Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors and the leading cause of death around the world
(1). Despite many advances in the
field of cancer therapeutics, chemotherapy remains the main
therapeutic approach for patients with advanced CRC. However, drug
resistance and toxicity against normal cells limit the
effectiveness of currently-used chemotherapies for CRC (2–4). Thus
it is necessary to develop novel anticancer agents. Compared to
modern chemotherapeutics natural products contain relatively fewer
side effects and have been shown to possess beneficial therapeutic
effects for cancer (5–7). Therefore, identifying naturally
occurring agents is a promising approach for anticancer
treatment.
Cancer cells are characterized by an unregulated
increase in cell proliferation (8).
Besides its significance for tumor biology, the uncontrolled
proliferation is an important prognostic indicator for various
cancers. Eukaryotic cell proliferation is regulated by the cell
cycle, and G1 to S transition is one of the two main checkpoints
used by cells to control the cell cycle progress (9). G1/S progression is highly regulated by
cyclin D1 and cyclin-dependent kinase 4 (CDK4) (10,11).
An unchecked or hyperactivated cyclin D1/CDK4 complex often leads
to uncontrolled cell division and malignancy (12,13).
As a proliferation inhibitor, p21 protein plays a role in G1 arrest
by binding to and inhibiting the activity of Cyclin-CDK complexes
(14). In addition, p21 also binds
to proliferating cell nuclear antigen (PCNA), a processivity factor
for DNA polymerase, inhibiting PCNA-dependent DNA replication
(15). The decrease of p21
expression is associated with the promotion of tumor formation and
a poor prognosis in many types of cancer (16).
The process of cell cycle is mediated by multiple
intracellular signaling transduction cascades including Akt and p53
pathways. PI3K-dependent Akt pathway is essential for cell
proliferation and survival and has been shown to be activated in
several cancer types (17–22). After activation by extracellular
stimuli, PI3K is able to phosphorylate PI(4)P and PI(4,5)P2 to
generate PI(3,4)P2 and PI(3,4,5)P3, respectively. These lipids
serve as plasma membrane docking sites for proteins containing
pleckstrin-homology (PH) domains, such as Akt and its upstream
activator PDK1. The colocalization of PDK1 and Akt in plasma
membrane results in the phosphorylation of Akt leading to its
activation (23). Akt promotes cell
survival by inhibiting apoptosis and/or by promoting cell cycle
progression (24,25). Akt upregulates the expression of
cyclin D1 through phosphorylating GSK3β. Phosphorylation of GSK3β
decreases its kinase activity on cyclin D1, which subsequently
prevents the nuclear export and the cytoplasmic proteasomal
degradation of cyclin D1 (26,27).
In addition, activation of Akt pathway negatively regulates p21
expression (28). The tumor
suppressor p53 is a transcription factor that responds to certain
stresses to preserve genomic integrity by arresting cell cycle
progression (29,30). p53 normally is a short-lived protein
that is maintained at low levels in cytoplasm, but in response to
DNA-damaging agents and nucleotide depletion, the p53 protein is
phosphorylated and accumulates in the nucleus, in which it induces
the expression of various critical genes such as p21. Therefore,
inhibition of excessive cell proliferation via modulation of Akt
and p53 pathways and the expression of the downstream cell
cycle-related genes (31) has
become a major focus for cancer chemotherapies.
As a well-known traditional Chinese folk medicine,
Scutellaria barbata D. Don (SB) has long been used as an
important component in many Chinese medicine formulas to treat
various types of cancer (32–35).
Previous studies proposed that extracts of SB (ESB) possess
antitumor activity to suppress the growth of many types of cancer
including CRC both in vitro and in vivo(36–42).
In addition, we recently reported that ESB is able to induce cancer
cell apoptosis via activation of the mitochondrion-dependent
pathway and inhibit tumor angiogenesis through suppression of
Hedgehog signaling (43–45). To further elucidate the precise
mechanism of the potential tumoricidal activity of SB, in the
present study we investigated its effect on the proliferation of
human colon carcinoma HT-29 cells and investigated the underlying
molecular mechanism.
Materials and methods
Materials and reagents
Dulbecco’s modified eagle medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA and
TRIzol reagent were purchased from Invitrogen (Carlsbad, CA, USA).
SuperScript II reverse transcriptase was obtained from Promega
(Madison, WI, USA). Cyclin D1, CDK4, p21, PCNA antibodies,
horseradish peroxidase (HRP)-conjugated secondary antibodies were
obtained from Cell Signaling (Beverly, MA, USA). Bio-Plex
phosphoprotein assay kits were purchased from Bio-Rad (Hercules,
CA, USA). BCA Protein Assay kit was purchased from Tiangen Biotech
Co., Ltd. (Beijing, China). All the other chemicals, unless
otherwise stated, were obtained from Sigma Chemicals (St. Louis,
MO, USA).
Preparation of ethanol extract of
Scutellaria barbata D. Don (EESB)
EESB was prepared as previously described (43). Stock solutions of EESB were prepared
by dissolving the EESB powder in 50% DMSO to a concentration of 500
mg/ml, and stored at −20°C. The working concentrations of EESB were
made by diluting the stock solution in the culture medium. The
final concentrations of DMSO in the medium were <0.5%.
Cell culture
Human colon carcinoma HT-29 cells were obtained from
American Type Culture Collection (ATCC, Manassas, VA, USA). Cells
were grown in DMEM containing 10% (v/v) FBS, 100 U/ml penicillin
and 100 μg/ml streptomycin in a 37°C humidified incubator with 5%
CO2.
Cell viability evaluation
Viability of HT-29 cells was assessed by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay. Cells were seeded into 96-well plates at a
density of 1×104 cells/well in 100 μl medium. The cells
were treated with various concentrations of EESB for different
periods of time. At the end of the treatment, 10 μl MTT (5 mg/ml in
phosphate buffered saline, PBS) was added to each well, and the
samples were incubated for an additional 4 h at 37°C. The
purple-blue MTT formazan precipitate was dissolved in 100 μl DMSO.
The absorbance was measured at 570 nm using an ELISA reader, model
ELX800 (BioTek, USA).
Colony formation assay
HT-29 cells (2×105) were seeded into
6-well plates in 2 ml medium and treated with various
concentrations of EESB for 24 h. The cells were then diluted in
fresh medium in the absence of EESB and reseeded into 6-well plates
at a density of 1.5×103 cells/well. After incubation for
7 days in a 37°C humidified incubator with 5% CO2, the
colonies were counted under a microscope. Cell survival was
calculated by normalizing the survival of the control cells as
100%.
Cell cycle analysis by flow
cytometry
The cell cycle analysis was carried out by flow
cytometry using a fluorescence-activated cell sorting (FACS)
caliber (Becton Dickinson, CA, USA) and Propidium iodide (PI)
staining. After treated with indicated concentrations of EESB for
24 h, HT-29 cells were harvested and adjusted to a density of
1×106 cells/ml, and fixed in 70% ethanol at 4°C
overnight. The fixed cells were washed twice with cold PBS, and
then incubated for 30 min with RNase (8 μg/ml) and PI (10 μg/ml).
The fluorescent signal was detected through the FL2 channel and the
proportion of DNA in different phases was analyzed using ModfitLT
version 3.0 (Verity Software House Inc., Topsham, ME, USA).
RT-PCR analysis
HT-29 cells were seeded into 6-well plates at a
density of 2×105 cells/well and treated with various
concentrations of EESB for 24 h. Total RNA was isolated with TriZol
reagent. Oligo(dT)-primed RNA (1 μg) was reverse-transcribed with
SuperScript II reverse transcriptase (Promega) according to the
manufacturer’s instructions. The obtained cDNA was used to
determine the mRNA amount of cyclin D1, CDK4, PCNA and p21 by PCR.
GAPDH was used as an internal control. The sequences of the primers
of cyclin D1, CDK4, PCNA, p21 and GAPDH were: cyclin D1 forward
5′-TGG ATG CTG GAG GTC TGC GAG GAA-3′ and reverse 5′-GGC TTC GAT
CTG CTC CTG GCA GGC-3′ (Tm=55°C, 573 bp); CDK4 forward 5′-CAT GTA
GAC CAG GAC CTA AGC-3′ and reverse 5′-AAC TGG CGC ATC AGA TCC
TAG-3′ (Tm=58°C, 206 bp); PCNA forward 5′-GCT GAC ATG GGA CAC
TTA-3′ and reverse 5′-CTC AGG TAC AAA CTT GGT G-3′ (Tm=56°C, 610
bp); p21 forward 5′-GCG ACT GTG ATG CGC TAA TGG-3′, reverse 5′-TAG
AAA TCT GTC ATG CTG GTC TGC-3′ (Tm=55°C, 358 bp); GAPDH forward
5′-CG ACC ACT TTG TCA AGC TCA-3′ and reverse 5′-AG GGG TCT ACA TGG
CAA CTG-3′ (Tm=58°C, 240 bp). Samples were analyzed by gel
electrophoresis (1.5% agarose).
Western blot analysis
HT-29 cells (5×105) were seeded into
culture flask and treated with various concentrations of EESB for
24 h. The treated cells were lysed with cell lysis buffer and
centrifuged at 15,000 × g for 15 min followed by determination of
protein concentration in supernatants. Equal protein per lysate was
resolved on Tris-glycine gel, transferred onto PVDF membrane, and
blocked for 2 h with 5% nonfat dry milk. Membranes were incubated
with desired primary antibody cyclin D1, CDK4, p21, PCNA and
β-actin (at a dilution of 1:1000) overnight at 4°C and then with
appropriate HRP-conjugated secondary antibody followed by enhanced
chemiluminescence detection.
Bio-Plex phosphoprotein assay
HT-29 cells (2.5×105) were seeded into 25
cm2 flasks in 5 ml medium and treated with 1.5 mg/ml of
EESB for 24 h. Treated cells were lysed using a commercially
available lysis kit and centrifuged at 14,000 × g for 15 min. The
protein extracts were quantified by BCA protein assay. The presence
of p-AKT, p-p53 was detected using a bead-based multiplex assay for
phosphoproteins according to the manufacturer’s protocol (Bio-Rad).
Data were collected and analyzed using the Bio-Plex 200 suspension
array system (Bio-Rad).
Statistical analysis
Data were analyzed using the statistical software
SPSS13.0. Statistical analysis of the data was performed with
Student’s t-test and One-way analysis of variance (ANOVA). P-values
<0.05 was considered as significant.
Results
EESB suppressed HT-29 cell
proliferation
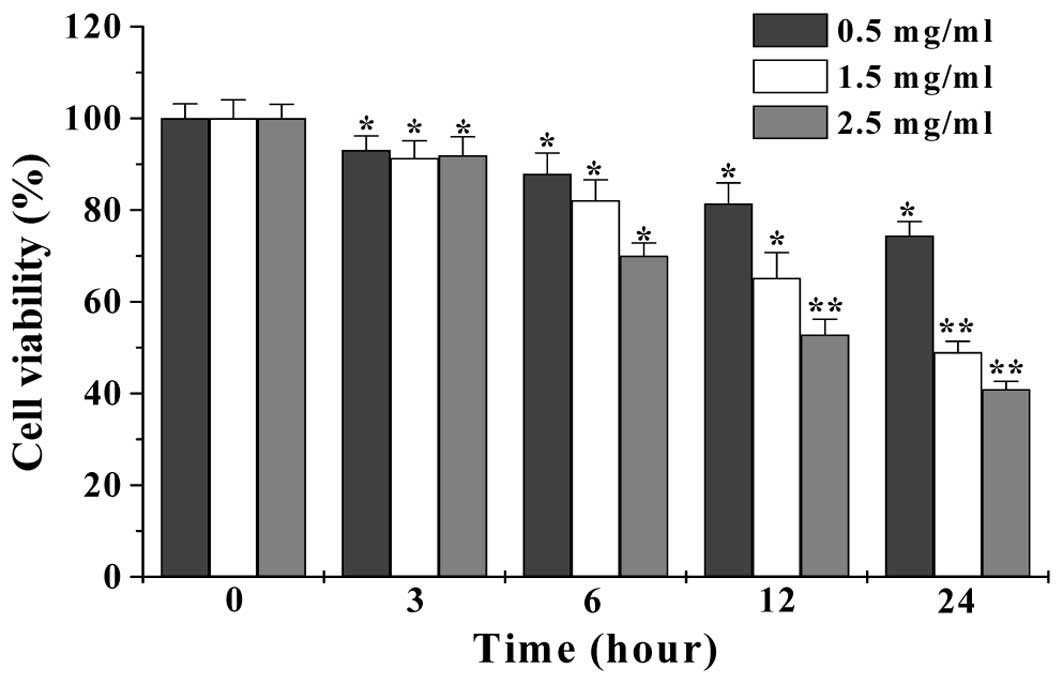
We first performed MTT assay to examine the effect
of EESB on HT-29 cell viability. As shown in Fig. 1, treatment with 0.5–2.5 mg/ml of
EESB for 3–24 h, respectively reduced cell viability by 6.92–25.59,
8.65–51 or 8.07–59.1%, compared to untreated control cells
(P<0.01 or 0.05). We further verified these results using a
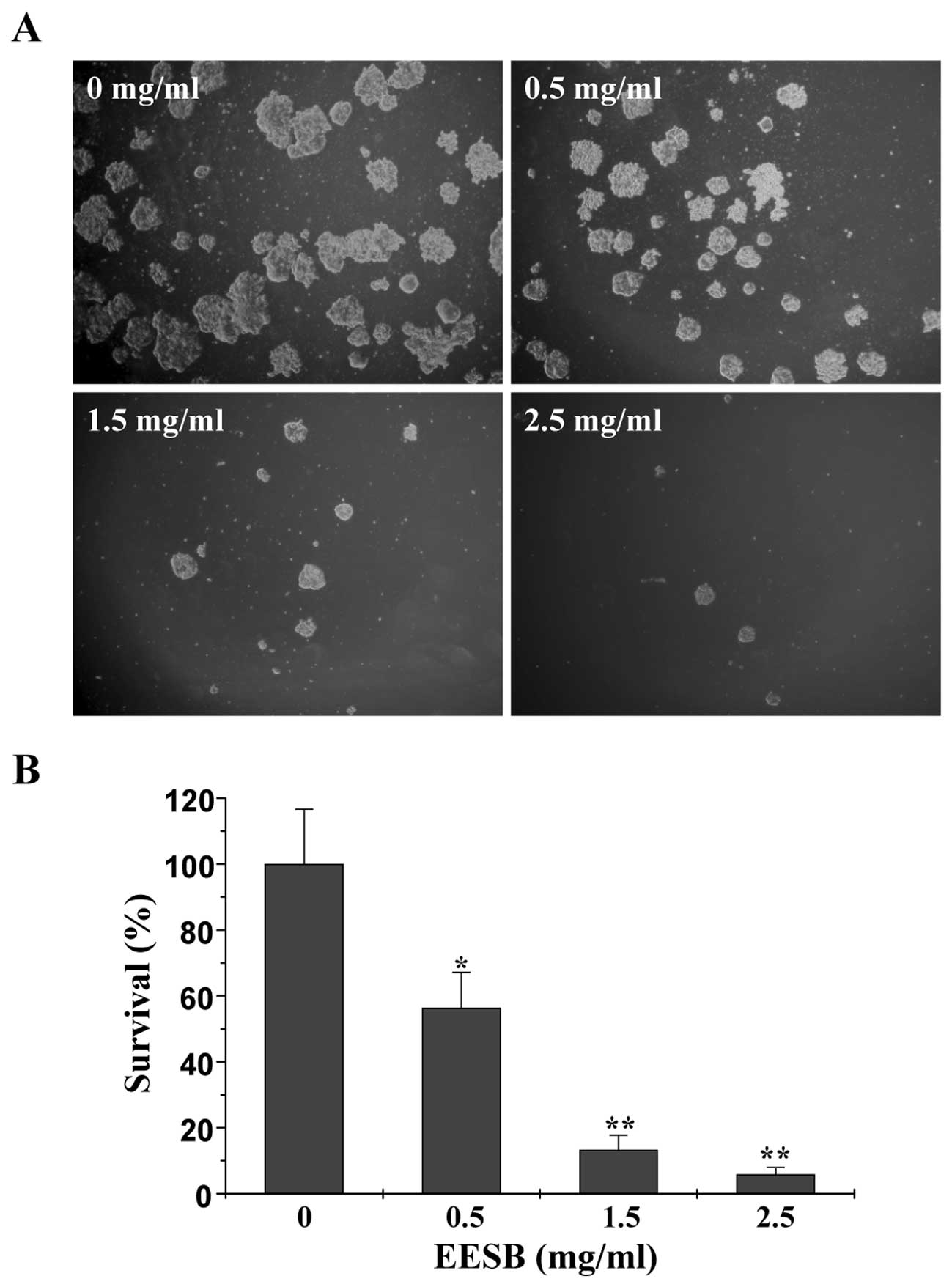
colony formation assay. As shown in Fig. 2A and B, treatment with 0.5, 1.5 and
2.5 mg/ml of EESB for 24 h reduced the cell survival rate by 43.70,
86.67 and 94.07% (P<0.01 or 0.05, vs. control). Thus, EESB
inhibits CRC cell proliferation in a dose- and time-dependent
manner.
EESB inhibited G1/S cell cycle
progression in HT-29 cells
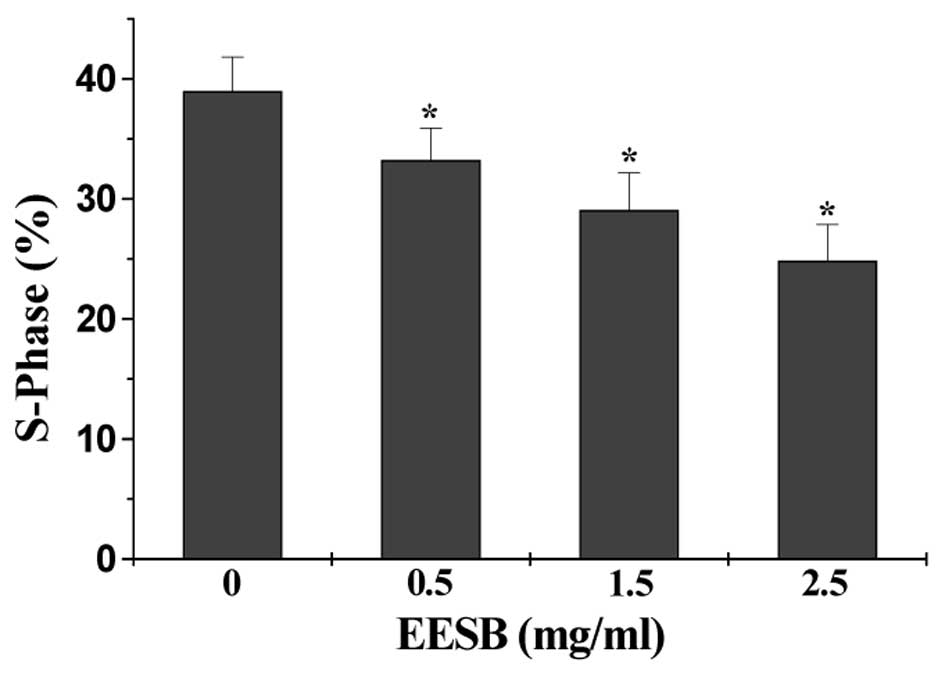
The effect of EESB on cell cycle was evaluated by
FACS analysis with PI staining. As shown in Fig. 3, the percentage of HT-29 cells in
S-phase following treatment with 0, 0.5, 1.5 or 2.5 mg/ml of EESB
was 38.97, 33.22, 29.06 or 24.85%, respectively (P<0.05),
suggesting that EESB-caused inhibition of HT-29 cell proliferation
is mediated by the blockade of cell cycle G1-S progression.
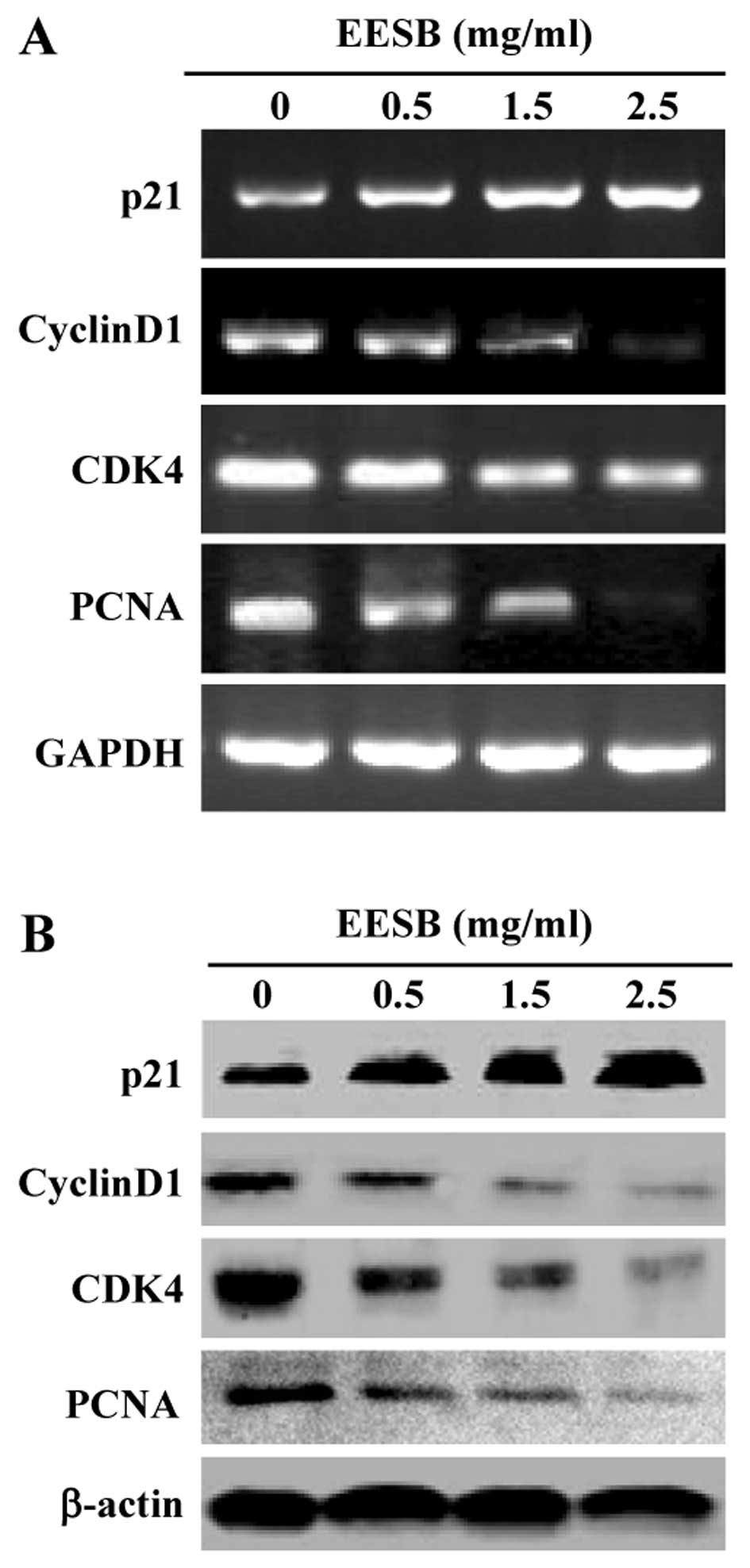
EESB altered the expression of cell
cycle-regulatory factor in HT-29 cells
We next examined the effect of EESB on the
expression of cell cycle-regulatory factors. Data from RT-PCR and
western blot analysis showed that EESB treatment profoundly
enhanced antiproliferative p21 expression, but suppressed the
expression of pro-proliferative PCNA, cyclin D1 and CDK4 in HT-29
cells, at both transcriptional and translational levels (Fig. 4).
EESB modulated Akt and p53 pathways in
HT-29 cells
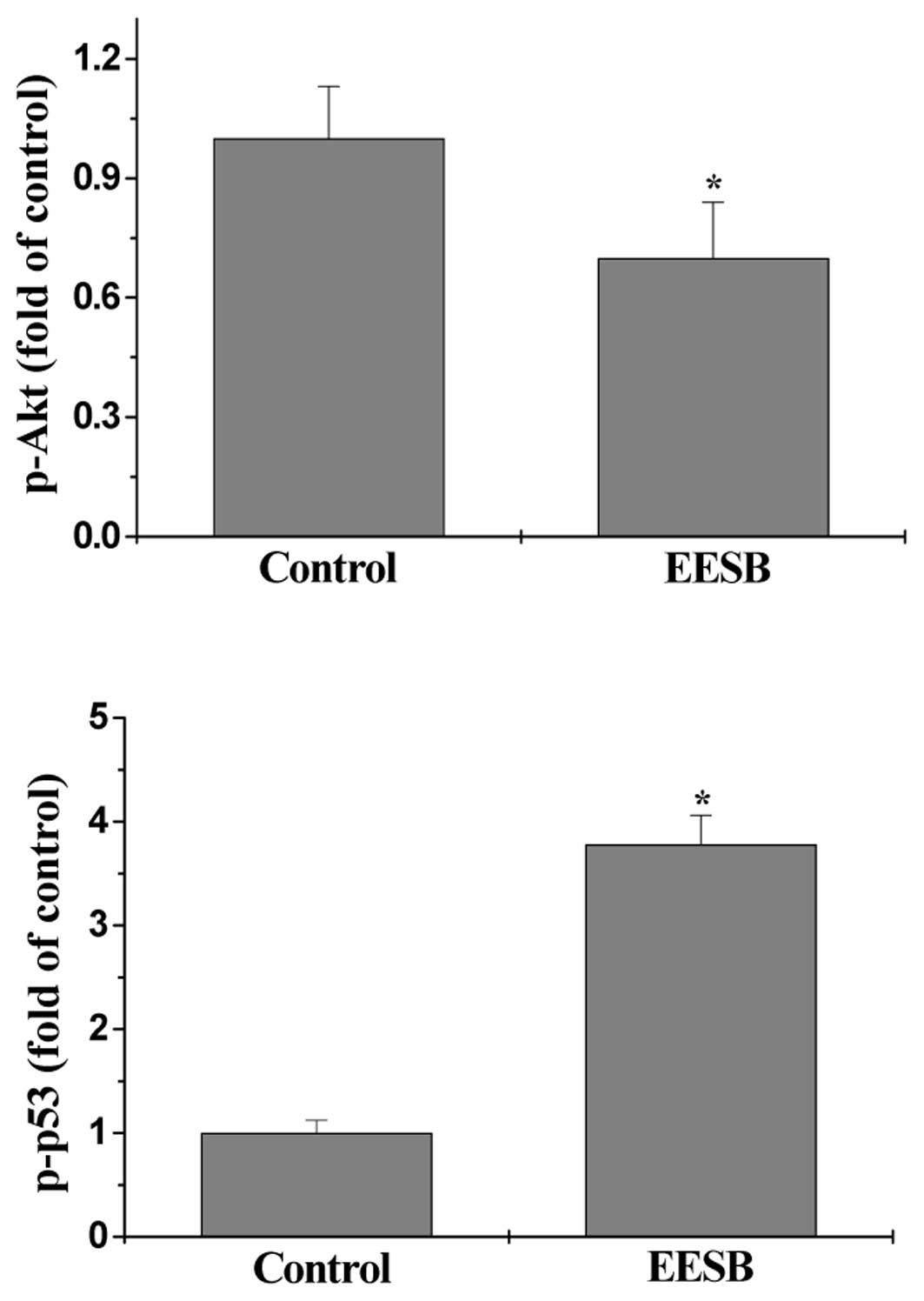
The activation (phosphorylation) of Akt and p53 was
determined by Bio-Plex Phospho-protein assay. As shown in Fig. 5A and B, after EESB treatment the
phosphorylation level of Akt in HT-29 cells was significantly
decreased, whereas that of p53 was significantly increased, as
compared to controls (P<0.05). These data suggest that EESB
modulates the activation of multiple cell cycle-related signaling
pathways.
Discussion
Natural products have been used in China for
thousands of years as alternative remedies for a variety of
diseases including cancer. Among Chinese traditional medicinal
plants, Scutellaria barbata D. Don (SB) has been
traditionally used for the treatment of inflammation, such as
hepatitis, osteomyelitis, gynecological diseases, due to its
antibacterial activity. Recently, SB has gained increasingly
attention to its usage as an antitumor herb (32–42).
Similar to other medicinal herbs, SB is considered to be a
multi-target agent that exerts therapeutic function in a holistic
way. Previously, we reported that SB promotes the apoptosis of
human colorectal carcinoma cells in vitro and inhibits tumor
angiogenesis in vivo via suppression of the Hedgehog pathway
(43–45). To further elucidate the mechanism of
the tumoricidal activity of SB, herein we investigated its effect
on the proliferation of human colon carcinoma HT-29 cells.
In the present study, we found that ethanol extract
of SB (EESB) inhibited proliferation of HT-29 cells in a dose- and
time-dependent manner. Eukaryotic cell proliferation is regulated
by the cell cycle, which consists of four periods: S phase (DNA
synthesis phase), M phase (mitosis), G1 and G2 phase. At different
phases, passage through the cell cycle is governed by sequential
activation and subsequent inactivation of a series of
cyclin-dependent kinases (CDKs), whose activity depends on
interactions with timely expressed cyclins and cyclin-dependent
kinase inhibitors (CDKIs). By using FACS analysis with PI staining
we found that the inhibitory effect of EESB on HT-29 cell
proliferation was associated with the blockage of G1 to S
progression. As one of the main checkpoints of cell cycle, G1/S
transition is responsible for initiation and completion of DNA
replication (9), which is strongly
regulated by the combined activity of the cyclin D1/CDK4 complex
(10,11). The proliferation inhibitor p21 plays
an inhibitory role in G1/S progression by inhibiting the activity
of cyclin-CDK complexes as well as the PCNA-dependent DNA
replication (14,15). Consistent with the effect on G1/S
arrest, EESB upregulated p21 expression and downregulated the
expression of PCNA, cyclin D1 and CDK4 in HT-29 cells. The process
of cell cycle is mediated by multiple intracellular signaling
transduction cascades including Akt and p53 pathways. Activation of
Akt pathway promotes cell proliferation by positively regulating
cyclin D1 expression and downregulating the expression of p21
(26–28). In response to DNA-damaging agents,
the tumor suppressor p53 protein is phosphorylated and induces the
expression of various critical genes including p21. By using
Bio-plex cytokine assay, herein we found that EESB treatment
significantly suppressed that activation of Akt but increased the
phosphorylation level of p53 in HT-29 cells.
In conclusion, we demonstrated that EESB inhibited
the proliferation of HT-29 cells via G1/S cell cycle arrest, which
was mediated by the modulation of p53 and Akt pathways. Together
with our previous studies, it is suggested that Scutellaria
barbata D. Don inhibits cancer progression via multiple
mechanisms, including induction of cancer cell apoptosis,
inhibition of cell proliferation and tumor angiogenesis.
Acknowledgements
This work was sponsored by the National Natural
Science Foundation of China (81073097), Natural Science Foundation
of Fujian Province of China (2010J01195) and Youth Science
Foundation of Health Department of Fujian Province (2012-2-60).
Abbreviations:
|
EESB
|
ethanol extract of Scutellaria
barbata D. Don
|
|
CRC
|
colorectal cancer
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
3-(4,
5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Gorlick R and Bertino JR: Drug resistance
in colon cancer. Semin Oncol. 26:606–611. 1999.
|
|
3
|
Grau MV, Rees JR and Baron JA:
Chemoprevention in gastrointestinal cancers: current status. Basic
Clin Pharmacol Toxicol. 98:281–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
5
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harvey AL: Natural products in drug
discovery. Drug Discov Today. 13:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nurse P: Ordering S phase and M phase in
the cell cycle. Cell. 79:5471994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Robles AI, Martinez LA, Liu F,
Gimenez-Conti IB and Conti CJ: Expression of G1 cyclins,
cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in
androgen-induced prostate proliferation in castrated rats. Cell
Growth Differ. 7:1571–1578. 1996.PubMed/NCBI
|
|
11
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
12
|
Kouraklis G, Theocharis S, Vamvakas P, et
al: Cyclin D1 and Rb protein expression and their correlation with
prognosis in patients with colon cancer. World J Surg Oncol.
4:52006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zafonte BT, Hulit J, Amanatullah DF, et
al: Cell-cycle dysregulation in breast cancer: breast cancer
therapies targeting the cell cycle. Front Biosci. 5:D938–D961.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harper JW, Elledge SJ, Keyomarsi K, et al:
Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell.
6:387–400. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waga S, Hannon GJ, Beach D and Stillman B:
The p21 inhibitor of cyclin-dependent kinases controls DNA
replication by interaction with PCNA. Nature. 369:574–578. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Domagala W, Welcker M, Chosia M, et al:
p21/WAF1/Cip1 expression in invasive ductal breast carcinoma:
relationship to p53, proliferation rate, and survival at 5 years.
Virchows Arch. 439:132–140. 2001.PubMed/NCBI
|
|
17
|
Franke TF, Kaplan DR, Cantley LC and Toker
A: Direct regulation of the Akt proto-oncogene product by
phosphatidylinositol-3, 4-bisphosphate. Science. 275:665–668. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang F, Lee JT, Navolanic PM, et al:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: a target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clarke RB: p27KIP1 phosphorylation by
PKB/Akt leads to poor breast cancer prognosis. Breast Cancer Res.
5:162–163. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franke TF, Kaplan DR and Cantley LC: PI3K:
downstream AKTion blocks apoptosis. Cell. 88:435–437. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burqering BM and Coffer PJ: Protein kinase
B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction.
Nature. 376:599–602. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Franke TF, Yang SI, Chan TO, et al: The
protein kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alessi DR, Andjelkovic M, Caudwell B, et
al: Mechanism of activation of protein kinase B by insulin and
IGF-1. EMBO J. 15:6541–6551. 1996.PubMed/NCBI
|
|
24
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rommel C, Clarke BA, Zimmermann S, et al:
Differentiation stage-specific inhibition of the Raf-MEK-ERK
pathway by Akt. Science. 286:1738–1741. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH
and Hung MC: Cytoplasmic localization of p21Cip1/WAF1 by
Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat
Cell Biol. 3:245–252. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Levine AJ: p53, the cellular gatekeeper
review for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agarwal ML, Taylor WR, Chernov MV,
Chernova OB and Stark GR: The p53 network. J Biol Chem. 273:1–4.
1998. View Article : Google Scholar
|
|
31
|
Schwartz GK and Shah MA: Targeting the
cell cycle: a new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiangsu New Medical College. Dictionary of
Chinese Materia Medica. Shanghai Sci Techno Press; Shanghai:
1997
|
|
33
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the Peoples Republic of China. Chin Med Sci
Technol Press. 1:109–110. 2010.
|
|
34
|
Tan P, Lu BZ and Bao WL: Analysis on the
clinical application of Scutellaria barbata D. Don in the
anti-cancer therapy. J Jiangxi Tradit Chin Med. 37:57–58. 2006.
|
|
35
|
Qian B: Clinical Effect of Anticancer
Chinese Medicine. Shanghai Transl Publ House; Shanghai: pp.
111–112. 1987
|
|
36
|
Cha YY, Lee EO, Lee HJ, et al: Methylene
chloride fraction of Scutellaria barbata induces apoptosis
in human U937 leukemia cells via the mitochondrial signaling
pathway. Clin Chim Acta. 348:41–48. 2004.PubMed/NCBI
|
|
37
|
Dai ZJ, Liu XX, Tang W, et al: Antitumor
and immune-modulating effects of Scutellaria barbata extract
in mice bearing hepatocarcinoma H22 cells-derived tumor. Nan Fang
Yi Ke Da Xue Xue Bao. 28:1835–1837. 2008.(in Chinese).
|
|
38
|
Goh D, Lee YH and Ong ES: Inhibitory
effects of a chemically standardized extract from Scutellaria
barbata in human colon cancer cell lines, LoVo. J Agric Food
Chem. 53:8197–8204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marconett CN, Morgenstern TJ, San Roman
AK, Sundar SN, Singhal AK and Firestone GL: BZL101, a phytochemical
extract from the Scutellaria barbata plant, disrupts
proliferation of human breast and prostate cancer cells through
distinct mechanisms dependent on the cancer cell phenotype. Cancer
Biol Ther. 10:397–405. 2010.PubMed/NCBI
|
|
40
|
Suh SJ, Yoon JW, Lee TK, et al:
Chemoprevention of Scutellaria barbata on human cancer cells
and tumorigenesis in skin cancer. Phytother Res. 21:135–141.
2007.
|
|
41
|
Wong BYY, Nguyen DL, Lin T, et al: Chinese
medicinal herb Scutellaria barbata modulates apoptosis and
cell survival in murine and human prostate cancer cells and tumor
development in TRAMP mice. Eur J Cancer Prev. 18:331–341. 2009.
|
|
42
|
Zhao Z, Holle L, Song W, Wei Y, Wagner TE
and Yu X: Antitumor and anti-angiogenic activities of
Scutellaria barbata extracts in vitro are partially
mediated by inhibition of Akt/protein kinase B. Mol Med Rep.
5:788–792. 2011.
|
|
43
|
Wei LH, Chen YQ, Lin JM, et al:
Scutellaria barbata D. Don induces apoptosis of human colon
carcinoma cell through activation of the mitochondrion-dependent
pathway. J Med Plants Res. 5:1962–1970. 2011.
|
|
44
|
Wei LH, Lin JM, Xu W, Hong ZF, Liu XX and
Peng J: Inhibition of tumor angiogenesis by Scutellaria
barbata D. Don via suppressing proliferation, migration and
tube formation of endothelial cells and downregulation of the
expression of VEGF-A in cancer cells. J Med Plants Res.
5:3260–3268. 2011.
|
|
45
|
Wei LH, Lin JM, Xu W, et al:
Scutellaria barbata D. Don inhibits tumor angiogenesis via
suppression of Hedgehog pathway in a mouse model of colorectal
cancer. Int J Mol Sci. 13:9419–9430. 2012. View Article : Google Scholar
|