Introduction
Telomeres, telomerase and tankyrase (TNKS) have an
extremely important and special association with human ‘cell aging’
and ‘immortalized cells’, offering new opportunities for the
research of senescence and cancer. Telomere is a short DNA-protein
complex that presents in the linear chromosome ends of eukaryotic
cells and constitutes a special hat-like structure along with
telomere-binding proteins to maintain the integrity of chromosomes.
Telomerase is a ribonucleoprotein complex of RNA and protein
composition, and belongs to the reverse transcriptases. Subunits of
the human telomerase gene include telomerase RNA (hTR), telomerase
binding protein (hTP1) and telomerase activity catalytic unit
(hTERT). The main function of telomerase is to maintain telomere
length. Telomeres can use the 3′ end as primers, their own RNA as a
template to synthesize telomere repeat sequences of TTAGGG, and add
these to chromosome ends to maintain the original length of the
telomere. Telomerase activity has been found in germ and stem
cells, which have self-renewal capacity, and in 80–95% of tumors,
yet it is weak or has no activity in normal somatic cells (1). Telomerase activity gradually
disappears with the differentiation and maturation of cells.
TNKS, an adenosine diphosphate ribose polymerase,
consists of two members: tankyrase 1 (TNKS1) and tankyrase 2
(TNKS2). TNKS1 is located on chromosome 8 (p23.1), and its protein
consists of 1,237 amino acid residues (2). There are four main domains of TNKS1,
including HPS, ankyrin (ANK), SAM and the polyADP-ribose polymerase
(PARP) domain (3–6). Therefore, TNKS1 has specific PARP
activity. However, TNKS1 does not directly affect the activity of
telomerase, but functions by the ribosylation of telomere-binding
protein telomere repeat-binding factor 1 (TRF1). Thus, the effect
of binding telomeric DNA is relieved and the telomere is in a kind
of ‘open’ state, which facilitates the access of telomerase as well
as other factors to the ends of telomeres (7). Subsequently, the stability of telomere
length in cancer cells is maintained, and the continuous
proliferation of cancer cells is ensured. The overexpression of
TNKS1 results in telomere elongation and its suppression induces
telomere shortening in the presence of telomerase, indicating that
TNKS1 may be a prospective therapeutic target for cancer therapy
(8–10). Therefore, TNKS1 is a positive
regulator of telomerase activation and telomere extension in the
human body (11).
Telomerase inhibitors have been used for cancer
therapy and have achieved a certain effect. However, conventional
telomerase inhibitors require long treatment periods for telomere
shortening to a critical length (12), and cancer cells are prone to drug
resistance. In addition, normal tissues with telomerase activity
can be affected, such as bone marrow and reproductive system
tissues. Moreover, the presence of TNKS1 may alleviate the effect
of telomerase inhibitors by accelerating the access of residual
telomerase activity to telomeres (13). Therefore, the therapeutic effect is
not satisfactory. Previous studies found that XAV939, a TNKS1
inhibitor, inhibited the proliferation of colon cancer and
neuroblastoma (NB) cells, presumably by preventing PARsylation of
TRF1 (8) or by inhibiting the
Wnt/β-catenin signaling pathway (14,15)
and causing a downstream DNA damage response (15,16).
At present, it is not clear whether XAV939 affects telomere length
and telomerase activity in NB cells.
In the present study, we initially treated SH-SY5Y
cells with XAV939 and RNA interference-TNKS1 (RNAi-TNKS1). We then
measured the telomere length using quantitative real-time
polymerase chain reaction (qPCR) assay, detected telomerase
activity using the ELISA kit, observed cell apoptotic morphology by
transmission electron microscopy (TEM), determined the percentage
of apoptotic cells with flow cytometry (FCM) and Hoechst 33342
staining, and determined the invasive ability by a cell invasion
assay. Furthermore, the mechanism of apoptosis was explored, which
provides experimental basis for the clinical application of
small-molecule inhibitors for achieving a cure for NB.
Materials and methods
Cell culture and the TNKS1 inhibitor
Human NB SH-SY5Y cells were purchased from the
American Type Culture Collection (ATCC; Rockville, MD, USA) and
cultured in Dulbecco’s modified Eagle’s medium-F12 (DMEM-F12;
HyClone), supplemented with 10% fetal bovine serum (FBS; Gibco),
100 U/ml penicillin and 100 μg/ml streptomycin (Sigma Chemical Co.,
St. Louis, MO, USA) at 37°C in a humidified 5% CO2
incubator. The TNKS1 inhibitor XAV939 was purchased from
Sigma-Aldrich.
XAV939 treatment and RNAi
SH-SY5Y cells were cultured and proliferated for
XAV939 and RNAi treatment. XAV939 was dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich), and the final concentration was 1
μM based on our previous study results (14). The control group was treated with
DMSO only. The TNKS1 gene was knocked down by RNAi method, and the
related products were purchased from GeneChem Co., Ltd. (Shanghai,
China). Three specific sequences of short hairpin RNAs (shRNAs)
targeting different regions of the human TNKS1 mRNA sequence
(shRNA-1, −2 and −3, designed to choose the best sequence for the
RNAi effect) and a scrambled shRNA (SCR), were constructed into the
GV118 lentiviral vector, which had a GFP tag. Then the lentivirus
was packaged and amplified in HEK293T cells. The SH-SY5Y cells were
infected at an MOI of 10 on the basis of a preliminary experiment.
The shRNA sequences targeting human TNKS1 and SCR are listed below
(5′–3′):
shRNA-1, GCCAGGGATAACTGGAACTAT -
ATAGTTCCAGTTATCCCTGGC
shRNA-2, GCTCCAGAAGATAAAGAATAT -
ATATTCTTTATCTTCTGGAGC
shRNA-3, CGACTCTTAGAGGCATC TAAA -
TTTAGATGCCTCTAAGAGTCG
SCR, TTCTCCGAACGTGTCACGT.
The effect of RNAi was then verified by quantitative
real-time RT-PCR (qRT-PCR) and western blotting, and the optimal
sequence was selected for follow-up assays. Following XAV939
treatment or RNAi-TNKS1 for 72 h, all groups were subjected to
detection of telomere length, telomerase activity, apoptosis
evaluation and cell invasion assay.
DNA extraction
The Genomic DNA extraction kit (Dingguo
Biotechnology, Beijing, China) was used to extract total DNA
according to the manufacturer’s instructions. The DNA quality and
quantity were checked and quantified using NanoDrop 1000 (NanoDrop,
Wilmington, DE, USA).
Quantitative real-time polymerase chain
reaction (qPCR)
The telomere length was determined using qPCR as
previously described by Cawthon (18,19).
This method determines the average ratio of telomere repeat copy
numbers to single-copy gene numbers (the relative T/S ratio) in
each sample. The T/S ratio is proportional to the average telomere
length, thus the relative telomere length can be calculated
quantitatively. qPCR reactions were performed using an ABI 7500
(Applied Biosystems). Triplicate DNA samples were amplified in
parallel in 20 μl PCR reactions containing 60 ng of sample DNA, 10
μl 2X SYBR® Prime Ex Taq™ (Takara), 100 nM of
forward telomere primer (CGGTTTGTTTGGGTTTGGGT TTGGGTTTGGGTTTGGGTT)
and 900 nM of reverse telomere primer (GGCTTGCCTTACCCTTACCCTTACCCT
TACCCTTACCCT) and 0.4 μl 50X ROX Fluorescent Dye II. The reaction
proceeded for one cycle at 95°C for 10 min, followed by 30 cycles
at 95°C for 15 sec and 56°C for 1 min. The β-globin reaction
mixture consisted of 60 ng of sample DNA, 10 μl 2X SYBR®
Prime Ex Taq™, 300 nM of forward β-globin primer
(GCTTCTGACACAACTGTGTTCACT AGC) and 700 nM of reverse β-globin
primer (CACCAA CTTCATCCACGTTCACC) and 0.4 μl 50X ROX Fluorescent
Dye II. The β-globin reaction proceeded for one cycle at 95°C for
10 min, followed by 35 cycles at 95°C for 15 sec and 54°C for 1
min. The data were then analyzed with the ABI SDS software to
generate the standard curve for each plate. Relative telomere
length was calculated from the T/S ratio = 2−ΔCt, where
ΔCt = Cttelomere − Ctβ-globin.
Measurement of telomerase activity
Telomerase activity was measured by a combination of
telomeric repeat amplification protocol (TRAP) and the human
telomerase ELISA kit (R&D Systems) according to the
manufacturers’ protocol. The assay was performed 96 h after XAV939
treatment or RNAi-TNKS1.
Observation of apoptotic morphology
The morphological cell change characteristic of
apoptosis was observed by TEM. Following XAV939 treatment and
RNAi-TNKS1, the SH-SY5Y cells were collected and fixed in 2.5%
glutaraldehyde for 24 h. The cells were then washed with
phosphate-buffered saline (PBS) 3 times and fixed in 1% osmic acid
for 2–3 h. Subsequently, the samples were dehydrated in ascending
grades of ethanol followed by clearing in propylene oxide, replaced
with acetone, and embedded in araldite. The pellet was cut into
1-μm slices and immersed in 3% uranyl acetate-lead citrate for
double staining and viewed by TEM (JEM-1200EX).
Detection of apoptosis using Annexin V/PI
staining
SH-SY5Y cell apoptosis was quantified by FCM using
the Annexin V/FITC apoptosis detection kit (KeyGen Biotech,
Nanjing, China) following the manufacturer’s protocol. The cells
were seeded in 6-well plates (1×105 cells/well) and
treated with DMSO or 1 μM XAV939 or RNAi-TNKS1. Then, the cells
were harvested, washed with cold PBS and double-stained with
Annexin V-FITC as well as propidium iodide (PI) in the dark. Each
sample was then analyzed by fluorescence-activated cell sorting
(FACS) (BD, San Jose, CA, USA). At least 10,000 cells were
analyzed. Alternatively, apoptosis was also determined using
Hoechst 33342 staining. At the indicated time points after
treatment, cells were washed with PBS and stained with Hoechst
33342 (10 μg/ml; Sigma-Aldrich). The cells were then observed by a
fluorescence microscope (Olympus inverted fluorescence microscope,
IX71) with excitation at 340 nm, and ~100 cells from five random
microscopic fields were counted. The percentage of apoptotic cells
was calculated as the ratio of apoptotic to total cells. The mean
and standard error were calculated for each time point and
treatment group (14).
Cell invasion assay
Cell invasion assays were performed using BD
Matrigel Basement Membrane Matrix (BD Biosciences, Franklin Lakes,
NJ, USA) according to the manufacturer’s instructions. The diluted
Matrigel was added to the upper chamber of a 24-well Transwell
plate (Corning Company, Corning, NY, USA) and incubated at 37°C for
4–5 h for gelling. Transfected and untransfected SH-SY5Y cells were
harvested from the culture dishes by trypsin, washed and
resuspended in serum-free medium (containing XAV939), and added to
the upper wells at a density of 2×105 cells/well in 100
μl DMEM/F12 medium, while 600 μl medium containing 20% FBS was
added to the lower wells. The Transwell plates were incubated at
37°C in a humidified atmosphere containing 5% CO2 in air
for 24 h, and then the cells remaining on the upper surface of the
membrane were removed with cotton swabs. Migrated cells, which
remained on the lower face of the porous membrane, were fixed with
90% ethanol for 30 min and stained with 0.1% crystal violet dye for
10 min. Cells that had invaded the lower surface of the filter were
counted under an inverted microscope, and cells in nine fields per
well were counted.
Statistical analysis
All quantitative variables are expressed as means ±
standard deviation (SD) and tested for normality using homogeneity
variances prior to further statistical analysis. Each experiment
was repeated three times. The data were analyzed by one-way ANOVA
followed by Tukey’s post-test using SPSS software, version 16.0.
Differences were considered to be statistically significant at
P<0.05.
Results
Optimal sequence for RNAi-TNKS1
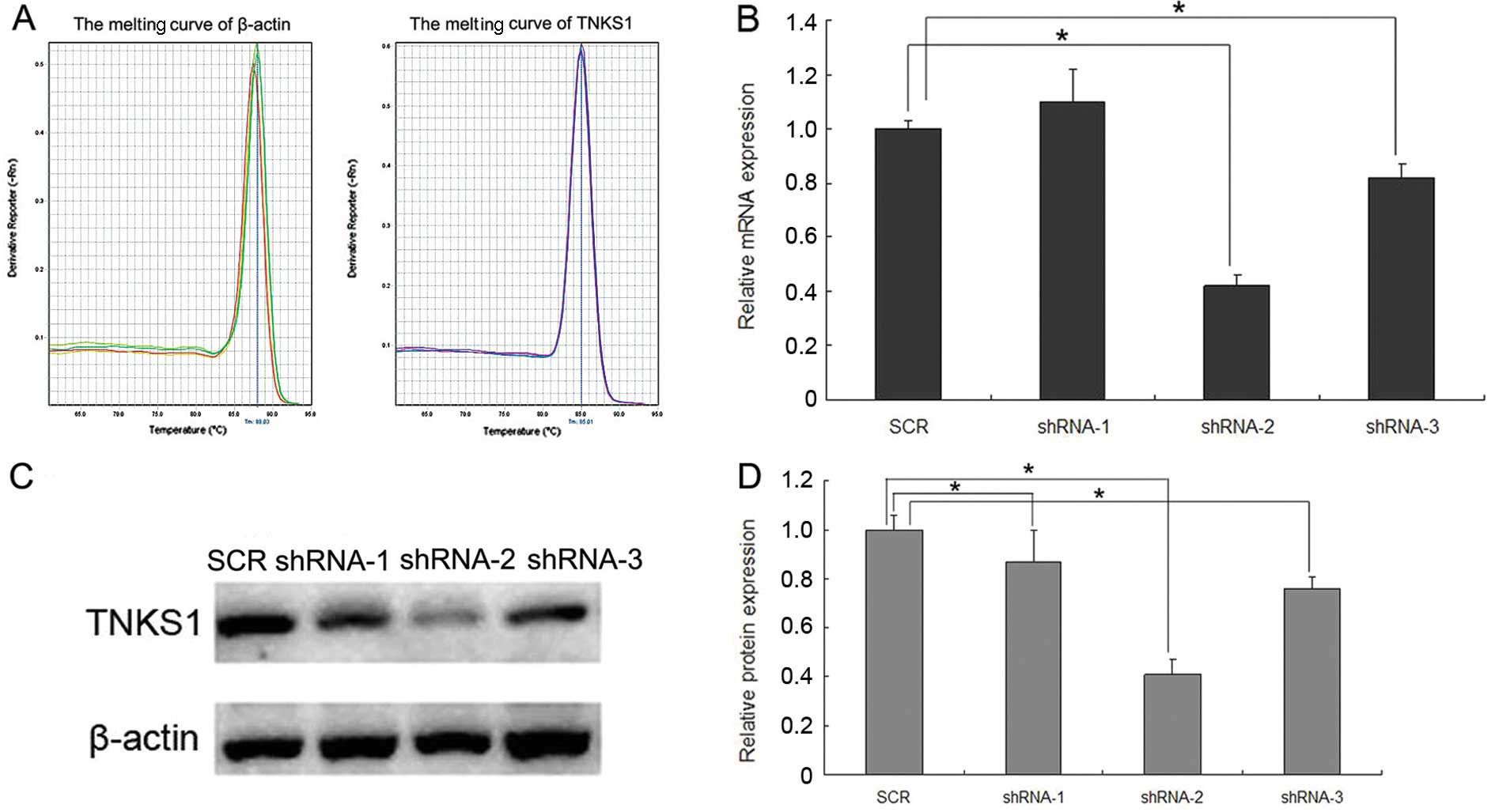
Both qPCR and western blotting were used to detect
the optimal sequence for RNAi-TNKS1. The results of qRT-PCR showed
that the melting curve of β-actin and TNKS1 were standard single
peaks, indicating specific amplification products (Fig. 1A). After transfection with shRNA-1,
−2, −3 and SCR, the relative expression of TNKS1 mRNA was
1.10±0.12, 0.42±0.04, 0.82±0.05 and 1.00±0.03, respectively
(Fig. 1B, P<0.05), while the
relative expression of TNKS1 protein was 0.87±0.13, 0.41±0.06,
0.76±0.05 and 1.00±0.03, respectively (Fig. 1C and D, P<0.05). The results
indicated that transfection with shRNA-2 most significantly reduced
both the expression of TNKS1 mRNA and protein, and the inhibition
ratio was ~60%, which reached the standard for the interference
effect. Thus, shRNA-2 was chosen as the optimal sequence for
RNAi-TNKS1 for the follow-up assays.
The telomere length of SH-SY5Y cells is
shortened after XAV939 and RNAi-TNKS1
Results of qPCR showed that the melting curves of
β-globin and telomere presented standard single peaks, indicating
specific amplification products (Fig.
2A). Following treatment with XAV939 or RNAi-TNKS1, the
relative telomere lengths were 0.60±0.05 and 0.46±0.08,
respectively, which were both lower than these lengths in the
control and SCR groups (Fig. 2B,
P<0.05). The results indicate that treatment with XAV939 and
RNAi-TNKS1 significantly shortened the telomere length compared
with that of the control groups.
XAV939 treatment and RNAi-TNKS1 have no
effect on telomerase activity
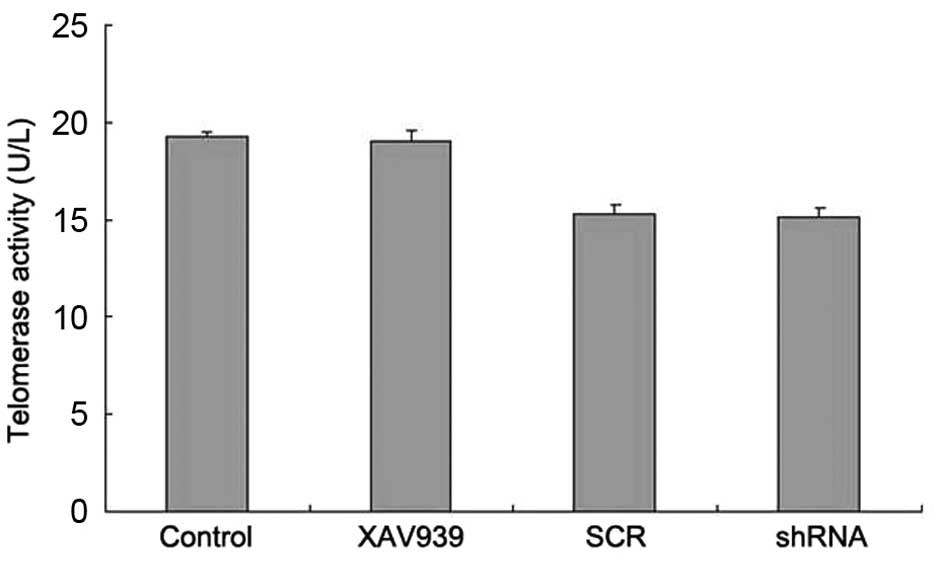
Telomerase was extracted by the TRAP-PCR kit and
measured by the ELISA kit. The results showed that the telomerase
activity in the control, XAV939 treatment, SCR and shRNA groups was
19.25±0.30, 19.01±0.57, 15.30±0.50 and 15.13±0.52 U/l, respectively
(Fig. 3). Compared with the
respective control groups, the telomerase activity in the cell
groups treated with XAV939 or RNAi-TNKS1 was not significantly
different (Fig. 3, P>0.05),
indicating that XAV939 treatment and RNAi-TNKS1 had no effect on
telomerase activity.
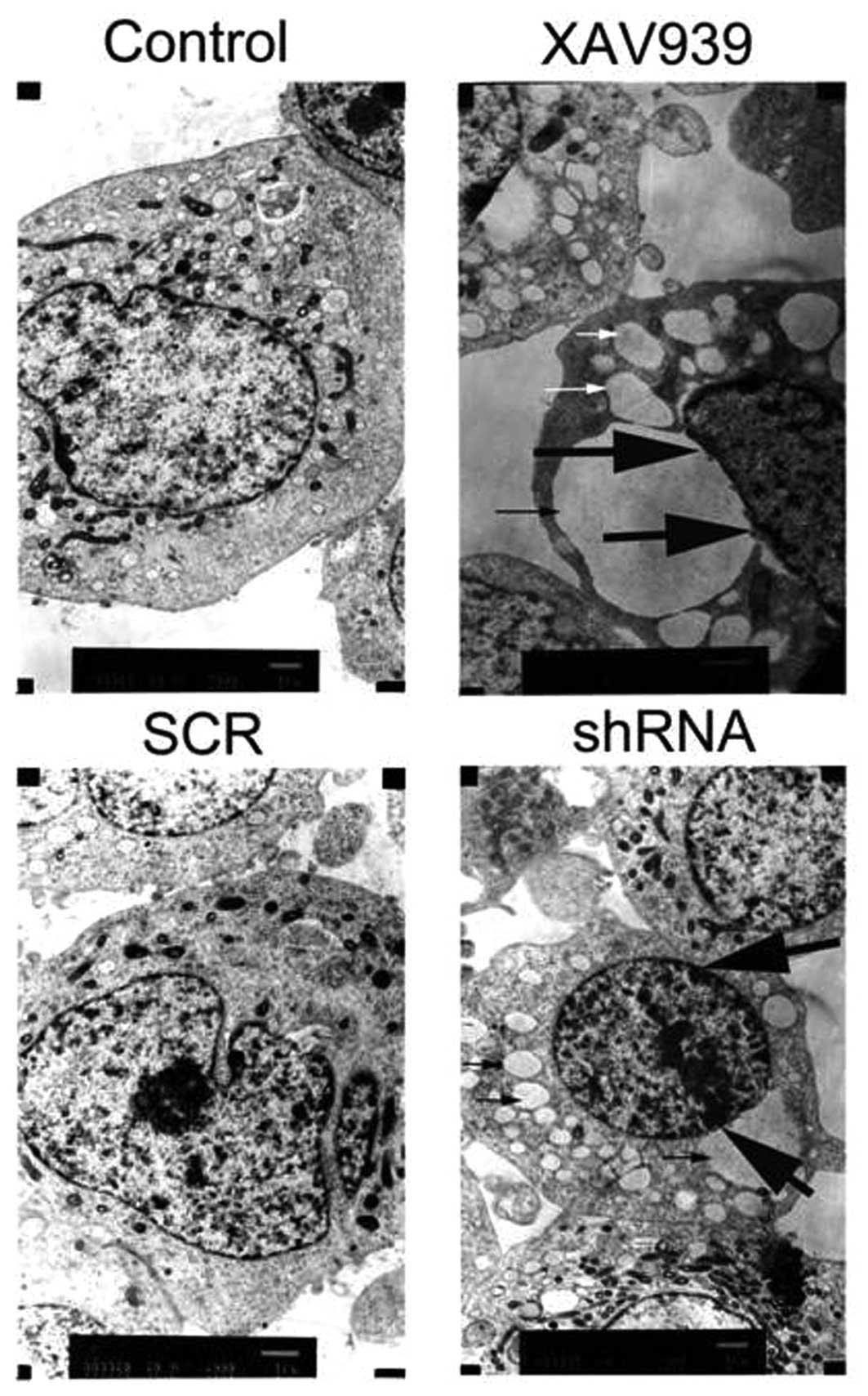
Apoptotic morphology of the SH-SY5Y
cells
After treatment with XAV939 or RNAi-TNKS1, the TEM
results showed that the mitochondria were swollen and formed many
vacuoles (Fig. 4, indicated by thin
arrows). Other organelle structure was unclear or disappeared, and
chromatin was condensed at the edge of the nuclear membrane, which
are typical morphologic alterations of apoptotic or necrotic cells
(Fig. 4, indicated by thick
arrows).
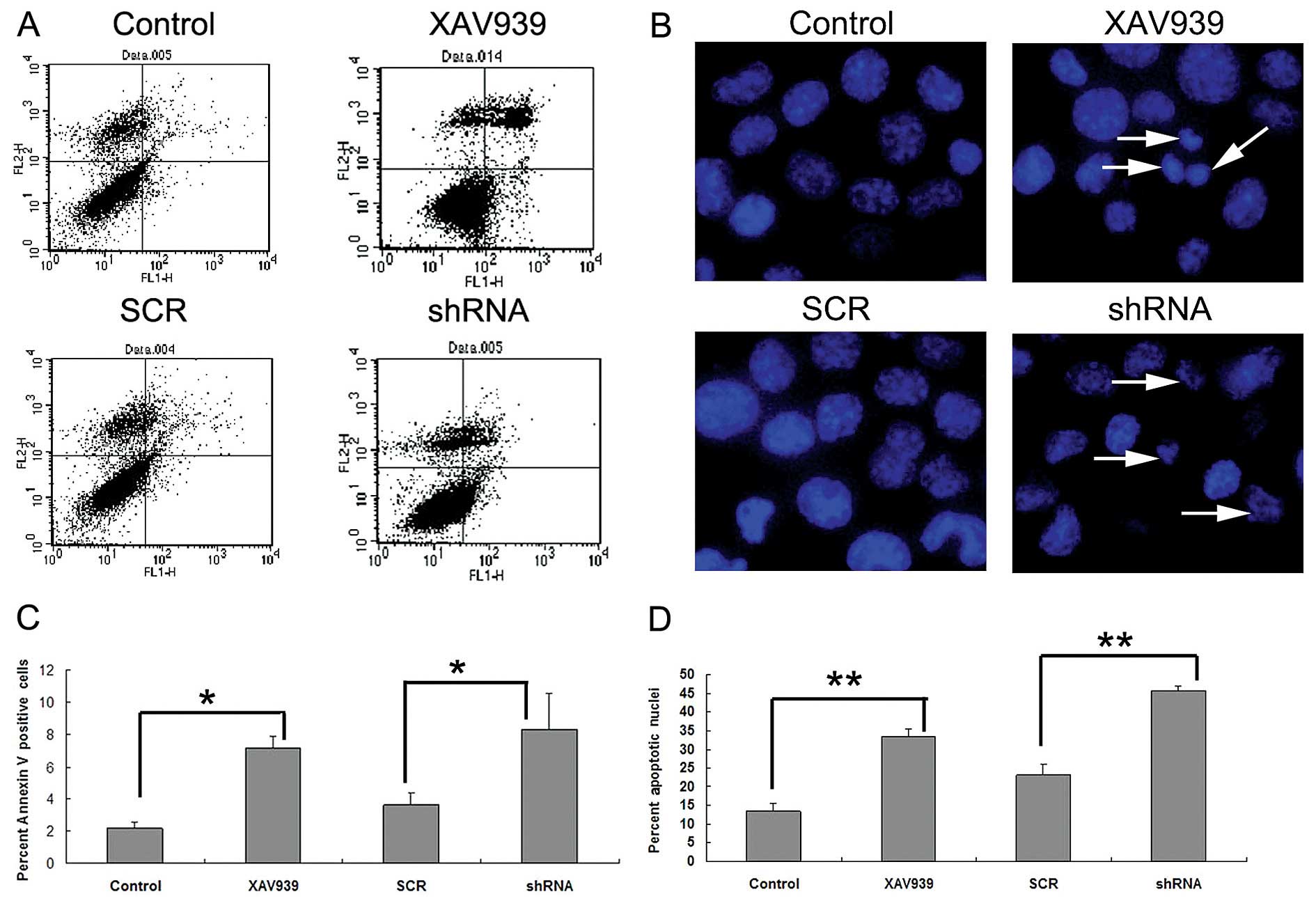
Treatment with XAV939 or RNAi-TNKS1
induces apoptosis in SH-SY5Y cells
Early apoptotic cells stained by Annexin V, are
noted in the right lower quadrant in Fig. 5A. The results showed that the
percentages of apoptotic cells in the control and SCR groups were
2.15±0.38 and 3.63±0.69%, while that in the XAV939-treated or
RNAi-TNKS1 group was 7.19±0.74%, respectively (Fig. 5C), which were significantly higher
than the percentages in the relevant control group (P<0.05,
Fig. 5C). To further confirm that
TNKS1 inhibition promoted apoptosis in SH-SY5Y cells, we studied
the nuclear morphology of cells following Hoechst 33342 staining
(Fig. 5B). As depicted in Fig. 5B, control cells without XAV939
treatment had normal and intact cell membrane, and were stained
uniformly and displayed equally disseminated chromatin. In
contrast, cells that were treated with XAV939 or RNAi-TNKS1 showed
varying degrees of archetypal characteristics of apoptotic cells,
such as the condensation of chromatin, shrinkage of nuclei and the
presence of apoptotic bodies with intense blue fluorescence. The
major findings are indicated by arrows in Fig. 5B. The percentage of cells with
apoptotic nuclei following XAV939 treatment or RNAi-TNKS1 increased
from 13.47 to 33.59% and from 23.08 to 45.56%, respectively, which
demonstrated the promotion of apoptosis following TNKS1 inhibition
(P<0.05, Fig. 5D). Collectively,
these results suggest that apoptosis is promoted by TNKS1
inhibition in NB.
XAV939 treatment or RNAi-TNKS1 inhibits
the invasive ability of SH-SY5Y cells
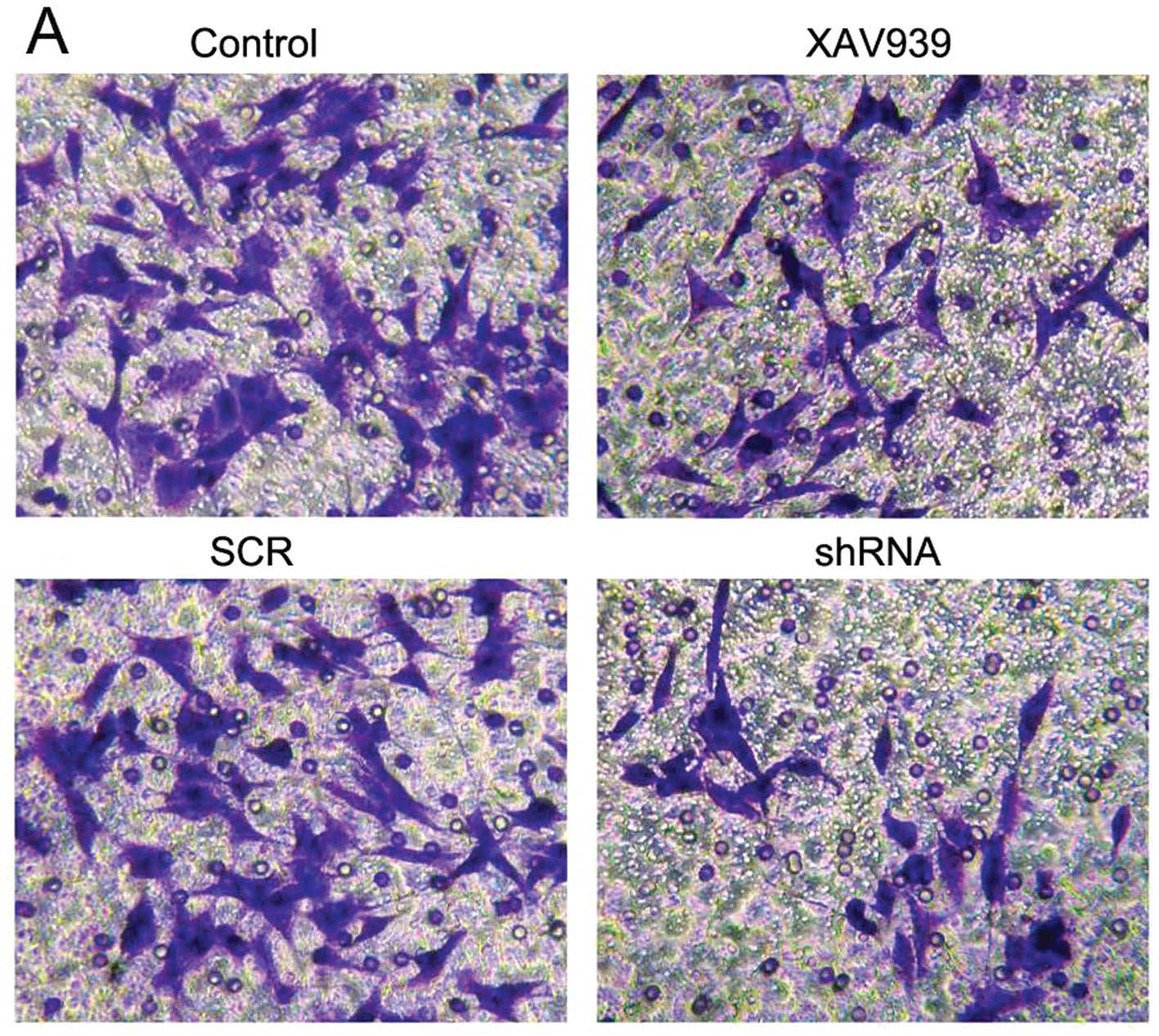
SH-SY5Y cells that migrated into the lower chamber
were stained with crystal violet (Fig.
6A). The results showed that the numbers of migrated cells in
the control, XAV939 treatment, SCR and shRNA groups were 58.0±3.0,
30.2±2.1, 60.1±3.5 and 28.1±1.6, respectively (Fig. 6B). Following treatment with XAV939
and RNAi-TNKS1, the invasion rates decreased 47.9 and 53.2%
respectively, compared with the control groups (Fig. 6B, P<0.05). It can be concluded
that both XAV939 treatment and RNAi-TNKS1 inhibit the invasiveness
of SH-SY5Y cells.
Discussion
The widespread presence of telomerase in human
tumors but its absence in normal somatic cells makes it an
attractive therapeutic target. It has been found that telomerase
activity is increased in a variety of tumor tissues, such as
well-differentiated non-Hodgkin’s lymphoma, colon and liver cancer,
and high expression of TNKS1 was detected simultaneously (19–23),
suggesting that the expression of TNKS1 is positively correlated
with telomerase activity. It was also reported that the presence of
TNKS1 undermined the role of telomerase inhibitors, and
consequently the telomere became more tolerant to telomerase
inhibitors in the progressive process of telomere shortening
(13). Thus, the inhibition of
TNKS1 is expected to compensate for incomplete inhibition of
telomerase and to shorten the time period of drug treatment and
telomere crisis, thus reducing the potential risk of drug
resistance. Additionally, TNKS1 inhibitors have no effect on the
telomere length of normal fibroblasts (13) and selectively play a role in
telomerase-positive cells, suggesting the use of TNKS1 as a
rational target for telomere-directed cancer therapy (7). Most types of NB are
telomerase-positive (24), and our
previous study demonstrated that SH-SY5Y cells overexpressed TNKS1
(unpublished data). Thus, the inhibition of TNKS1 is necessary and
effective for the treatment of NB.
The present study showed that treatment with XAV939
or RNAi-TNKS1 can shorten telomere length and promote cell
apoptosis in NB SH-SY5Y cells, which is in accordance with the
report that the RNAi approach could quickly lead to the growth
inhibition of tumor cells dependent on telomere shortening
(25). The advantage of the RNAi
technique is that it can greatly reduce the interval between
telomere shortening and the growth inhibition of tumor cells. The
classical and most widely applied method for telomere length
measurement is the DNA blot hybridization, also called the ‘gold
standard’ (26). However, this
method requires a large number of DNA samples and a long test
period. Moreover, the result obtains part of the sub-telomeric
length in addition to the average telomere length of all nucleated
cells in a representative sample, thus its further application is
hindered. Other methods, such as quantitative fluorescence in
situ hybridization (Q-FISH) and its evolved flow fluorescence
in situ hybridization (Flow-FISH) (27,28),
are both hindered by serious technical difficulties, high
laboratory equipment requirements and a long test cycle as well as
other issues. In 2002, Cawthon proposed the method of qPCR for
detecting telomere length, and solved the problems of processing
large numbers of samples in a simple and rapid high-throughput
manner (18). The ratio of telomere
(T) repeat copy number to single copy gene number (S), namely T/S,
is proportional to telomere length, and telomere length can be
determined according to T/S. Thus, we applied this method for
measuring telomere length.
Previous research found that RNAi-TNKS1 did not
affect telomerase activity in gastric cancer cell lines, yet
decreased the mRNA levels of hTERT and hTR, and the related
mechanism still requires further study (29). The present study also demonstrated
that XAV939 or RNAi-TNKS1 did not affect the telomerase activity. A
number of new modulators of telomerase, including extracellular
signal-regulated kinase 8 (ERK8), were discovered by applying
high-throughput functional RNAi for widespread screening of
telomerase activity. The inhibition of ERK8 reduced the telomerase
activity and stimulated telomere dysfunction. The synergistic
effect of RNAi-ERK8 and XAV939 treatment accelerated the appearance
of telomere crisis and reduced the interval between telomerase
inhibition and cell death (30).
Thus, the combined inhibition of TNKS1 and telomerase activity
would be a more rational treatment strategy in cancer therapy
targeting the telomere.
Various associations also exist between telomere
shortening and certain signaling pathways. Studies have shown that
targeted inhibition of GSK-3β protein greatly affected cell
proliferation and telomere length, suggesting a relationship
between telomere maintenance and Wnt signaling pathways (31). Our previous study also demonstrated
that XAV939 induced the apoptosis of NB cells as detected by
Annexin V and Hoechst 33342 staining as well as decreased
expression of Bcl-2 (14). In
agreement with previous research, the cell apoptosis was confirmed
by TEM and FCM, and decreased invasive ability was detected by
invasion assay following XAV939 treatment or RNAi-TNKS1. This
suggests that the combined application of telomerase inhibitors,
TNKS inhibitors and related signaling pathway inhibitors for
multi-targeted therapy of malignant tumors is expected to offer
superior effects (32,33). However, TNKS inhibitors may cause
side-effects in clinical treatment, such as intestinal toxicity,
and this warrants further investigation (34). One major cause is that TNKS is not
only found in telomeres, but is also distributed in other parts of
the cell, such as the central body and the distribution is
associated with the cell cycle (23). Kim and Smith reported that XAV939
treatment or TNKS1 inhibition by siRNA both induced excess sister
chromatid cohesion at telomeres leading to prolonged anaphase in
normal human and cancer cells, which contributes to the explanation
of the mechanism of telomere damage (35). Moreover, our present and previous
results showed that the inhibition of TNKS1 promoted cancer cell
apoptosis by two distinct mechanisms, telomere length shortening
and mitotic arrest, which hastened cell death (14). These results were consistent with
studies by Dynek and Smith (36)
and Chang et al (37).
In conclusion, the present study demonstrated that
both XAV939 and RNAi-TNKS1 promote cell apoptosis and reduce cell
invasion, accompanied by telomere shortening and unaltered
telomerase activity in NB SH-SY5Y cells. We speculate that the cell
apoptosis was due to the impaired telomere length, causing
chromosomal instability. However, more experiments should be
conducted to clarify the exact mechanisms. The present study may
contribute to achieving a cure for malignant NB by multi-targeted
therapy using small-molecule agents.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (30772215). The authors would like to
thank the Departments of Developmental Biology and Pathophysiology,
China Medical University, and the individuals who assisted our
research.
References
|
1
|
Ramirez R, Carracedo J, Jiménez R, et al:
Massive telomere loss is an early event of DNA damage-induced
apoptosis. J Biol Chem. 278:836–842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lyons RJ, Deane R, Lynch DK, Ye ZS,
Sanderson GM, Eyre HJ, Sutherland GR and Daly RJ: Identification of
a novel human tankyrase through its interaction with the adaptor
protein Grbl4. J Biol Chem. 276:17172–17180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Rycker M, Venkatesan RN, Wei C and
Price CM: Vertebrate tankyrase domain structure and sterile α motif
(SAM)-mediated multimerization. Biochem J. 372:87–96. 2003.
|
|
4
|
Seimiya H, Muramatsu Y, Smith S and Tsuruo
T: Functional subdomain in the ankyrin domain of tankyrase 1
required for poly(ADP-ribosyl)ation of TRF1 and telomere
elongation. Mol Cell Biol. 24:1944–1955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Rycker M and Price CM: Tankyrase
polymerization is controlled by its sterile α motif and
poly(ADP-ribose) polymerase domains. Mol Cell Biol. 24:9802–9812.
2004.PubMed/NCBI
|
|
6
|
Kaminker PG, Kim SH, Taylor RD,
Zebarjadian Y, Funk WD, Morin GB, Yaswen P and Campisi J: TANK2, a
new TRF1-associated poly(ADP-ribose) polymerase, causes rapid
induction of cell death upon overexpression. J Biol Chem.
276:35891–35899. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seimiya H: The telomeric PARP, tankyrases,
as targets for cancer therapy. Br J Cancer. 94:341–345. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith S, Giriat I, Schmitt A and de Lange
T: Tankyrase, a poly(ADP-ribose) polymerase at human telomeres.
Science. 282:1484–1487. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsiao SJ and Smith S: Sister telomeres
rendered dysfunctional by persistent cohesion are fused by NHEJ. J
Cell Biol. 184:515–526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Papeo G, Forte B, Orsini P, Perrera C,
Posteri H, Scolaro A and Montagnoli A: Poly(ADP-ribose) polymerase
inhibition in cancer therapy: are we close to maturity? Expert Opin
Ther Pat. 19:1377–1400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muramatsu Y, Ohishi T, Sakamoto M, Tsuruo
T and Seimiya H: Cross-species difference in telomeric function of
tankyrase 1. Cancer Sci. 98:850–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu R, Pal J, Buon L, et al: Targeting
homologous recombination and telomerase in Barrett’s
adenocarcinoma: impact on telomere maintenance, genomic instability
and tumor growth. Oncogene. 33:1495–1505. 2014.PubMed/NCBI
|
|
13
|
Seimiya H, Muramatsu Y, Ohishi T and
Tsuruo T: Tankyrase 1 as a target for telomere-directed molecular
cancer therapeutics. Cancer Cell. 7:25–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian XH, Hou WJ, Fang Y, Fan J, Tong H,
Bai SL, Chen Q, Xu H and Li Y: XAV939, a tankyrase 1 inhibitior,
promotes cell apoptosis in neuroblastoma cell lines by inhibiting
Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res.
32:1002013.PubMed/NCBI
|
|
15
|
Huang SM, Mishina YM, Liu S, et al:
Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dregalla RC, Zhou J, Idate RR, Battaglia
CL, Liber HL and Bailey SM: Regulatory roles of tankyrase 1 at
telomeres and in DNA repair: suppression of T-SCE and stabilization
of DNA-PKcs. Aging. 2:691–708. 2010.PubMed/NCBI
|
|
17
|
Sun J, Huang H and Zhu YY: Study on the
expression of tankyrase in malignant hematopoietic cells and its
relation with telomerase activity. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 12:11–15. 2004.(In Chinese).
|
|
18
|
Cawthon RM: Telomere measurement by
quantitative PCR. Nucleic Acids Res. 30:e472002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cawthon RM: Telomere length measurement by
a novel monochrome multiplex quantitative PCR method. Nucleic Acids
Res. 37:e212009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gelmini S, Poggesi M, Distante V, Bianchi
S, Simi L, Luconi M, Raggi CC, Cataliotti L, Pazzagli M and Orlando
C: Tankyrase, a positive regulator of telomere elongation, is
overexpressed in human breast cancer. Cancer Lett. 216:81–87. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gelmini S, Quattrone S, Malentacchi F,
Villari D, Travaglini F, Giannarini G, Della Melina A, Pazzagli M,
Nicita G, Selli C and Orlando C: Tankyrase-1 mRNA expression in
bladder cancer and paired urine sediment: preliminary experience.
Clin Chem Lab Med. 45:862–866. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poonepalli A, Banerjee B, Ramnarayanan K,
Palanisamy N, Putti TC and Hande MP: Telomere-mediated genomic
instability and the clinico-pathological parameters in breast
cancer. Genes Chromosomes Cancer. 47:1098–1109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gelmini S, Poggesi M, Pinzani P, Mannurita
SC, Cianchi F, Valanzano R and Orlando C: Distribution of
Tankyrase-1 mRNA expression in colon cancer and its prospective
correlation with progression stage. Oncol Rep. 16:1261–1266.
2006.PubMed/NCBI
|
|
24
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andrews LG and Tollefsbol TO: Methods of
telomerase inhibition. Methods Mol Biol. 405:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aubert G and Lansdorp PM: Telomeres and
aging. Physiol Rev. 88:557–579. 2008. View Article : Google Scholar
|
|
27
|
Baerlocher GM, Vulto I, de Jong G and
Lansdorp PM: Flow cytometry and FISH to measure the average length
of telomeres (flow FISH). Nat Protoc. 1:2365–2376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poon SS and Lansdorp PM: Measurements of
telomere length on individual chromosomes by image cytometry.
Methods Cell Biol. 64:69–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Yang MH, Zhao JJ, Chen L, Yu ST,
Tang XD, Fang DC and Yang SM: Inhibition of tankyrase 1 in human
gastric cancer cells enhances telomere shortening by telomerase
inhibitors. Oncol Rep. 24:1059–1065. 2010.PubMed/NCBI
|
|
30
|
Cerone MA, Burgess DJ, Naceur-Lombardelli
C, Lord CJ and Ashworth A: High-throughput RNAi screening reveals
novel regulators of telomerase. Cancer Res. 71:3328–3340. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bilsland AE, Hoare S, Stevenson K, et al:
Dynamic telomerase gene suppression via network effects of GSK3
inhibition. PLoS One. 4:e64592009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohishi T, Tsuruo T and Seimiya H:
Evaluation of tankyrase inhibition in whole cells. Methods Mol
Biol. 405:133–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu D, Li H and Liu JP: Inhibition of
telomerase by targeting MAP kinase signaling. Methods Mol Biol.
405:147–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lau T, Chan E, Callow M, Waaler J, Boggs
J, Blake RA, Magnuson S, Sambrone A, Schutten M, Firestein R,
Machon O, Korinek V, Choo E, Diaz D, Merchant M, Polakis P,
Holsworth DD, Krauss S and Costa M: A novel tankyrase
small-molecule inhibitor suppresses APC mutation-driven
colorectal tumor growth. Cancer Res. 73:3132–3144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim MK and Smith S: Persistent telomere
cohesion triggers a prolonged anaphase. Mol Biol Cell. 25:30–40.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dynek JN and Smith S: Resolution of sister
telomere association is required for progression through mitosis.
Science. 304:97–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang P, Coughlin M and Mitchison TJ:
Tankyrase-1 polymerization of poly(ADP-ribose) is required for
spindle structure and function. Nat Cell Biol. 7:1133–1139. 2005.
View Article : Google Scholar : PubMed/NCBI
|