Introduction
Gastric cancer is a significant worldwide health
issue, accounting for approximately one million new cases and more
than 700,000 cancer-related deaths in 2008 (1). The majority of gastric cancer cases
occurs in developing countries, and these regional variations may
in part reflect differences in dietary patterns and the prevalence
of Helicobacter pylori infection (2). Gastric cancer progression during the
late stages significantly contributes to treatment failure, similar
to most other cancers. Previous studies have shown that cancer
cells obtain an interstitial cell phenotype and characteristics
associated with invasive capacity via epithelial-mesenchymal
transition (EMT), leading to invasion into surrounding tissues or
distant organs (cancer metastasis) (3). However, to date, it remains to be
defined how gastric cancer cells acquire EMT and malignant
transformation. Thus, research on this topic could provide novel
targets for gastric cancer treatment and prevention.
Helicobacter pylori infection plays a
significant role in gastric cancer development and progression
(4), while cluster of
differentiation 14 (CD14) functions as a co-receptor with either
Toll-like receptor TLR4 or MD-2 in the detection of bacterial
lipopolysaccharide (LPS) and plays a role in the innate immune
system (5,6). Another study showed that LPS, the main
component of Gram-negative bacteria endotoxin, is able to induce
EMT of cancer cells (7).
Specifically, membrane CD14, together with TLR4, binds to LPS and
transmits signals into the nucleus, activating the release of a
series of cytokines (8,9), which may promote gastric
carcinogenesis. For example, a previous study found that certain
polymorphisms of the CD14 gene promoter region are associated with
the susceptibility to gastric cancer, which could be due to
alterations in CD14 expression (10).
CD14 also affects the apoptosis of cancer cells and
regulates cell cycle distribution through nuclear transcription
factors (11,12). These data clearly indicate that CD14
plays a role in gastric carcinogenesis; however, the underlying
mechanisms remain to be determined. Thus, in the present study we
aimed to ascertain whether CD14 affects gastric cancer cell EMT and
invasion using shRNA technology and the underlying mechanisms
responsible for CD14-mediated tumor cell EMT and invasion. These
studies could provide experimental evidence for future treatment
and prognosis of gastric cancer.
Materials and methods
Cell lines and culture
Gastric cancer cell lines (SGC-7901, MGC-803,
BGC-823 and MKN-28) were provided by the Institute of Biochemistry
and Cell Biology, Chinese Academy of Sciences (Shanghai, China) and
were cultured in RPMI-1640 medium containing 10% fetal bovine serum
(FBS), 100 U/ml penicillin and 100 mg/l streptomycin (Solarbio,
Beijing, China) at 37°C in a humidified incubator with 5%
CO2 and 95% air. The cells were passaged every 2–3 days
with 0.05% trypsin-EDTA.
RNA isolation and RT-PCR
Total RNA was extracted from cultured cells using
TRIzol reagent, and reversely transcribed into cDNA using the cDNA
First Strand synthesis kit (both from Beijing Tiangen Company,
Beijing, China) according to the manufacturer’s instructions. PCR
was performed in a 20 μl volume containing 1 μl of cDNA template, 1
μl of upstream and downstream primers, 10 μl of 2X TaqPCR Master
Mix (Beijing Tiangen Company) and 7 μl of water from the Ultrapure
Water Polishing System (Heal Force, Hong Kong, China). The primers
used in this study are listed in Table
I and were synthesized by Sangon Company (Shanghai, China). PCR
conditions were set at an initial 95°C for 5 min, followed by 30
cycles of 95°C for 20 sec, 58°C for 20 sec, and 72°C for 30 sec,
and a final extension at 72°C for 5 min. The PCR products were then
separated using electrophoresis on a 1.5% agarose gel. β-actin mRNA
was used as an internal control for semi-quantitative analyses of
target genes.
 | Table IPCR primers used in the present
study. |
Table I
PCR primers used in the present
study.
| Gene | Sequences | Expected PCR product
size (bp) |
|---|
| CD14 |
5′-TCAGAGGTTCGGAAGACTTATCG-3′
5′-CTTTAGAAACGGCTCTAGGTTGAGA-3′ | 239 |
| E-cadherin |
5′-ATGCCGCCATCGCTTACAC-3′
5′-CGACGTTAGCCTCGTTCTCA-3′ | 285 |
| N-cadherin |
5′-CAACACACTCGCAGACGCTCA-3′
5′-AAGACGGCTCCAGGCAGTTT-3′ | 228 |
| Vimentin |
5′-CAACACACTCGCAGACGCTCA-3′
5′-AAGACGGCTCCAGGCAGTTT-3′ | 160 |
| TNF-α |
5′-GTCTCCTACCAGACCAAGGTCAAC-3′
5′-CACAGGGCAATGATCCCAAAGTAG-3′ | 221 |
| TGF-β |
5′-CAACAATTCCTGGCGATACC-3′
5′-GCTAAGGCGAAAGCCCTCAAT-3′ | 136 |
Protein extraction and western
blotting
Total cellular protein was extracted from the cells
in RIPA lysis buffer, and the protein concentration was quantified
using the BCA method. Protein lysis containing 40 μg protein was
separated using sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto PVDF membranes
(Millipore, Bedford, MA, USA). The membranes were then incubated
with 5% skim milk solution in phosphate-buffered saline (PBS) for
50 min and then with the primary antibody (i.e., CD14 at a dilution
of 1:500; TNF-α at 1:1,000; TGF-β1 at 1:500; E-cadherin at 1:800;
N-cadherin at 1:1,000; vimentin at 1:600) at 4°C overnight.
Anti-TNF-α antibody was purchased from R&D Systems
(Minneapolis, MN, USA), anti-CD14, anti-vimentin and anti-TGF-β1
antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA, USA), and anti-TNF-α, anti-E-cadherin and anti-N-cadherin
antibodies were obtained from Abcam (Cambridge, MA, USA). On the
following day, the membranes were washed with PBS/Tween-20 and then
incubated with a secondary antibody at a dilution of 1:4,000 for 45
min at 37°C. Following washing with PBS-Tween-20 in triplicate, the
membranes were incubated with enhanced chemiluminescence (ECL)
substrate for visualizing the positive protein bands following
expose to X-ray film. The membranes were then stripped and
re-blotted with anti-β-actin antibody as the internal control.
Construction of the CD14 shRNA vector and
gene transfection
In the present study, we designed 4 siRNA primers to
knockdown CD14 expression and 1 unrelated interference sequence
according to a previously published method (13), while GAPDH interference sequences
were used as the positive control. These DNA sequences were
synthesized by GenePharma (Shanghai, China) and were used for
transfection into MGC-803 cells. The interference efficiency was
measured 48 h following transfection using western blotting (data
not shown). The CD14 siRNA with the highest interference efficiency
was selected for expression vector construction, i.e., the DNA
sequences (5′-GGTACTGAGCATTGCCCAA-3′) were located at 898
nucleotides of CD14 mRNA (NM_000591) and used for vector
construction. The corresponding sense and antisense
oligonucleotides were: 5′-GATCCCCGGTACTG
AGCATTGCCCAATTCAAGAGATTGGGCAATGCTCA GTACCTTTTT-3′ and
5′-AGCTAAAAAGGTACTGAG CATTGCCCAATCTCTTGAATTGGGCAATGCTCAGTA
CCGGG-3′, respectively. These 2 oligonucleotides were digested with
BamHI and HindIII (Takara, Dalian, China) and then
annealed and ligated into pGCsi-H1/Neo (GenePharma). Following
amplification and DNA sequencing, this vector plus negative control
vectors were used for the subsequent experiments. To knockdown CD14
expression, gastric cancer MGC-803 cells were seeded onto 6-well
plates with RPMI-1640 media containing 10% FBS and grown overnight
to reach 80–90% confluence. On the following day, cells were
transfected with CD14 shRNA and negative control plasmids, using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. After 5 h of
transfection, the media were replaced with RPMI-1640 media
containing 20% FBS. Transfection efficiency was then determined 48
h later using a fluorescence microscope. The cells were further
cultured in a complete media containing 800 μg/ml G418 (Invitrogen
Life Technologies) and the media were replaced every 2–3 days for 2
weeks. G418 concentration was reduced to 400 μg/ml. Once the cells
had a strong expression of green fluorescence, the cells were
expanded and then used for the experiments.
Lipopolysaccharides and TNF-α
treatment
The stable CD14-knockdown and negative control cells
were cultured routinely in RPMI-1640 media containing 10% FBS, 100
U/ml penicillin, 100 mg/l streptomycin and 400 μg/ml G418. Cells at
a logarithmic growth phase were then treated with
lipopolysaccharides (LPS at 1 μg/ml) for 4 h. The cells were
grouped for the following experiments. The treatment groups were as
follows: control cells, sh-CD14-alone cells, LPS-alone cells, LPS +
sh-CD14 cells, LPS + sh-CD14 + TNF-α cells.
Tumor cell Transwell invasion assay
To determine the effects of CD14 knockdown on the
invasive ability of the cells with or without LPS treatment, we
inoculated cells (2×105/well) into the upper chamber of
Transwell plates (Corning Incorporated, Corning, NY, USA) coated
with Matrigel (BD Biosciences, San Jose, CA, USA). In the lower
chamber, RPMI-1640 medium containing 10% FBS was added, and the
cells were cultured for 48 h. The cells remaining on the surface of
the upper chamber were removed using cotton swaps, and the cells
that had invaded onto the bottom surface were fixed with 40 ml/l
formaldehyde for 15 min and stained with hematoxylin for 2 min. The
cells were evaluated and counted under a light microscope. Five
fields were chosen for each well, and the numbers of invaded cells
were recorded.
Statistical analyses
Statistical analyses were performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). The experimental data are
expressed as means ± SD. The Student’s t-test was used for
comparison between 2 groups. P<0.05 was considered to indicate a
statistically significant result.
Results
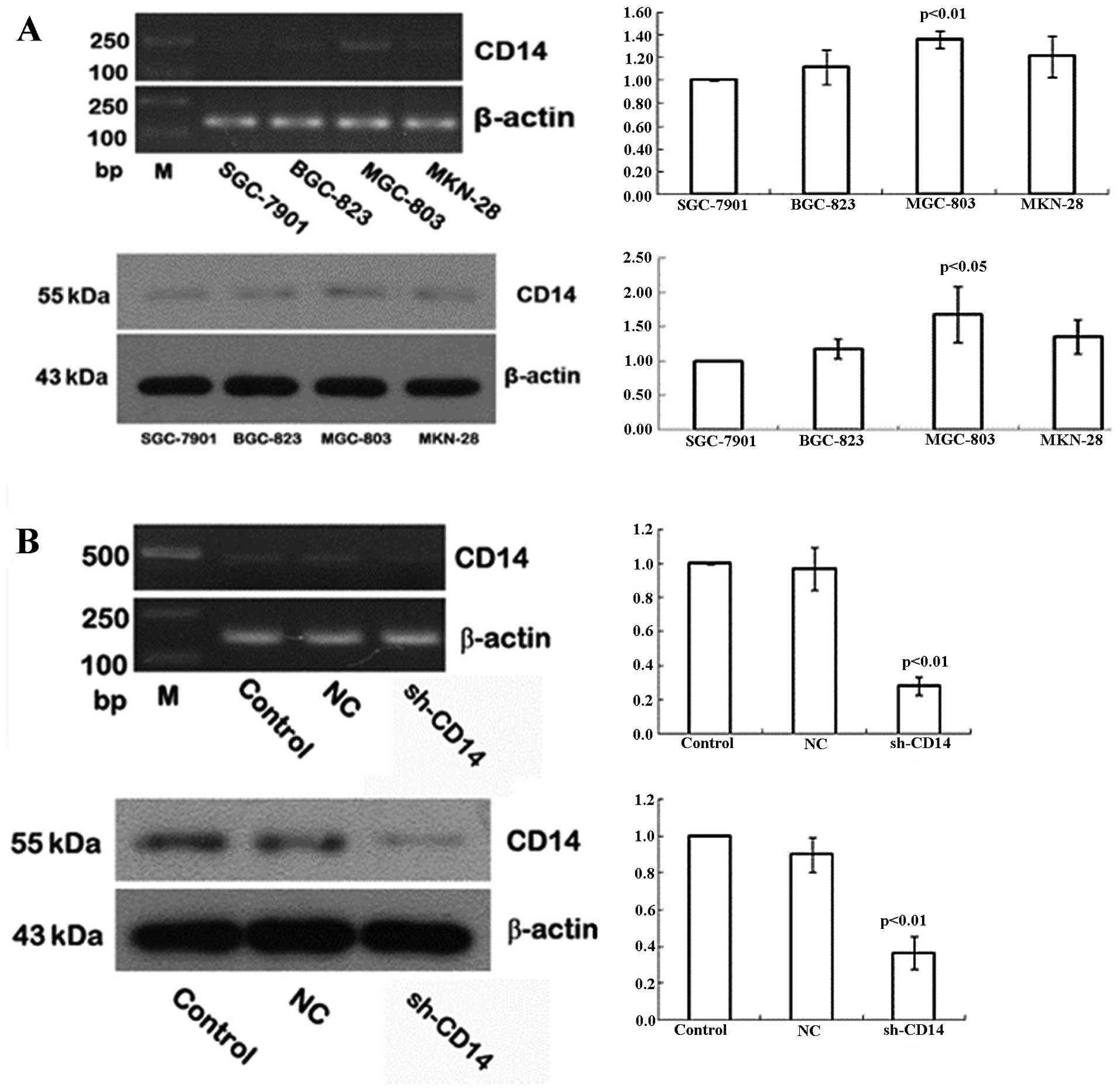
Expression of CD14 in gastric cancer cell
lines
In the present study, we assessed CD14 expression in
the different gastric cancer cell lines using semi-quantitative
RT-PCR and western blotting. We found that the highest levels of
CD14 mRNA and protein were detected in MGC-803 cells, followed by
MKN-28 and BGC-823, whereas SGC-7901 had the lowest levels of CD14
mRNA and protein (Fig. 1A). Thus,
we selected the MGC-803 cell line for subsequent CD14-knockdown
experiments.
Establishment of stable CD14-knockdown
cells
To assess the role of CD14 protein in gastric
cancer, we used CD14 shRNA to knockdown CD14 expression in the
MGC-803 gastric cancer cell line and negative control shRNA was
used as a control. Following 48 h of gene transfection, green
fluorescent-positive cells were identified as CD14-shRNA. After 4
weeks of G418 selection, 90% of the cells produced green
fluorescence, indicating that cells achieved stable transfection of
CD14-shRNA. Expression of CD14 at the mRNA and protein levels was
then detected in the stable cells, and the data showed that the
levels were reduced by 72 and 63%, respectively, when compared to
the control cells (Fig. 1B).
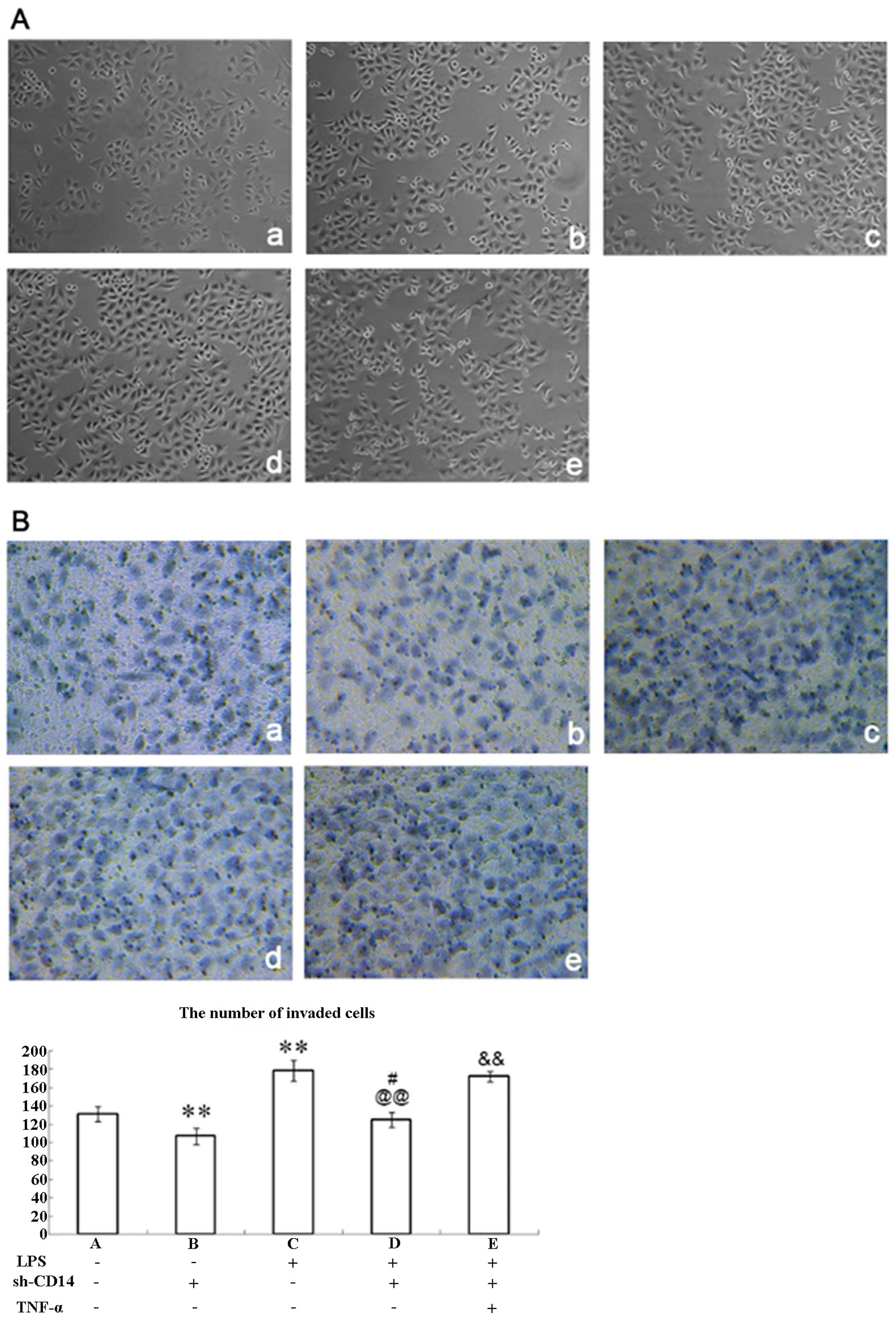
Changes in cell morphology following CD14
knockdown
Parental MGC-803 cells exhibited prismatic or
polygonal morphology with adherent growth (Fig. 2A-a), while stable CD14-knockdown
cells did not show significant changes in morphology (Fig. 2A-b). Moreover, previous studies have
demonstrated that LPS can bind to CD14 and promote TNF-α expression
in cells, which in turn increases TGF expression and induces tumor
cells to EMT (14,15). Therefore, we treated these
CD14-knockdown cells with LPS for 4 h and found that these cells
were altered to a sporadic long spindle shape (Fig. 2A-c and -d) when compared to the
parental or negative control vector-transfected cells. In addition,
TNF-α treated cells were further elongated, connections between
cells were reduced, the gap between the cells was increased, and
the cells were transformed into mesenchymal cells when compared to
the LPS + sh-CD14 cells (Fig.
2A-e).
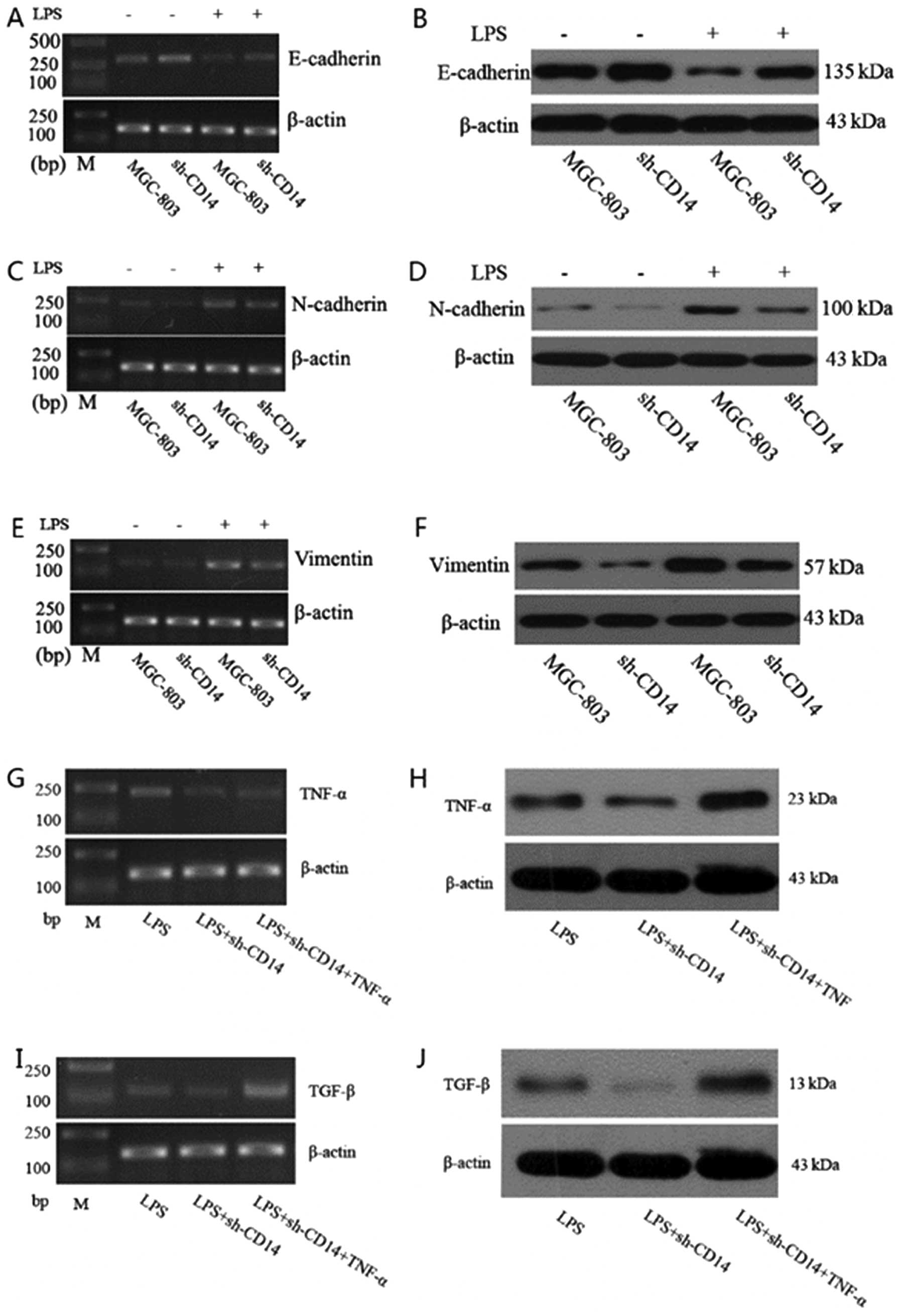
Effects of CD14 knockdown on regulation
of LPS-induced EMT in gastric cancer cells
We further confirmed the effects of CD14 knockdown
on gastric cancer cells. We found that CD14 knockdown increased
expression of E-cadherin protein (P<0.05), but reduced
expression of N-cadherin and vimentin proteins (P<0.05). Since
LPS was able to activate CD14 protein, we treated these cells with
LPS for 4 h and found that LPS treatment of parental MGC-803 cells
significantly reduced E-cadherin expression (P<0.01), whereas
N-cadherin and vimentin expression were significantly increased
(P<0.01) compared to the untreated cells, indicating that LPS
promotes EMT in gastric cancer cells. However, in the stable
CD14-knockdown cells, LPS treatment significantly increased
E-cadherin expression (P<0.05), while N-cadherin and vimentin
expression was significantly reduced (P<0.05) compared to the
cells treated with LPS only, indicating that in the event of lack
of CD14 expression, LPS treatment does not induce EMT (Fig. 3A–F).
Effect of CD14 knockdown on expression of
TNF-α and TGF-β
We determined the effects of CD14 knockdown on
expression of TNF-α and TGF-β. Compared to LPS-treated parental
MGC-803 cells, expression of TNF-α and TGF-β in the LPS-treated
CD14-knockdown cells was significantly reduced (P<0.05).
Moreover, expression of TNF-α and TGF-β was significantly higher in
the LPS and TNF-α-treated CD14-knockdown cells than that of the
LPS-treated parental shRNA-transfected cells (Fig. 3G–J).
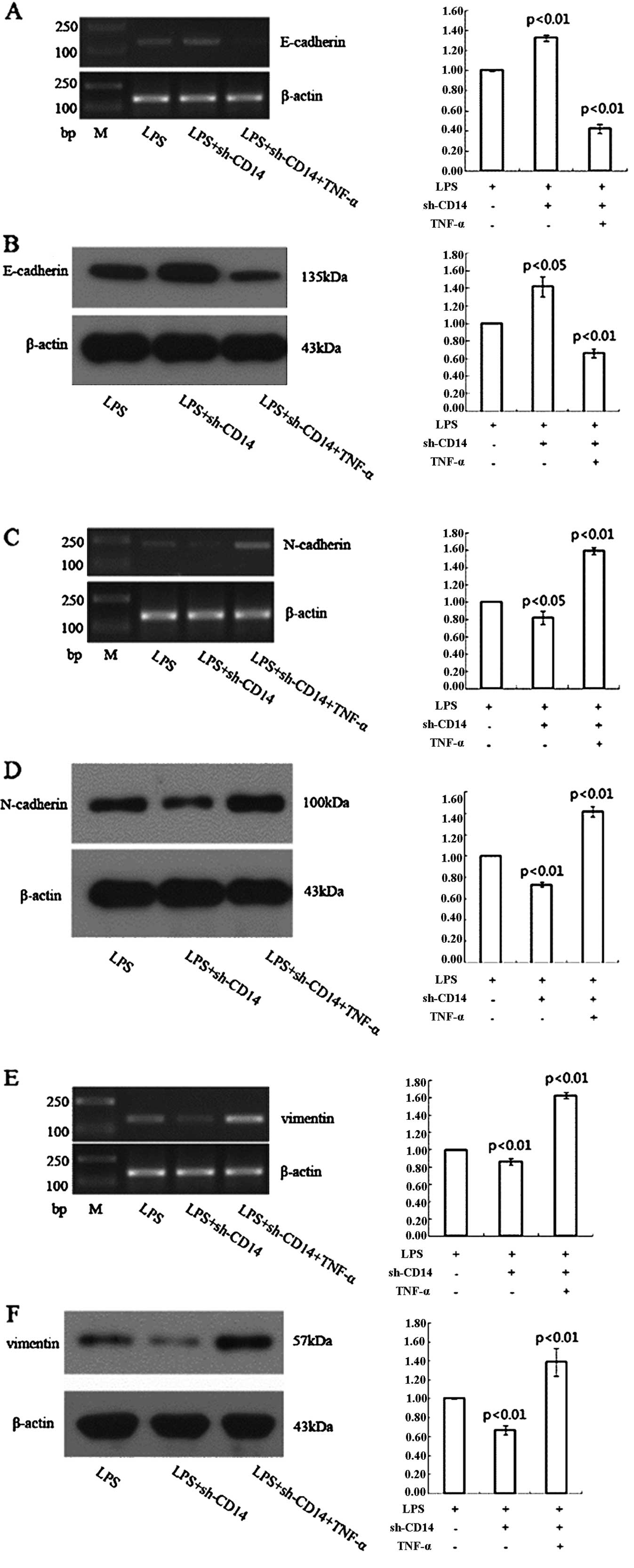
Effect of CD14 knockdown on the
regulation of gastric cancer cell EMT through TNF-α
LPS treatment of the CD14 knockdown MGC-803 cells
resulted in a higher expression of E-cadherin and lower expression
of N-cadherin and vimentin when compared to the LPS-treated
negative control shRNA-transfected cells (Fig. 4). However, TNF-α treatment of these
cells significantly reduced E-cadherin expression and increased
expression of N-cadherin and vimentin compared to LPS-treated
CD14-knockdown MGC-803 cells (Fig.
4).
Effect of CD14 knockdown on the
regulation of gastric cancer cell invasion
Tumor cell EMT usually results in cell migration and
invasion. Therefore, we assessed tumor cell invasion capacity
following CD14 knockdown and LPS treatment. Indeed, the invasive
capacity of CD14-knockdown cells was significantly lower than that
of the negative control shRNA-transfected MGC-803 cells (P<0.01;
Fig. 2B-a and -b). LPS-treated
parental MGC-803 cells had significantly higher invasive capacity
than that of the control cells without LPS treatment (P<0.05;
Fig. 2B-c), whereas LPS-treated
CD14-knockdown cells showed significantly lower levels of tumor
cell invasion than that of the LPS-treated parental MGC-803 cells
(P<0.01; Fig. 2B-d).
Nevertheless, addition of TNF-α to LPS-treated CD14-knockdown cells
significantly increased invasion (P<0.01; Fig. 2B-e). These results indicate that
CD14 can facilitate cancer cell invasion via upregulation of
TNF-α.
Discussion
Cancer invasion and metastases contribute to
advanced stage disease and increased cancer-related deaths.
Molecularly, they are the consequence of multiple factorial
interactions. For cancer metastases, induced tumor cell migration
and invasion through cell connection disruption are essential
steps. Although EMT is a normal physiological process during
embryonic development and organ formation or even during fibrosis,
induction of tumor cell EMT can cause tumor migration, invasion and
distant metastasis (16).
Molecularly, E-cadherin plays an important role in maintenance of
epithelial cell polarity and tight junctions between cells.
Reduction in E-cadherin expression may promote EMT. In contrast to
E-cadherin, N-cadherin is commonly found in cancer cells and
provides a mechanism for transendothelial migration; thus promoting
tumor cell invasion and metastasis (17). A previous study hypothesized that
conversion of E-cadherin to N-cadherin leads to EMT (18). Moreover, vimentin is a marker of
mesenchymal cells and is normally expressed at a low level or is
absent in epithelial cells (19).
However, vimentin is highly expressed in invasive tumor cells or
during the EMT process. Therefore, downregulation of E-cadherin or
upregulation of N-cadherin and vimentin are considered markers of
EMT. In the present study, we found that knockdown of CD14
expression increased E-cadherin expression, but decreased
N-cadherin and vimentin expression. Our data indicate that CD14
protein is positively associated with EMT of gastric cancer cells.
Further study showed that knockdown of CD14 expression reduced
gastric cancer cell invasive capacity, even following LPS
treatment.
LPS is the main component of endotoxin in
Gram-negative bacteria, e.g., H. pylori infection is a major
cause of chronic gastritis and is an initial factor in gastric
cancer (20). In the present study,
we found that LPS treatment reduced E-cadherin expression and
enhanced N-cadherin and vimentin expression in gastric cancer
cells, indicating that LPS promotes EMT of gastric cancer cells.
Furthermore, CD14 normally binds to LPS to form a
LPS-CD14//TLR4-MD2 complex, which results in the activation NF-κB
and other transcriptional factors (21–23).
However, our present data demonstrated that CD14 knockdown enhanced
E-cadherin but reduced N-cadherin and vimentin expression in the
LPS-treated cells and in turn inhibited LPS-induced EMT. Thus, the
role of CD14 in LPS-induced EMT may play an important role in
gastric cancer development and progression.
TNF-α, an important inflammatory factor, was
reported to induce EMT and promote tumor cell migration and
invasion through stabilization of Snail and the NF-κB pathway
(24). To further clarify the
mechanisms of CD14 in promoting EMT of gastric cancer cells, our
present data showed that CD14 knockdown significantly reduced TNF-α
expression, which was associated with enhanced expression of
E-cadherin and decreased expression of N-cadherin and vimentin.
Most importantly, CD14 knockdown did not affect expression of
E-cadherin, N-cadherin and vimentin in cells treated with TNF-α.
These data indicate that TNF-α expression may mediate the effects
of CD14 knockdown on inhibition of LPS-induced EMT. Furthermore,
TGF-β is a potent EMT-promoting factor in a variety of epithelial
and cancer cells (25). TGF-β
inhibits tumor growth and promotes apoptosis in the early stages of
tumor development, but during the late stages, cancer cells lose
the sensitivity to TGF-β and secrete a large amount of TGF-β,
leading to tumor invasion via EMT (26). Our present data showed that CD14
knockdown significantly reduced TGF-β expression, which suggests
that TGF-β participates in CD14-induced gastric cancer cell EMT
process. However, further study is needed to verify how these genes
are activated by CD14. In addition, although CD14 is a high
affinity receptor for LPS, it does not have transmembrane regions
and requires the TLR4 to transmit the signals into the nucleus.
Previous studies have shown that TLR4-induced liver fibrosis and
immune escape of lung cancer cells were mediated by promotion of
TGF-β expression (27,28). A previous study also demonstrated
that supernatant from CD14-overexpressing cells significantly
promoted tumor cell invasion (29).
Thus, we suspect that the impact of CD14 on EMT may also be
associated with the TLR4 pathway, but we did not provide
experimental data to support it, which is a limitation of this
study. Expression of CD14 and TLR4 was found to be significantly
higher in highly invasive cancer cells (e.g., prostate cancer PC3
cells) than levels in cells with low invasiveness (30). These data indicate that CD14 needs
to work with TLR4 for functionality.
In summary, the present study is a
proof-of-principle study to demonstrate the role of CD14 in
mediating the EMT and invasion of gastric cancer cells in
vitro. Future studies will provide mechanistic data to support
the current findings in vitro and in vivo.
Acknowledgements
This study was supported in part by a grant from the
National Natural Science Foundation of China (no. 81060165).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji J: Chinese guidelines for diagnosis and
treatment of gastric cancer (2011 edition). Transl Gastrointest
Cancer. 1:103–114. 2012.
|
|
3
|
Thiery JP: Epithelial-mesenchymal
transitions in tumor progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herrera V and Parsonnet J: Helicobacter
pylori and gastric adenocarcinoma. Clin Microbiol Infect.
15:971–976. 2009. View Article : Google Scholar
|
|
5
|
Wright SD, Ramos RA, Tobias PS, Ulevitch
RJ and Mathison JC: CD14, a receptor for complexes of
lipopolysaccharide (LPS) and LPS binding protein. Science.
249:1431–1433. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pugin J, Heumann ID, Tomasz A, Kravchenko
VV, Akamatsu Y, Nishijima M, Glauser MP, Tobias PS and Ulevitch RJ:
CD14 is a pattern recognition receptor. Immunity. 1:509–516. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC
and Lee CH: Resveratrol inhibits LPS-induced epithelial-mesenchymal
transition in mouse melanoma model. Innate Immun. 18:685–693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gioannini TL and Weiss JP: Regulation of
interactions of Gram-negative bacterial endotoxins with mammalian
cells. Immunol Res. 39:249–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyake K: Innate immune sensing of
pathogens and danger signals by cell surface Toll-like receptors.
Semin Immunol. 19:3–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao D, Sun T, Zhang X, Guo Y, Yu D, Yang
M, Tan W, Wang G and Lin D: Role of CD14 promoter polymorphisms in
Helicobacter pylori infection-related gastric carcinoma.
Clin Cancer Res. 13:2362–2368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanczkowski W, Tymoszuk P,
Ehrhart-Bornstein M, Wirth MP, Zacharowski K and Bornstein SR:
Abrogation of TLR4 and CD14 expression and signaling in human
adrenocortical tumors. J Clin Endocrinol Metab. 95:E421–E429. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zanoni I, Ostuni R, Capuano G, Collini M,
Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G,
Costa B, Zaza A, Ricciardi-Castagnoli P and Granucci F: CD14
regulates the dendritic cell life cycle after LPS exposure through
NFAT activation. Nature. 460:264–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reynolds A, Leake D, Boese Q, Scaringe S,
Marshall WS and Khvorova A: Rational siRNA design for RNA
interference. Nat Biotechnol. 22:326–330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawata M, Koinuma D, Ogami T, Umezawa K,
Iwata C, Watabe T and Miyazono K: TGF-β-induced
epithelial-mesenchymal transition of A549 lung adenocarcinoma cells
is enhanced by pro-inflammatory cytokines derived from RAW 264.7
macrophage cells. J Biochem. 151:205–216. 2012.
|
|
15
|
Bates RC and Mercurio AM: Tumor necrosis
factor-α stimulates the epithelial-to-mesenchymal transition of
human colonic organoids. Mol Biol Cell. 14:1790–1800. 2003.
|
|
16
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada
M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, Kawaguchi Y,
Fujimoto K, Hosotani R and Imamura M: N-cadherin expression and
epithelial-mesenchymal transition in pancreatic carcinoma. Clin
Cancer Res. 10:4125–4133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cavallaro U, Schaffhauser B and
Christofori G: Cadherins and the tumour progression: is it all in a
switch? Cancer Lett. 176:123–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lang SH, Hyde C, Reid IN, Hitchcock IS,
Hart CA, Bryden AA, Villette JM, Stower MJ and Maitland NJ:
Enhanced expression of vimentin in motile prostate cell lines and
in poorly differentiated and metastatic prostate carcinoma.
Prostate. 52:253–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polk DB and Peek RM Jr: Helicobacter
pylori: gastric cancer and beyond. Nature Rev Cancer.
10:403–414. 2010. View
Article : Google Scholar
|
|
21
|
Triantafilou M and Triantafilou K: The
dynamics of LPS recognition: complex orchestration of multiple
receptors. J Endotoxin Res. 11:5–11. 2005.PubMed/NCBI
|
|
22
|
Le Roy D, Di Padova F, Adachi Y, Glauser
MP, Calandra T and Heumann D: Critical role of
lipopolysaccharide-binding protein and CD14 in immune responses
against gram-negative bacteria. J Immunol. 167:2759–2765.
2001.PubMed/NCBI
|
|
23
|
Schimke J, Mathison J, Morgiewicz J and
Ulevitch RJ: Anti-CD14 mAb treatment provides therapeutic benefit
after in vivo exposure to endotoxin. Proc Natl Acad Sci USA.
95:13875–13880. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of snail by NF-κB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009.
|
|
25
|
Iwano M: EMT and TGF-beta in renal
fibrosis. Front Biosci. 2:229–238. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zavadil J and Böttinger EP: TGF-β and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005.
|
|
27
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-β
signaling and hepatic fibrosis. Nat Med. 13:1324–1332. 2007.
|
|
28
|
He W, Liu Q, Wang L, Chen W, Li N and Cao
X: TLR4 signaling promotes immune escape of human lung cancer cells
by inducing immunosuppressive cytokines and apoptosis resistance.
Mol Immunol. 44:2850–2859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song MN and Cho SY: CD14 acts as an
angiogenic factor by inducing basic fibroblast growth factor
(bFGF). Bull Korean Chem Soc. 28:1613–1614. 2007. View Article : Google Scholar
|
|
30
|
Hua D, Liu MY, Cheng ZD, Qin XJ, Zhang HM,
Chen Y, Qin GJ, Liang G, Li JN, Han XF and Liu DX: Small
interfering RNA-directed targeting of Toll-like receptor 4 inhibits
human prostate cancer cell invasion, survival and tumorigenicity.
Mol Immunol. 46:2876–2884. 2009. View Article : Google Scholar : PubMed/NCBI
|