Introduction
The incidence of malignant melanoma is increasing
worldwide (1). Although melanoma
accounts for only 4% of skin cancer cases (2), it accounts for 79% of skin
cancer-related deaths (3). Melanoma
can be cured if excised at an early stage. However, once tumors
have disseminated to distant organs, systemic chemotherapy induces
a complete response in less than 2% of cases and the median
survival time is 9 months (4).
Novel therapies were recently approved for advanced melanoma by the
US Food and Drug Administration (5), including vemurafenib, a BRAF
inhibitor, for patients positive for the BRAFV600E mutation, and
ipilimumab, an inhibitor of CTLA-4 (cytotoxic T
lymphocyte-associated antigen 4), that indirectly activates T-cell
mediated antitumor immune responses (6). Furthermore, several drugs targeting
specific oncogenes or signaling pathways, including cKIT, NRAS,
PI3K and MITF, are now in clinical development (7). Further studies aimed at developing
novel therapeutic approaches for melanomas are expected to improve
the prognosis of this disease.
Nestin, a class VI intermediate filament protein,
was originally described as a neural stem cell marker expressed
during the development of the central nervous system (8). Nestin is expressed throughout the
dermis in the early embryo, but it is subsequently restricted to
the follicular connective tissue sheaths later in development and
to hair follicles after birth (9).
Nestin-positive hair follicle cells located just above the bulge
area can differentiate into various cell types during wound healing
(9,10) and can regulate the
neovascularization of the dermis in association with the hair
growth cycle (11). Thus,
nestin-positive cells have been considered to be stem cells of the
follicular mesenchyme and nestin has been shown to have an
important regulatory role in dermal homeostasis and cutaneous
neovasculogenesis (9).
Nestin has also been reported to be present in
various neoplasms, including pancreatic cancer (12,13),
prostate cancer (14), breast
cancer (15), glioblastomas
(16), gastrointestinal stromal
tumors (17), trichoblastoma
(18), trichilemmoma (19), squamous cell carcinoma of the skin
(20), angiosarcoma (17), dermatofibrosarcoma (9,21) and
malignant melanomas (22). A number
of studies have shown that nestin expression in human melanomas
correlates with a poor prognosis (17,23–26).
Nestin has been reported to be overexpressed in advanced stages of
melanoma (25), at the invading
front (24) and at sites of
melanoma metastases (22,23,27).
Furthermore, nestin- and CD133-positive circulating melanoma cells
were detected in the peripheral blood of patients with
advanced-stage melanoma (28) and
the number of nestin-positive circulating melanoma cells was
associated with short overall survival time (29). These data indicate that nestin may
be significantly involved in the invasion and distant metastasis of
melanomas.
We hypothesized that nestin could be a marker of
melanocyte immaturity and a novel therapeutic target for melanoma.
In this study, we examined nestin expression in melanocytic nevi
and at different stages of malignant melanoma in order to evaluate
its potential as a marker of melanocytic neoplasms and as a
therapeutic target for malignant melanomas.
Materials and methods
Materials
The following reagents were used for
immunohistochemistry: mouse monoclonal anti-nestin antibody from
R&D Systems, Inc. (Westerville, OH, USA); Histofine Simple
Stain MAX PO (M) kits from Nichirei (Tokyo, Japan); and New Silane
II and the malinol mounting medium from Mutoh Chemical Co. (Tokyo,
Japan). All other chemicals and reagents were purchased from
Sigma-Aldrich Corp. (St. Louis, MO, USA).
Patients and tissues
For this study, we used tissues from patients (nevi,
N=53; malignant melanomas, N=17) who received treatment at the
Nippon Medical School Hospital (Bunkyo-ku, Tokyo, Japan) between
2004 and 2012. The melanoma patients comprised 10 men and 7 women
whose median age was 68 years (range 42–84 years). The nevus
patients comprised 13 men and 40 women whose median age was 37
years (range 14–71 years). The clinicopathological stage was
determined according to the TNM classification system (30). This study was carried out in
accordance with the Declaration of Helsinki 2008, and informed
consent for the use of melanoma and nevus tissues was obtained from
all patients.
Immunohistochemistry
Paraffin-embedded tissue sections (3 μm) were
immunostained using Histofine Simple Stain MAX PO (M) kits.
Following deparaffinization, endogenous peroxidase activity was
blocked by incubating sections with 0.3% hydrogen peroxide in
methanol for 30 min. Sections were then incubated overnight at 4°C
in the absence (negative controls) or presence of monoclonal
anti-nestin antibody (diluted 1:200). Bound antibodies were
detected with Histofine kits, using
diaminobenzidine-tetrahydrochloride as a chromogen.
Immunohistochemically stained tissues were considered positive for
nestin expression when staining was noted in the cytoplasm of
>10% of the cells, regardless of the intensity of staining
(13). Two investigators (Michiko
Akiyama and Yoko Matsuda) separately evaluated all the specimens in
a blinded manner.
In situ hybridization
A 235-bp BamHI-EcoRI cDNA fragment,
corresponding to nucleotides 1045–1227 of the human nestin cDNA
sequence, was subcloned into the pGEM-T vector and the presence of
the insert was confirmed by sequencing. Probes were labeled with
DIG-UTP using SP6 or T7 RNA polymerase and the DIG RNA-labeling
kit. In situ hybridization was carried out as previously
described (13). Tissue sections
were deparaffinized and incubated with 0.2 M HCl for 20 min at room
temperature (RT) and then with 100 μg/ml proteinase K for 15 min at
37°C. The sections were postfixed in phosphate-buffered saline
(PBS) containing 4% paraformaldehyde for 5 min, then incubated
twice with PBS containing 2 mg/ml glycine for 15 min each and then
incubated once with 2X standard saline citrate (SSC) containing 50%
formamide for 1 h, prior to the initiation of the hybridization
reaction. Hybridization was carried out in a moist chamber for 16 h
at 42°C. The sections were then washed sequentially with 2X SSC for
20 min at 42°C and with 0.2X SSC for 20 min at 42°C. The DIG
nucleic acid detection kit was used for immunological detection.
The sections were washed briefly with buffer 1 (100 mM Tris-HCl and
150 mM NaCl, pH 7.5), incubated with 1% (w/v) blocking reagent in
buffer 1 for 1 h at RT and then incubated with alkaline
phosphatase-conjugated polyclonal sheep anti-DIG Fab fragment at a
1:2000 dilution for 1 h at RT. The sections were then washed 3
times with buffer 1 containing 0.2% Tween-20 for 15 min at RT,
equilibrated in buffer 3 (100 mM Tris-HCl, 100 mM NaCl and 50 mM
MgCl2, pH 9.5) for 2 min and incubated with the staining
solution containing nitroblue tetrazolium and X-phosphate in a dark
box for 2–3 h. The reaction was stopped by the addition of
Tris-EDTA buffer (10 mM Tris-HCl and 1 mM EDTA, pH 8.0) and the
sections were mounted in an aqueous mounting medium.
Statistical analysis
All quantitative data are presented as the means ±
SEM. The association of nestin expression levels and
clinicopathological features was assessed by the χ2
test. Data for 2 groups were compared using the Student’s t-test.
P<0.05 was considered to indicate statistically significant
differences. Computations were performed using the StatView J
version 5.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Immunohistochemical analysis of nestin in
nevi
Immunohistochemical analysis was used to examine the
localization of nestin expression in nevi (N=53). We divided these
nevi into 2 groups: junctional, nevus cells located within the
epidermis; and compound, nevus cells in the dermis or in both the
dermis and epidermis. The junctional nevi consisted of Clark type
and the compound nevi consisted of Unna, Miescher and Clark type
nevi according to the classification by Ackerman and Magana-Garcia
(31). Expression of nestin was
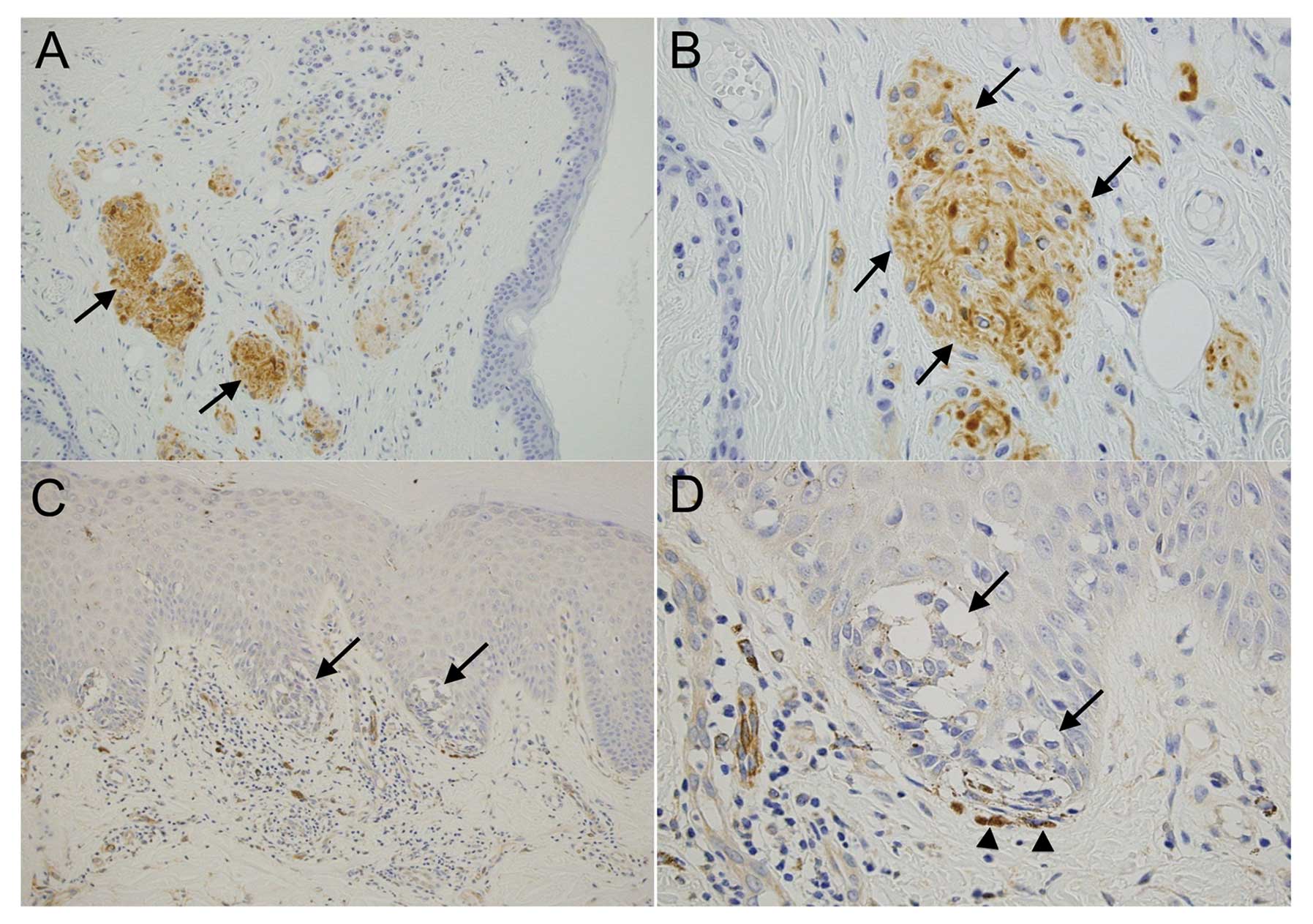
detected in all the compound nevi (N=27, 100%) and was particularly
high in the nevus nest, which showed neural differentiation
(Fig. 1A and B), whereas only 4/26
junctional nevi were positive for nestin (15.4%) (Fig. 1C and D). The χ2 test
showed that difference in the percentage of nestin-positive nevi
was statistically significant between the compound and junctional
types (P<0.05) (Table I). Thus,
nevus cells in the epidermal or superficial dermal area tended to
be nestin-negative, whereas nevus cells in the deep dermis were
nestin-positive.
 | Table ISummary of the immunohistochemical
analysis of nestin expression in nevi and melanomas. |
Table I
Summary of the immunohistochemical
analysis of nestin expression in nevi and melanomas.
| No. | Nestin (+) | Nestin (−) | Percentage of nestin
(+) cases |
|---|
| Nevus |
| Total | 53 | 31 | 22 | 58.5 |
| Junctional | 26 | 4 | 22 | 15.4 |
| Compound | 27 | 27a | 0 | 100 |
| Melanoma |
| Total | 17 | 14 | 3 | 82.4 |
| Tis, T1, T2 | 6 | 3 | 3 | 50 |
| T3, T4 | 11 | 11a | 0 | 100 |
Immunohistochemical analysis of nestin in
malignant melanoma
Immunohistochemical analysis was performed to
examine the localization of nestin expression in malignant
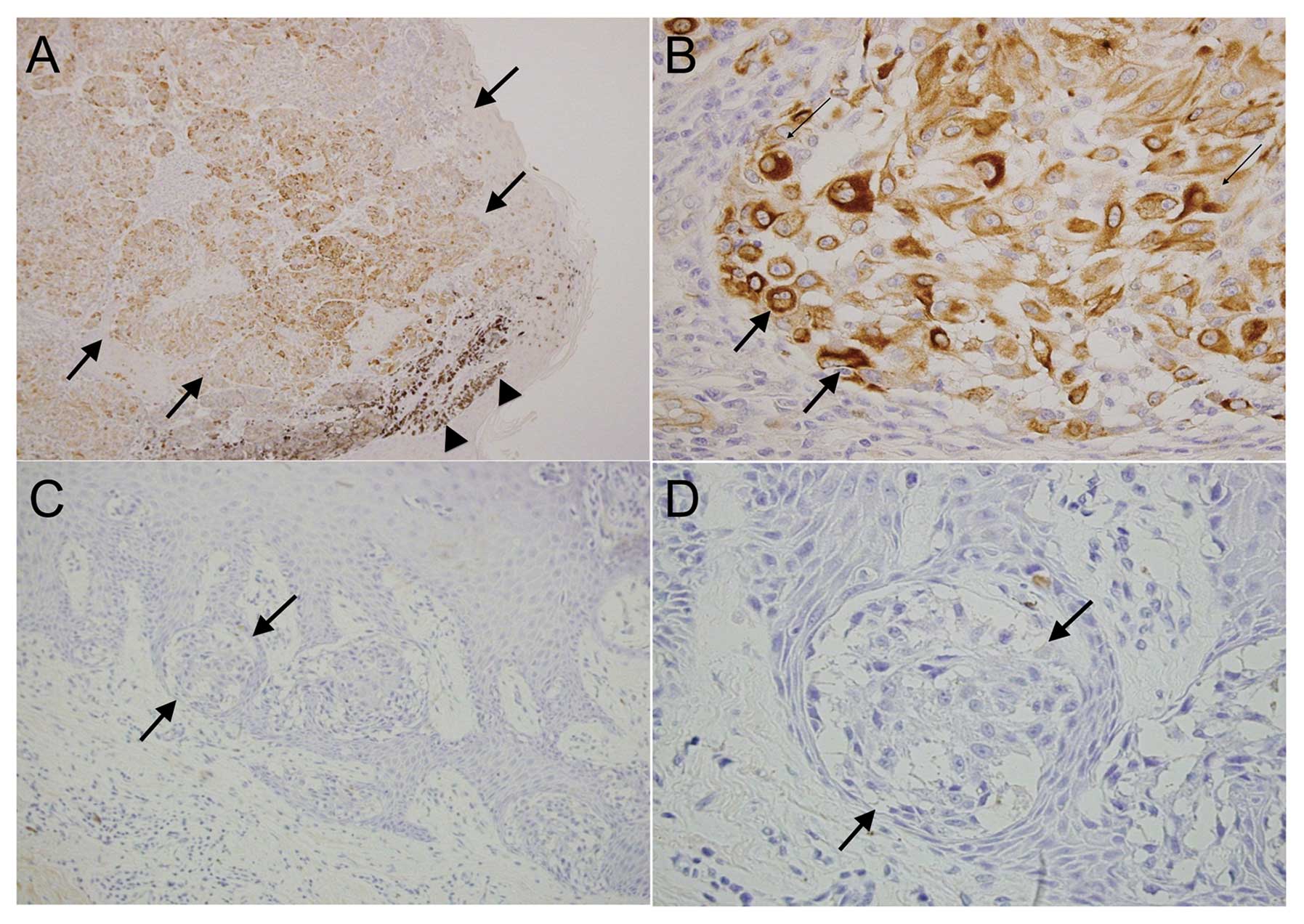
melanomas (N=17). Nestin was present in the cytoplasm of tumor
cells in 14 of the 17 melanoma cases (82.4%) (Fig. 2A and B and Table I). Melanoma cells in epidermal or
superficial dermal areas tended to be nestin-negative (Fig. 2C and D), whereas melanoma cells in
the deep dermis or thickened melanoma were nestin-positive.
Consistent with previous reports, all cases of stage IV (N=8, 100%)
and stage III melanoma (N=3, 100%) were nestin-positive, whereas 2
of the 3 stage II melanoma cases (66.7%) and 1 of the 3 stage I and
Tis melanoma cases (33.3%) were nestin-positive. The χ2
test indicated a statistically significant relationship between
melanoma T stage (Tis-T2 vs. T3–T4) and nestin expression
(P<0.05) (Table I).
Immunohistochemical and in situ
hybridization analyses of nestin expression in malignant
melanoma
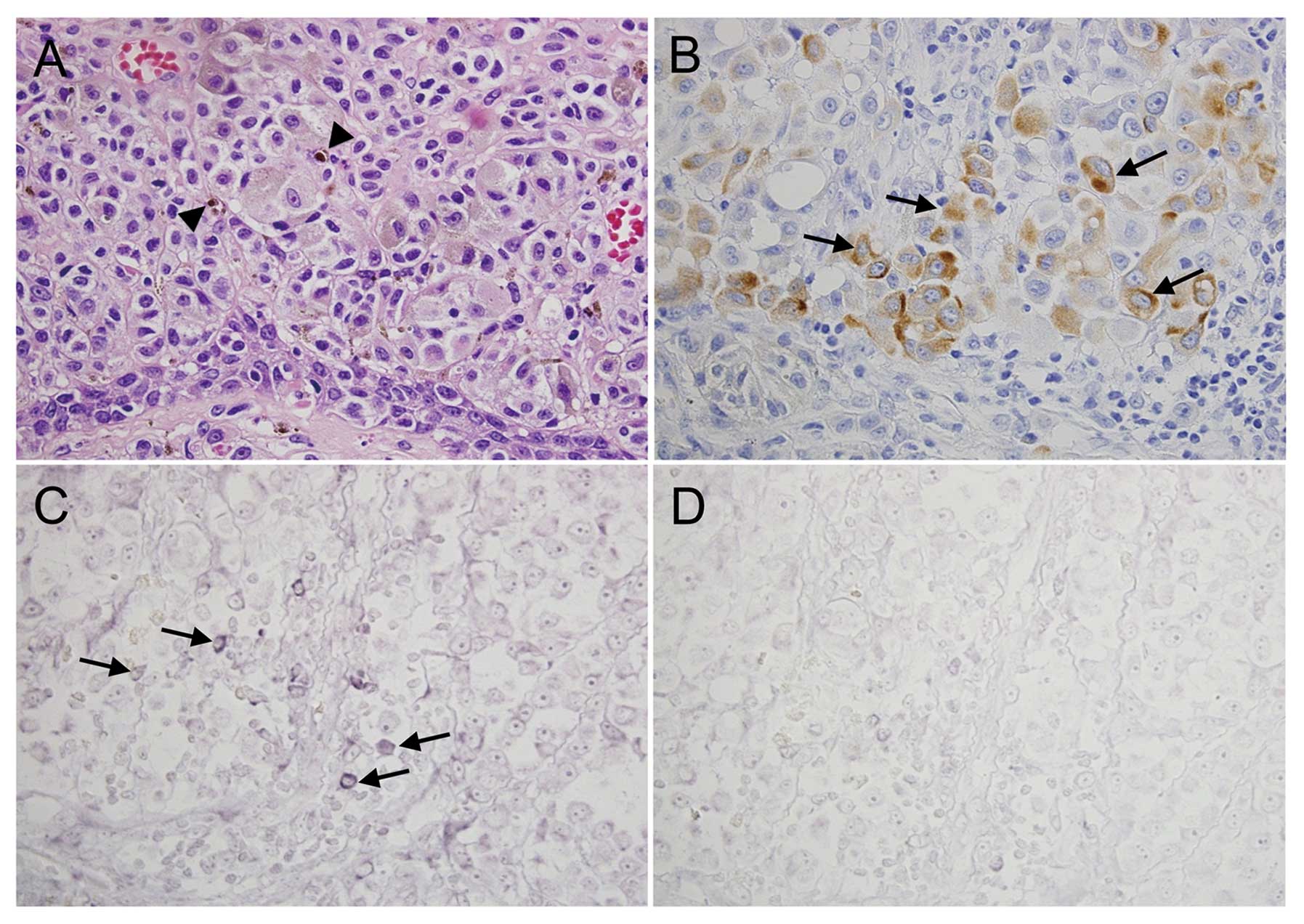
Subsequently, we analyzed serial sections of human
melanomas for nestin expression by using in situ
hybridization. These experiments revealed that nestin protein and
mRNA were strongly expressed in some of the melanoma cells
(Fig. 3B and C, arrows). The
control sense probe did not produce a signal (Fig. 3D). Serial sections from 3 additional
melanoma tissues were analyzed for nestin expression by using
immunohistochemical staining and in situ hybridization and
showed similar expression patterns (data not shown).
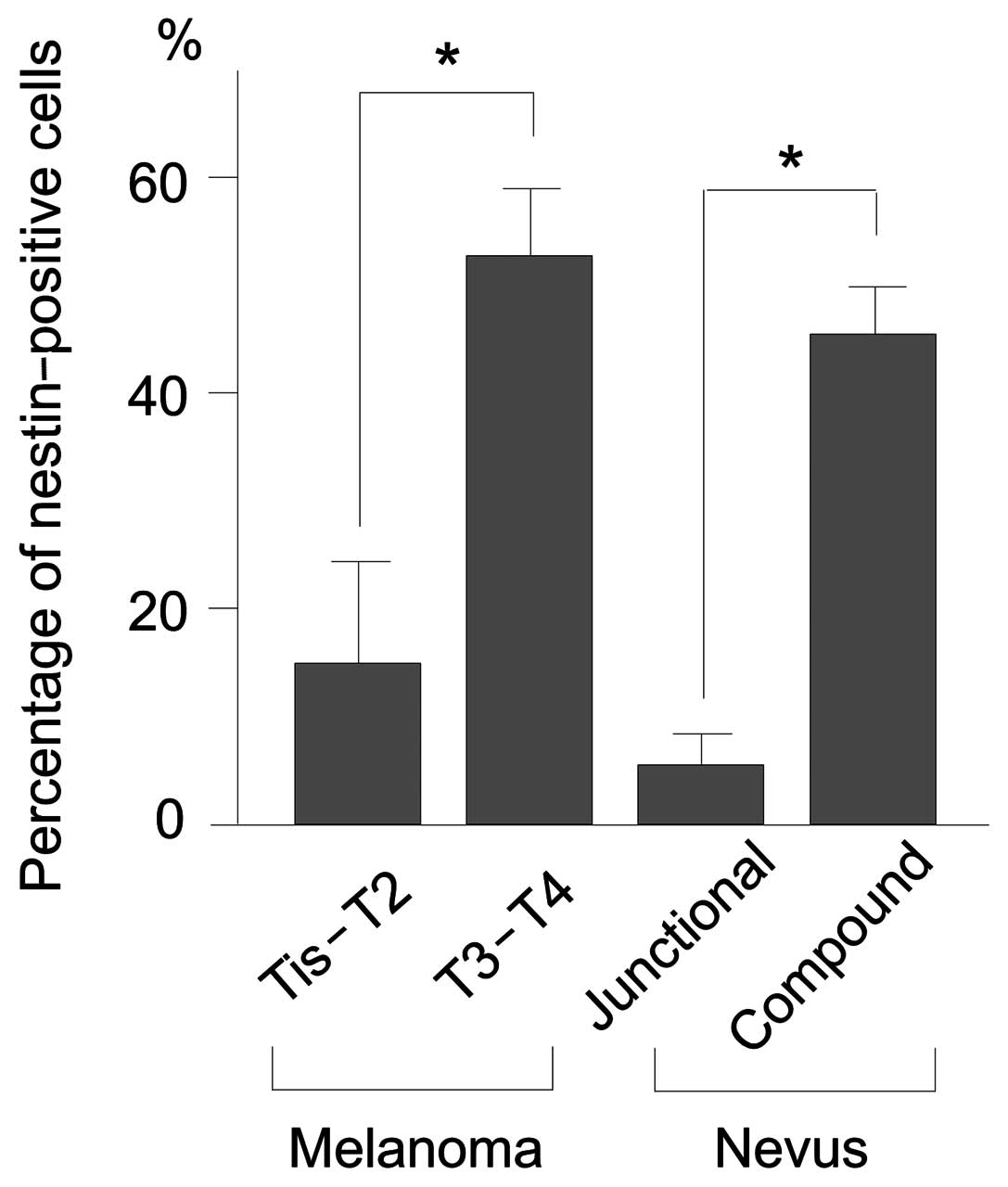
Percentage of nestin-positive cells in
melanomas and nevi
We divided melanoma cases into 2 groups: Tis-T2 and
T3–T4. Nestin expression was significantly higher in T3–T4
melanomas, in which tumor thickness is >2 mm, than in Tis-T2
melanomas, in which tumor thickness is ≤2 mM (P<0.05) (Fig. 4). Nestin expression was also
significantly higher in compound than in junctional nevi
(P<0.05) (Fig. 4).
Discussion
In this study, nestin was expressed in all the
compound nevi, which have dermal nests of nevus cells, whereas only
a small number of junctional nevi showed nestin expression. Of
note, nestin was strongly expressed in the nevus nests in the
dermal area that show what is referred to as neurotization
(32). Nevus cells are derived from
neural crest cells, which give rise to diverse cellular phenotypes,
including Schwann, glia and melanocytes (33). It has been suggested that nevi with
neuroid changes are transformed from Schwann cells, or,
alternatively, may result from a natural regression of nevus cells
(32). Thus, the strong nestin
expression in neurotized nevus cells may relate to it being a
neural stem/progenitor cell marker.
In malignant melanoma, nestin expression was
observed in all T3 and T4 melanomas, in which tumor thickness is
more than 2 mm, but only in half of the T2, T1 and Tis tumors, in
which tumor thickness is 2 mm or less. This stepwise increase in
nestin expression is consistent with previous reports (23,25,26).
We also observed strong nestin expression in the peripheral area or
invading front of the tumor in some T4 cases, a finding that is in
accordance with previous results (24,25).
A critical question that has yet to be clarified is
whether, in melanoma tumorigenesis, the cell that initiates
melanoma is a cancer stem cell. Herein, we showed that nestin was
expressed in both malignant melanomas and nevi, an observation that
is significant with respect to the origin and/or behavior of these
tumors. Consistent with previous reports, we also showed that the
expression of nestin was increased in advanced-stage melanomas
(25). The mechanism that underlies
this increase remains unknown, but it may be associated with the
increase in stem/progenitor-like cells caused by the
dedifferentiation of melanoma cells or the accumulation of immature
phenotype cells observed in patients with advanced-stage
melanoma.
In conclusion, nestin was expressed in advanced
melanoma tissues and neurotized nevi. Nestin is therefore a
potentially important marker of melanocytic neoplasms; however,
further studies are required to elucidate the molecular processes
that regulate nestin expression and to evaluate the potential of
nestin-targeted therapy for malignant melanomas.
Acknowledgements
The authors thank Ms. Yoko Kawamoto and Ms. Taeko
Suzuki (Departments of Pathology and Integrative Oncological
Pathology) for their technical assistance and Dr Shin-ichi Tsuchiya
(Division of Surgical Pathology, Nippon Medical School Hospital)
for preparing tissue blocks. This study was supported by Leave a
Nest Co., Ltd., a Grant-in-Aid for Scientific Research (M. Akiyama)
and a Grant-in-Aid for Young Scientific Research (A, no. 22689038
to Y. Matsuda).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Essner R, Belhocine T, Scott AM and
Even-Sapir E: Novel imaging techniques in melanoma. Surg Oncol Clin
North Am. 15:253–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kochhar R, Ali H, Mak S and Manoharan P:
Metastatic cutaneous malignant melanoma: spectrum of imaging
findings and the role of multimodality imaging. J Med Imaging
Radiat Oncol. 53:467–478; quiz 478–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee ML, Tomsu K and Von Eschen KB:
Duration of survival for disseminated malignant melanoma: results
of a meta-analysis. Melanoma Res. 10:81–92. 2000.PubMed/NCBI
|
|
5
|
Goozner M: Drug approvals 2011: focus on
companion diagnostics. J Natl Cancer Inst. 104:84–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Finn L, Markovic SN and Joseph RW: Therapy
for metastatic melanoma: the past, present and future. BMC Med.
10:232012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flaherty KT and Fisher DE: New strategies
in metastatic melanoma: oncogene-defined taxonomy leads to
therapeutic advances. Clin Cancer Res. 17:4922–4928. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sellheyer K and Krahl D: Spatiotemporal
expression pattern of neuroepithelial stem cell marker nestin
suggests a role in dermal homeostasis, neovasculogenesis and tumor
stroma development: a study on embryonic and adult human skin. J Am
Acad Dermatol. 63:93–113. 2010. View Article : Google Scholar
|
|
10
|
Amoh Y, Kanoh M, Niiyama S, et al: Human
and mouse hair follicles contain both multipotent and monopotent
stem cells. Cell Cycle. 8:176–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amoh Y, Li L, Yang M, et al: Nascent blood
vessels in the skin arise from nestin-expressing hair-follicle
cells. Proc Natl Acad Sci USA. 101:13291–13295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuda Y, Naito Z, Kawahara K, Nakazawa
N, Korc M and Ishiwata T: Nestin is a novel target for suppressing
pancreatic cancer cell migration, invasion and metastasis. Cancer
Biol Ther. 11:512–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawamoto M, Ishiwata T, Cho K, et al:
Nestin expression correlates with nerve and retroperitoneal tissue
invasion in pancreatic cancer. Hum Pathol. 40:189–198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kleeberger W, Bova GS, Nielsen ME, et al:
Roles for the stem cell associated intermediate filament Nestin in
prostate cancer migration and metastasis. Cancer Res. 67:9199–9206.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Cherukuri P, Li N, et al: Nestin is
expressed in the basal/myoepithelial layer of the mammary gland and
is a selective marker of basal epithelial breast tumors. Cancer
Res. 67:501–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishiwata T, Teduka K, Yamamoto T, Kawahara
K, Matsuda Y and Naito Z: Neuroepithelial stem cell marker nestin
regulates the migration, invasion and growth of human gliomas.
Oncol Rep. 26:91–99. 2011.PubMed/NCBI
|
|
17
|
Yang XH, Wu QL, Yu XB, et al: Nestin
expression in different tumours and its relevance to malignant
grade. J Clin Pathol. 61:467–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Misago N, Mori T and Narisawa Y: Nestin
expression in stromal cells of trichoblastoma and basal cell
carcinoma. J Eur Acad Dermatol Venereol. 24:1354–1358. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanoh M, Amoh Y, Tanabe K, Maejima H,
Takasu H and Katsuoka K: Nestin is expressed in HMB-45 negative
melanoma cells in dermal parts of nodular melanoma. J Dermatol.
37:505–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abbas O and Bhawan J: Expression of stem
cell markers nestin and cytokeratin 15 and 19 in cutaneous
malignancies. J Eur Acad Dermatol Venereol. 25:311–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mori T, Misago N, Yamamoto O, Toda S and
Narisawa Y: Expression of nestin in dermatofibrosarcoma protuberans
in comparison to dermatofibroma. J Dermatol. 35:419–425. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Florenes VA, Holm R, Myklebost O, Lendahl
U and Fodstad O: Expression of the neuroectodermal intermediate
filament nestin in human melanomas. Cancer Res. 54:354–356.
1994.PubMed/NCBI
|
|
23
|
Klein WM, Wu BP, Zhao S, Wu H,
Klein-Szanto AJ and Tahan SR: Increased expression of stem cell
markers in malignant melanoma. Mod Pathol. 20:102–107. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piras F, Perra MT, Murtas D, et al: The
stem cell marker nestin predicts poor prognosis in human melanoma.
Oncol Rep. 23:17–24. 2010.PubMed/NCBI
|
|
25
|
Brychtova S, Fiuraskova M, Hlobilkova A,
Brychta T and Hirnak J: Nestin expression in cutaneous melanomas
and melanocytic nevi. J Cutan Pathol. 34:370–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanabe K, Amoh Y, Kanoh M, et al:
Prognostic significance of the hair follicle stem cell marker
nestin in patients with malignant melanoma. Eur J Dermatol.
20:283–288. 2010.PubMed/NCBI
|
|
27
|
Mihic-Probst D, Kuster A, Kilgus S, et al:
Consistent expression of the stem cell renewal factor BMI-1 in
primary and metastatic melanoma. Int J Cancer. 121:1764–1770. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fusi A, Ochsenreither S, Busse A, Rietz A
and Keilholz U: Expression of the stem cell marker nestin in
peripheral blood of patients with melanoma. Br J Dermatol.
163:107–114. 2010.PubMed/NCBI
|
|
29
|
Fusi A, Reichelt U, Busse A, et al:
Expression of the stem cell markers nestin and CD133 on circulating
melanoma cells. J Invest Dermatol. 131:487–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balch CM, Gershenwald JE, Soong SJ, et al:
Final version of 2009 AJCC melanoma staging and classification. J
Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ackerman AB and Magana-Garcia M: Naming
acquired melanocytic nevi. Unna’s, Miescher’s, Spitz’s Clark’s. Am
J Dermatopathol. 12:193–209. 1990.
|
|
32
|
Van Paesschen MA, Goovaerts G and Buyssens
N: A study of the so-called neurotization of nevi. Am J
Dermatopathol. 12:242–248. 1990.PubMed/NCBI
|
|
33
|
Misago N: The relationship between
melanocytes and peripheral nerve sheath cells (Part I): melanocytic
nevus (excluding so-called ‘blue nevus’) with peripheral nerve
sheath differentiation. Am J Dermatopathol. 22:217–229.
2000.PubMed/NCBI
|