Introduction
Allyl isothiocyanate (AITC) is a cancer
chemopreventive phytochemical agent found in naturally occurring
dietary isothiocyanates (ITCs) (1–3). AITC
was found to have a major glucosinolate in several commonly
consumed cruciferous vegetables (cabbage, cauliflower and kale as
well as Brussels sprouts) (4,5).
Previous studies showed that AITC inhibited the growth of various
human cancer cell lines, such as colorectal carcinoma (5,6), lung
cancer (7), leukemia (4), breast adenocarcinoma (8), bladder cancer (5,9), brain
malignant glioma (10),
neuroblastoma (11), hepatoma
(12,13) and prostate cancer cells (2,14,15).
Of note, the half maximal inhibitory concentration
(IC50) values for the anticancer cell growth appear at
the low micromolar ranges of AITC (9). On the other hand, AITC appears to be
significantly less toxic to normal human bladder epithelial cells
(5,9). AITC is likely to attenuate tumor cell
growth by causing cell cycle arrest (8,10),
induction of cell apoptosis (1,2) and to
suppress metastasis via inhibition of invasion and migration in
neoplastic cells (5,16). The earlier studies in our laboratory
also demonstrated that AITC triggered G2/M phase arrest and
provoked apoptosis in MDA-MB-468 human breast adenocarcinoma cells
(8) and GBM 8401 human brain
glioblastoma multiforme cells (10).
Matrix metalloproteinases (MMPs) play key roles
during cancer cell metastasis (5,16). The
levels of cell adhesion, invasion and migration were suppressed
through the transcription of MMP-2/-9 by AITC at 0.1–5 μM in
SK-Hep-1 human liver cancer cells (16). However, few studies have addressed
the AITC-inhibited cell metastasis in epidermal growth factor
(EGF)-stimulated HT29 human colorectal cancer cells. The aim of the
present research was to determine the decrease of cell metastasis
in EGF-treated HT29 cells by AITC. The present study indicated that
AITC suppressed the extracellular signal-regulated kinase (ERK),
p38, c-Jun N-terminal kinase (JNK) mitogen-activated protein
kinase (MAPK) pathways and, thus, reduced the MMP-2 and -9, leading
to the inhibition of metastatic effects in EGF-stimulated HT29
cells in vitro. Furthermore, we examined the antimetastatic
effects of AITC by altering the cell cycle response-related gene
expression utilizing DNA microarray analysis. Our study is the
first to report that MAPK signals are pivotal for the
antimetastatic response of HT29 human colorectal adenocarcinoma
cells induced by AITC.
Materials and methods
Materials and reagents
RPMI-1640 medium, fetal bovine serum (FBS),
penicillin/streptomycin and trypsin-EDTA were bought from Gibco by
Life Technologies (Carlsbad, CA, USA). Millicell Hanging Cell
Culture Inserts (polyethylene terephthalate filters with 8 μm pore
size) were purchased from Merck Millipore Corp. (Billerica, MA,
USA). All primary antibodies for immunoblotting and horseradish
peroxidase (HRP)-conjugated secondary antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). AITC and
all other chemicals were purchased from Sigma-Aldrich Corp. (St.
Louis, MO, USA) unless otherwise specified.
Cell culture
The human colorectal adenocarcinoma HT29 cell line
was obtained from the Bioresource Collection and Research Center
(BCRC) of Food Industry Research and Development Institute
(Hsinchu, Taiwan). Cells were plated into 75-cm2 tissue
culture flasks and cultured in RPMI-1640 medium with 10% FBS, 100
U/ml penicillin and 100 μg/ml streptomycin in a humidified
atmosphere of 5% CO2 and 95% air at 37°C as previously
described (17,18). Cells were detached by 0.25%
trypsin/0.02% EDTA to keep cell growth.
Transwell cell invasion assay
HT29 cells at a density of 1×104
cells/0.4 ml serum-free RPMI-1640 medium were seeded with or
without (basal) 10 ng/ml EGF into the upper chambers (Millicell
Hanging Cell Culture Inserts; Merck Millipore Corp.) after
pre-coating with Matrigel (BD Biosciences, San Jose, CA, USA) in
the presence or absence of AITC at 5 and 10 μM. Each lower chamber
was filled with 600 μl of 10% FBS medium. After incubation for 24
h, the chambers were removed from the wells to measure invasive
ability and then fixed with methanol for 15 min before the
non-invasive cells were wiped with a cotton swab. Consequently,
cells were stained with 2% crystal violet for 10 min, and the
invaded cells were then photographed under a phase-contrast
microscope. The number of invasive cells was presented in the
membrane/filter of three random fields as previously described
(19,20). The invasion assay was performed with
a 100% support value of basal cells, and invasive cells were
quantified using NIH ImageJ 1.47 program.
Wound healing assay
HT29 cells in 6-well plates (~5×105
cells/well) were grown to 80% confluence, and cell monolayer was
scratched by a 200-μl pipette tip to create a gap of constant
width. Subsequently, cells were washed with PBS twice to remove
floating cells. Cells were exposed in the presence or absence of 10
ng/ml EGF and then treated with or without 5 and 10 μM of AITC in
serum-free RPMI-1640 medium for up to 24 h. The number of migrating
cells in the gap was captured under a phase-contrast microscope as
described elsewhere (21,22). Images for the scratch area of each
well were counted in three random fields from each triplicate
treatment. The number of migrated cells in EGF-untreated cells
(basal) was expressed at 100% and these in treated cells showed
related to the basal cells.
Total protein extraction and western blot
analysis
HT29 cells were incubated with or without 10 ng/ml
EGF and individually exposed to 5 and 10 μM of AITC. After a 24-h
treatment, each whole-cell protein extract was harvested and lysed
in the PRO-PREP protein extraction solution (Intron Biotechnology,
Seongnam-si, Gyeonggi-do, Korea). The protein concentration of the
cell lysate was estimated with the Bio-Rad protein assay kit
(Bio-Rad Laboratories, Hercules, CA, USA), and the total proteins
(40 μg) were electrophoresed by 10–12% SDS-PAGE before being
transferred and blotted using iBlot dry blotting system with
polyvinylidene difluoride (PVDF) membrane (Invitrogen by Life
Technologies). The membranes were blocked and probed first with
specific antibodies in blocking buffer at 4°C overnight as
previously described (23,24), followed by incubation with the
appropriate horseradish peroxide (HRP)-linked secondary antibodies.
Immobilon Western Chemiluminescent HRP substrate (Millipore) and
X-ray film (GE Healthcare, Piscataway, NJ, USA) were applied to
visualize, and the membranes were stripped and reprobed with
β-actin to normalize to the protein expression as described
elsewhere (25).
RNA extraction and expression
microarray
HT29 cells (1×106 cells/well) were
maintained in RPMI-1640 medium with 10 ng/ml EGF and treated with
or without 10 μM AITC for 24 h. After exposure, cell pellets were
subsequently harvested, and the total RNA from each treatment was
purified using the Qiagen RNeasy Mini kit (Qiagen, Valencia, CA,
USA) as previously described (23,26).
The RNA purity was determined to check the quality at 260 nm and
280 nm using a NanoDrop 1000 Spectrophotometer (Thermo Fisher
Scientific, Wilmington, DE, USA) (26,27).
Each sample (300 ng) was amplified and labeled using the GeneChip
WT Sense Target Labeling and Control Reagents kit (Affymetrix,
Santa Clara, CA, USA) for expression analysis. The synthesized cDNA
was labeled with fluorescence, and then hybridized for 17 h at 45°C
and 60 rpm using Affymetrix GeneChip Human Gene 1.0 ST array
(Affymetrix) to determine microarray hybridization following the
manufacturer’s recommendations.
The arrays were subsequently washed by Fluidics
Station 450 (Affymetrix) and stained with
streptavidin-phycoerythrin (GeneChip Hybridization, Wash and Stain
kit, Affymetrix), and were scanned on a GeneChip Scanner 3000
(Affymetrix). The localized concentrations of fluorescent molecules
were quantitated and analyzed using Expression Console software
(Affymetrix) with default RMA parameters as previously described
(28,29). The gene expression level of a
1.8-fold-change altered by AITC was considered a difference.
Statistical analysis
Each experiment was performed in triplicate and
expressed as the means ± standard deviation (SD) of analysis. The
results were assessed by one-way ANOVA followed by Bonferroni’s
multiple comparison test, and the value of P<0.05 indicated
statistically significant differences from other treatments.
Results
AITC suppresses EGF-stimulated invasion
of HT29 cells
To investigate the effects of AITC on HT29 cell
invasion in vitro, Transwell cell invasion assay was
performed to determine the invasive ability of the HT29 cells with
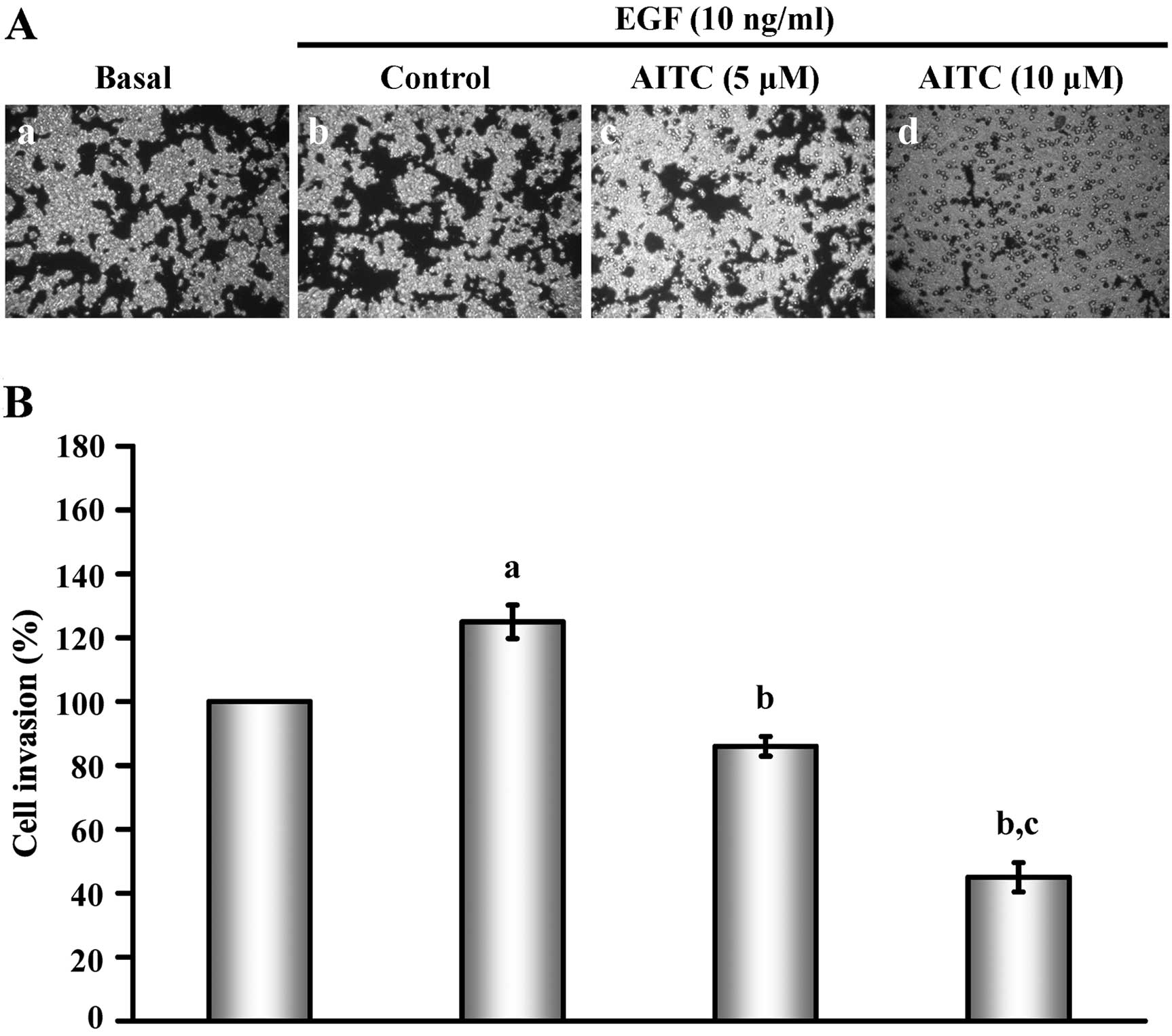
or without EGF stimulation. Results in Fig. 1A show that AITC at 5 and 10 μM
markedly decreased the invasion of HT29 cells induced by EGF
relative to the control group. In addition, the invasive ability
was reduced in a concentration-dependent manner (Fig. 1B). Our data demonstrated that AITC
may effectively inhibit the invasion of HT29 cells in
vitro.
AITC inhibits EGF-triggered HT29 cell
migration
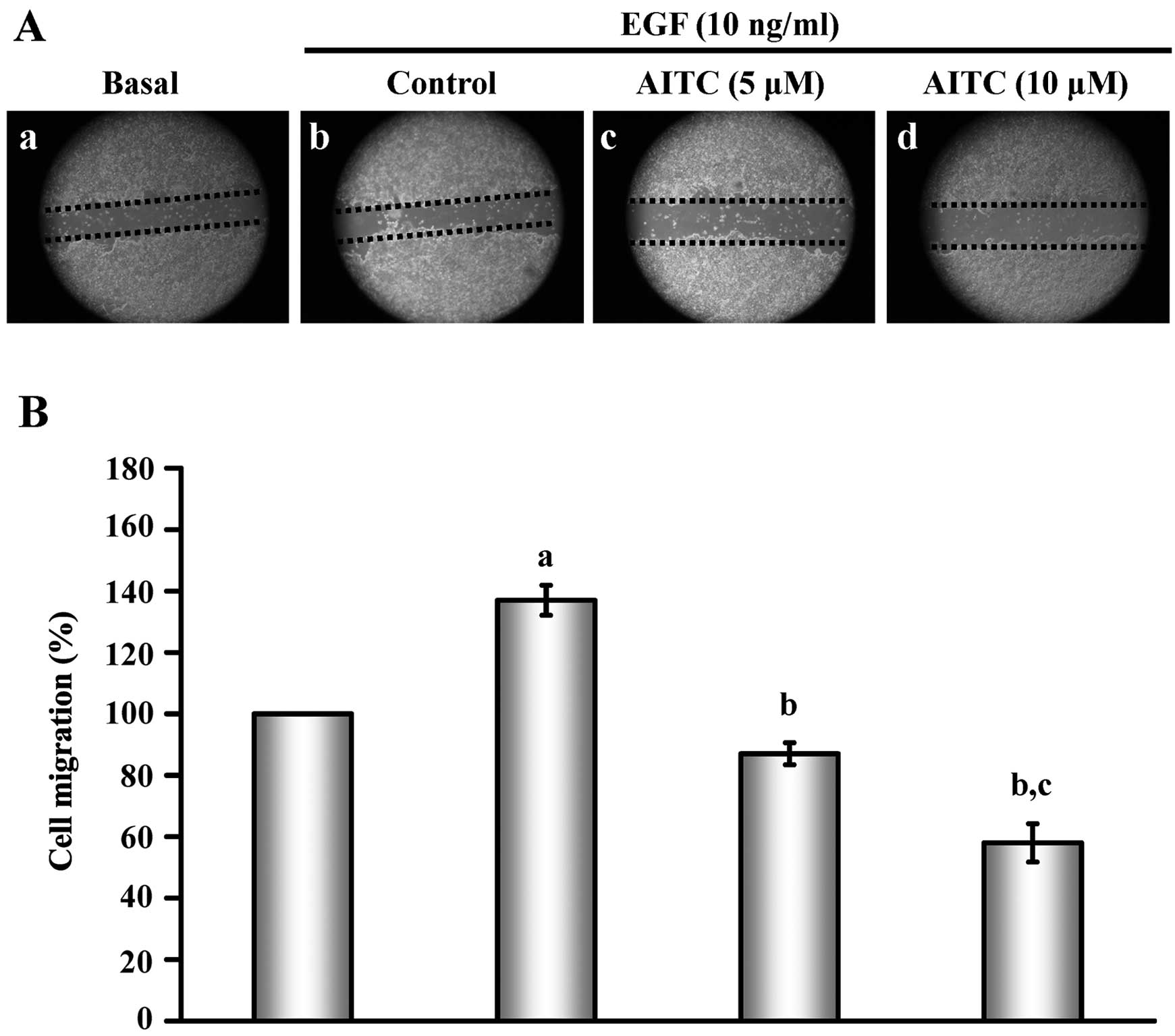
As shown in Fig. 2,
using the wound healing assay, the concentrations of 5 and 10 μM of
AITC markedly inhibited HT29 cell migration (Fig. 2A) by ~48 and 81%, respectively,
(Fig. 2B) after a 24-h incubation
when compared with EGF-induced migration of HT29 cells. Based on
these findings, we concluded that the migratory ability occurred in
AITC-treated HT29 cells.
AITC alters the abundance of protein
level with metastatic response in HT29 cells
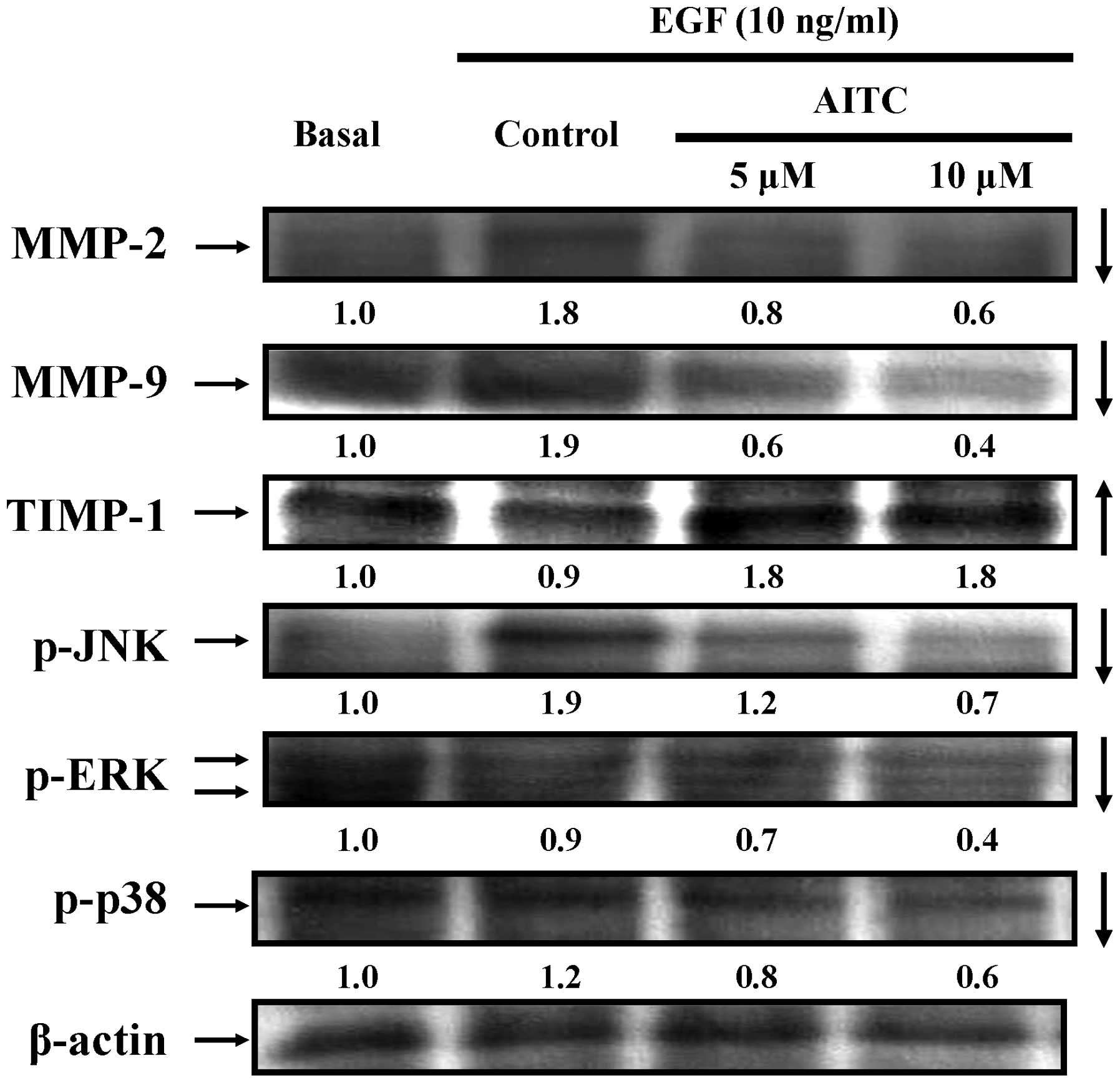
Next, we clarified if AITC-suppressed migration and
invasion is mediated through downregulation of associated protein
signals. Treatment of EGF-treated HT29 cells with 5 and 10 μM of
AITC for 24 h, and data shown in Fig.
3 revealed that AITC decreased the protein expressions of MMP-2
and MMP-9 in EGF-treated HT29 cells. Alternatively, the level of
the tissue inhibitor of metalloproteinase-1 (TIMP-1) was increased
in examined HT29 cells (Fig. 3). We
further determined the effect of AITC on the MAPK signaling
pathways. Our results indicated that AITC inhibited the
phosphorylation of JNK, ERK and p38 signaling in HT29 cells after
EGF exposure (Fig. 3). Based on
these results, we found that AITC affected MMP-9/-2, TIMP-1 and
MAPKs signaling in EGF-treated HT29 cells in vitro.
Microarray analysis
Data were analyzed to examine the expressed genes in
EGF-stimulated HT29 cells treated with or without AITC as can be
seen in Table I. We showed that the
transcripts of 58 genes were upregulated, while these of 24 genes
were downregulated in HT29 cells exposed to AITC after EGF
induction, respectively. Our results revealed that AITC regulated
the expression of important genes that control cell growth (AKR1C2,
AKR1C1, AKR1C1, ALDH3A1, TXNRD1 and ANKRD11), G-protein coupled
receptor (AKR1C1 and AKR1C3), angiogenesis (HMOX1, MT1G and VEZF1),
cell adhesion (TROAP, ITGB1 and EZR), cell cycle and mitosis
(CSNK1A1, CDC20, BIRC5, KIF20A, CSNK1A1, ITGB1 and LIMA1), cell
migration (HMOX1 and ITGB1), cytoskeleton organization (KIF20A and
LIMA1), DNA damage and repair (SRXN1, G6PD, PTGR1, UNG, USP10 and
PRKDC) and transcription and translation (EIF4A1, TRIM16 and
ZKSCAN1) as listed in Table I. The
Gene to GO BP test for over-representation and pathways in HT29
cells after AITC 24 h-treatment identified by DNA microarray are
listed in Table II. AITC altered
the expression of negative regulation of protein kinase activity on
EGFR and PKB/mTOR signaling genes (AKR1C2, AKR1C1, AKR1C1 and
AKT1S1) in examined HT29 cells.
 | Table IThe genes with more than 1.8-fold
changes in mRNA levels in HT29 cells after AITC (5 μM) 24-h
treatment identified by DNA microarray. |
Table I
The genes with more than 1.8-fold
changes in mRNA levels in HT29 cells after AITC (5 μM) 24-h
treatment identified by DNA microarray.
| Fold-change | Representative
public ID | Gene symbol | Gene Ontology
biological process |
|---|
| 7.649 | NM_205845.1 | AKR1C2 | Positive regulation
of cell proliferation; positive regulation of protein kinase B
signaling cascade |
| 7.423 | NM_001353.5 | AKR1C1 | G-protein coupled
receptor signaling pathway; positive regulation of protein kinase B
signaling cascade |
| 6.365 | NM_001080538.1 | AKR1B10 | Cellular aldehyde
metabolic process; steroid metabolic process |
| 5.879 | NM_020299.4 | AKR1B10 | Cellular aldehyde
metabolic process; steroid metabolic process |
| 5.682 | NM_001080538.1 | AKR1B15 | Oxidation-reduction
process; inferred from electronic annotation |
| 4.953 | NM_020299.4 | AKR1B10 | Cellular aldehyde
metabolic process; steroid metabolic process |
| 3.708 | BM907551 | AKR1C1 | G-protein coupled
receptor signaling pathway |
| 3.655 | NM_001135168.1 | ALDH3A1 | Positive regulation
of cell proliferation |
| 2.585 | NM_002061.2 | GCLM | Response to
oxidative stress; negative regulation of apoptotic process |
| 2.452 | AF121775.1 | ANKRD11 | Multicellular
organism growth |
| 2.412 | NM_002133.1 | HMOX1 | Angiogenesis;
negative regulation of cell migration; intracellular protein kinase
cascade |
| 2.367 | BC022241.1 | AKT1S1 | Negative regulation
of protein kinase activity; EGFR signaling pathway; negative
regulation of TOR signaling |
| 2.285 | NM_012212.3 | PTGR1 | Oxidation-reduction
process |
| 2.280 | NM_080725.1 | SRXN1 | Response to
oxidative stress |
| 2.142 | AW003954 | TROAP | Cell adhesion |
| 2.140 | AK293322.1 | TXNRD1 | Signal
transduction; cell proliferation |
| 2.138 | NM_003739.4 | AKR1C3 | G-protein coupled
receptor signaling; cellular response to starvation; positive
regulation of cell death |
| 2.114 | U79273.1 | EIF4A1 | Translational
initiation |
| 2.044 | NM_003900.4 | SQSTM1 | Ubiquitin-dependent
protein catabolic process; autophagy; apoptotic process; regulation
of I-κB kinase/NF-κB cascade |
| 2.040 | NM_000187.2 | HGD | Oxidation-reduction
process |
| 1.997 | NM_000402.3 | G6PD | Cellular response
to oxidative stress |
| 1.969 | NM_006470.3 | TRIM16 | Positive regulation
of transcription |
| 1.929 | AK304288.1 | GCLC | Negative regulation
of neuron apoptotic process |
| 1.920 | AI377389 | CSNK1A1 | Protein
phosphorylation; cell cycle; mitosis |
| 1.906 | BC053576.1 | UGT1A1 | Response to
starvation; cellular response to glucocorticoid stimulus |
| 1.905 | DQ364250.1 | UGT1A1 | Metabolic
process |
| 1.898 | NM_080489.3 | FKBP1A-SDCBP2 | Intracellular
signal transduction |
| 1.888 | NM_001255.2 | CDC20 | Cell cycle
checkpoint ; M phase of mitotic cell cycle |
| 1.876 | BC041809.1 | GCLM | Cysteine metabolic
process; negative regulation of apoptotic process |
| 1.864 | AL519718 | BIRC5 | G2/M transition of
mitotic cell cycle; M phase of mitotic cell cycle regulation of
apoptotic process |
| 1.859 | M90656.1 | GCLC | Amino acid
metabolic process; negative regulation of apoptotic process |
| 1.823 | AK316437.1 | ARHGAP11A | Small GTPase
mediated signal transduction; positive regulation of GTPase
activity |
| 1.823 | BC012999.2 | KIF20A | M phase of mitotic
cell cycle; microtubule bundle formation |
| 1.819 | AK297737.1 | UGDH | UDP-glucuronate
biosynthetic process |
| −1.897 | AK290883.1 | SAV1 | Positive regulation
of apoptotic process; negative regulation of epithelial cell
proliferation |
| −1.900 | NM_000492.3 | CFTR | ATP catabolic
process; positive regulation of vasodilation |
| −1.938 | BC040361.1 | NRIP1 | Regulation of
transcription; androgen receptor signaling pathway |
| −1.956 | BP319539 | MT1X | Cellular response
to erythropoietin |
| −1.966 | NM_001003954.1 | ANXA13 | Cell
differentiation |
| −2.007 | BC009894.2 | PAPSS2 | Small molecule
metabolic process |
| −2.010 | NM_005100.3 | AKAP12 | Signal
transduction; G-protein coupled receptor signaling pathway;
positive regulation of PKA signaling |
| −2.010 | BC125158.1 | ANXA13 | Cell
differentiation |
| −2.037 | AK299416.1 | UNG | DNA repair |
| −2.223 | NM_003439.1 | ZKSCAN1 | Regulation of
transcription |
| −2.234 | AK298206.1 | LYPLA1 | Fatty acid
metabolic process |
| −2.291 | AF291053.1 | LYPLA1 | Small molecule
metabolic process |
| −2.305 | NM_005950.1 | MT1G | Cellular response
to VEGF stimulus; negative regulation of growth |
| −2.424 | AK001650.1 | MOB1A | Hippo signaling
cascade |
| −2.502 | BC009079.1 | JAK1 | Protein
phosphorylation; intracellular signal transduction |
| −2.514 | CN349067 | ITGB1 | Mitotic cell cycle;
cell-cell adhesion; cell-matrix adhesion; integrin-mediated
signaling pathway; cell migration |
| −2.560 | AI479225 | CDC27 | Cell cycle; mitotic
metaphase/anaphase transition; cell division |
| −2.642 | U81607.1 | AKAP12 | G-protein coupled
receptor signaling pathway; positive regulation of PKA
signaling |
| −2.762 | BG338144 | EZR | Cell-cell adhesion;
regulation of cell shape |
| −2.780 | BG539466 | VEZF1 | Angiogenesis;
regulation of transcription |
| −3.000 | BG390445 | USP10 | DNA repair;
regulation of autophagy; DNA damage response |
| −3.005 | BF681396 | PRKDC | DNA repair;
apoptotic process |
| −3.067 | NM_001134439.1 | PHLDB2 | Intracellular
signal transduction |
| −3.631 | DA593848 | LIMA1 | Negative regulation
of actin filament depolymerization; actin filament bundle
assembly |
 | Table IIGene to GO BP test for
over-representation in HT29 cells after AITC (5 μM) 24-h treatment
identified by DNA microarray. |
Table II
Gene to GO BP test for
over-representation in HT29 cells after AITC (5 μM) 24-h treatment
identified by DNA microarray.
| Term | Count | Percentage | P-value |
|---|
| GO:0007049-cell
cycle | 114 | 10.73446 | 1.61E-18 |
| GO:0000278-mitotic
cell cycle | 69 | 6.497175 | 2.74E-16 |
| GO:0022403-cell
cycle phase | 73 | 6.873823 | 6.45E-16 |
| GO:0022402-cell
cycle process | 87 | 8.19209 | 3.02E-15 |
|
GO:0048285-organelle fission | 50 | 4.708098 | 9.32E-15 |
| GO:0000280-nuclear
division | 48 | 4.519774 | 3.52E-14 |
|
GO:0007067-mitosis | 48 | 4.519774 | 3.52E-14 |
| GO:0000087-M phase
of mitotic cell cycle | 48 | 4.519774 | 7.18E-14 |
| GO:0000279-M
phase | 60 | 5.649718 | 7.22E-14 |
| GO:0051301-cell
division | 54 | 5.084746 | 1.30E-12 |
Discussion
It is well documented that cell metastasis is one of
the major causes of colorectal cancer-related mortality (17,18,30).
Interference with human epidermal growth factor receptor (EGFR) or
downstream intracellular signaling provides a novel approach in
cancer chemotherapeutic agents (31–33).
Activation of the EGFR pathway promotes by EGF-stimulated
responsible for colorectal cancer cell growth, invasion, metastasis
and inhibition of cell death (34,35).
Following EGF stimulation, EGFR is autophosphorylated, which turns
on downstream intracellular signaling cascades such as MAPKs (ERK,
p38 and JNK) signaling pathways (36,37).
In the present study, we found that AITC was able to inhibit the
invasion and migration in EGF-stimulated HT29 cells and could have
a potential to treat colorectal cancer cell metastasis (Figs. 1 and 2). Cancer metastasis is a complicated
progression that involves the increase in cell invasion, migration
and degradation of extracellular matrix (ECM) then circulation in
the vascular and lymphatic systems, finally the residence in
distant organs (5,16,38).
The matrix metalloproteinases (MMPs) are responsible for ECM
degradation (39). The expression
levels of MMP-2 and MMP-9 in colorectal cancer cells are highly
related to the metastatic potential (40,41).
Herein, we indicated that AITC was involved in MMP-2 and -9 by
decreasing their protein level expression (Fig. 3). AITC also enhanced the expression
of TIMP-1 level (Fig. 3), leading
to the suppression of invasion and migration of EGF-stimulated HT29
cells. These findings also outlined the significance of MMP-2 and
-9 in colorectal cancer cell metastasis.
MAPK (ERK, p38 and JNK) signals are known to be
correlated with MMP-2 and -9 promoter induction through AP-1 and to
regulate the activities of MMP-2/-9 in colorectal cancer cells
(15,42). It was reported that inhibition of
MAPK/AP-1-mediated transcription resulted in reduced migration,
invasion, and metastasis in colorectal cancer cells (15). The present study indicated that AITC
inhibited the metastasis of EGF-stimulated HT29 cells through
reductions of the MAPK signaling pathways in vitro (Fig. 3).
In our previous studies, we demonstrated that AITC
induced cell cycle G2/M phase arrest in MDA-MB-468 cells and GBM
8401 cells (8,10). In addition, previous evidence
reported that AITC also inhibited cell growth of SW620 human
colorectal cancer cells (6), HL-60
leukemia cells (4) as well as
prostate cancer PC-3 and LNCaP cells (2) through induction of G2/M phase arrest.
In the current study, we examined the change of mRNA expression
profile in AITC-treated EGF-stimulated HT29 cells by DNA
microarray. Our data showed that cellular and molecular responses
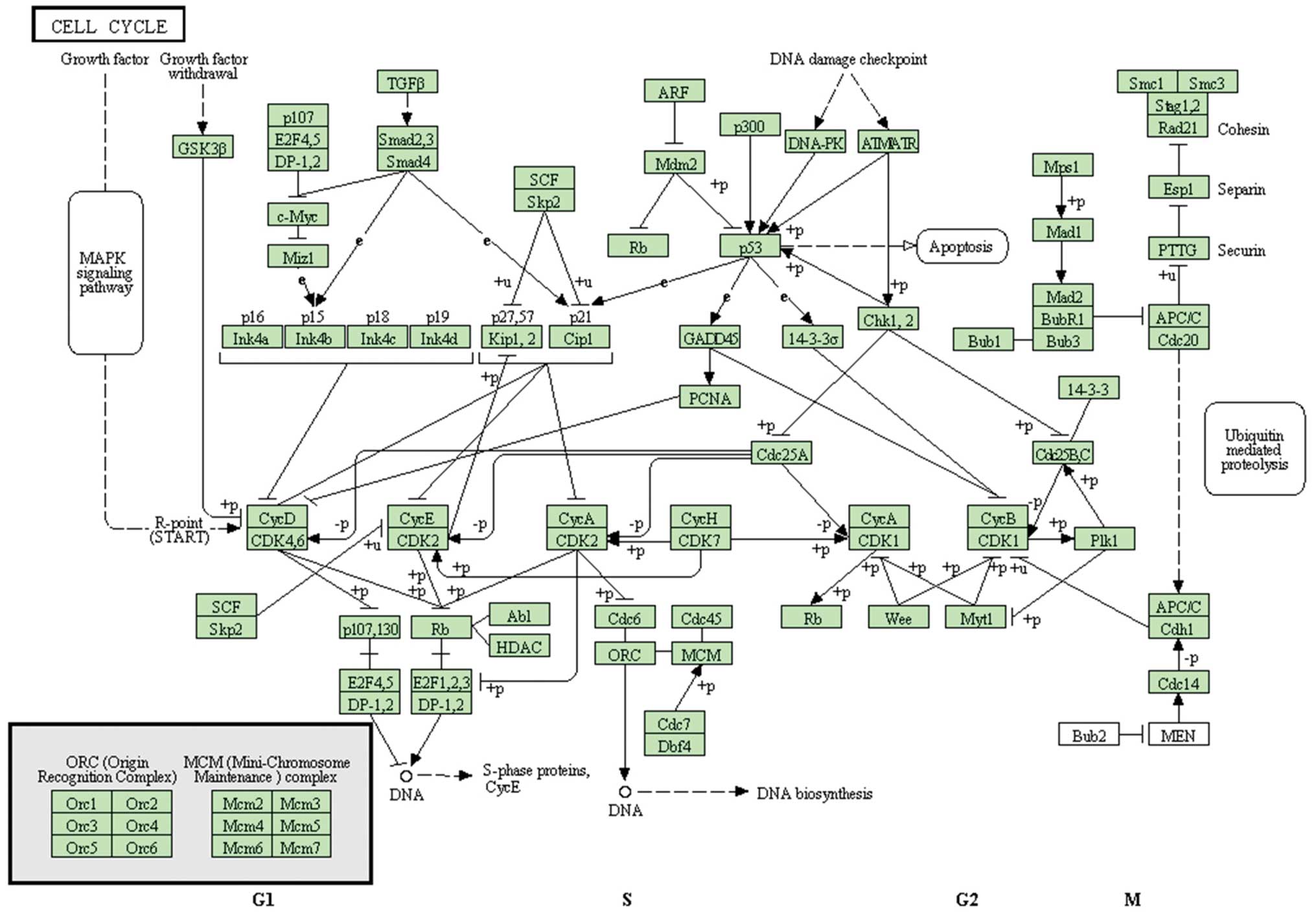
to AITC treatment were complicated and likely to be mediated
through a variety of regulatory pathways (Fig. 4). AITC regulated the expression of
important genes that control cell growth, G-protein coupled
receptor, angiogenesis, cell adhesion, cell cycle and mitosis, cell
migration, cytoskeleton organization, DNA damage and repair as well
as transcription and translation (Table
I). Regulation of these genes may be responsible for inhibiting
the cell metastasis and cell proliferation of EGF-stimulated HT29
cells. However, we also found that AITC altered the expression of
negative regulation of protein kinase activity on EGFR and PKB/mTOR
signaling genes. We suggested that PKB/mTOR signaling genes are
also the regulators of translation initiation in MMPs. Inhibition
of the PKB/mTOR pathways might have the potential to prevent
EGF-stimulated HT29 cell invasion and migration. The molecular
signaling pathways involved in the effects of AITC on
EGF-stimulated HT29 cells are summarized in Fig. 4.
In conclusion, this is the first study to
demonstrate that AITC inhibits the metastatic potential actions,
including invasion and migration of EGF-stimulated HT29 cells. AITC
may be a promising agent in the therapy of colorectal cancer and
metastasis of the disease.
Acknowledgements
The present study was supported by a research grant
to K-C.L. awarded by China Medical University Beigang Hospital
(CMUBH R101-010).
References
|
1
|
Geng F, Tang L, Li Y, et al: Allyl
isothiocyanate arrests cancer cells in mitosis, and mitotic arrest
in turn leads to apoptosis via Bcl-2 protein phosphorylation. J
Biol Chem. 286:32259–32267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao D, Srivastava SK, Lew KL, et al:
Allyl isothiocyanate, a constituent of cruciferous vegetables,
inhibits proliferation of human prostate cancer cells by causing
G2/M arrest and inducing apoptosis. Carcinogenesis. 24:891–897.
2003. View Article : Google Scholar
|
|
3
|
Wu CL, Huang AC, Yang JS, et al: Benzyl
isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated
generation of reactive oxygen species causes cell cycle arrest and
induces apoptosis via activation of caspase-3, mitochondria
dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2
OS cells. J Orthop Res. 29:1199–1209. 2011.
|
|
4
|
Xu K and Thornalley PJ: Studies on the
mechanism of the inhibition of human leukaemia cell growth by
dietary isothiocyanates and their cysteine adducts in vitro.
Biochem Pharmacol. 60:221–231. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhattacharya A, Li Y, Wade KL, Paonessa
JD, Fahey JW and Zhang Y: Allyl isothiocyanate-rich mustard seed
powder inhibits bladder cancer growth and muscle invasion.
Carcinogenesis. 31:2105–2110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lau WS, Chen T and Wong YS: Allyl
isothiocyanate induces G2/M arrest in human colorectal
adenocarcinoma SW620 cells through down-regulation of Cdc25B and
Cdc25C. Mol Med Rep. 3:1023–1030. 2010.PubMed/NCBI
|
|
7
|
Schaefer EA, Stohr S, Meister M, Aigner A,
Gudermann T and Buech TR: Stimulation of the chemosensory TRPA1
cation channel by volatile toxic substances promotes cell survival
of small cell lung cancer cells. Biochem Pharmacol. 85:426–438.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai SC, Huang WW, Huang WC, et al:
ERK-modulated intrinsic signaling and G2/M phase arrest
contribute to the induction of apoptotic death by allyl
isothiocyanate in MDA-MB-468 human breast adenocarcinoma cells. Int
J Oncol. 41:2065–2072. 2012.PubMed/NCBI
|
|
9
|
Bhattacharya A, Li Y, Geng F, Munday R and
Zhang Y: The principal urinary metabolite of allyl isothiocyanate,
N-acetyl-S-(N-allylthiocarbamoyl)cysteine, inhibits the growth and
muscle invasion of bladder cancer. Carcinogenesis. 33:394–398.
2012. View Article : Google Scholar
|
|
10
|
Chen NG, Chen KT, Lu CC, et al: Allyl
isothiocyanate triggers G2/M phase arrest and apoptosis in human
brain malignant glioma GBM 8401 cells through a
mitochondria-dependent pathway. Oncol Rep. 24:449–455. 2010.
|
|
11
|
Louhivuori LM, Bart G, Larsson KP, et al:
Differentiation dependent expression of TRPA1 and TRPM8 channels in
IMR-32 human neuroblastoma cells. J Cell Physiol. 221:67–74. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang ES and Kim GH: Allyl isothiocyanate
influences cell adhesion, migration and metalloproteinase gene
expression in SK-Hep1 cells. Exp Biol Med (Maywood). 234:105–111.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garcia A, Haza AI, Arranz N, Rafter J and
Morales P: Protective effects of isothiocyanates alone or in
combination with vitamin C towards N-nitrosodibutylamine or
N-nitrosopiperidine-induced oxidative DNA damage in the single-cell
gel electrophoresis (SCGE)/HepG2 assay. J Appl Toxicol. 28:196–204.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srivastava SK, Xiao D, Lew KL, et al:
Allyl isothiocyanate, a constituent of cruciferous vegetables,
inhibits growth of PC-3 human prostate cancer xenografts in
vivo. Carcinogenesis. 24:1665–1670. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu C, Shen G, Yuan X, et al: ERK and JNK
signaling pathways are involved in the regulation of activator
protein 1 and cell death elicited by three isothiocyanates in human
prostate cancer PC-3 cells. Carcinogenesis. 27:437–445. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang ES and Lee HJ: Allyl isothiocyanate
and its N-acetylcysteine conjugate suppress metastasis via
inhibition of invasion, migration, and matrix
metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells.
Exp Biol Med (Maywood). 231:421–430. 2006.
|
|
17
|
Lai KC, Hsu SC, Kuo CL, et al: Phenethyl
isothiocyanate inhibited tumor migration and invasion via
suppressing multiple signal transduction pathways in human colon
cancer HT29 cells. J Agric Food Chem. 58:11148–11155. 2010.
View Article : Google Scholar
|
|
18
|
Lan YH, Wu YC, Wu KW, et al: Death
receptor 5-mediated TNFR family signaling pathways modulate
γ-humulene-induced apoptosis in human colorectal cancer HT29 cells.
Oncol Rep. 25:419–424. 2011.PubMed/NCBI
|
|
19
|
Lin HJ, Su CC, Lu HF, et al: Curcumin
blocks migration and invasion of mouse-rat hybrid retina ganglion
cells (N18) through the inhibition of MMP-2, -9, FAK, Rho A and
Rock-1 gene expression. Oncol Rep. 23:665–670. 2010.PubMed/NCBI
|
|
20
|
Lu CC, Yang JS, Chiang JH, et al:
Inhibition of invasion and migration by newly synthesized
quinazolinone MJ-29 in human oral cancer CAL 27 cells through
suppression of MMP-2/9 expression and combined down-regulation of
MAPK and AKT signaling. Anticancer Res. 32:2895–2903.
2012.PubMed/NCBI
|
|
21
|
Chen YY, Chiang SY, Lin JG, et al: Emodin,
aloe-emodin and rhein inhibit migration and invasion in human
tongue cancer SCC-4 cells through the inhibition of gene expression
of matrix metalloproteinase-9. Int J Oncol. 36:1113–1120.
2010.PubMed/NCBI
|
|
22
|
Hong BH, Wu CH, Yeh CT and Yen GC:
Invadopodia-associated proteins blockade as a novel mechanism for
6-shogaol and pterostilbene to reduce breast cancer cell motility
and invasion. Mol Nutr Food Res. 57:886–895. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
24
|
Chiang JH, Yang JS, Lu CC, et al: Newly
synthesized quinazolinone HMJ-38 suppresses angiogenetic responses
and triggers human umbilical vein endothelial cell apoptosis
through p53-modulated Fas/death receptor signaling. Toxicol Appl
Pharmacol. 269:150–162. 2013. View Article : Google Scholar
|
|
25
|
Lu CC, Yang JS, Chiang JH, et al: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mozaffarieh M, Konieczka K, Hauenstein D,
Schoetzau A and Flammer J: Half a pack of cigarettes a day more
than doubles DNA breaks in circulating leukocytes. Tob Induc Dis.
8:142010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jacobs AT and Marnett LJ: HSF1-mediated
BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated
colon cancer cells via stabilization of anti-apoptotic Bcl-2
proteins. J Biol Chem. 284:9176–9183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu CY, Yang JS, Huang SM, et al: Smh-3
induces G2/M arrest and apoptosis through
calcium-mediated endoplasmic reticulum stress and mitochondrial
signaling in human hepatocellular carcinoma Hep3B cells. Oncol Rep.
29:751–762. 2013.PubMed/NCBI
|
|
29
|
Wu RS, Yu CS, Liu KC, et al: Citosol
(thiamylal sodium) triggers apoptosis and affects gene expressions
of murine leukemia RAW 264.7 cells. Hum Exp Toxicol. 31:771–779.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang YJ, Yang JS, Lin CF, et al:
Houttuynia cordata Thunb extract induces apoptosis through
mitochondrial-dependent pathway in HT-29 human colon adenocarcinoma
cells. Oncol Rep. 22:1051–1056. 2009.
|
|
31
|
Chen HJ, Jiang YL, Lin CM, et al: Dual
inhibition of EGFR and c-Met kinase activation by MJ-56 reduces
metastasis of HT29 human colorectal cancer cells. Int J Oncol.
43:141–150. 2013.PubMed/NCBI
|
|
32
|
Friedmann B, Caplin M, Hartley JA and
Hochhauser D: Modulation of DNA repair in vitro after
treatment with chemotherapeutic agents by the epidermal growth
factor receptor inhibitor gefitinib (ZD1839). Clin Cancer Res.
10:6476–6486. 2004.PubMed/NCBI
|
|
33
|
Chu E: Clinical colorectal cancer: the
epidermal growth factor receptor signaling pathway as a
chemotherapeutic target. Clin Colorectal Cancer. 2:202–203. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu Z, Jiang G, Blume-Jensen P and Hunter
T: Epidermal growth factor-induced tumor cell invasion and
metastasis initiated by dephosphorylation and downregulation of
focal adhesion kinase. Mol Cell Biol. 21:4016–4031. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toulany M, Baumann M and Rodemann HP:
Stimulated PI3K-AKT signaling mediated through ligand or
radiation-induced EGFR depends indirectly, but not directly, on
constitutive K-Ras activity. Mol Cancer Res. 5:863–872. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D
and Sarkar FH: Forkhead box M1 transcription factor: a novel target
for cancer therapy. Cancer Treat Rev. 36:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong J, Ramachandiran S, Tikoo K, Jia Z,
Lau SS and Monks TJ: EGFR-independent activation of p38 MAPK and
EGFR-dependent activation of ERK1/2 are required for ROS-induced
renal cell death. Am J Physiol Renal Physiol. 287:F1049–F1058.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: a dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hidalgo M and Eckhardt SG: Development of
matrix metalloproteinase inhibitors in cancer therapy. J Natl
Cancer Inst. 93:178–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Murray D, Morrin M and McDonnell S:
Increased invasion and expression of MMP-9 in human colorectal cell
lines by a CD44-dependent mechanism. Anticancer Res. 24:489–494.
2004.PubMed/NCBI
|
|
41
|
Kapral M, Wawszczyk J, Jurzak M, Dymitruk
D and Weglarz L: Evaluation of the expression of metalloproteinases
2 and 9 and their tissue inhibitors in colon cancer cells treated
with phytic acid. Acta Pol Pharm. 67:625–629. 2010.PubMed/NCBI
|
|
42
|
Jeong WS, Kim IW, Hu R and Kong AN:
Modulation of AP-1 by natural chemopreventive compounds in human
colon HT-29 cancer cell line. Pharm Res. 21:649–660. 2004.
View Article : Google Scholar : PubMed/NCBI
|