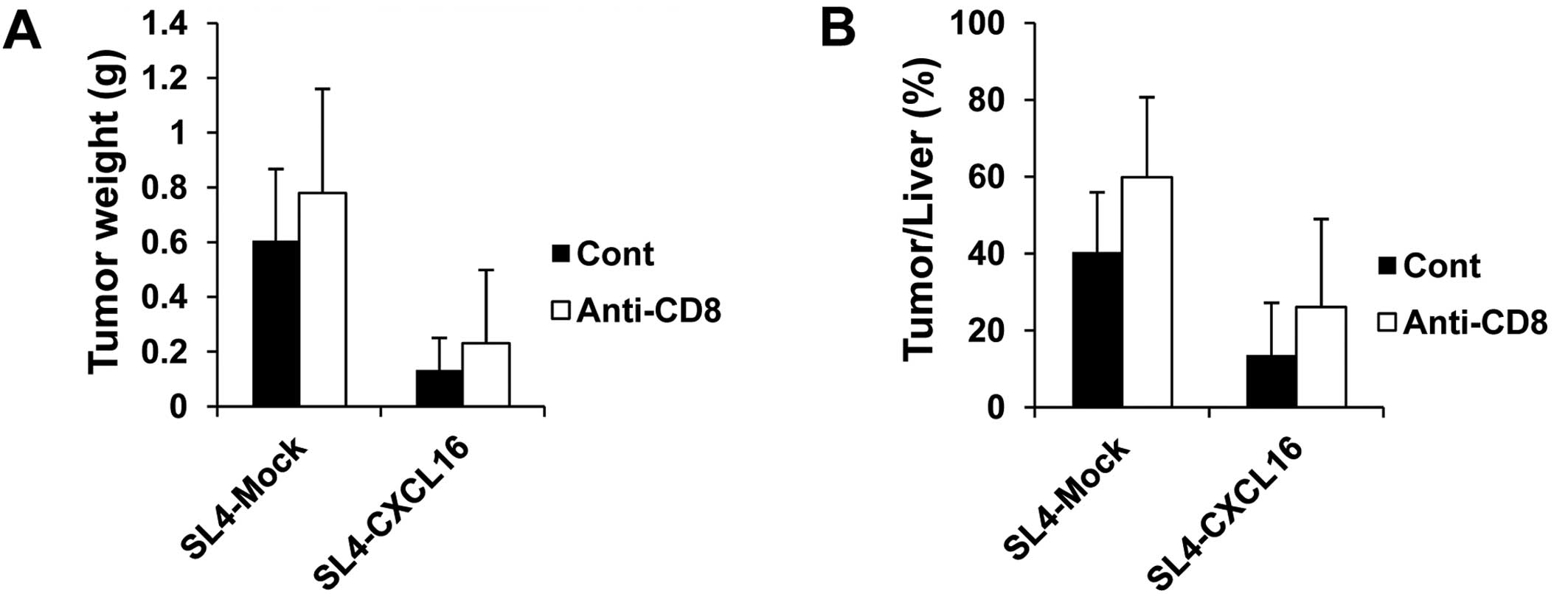
Thus, we examined the role of CD8 T cells in the inhibition of
metastasis by CXCL16 in CD8 T cell-depleted mice. To analyze
lymphocytes in tumor tissues, we injected SL4-Mock and SL4-CXCL16
cells (1.5×105 cells/200 μl PBS/mouse) and sacrificed
the animals at 17 days. In the CD8 T cell-depleted group, liver
metastasis was decreased by CXCL16 expression the same as in the
control group. Tumor weight and tumor/liver weight percentage were
not changed in the CD8 T cell-depleted mice (Fig. 4). These results suggested that CD8 T
cells were not related to the inhibition of liver metastasis by
CXCL16.
Inhibition of liver metastasis by CXCL16
is recovered in NKT cell-depleted mice
NKT cells present in the liver and the activation of
NKT cells regulate tumor surveillance in the liver microenvironment
and suppresses liver metastasis (24–30).
Thus, we examined whether NKT cells are involved in the inhibition
of metastasis by CXCL16. We injected SL4-Mock and SL4-CXCL16 cells
(1.5×105 cells/200 μl PBS/mouse) into C57BL/6 mice and
DPPE-PEG-treated mice. DPPE-PEG has been reported to be used to
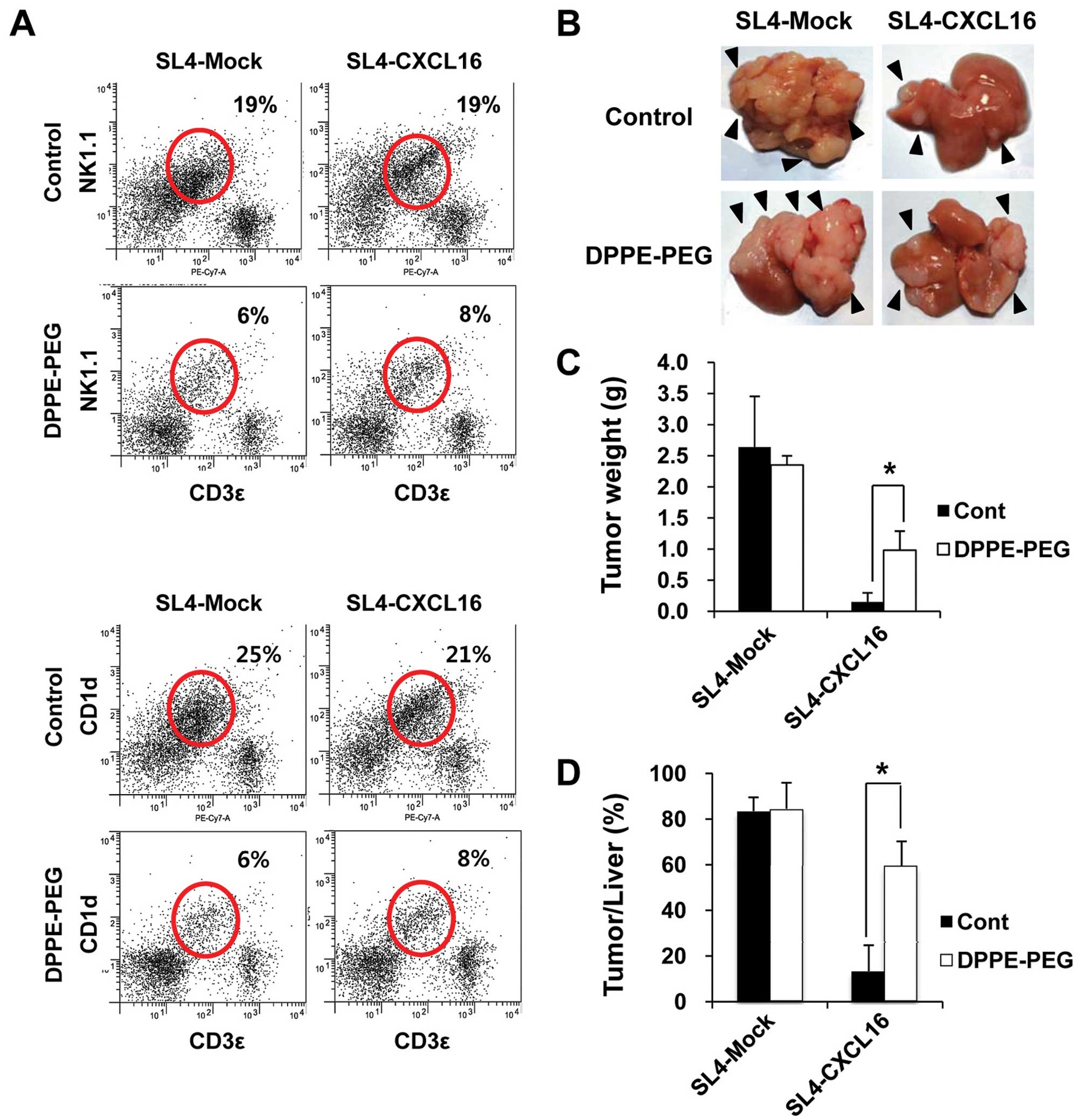
inhibit the action of NKT cells (38). The expression of NK1.1 and CD1d in
metastatic tumor tissues was measured by FACS analysis. Thus, we
used DPPE-PEG as a NKT cell-specific antagonist. As expected,
levels of NK1.1 and CD1d were decreased by the DPPE-PEG treatment
(Fig. 5A). In addition, the
inhibition of liver metastasis by CXCL16 recovered in NKT
cell-depleted mice (Fig. 5B). Tumor
weight and tumor/liver weight percentage were significantly
recovered in NKT cell-depleted mice (Fig. 5C and D). These results indicate that
NKT cells are necessary for the inhibition of liver metastasis by
CXCL16-expressing cancer cells.
Discussion
Previously, we reported a relationship between the
expression of CXCL16 and prognosis of CRC patients, therefore
CXCL16 is expected to be a new biomarker for CRC (15). From our report, a recent study found
CXCL16 and its receptor CXCR6 to be related to various types of
cancer, such as prostate cancer and epithelial ovarian carcinoma,
respectively (36,37). However, there is no appropriate
treatment for liver metastasis. Therefore, we determined the
relationship between CXCL16 and the liver metastasis of CRC
cells.
In this study, we investigated the role of
cancer-derived CXCL16 and the possibility of gene therapy using
CXCL16. We established cells stably overexpressing CXCL16 using the
mouse colon 38 SL4 cell line and inoculated SL4-Mock and SL4-CXCL16
cells into the intraportal vein to confirm the effect of CXCL16
expression on liver metastasis. Liver metastasis of CRC cells was
decreased by CXCL16 in tumor tissues (Fig. 2A and B). We carried out a microarray
assay to confirm the difference between WT and CXCL16-expressing
cells. The microarray data showed that there was no significant
difference in metastasis-related factors (data not shown).
Therefore, we focused on organ-specific immune cells which related
to CXCL16. CXCR6, a specific receptor of CXCL16, is expressed in
CD4 T cells, CD8 T cells and NKT cells. Real-time PCR data
suggested that CD8 T cells and NKT cells were generated in
CXCL16-expressing tumor tissues (Fig.
2C).
Since activated CD8 lymphocytes were recruited to
inflamed liver in a mouse model and human patients (39,40),
we focused on the involvement of CD8 T cells. Thus, we examined the
effect of CXCL16 on liver metastasis in CD8 T cell-depleted mice,
however, there was no difference from the CD8 T cell-depleted group
(Fig. 4). This is probably because
among the CD8 T cells, CD8+ Treg cells may be produced
and suppress the proliferation and function of effector T cells
(41).
Nevertheless, NKT cells make up approximately 1–2%
of the lymphocytes in the spleen and account for approximately 30%
of lymphocytes in the liver and these NKT cells express high levels
of CXCR6. The CXCL16 and CXCR6 axis has been reported to act as a
regulator of NKT cell functions such as inhibition of cancer and
metastasis (21,34,35).
It was reported that neutralization of CXCL16 enhanced metastasis
to the liver by B16 melanoma cells, but not lung metastasis
(28). These reports suggested that
CXCL16 and CXCR6-expressing NKT cells are involved in metastasis to
the liver. Therefore, we examined the effect of CXCL16 on the liver
metastasis of CRC cells in NKT cell-depleted mice.
Liver metastasis in the control SL4-CXCL16 group was
increased by NKT cell depletion (Fig.
5). However, this is an unsettled issue that remains to be
clarified in future studies. Markedly, liver metastasis was not
increased in the SL4-Mock group, whereas NKT cells were depleted by
DPPE-PEG. α-GalCer (NKT cell activator) treatment did not reduce
the progression of tumors, as Th1 response by NKT cells was reduced
in CXCL16−/− mice (28).
It is possible that NKT cells, recruited by CXCL16, are CXCR6
positive and invariant NKT cells which has cytotoxicity to the
tumor cells, conversely immature NKT cells did not effect to the
metastasis. Further studies are needed to investigate the
population and functions of NKT cells augmented by CXCL16-positive
CRC compared with CXCL16-negative CRC in the experimental model and
clinical samples.
Overexpression of chemokines in cancer can be used
to recruit immune cells for antitumor responses and cytotoxicity to
cancer cells. It has been reported that CCL21 and CX3CL1
overexpression reduced colon adenocarcinoma growth and inhibited
primary cancer and metastasis of neuroblastoma, respectively.
However, these results are conducted in an experimental model
(42). Reasonable evidence exists
that CXCL16 shows the possibility of application in antitumor
therapy, as we reported that spontaneous CXCL16 expression is
related to the pathology of CRC in clinical cases (15), not only in an experimental
model.
Herein we reported that cancer-derived CXCL16
suppresses the liver metastasis of CRC cells by augmenting NKT
cells to the metastatic organ. Therefore, our findings indicate
that a cancer gene therapy strategy supporting the accumulation of
NKT cells by CXCL16, might be an effective therapeutic approach
against liver metastasis in CRC.
Acknowledgements
This study was supported by a Grant-in-Aid for
Scientific Research (C) (no. 22501042).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Timmerman RD, Bizekis CS, Pass HI, Fong Y,
Dupuy DE, Dawson LA and Lu D: Local surgical, ablative, and
radiation treatment of metastases. CA Cancer J Clin. 59:145–170.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zlotnik A, Burkhardt AM and Homey B:
Homeostatic chemokine receptors and organ-specific metastasis. Nat
Rev Immunol. 11:597–606. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koizumi K, Hojo S, Akashi T, Yasumoto K
and Saiki I: Chemokine receptors in cancer metastasis and cancer
cell-derived chemokines in host immune response. Cancer Sci.
98:1652–1658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koizumi K, Kato S, Sakurai H, Hashimoto I,
Yasumoto K and Saiki I: Therapeutics target of CXCR4 and its
downstream in peritoneal carcinomatosis of gastric cancer. Front
Biosci (Schol Ed). 4:269–276. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matloubian M, David A, Engel S, Ryan JE
and Cyster JG: A transmembrane CXC chemokine is a ligand for HIV
coreceptor Bonzo. Nat Immunol. 1:298–304. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimaoka T, Kume N, Minami M, Hayashida K,
Kataoka H, Kita T and Yonehara S: Molecular cloning of a novel
scavenger receptor for oxidized low density lipoprotein, SR-PSOX,
on macrophages. J Biol Chem. 275:40663–40666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilbanks A, Zondlo SC, Murphy K, Mak S,
Soler D, Langdon P, Andrew DP, Wu L and Briskin M: Expression
cloning of the STRL33/BONZO/TYMSTR ligand reveals elements of CC,
CXC, and CX3C chemokines. J Immunol. 166:5145–5154. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abel S, Hundhausen C, Mentlein R, Schulte
A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S,
Muetze B, Schuster B, Kallen KJ, Saftig P, Rose-John S and Ludwig
A: The transmembrane CXC-chemokine ligand 16 is induced by
IFN-gamma and TNF-alpha and shed by the activity of the
disintegrin-like metalloproteinase ADAM10. J Immunol.
172:6362–6372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gough PJ, Garton KJ, Wille PT, Rychlewski
M, Dempsey PJ and Raines EW: A disintegrin and metalloproteinase
10-mediated cleavage and shedding regulates the cell surface
expression of CXC chemokine ligand 16. J Immunol. 172:3678–3685.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ludwig A, Hundhausen C, Lambert MH,
Broadway N, Andrews RC, Bickett DM, Leesnitzer MA and Becherer JD:
Metalloproteinase inhibitors for the disintegrin-like
metalloproteinases ADAM10 and ADAM17 that differentially block
constitutive and phorbol ester-inducible shedding of cell surface
molecules. Comb Chem High Throughput Screen. 8:161–171. 2005.
View Article : Google Scholar
|
|
12
|
Gutwein P, Schramme A, Sinke N,
Abdel-Bakky MS, Voss B, Obermuller N, Doberstein K, Koziolek M,
Fritzsche F, Johannsen M, Jung K, Schaider H, Altevogt P, Ludwig A,
Pfeilschifter J and Kristiansen G: Tumoural CXCL16 expression is a
novel prognostic marker of longer survival times in renal cell
cancer patients. Eur J Cancer. 10:1016–1023. 2008.PubMed/NCBI
|
|
13
|
Lu Y, Wang J, Xu Y, Koch AE, Cai Z, Chen
X, Galson DL, Taichman RS and Zhang J: CXCL16 functions as a novel
chemotactic factor for prostate cancer cells in vitro. Mol Cancer
Res. 6:546–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Darash-Yahana M, Gillespie JW, Hewitt SM,
Chen YY, Maeda S, Stein I, Singh SP, Bedolla RB, Peled A, Troyer
DA, Pikarsky E, Karin M and Farber JM: The chemokine CXCL16 and its
receptor, CXCR6, as markers and promoters of
inflammation-associated cancers. Plos One. 4:e66952009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hojo S, Koizumi K, Tsuneyama K, Arita Y,
Cui Z, Shinohara K, Minami T, Hashimoto I, Nakayama T, Sakurai H,
Takano Y, Yoshie O, Tsukada K and Saiki I: High-level expression of
chemokine CXCL16 by tumor cells correlates with a good prognosis
and increased tumor-infiltrating lymphocytes in colorectal cancer.
Cancer Res. 67:4725–4731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wågsäter D, Hugander A and Dimberg J:
Expression of CXCL16 in human rectal cancer. Int J Mol Med.
14:65–69. 2004.
|
|
17
|
Wente MN, Gaida MM, Mayer C, Michalski CW,
Haag N, Giese T, Felix K, Bergmann F, Giese NA and Friess H:
Expression and potential function of the CXC chemokine CXCL16 in
pancreatic ductal adenocarcinoma. Int J Oncol. 33:297–308.
2008.PubMed/NCBI
|
|
18
|
Ou DL, Chen CL, Lin SB, Hsu CH and Lin LI:
Chemokine receptor expression profiles in nasopharyngeal carcinoma
and their association with metastasis and radiotherapy. J Pathol.
210:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Held-Feindt J, Rehmke B, Mentlein R,
Hattermann K, Knerlich F, Hugo HH, Ludwig A and Mehdorn HM:
Overexpression of CXCL16 and its receptor CXCR6/Bonzo promotes
growth of human schwannomas. Glia. 56:764–774. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seidl H, Richtig E, Tilz H, Stefan M,
Schmidbauer U, Asslaber M, Zatloukal K, Herlyn M and Schaider H:
Profiles of chemokine receptors in melanocytic lesions: de novo
expression of CXCR6 in melanoma. Human Pathol. 38:768–780. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim CH, Kunkel EJ, Boisvert J, Johnston B,
Campbell JJ, Genovese MC, Greenberg HB and Butcher EC: Bonzo/CXCR6
expression defines type 1-polarized T-cell subsets with
extralymphoid tissue homing potential. J Clin Invest. 107:595–601.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim CH, Johnston B and Butcher EC:
Trafficking machinery of NKT cells: shared and differential
chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT
cell subsets with distinct cytokine-producing capacity. Blood.
100:11–16. 2002.PubMed/NCBI
|
|
23
|
Taniguchi M, Harada M, Kojo S, Nakayama T
and Wakao H: The regulatory role of Valpha14 NKT cells in innate
and acquired immune response. Annu Rev Immunol. 21:483–513. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui J, Shin T, Kawano T, Sato H, Kondo E,
Toura I, Kaneko Y, Koseki H, Kanno M and Taniguchi M: Requirement
for Valpha14 NKT cells in IL-12-mediated rejection of tumors.
Science. 278:1623–1626. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawano T, Cui J, Koezuka Y, Toura I,
Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y
and Taniguchi M: Natural killer-like nonspecific tumor cell lysis
mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl
Acad Sci USA. 95:5690–5693. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toura I, Kawano T, Akutsu Y, Nakayama T,
Ochiai T and Taniguchi M: Cutting edge: inhibition of experimental
tumor metastasis by dendritic cells pulsed with
alpha-galactosylceramide. J Immunol. 163:2387–2391. 1999.PubMed/NCBI
|
|
27
|
Smyth MJ, Thia KY, Street SE, Cretney E,
Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY and Godfrey
DI: Differential tumor surveillance by natural killer (NK) and NKT
cells. J Exp Med. 191:661–668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cullen R, Germanov E, Shimaoka T and
Johnston B: Enhanced tumor metastasis in response to blockade of
the chemokine receptor CXCR6 is overcome by NKT cell activation. J
Immunol. 183:5807–5815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa R, Nagafune I, Tazunoki Y, Ehara
H, Tomura H, Iijima R, Motoki K, Kamishohara M and Seki S:
Mechanisms of the antimetastatic effect in the liver and of the
hepatocyte injury induced by alpha-galactosylceramide in mice. J
Immunol. 166:6578–6584. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smyth MJ, Crowe NY, Pellicci DG,
Kyparissoudis K, Kelly JM, Takeda K, Yagita H and Godfrey DI:
Sequential production of interferon-gamma by NK1.1(+) T cells and
natural killer cells is essential for the antimetastatic effect of
alpha-galactosylceramide. Blood. 99:1259–1266. 2002.
|
|
31
|
Tachibana T, Onodera H, Tsuruyama T, Mori
A, Nagayama S, Hiai H and Imamura M: Increased intratumor
Valpha24-positive natural killer T cells: a prognostic factor for
primary colorectal carcinomas. Clin Cancer Res. 11:7322–7327. 2005.
View Article : Google Scholar
|
|
32
|
Thomas SY, Hou R, Boyson JE, Means TK,
Hess C, Olson DP, Strominger JL, Brenner MB, Gumperz JE, Wilson SB
and Luster AD: CD1d-restricted NKT cells express a chemokine
receptor profile indicative of Th1-type inflammatory homing cells.
J Immunol. 171:2571–2580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnston B, Kim CH, Soler D, Emoto M and
Butcher EC: Differential chemokine responses and homing patterns of
murine TCR alpha beta NKT cell subsets. J Immunol. 171:2960–2969.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Geissmann F, Cameron TO, Sidobre S,
Manlongat N, Kronenberg M, Briskin MJ, Dustin ML and Littman DR:
Intravascular immune surveillance by CXCR6+ NKT cells
patrolling liver sinusoids. PLoS Biol. 3:e1132005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Germanov E, Veinotte L, Cullen R,
Chamberlain E, Butcher EC and Johnston B: Critical role for the
chemokine receptor CXCR6 in homeostasis and activation of
CD1d-restricted NKT cells. J Immunol. 181:81–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ha HK, Lee W, Park HJ, Lee SD, Lee JZ and
Chung MK: Clinical significance of CXCL16/CXCR6 expression in
patients with prostate cancer. Mol Med Rep. 4:419–424.
2011.PubMed/NCBI
|
|
37
|
Guo L, Cui ZM, Zhang J and Huang Y:
Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph
node metastasis in epithelial ovarian carcinoma. Chin J Cancer.
30:336–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lombardi V, Stock P, Singh AK, Kerzerho J,
Yang W, Sullivan BA, Li X, Shiratsuchi T, Hnatiuk NE, Howell AR, Yu
KO, Porcelli SA, Tsuji M, Kronenberg M, Wilson SB and Akbari O: A
CD1d-dependent antagonist inhibits the activation of invariant NKT
cells and prevents development of allergen-induced airway
hyperreactivity. J Immunol. 184:2107–2115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sato T, Thorlacius H, Johnston B, Staton
TL, Xiang W, Littman DR and Butcher EC: Role for CXCR6 in
recruitment of activated CD8+ lymphocytes to inflamed
liver. J Immunol. 174:277–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heydtmann M, Lalor PF, Eksteen JA,
Hubscher SG, Briskin M and Adams DH: CXC chemokine ligand 16
promotes integrin-mediated adhesion of liver-infiltrating
lymphocytes to cholangiocytes and hepatocytes within the inflamed
human liver. J Immunol. 174:1055–1062. 2005. View Article : Google Scholar
|
|
41
|
Wang RF: CD8+ regulatory T
cells, their suppressive mechanisms, and regulation in cancer. Hum
Immunol. 69:811–814. 2008.
|
|
42
|
Slettenaar VI and Wilson JL: The chemokine
network: a target in cancer biology? Adv Drug Deliv Rev.
58:962–974. 2006. View Article : Google Scholar : PubMed/NCBI
|