Introduction
Colorectal cancer (CRC) remains a major cause of
cancer worldwide and accounts for approximately 9% of overall
cancer incidence (1–2). Although recent advances in
chemotherapy have prolonged the survival of patients with advanced
disease, the recurrence rates remain high (3). Thus, improved understanding of CRC
development may facilitate the identification of molecular targets
for therapeutic intervention and improve prognosis of the
disease.
Oxidative stress plays a major role in CRC
development and progression (4),
and results from an excess production of free radicals or
insufficient antioxidant defenses. The tumor-suppressor protein p53
has received attention mainly because the gene is mutated and/or
inactivated in the majority of human cancers, including CRC
(5–6). p53 protein accumulation and activity
are induced by genotoxic, oxidative and oncogenic stresses
(7). Many p53 target genes have
been thoroughly characterized and are involved in its tumor
suppressive functions (8). Among
these antioxidant genes activated by p53, sestrins are important
for the inhibition of reactive oxygen species (ROS) and protection
from oxidative stress, transformation and genomic instability
(9–10). Sestrins are members of a family of
highly conserved antioxidant proteins. Mammalian cells express
three members of this family, including sestrin 1, 2 and 3
(11–12). Sestrin 2, transcriptionally
regulated by p53, has a cytoprotective function based on
regeneration of the overoxidized peroxiredoxins (10), which are supposed to be involved in
CRC (13–14). Sestrin 2 emerges as a novel player
in autophagy induction and tumor suppression (6,15).
Upregulation of sestrin 2 expression via JNK pathway activation
contributes to autophagy induction in cancer cells (16). Wang et al found that
fangchinoline, a novel antitumor agent, induced autophagic cell
death via p53/sestrin2/AMPK signalling in human hepatocellular
carcinoma cells (17). Analysis of
gene expression has shown that sestrin 1 and 2 are downregulated in
lung cancers of different origin such as large cell carcinoma,
adenocarcinoma, squamous cell carcinoma and small cell lung
carcinoma (18–20). It is also reported that sestrin 2
interacted directly with AMPK and mediated sensitization of breast
cancer cells to ionizing radiation (21). These studies suggest that the
sestrin 2 may be important in tumorigenesis by regulating oxidative
stress. More recently, the upregulation of sestrin 2 was found to
induce apoptosis through the AMPK/p38 signaling pathway in HT-29
colon cancer cells, which are p53 mutant, treated with quercetin
(22). However, the role of sestrin
2 in CRC has to be elucidated.
In the present study, we reported the expression of
sestrin 2 in human CRC tissues and cell lines. The correlation
between pathological factors and protein expression of sestrin 2 in
human CRC tissues was examined, as well as the correlation between
protein expression and disease-free survival (DFS) and overall
survival (OS). To the best of our knowledge, this study provides
the first evidence that sestrin 2 may be involved in CRC.
Materials and methods
Human subjects and clinical data
The parafin-embedded tissue samples of the CRC
patients (130 males and 107 females) who underwent surgery between
2004 and 2008 were obtained from the Department of Gastrointestinal
Surgery of the following hospitals: The First Affiliated Hospital
of Chongqing Medical University and The Chongqing Three Gorges
Central Hospital. The patients did not receive chemo- or
radiotherapy prior to sample collection. The histological type was
independently determined by two pathologists in the study. The
paraffin-embedded tissue specimens from 32 normal mucosa, 22 polyp,
30 adenomas (24 cases with mild dysplasia, 4 cases with moderate
dysplasia, 2 cases with severe dysplasia) and 26 borderline tissues
were used as controls. Demographics (age and gender) and tumor
features (differentiation, TNM stage, lymphatic invasion, lymphatic
node metastasis, invasion, liver metastasis, peritoneal metastasis
and serum CEA) were obtained from clinical and pathological records
(Table I). Surgical staging was
determined using criteria based on International Union Against
Cancer (UICC). DFS was regarded as the interval between the day
that surgery was performed and the day that recurrence was
identified. If recurrence was not diagnosed, the date the patient
succumbed or that of last follow-up was used. OS was regarded as
the interval between the dates of surgery and death. After the
initial operation for the primary lesion there was a 5-year period
for DFS and OS.
 | Table IAssociation of sestrin 2 expression
with clinicopathological characteristics in 237 CRC patients. |
Table I
Association of sestrin 2 expression
with clinicopathological characteristics in 237 CRC patients.
| Clinicopathological
factors | No. of patients
(n=237) | Sestrin 2
expression | P-value |
|---|
|
|---|
| Low no. (%) | High no. (%) |
|---|
| Age (years) | | 171 | 660.762 | |
| ≥65 | 104 | 74 (71.2) | 30 (28.8) | |
| <65 | 133 | 97 (72.9) | 36 (27.1) | |
| Gender | | | | 0.601 |
| Male | 130 | 92 (70.8) | 38 (39.2) | |
| Female | 107 | 79 (73.8) | 28 (26.2) | |
| Tumor site | | | | 0.688 |
| Distal | 152 | 111 (73) | 41 (27) | |
| Proximal | 85 | 60 (70.6) | 25 (29.4) | |
| Histology | | | | 0.208 |
| Well | 96 | 65 (67.8) | 31 (32.2) | |
| Moderate/poor
(mucinous) | 141 | 106 (75.2) | 35 (24.8) | |
| TNM stage | | | |
<0.001 |
| I/II | 78 | 43 (55.1) | 35 (44.9) | |
| III/IV | 159 | 128 (80.5) | 31 (19.5) | |
| Lymphatic
invasion | | | | 0.004 |
| Yes | 149 | 117 (78.5) | 32 (21.5) | |
| No | 88 | 54 (61.4) | 34 (38.6) | |
| Lymph node
metastasis | | | | 0.006 |
| Yes | 106 | 86 (81.1) | 20 (18.9) | |
| No | 131 | 85 (64.9) | 46 (35.1) | |
| Vascular
invasion | | | | 0.012 |
| Yes | 32 | 29 (90.6) | 3 (9.4) | |
| No | 205 | 142 (69.3) | 63 (30.7) | |
| Liver
metastasis | | | | 0.006 |
| Yes | 35 | 32 (93.5) | 3 (6.5) | |
| No | 202 | 139 (68.9) | 63 (31.1) | |
| Peritoneal
metastasis | | | | 0.359 |
| Yes | 29 | 23 (79.3) | 6 (20.7) | |
| No | 208 | 148 (71.2) | 60 (28.8) | |
| Serum CEA level
(μg/l) | | | | 0.218 |
| ≥5 | 175 | 130 (74.3) | 45 (25.7) | |
| <5 | 62 | 41 (66.1) | 21 (33.9) | |
Forty-two fresh CRC tissues as well as 19 normal
mucosa, 20 polyp, 22 adenomas (18 cases with mild dysplasia, 3
cases with moderate dysplasia, and 1 case with severe dysplasia)
and 26 borderline tissues collected between 2013 and 2014 were
immediately placed in a cryovial and stored in liquid nitrogen
until subsequent use for western blot analysis. Clinical features
of the CRC patients are shown in Table
II.
 | Table IIAssociation of sestrin 2 protein
expression with clinicopathological characteristics in CRC
patients. |
Table II
Association of sestrin 2 protein
expression with clinicopathological characteristics in CRC
patients.
| Clinicopathological
factors | No. of patients
(n=42) | Sestrin 2
expression protein | P-value |
|---|
| Age (years) | | | 0.700 |
| ≥65 | 18 | 0.234±0.085 | |
| <65 | 24 | 0.223±0.102 | |
| Gender | | | 0.979 |
| Male | 23 | 0.230±0.097 | |
| Female | 19 | 0.229±0.087 | |
| Tumor site | | | 0.815 |
| Distal | 25 | 0.227±0.096 | |
| Proximal | 17 | 0.234±0.089 | |
| Histology | | | 0.340 |
| Well | 14 | 0.249±0.083 | |
| Moderate/poor
(mucinous) | 28 | 0.220±0.096 | |
| TNM stage | | | 0.005 |
| I/II | 13 | 0.287±0.077 | |
| III/IV | 29 | 0.204±0.087 | |
| Lymphatic
invasion | | | 0.008 |
| Yes | 24 | 0.198±0.084 | |
| No | 18 | 0.272±0.086 | |
| Lymph node
metastasis | | | 0.012 |
| Yes | 20 | 0.193±0.088 | |
| No | 22 | 0.263±0.083 | |
| Vascular
invasion | | | 0.037 |
| Yes | 9 | 0.174±0.072 | |
| No | 33 | 0.245±0.091 | |
| Liver
metastasis | | | 0.001 |
| Yes | 6 | 0.146±0.043 | |
| No | 36 | 0.244±0.090 | |
| Peritoneal
metastasis | | | 0.622 |
| Yes | 4 | 0.208±0.146 | |
| No | 38 | 0.232±0.087 | |
| Serum CEA level
(μg/l) | | | 0.413 |
| ≥5 | 28 | 0.238±0.093 | |
| <5 | 14 | 0.213±0.089 | |
The study was approved by the Medical Ethics Review
Committee of the First Affiliated Hospital of Chongqing Medical
University. Informed and written consent was obtained from the
patients or their relatives for the use of any data and tissues for
this study. The study was performed as per the Declaration of
Helsinki of the World Medical Association.
Immunohistochemistry
Tissue sections were deparaffinized in xylene,
immersed in graded ethanol series, and then incubated in 3%
hydrogen peroxide for 15 min. For the antigen retrieval, the
sections were heated in a microwave oven for 10 min at 92–98°C in
10 mmol/l sodium citrate buffer (pH 6.0). Non-specific binding was
blocked by incubating the sections with 10% goat serum (Zhongshan
Golden Bridge, Beijing, China) in 0.1 M phosphate-buffered saline
(PBS) at room temperature for 30 min as described previously
(14). The sections were then
incubated with primary sestrin 2 antibody (mouse monoclonal
antibody; 1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA; cat. no. sc-101249) overnight at 4°C followed by incubation
with goat anti-mouse antibody (Zhongshan Golden Bridge, Inc.,
Beijing, China) for 30 min at 37°C. Sections were then treated with
ABC solution (Zhongshan Golden Bridge, Inc.) at 37°C for 30 min and
washed with PBS. Immunoreactivity was detected with
3,3′-diaminobenzidine (DAB, Zhongshan Golden Bridge, Inc.) for 5
min. Counterstaining was performed using hematoxylin. For the
negative controls, the primary antibodies were replaced with PBS. A
LEICA DM6000B automatic-microscope (Leica, Solms, Germany) was
employed for collecting of images.
The cells with buffer stain in the cytoplasm were
considered to be positive. Ten random visual field images for each
sample were analyzed. Staining intensity was graded on a 0–3 scale
as: 0, absence of staining;, 1, weakly stained; 2, moderately
stained; and 3, strongly stained. The percentage of positive tumor
cells was scored as: 0, absence of positive cells; 1, <33%
positive tumor cells; 2, 33–66% positive tumor cells; and 3;
>66% positive tumor cells. The staining score, calculated as the
staining intensity score multiplied by the percentage score ranged
from 0 to 9 (23). Low and high
expression was regarded as a staining score of 0–4 and 5–9,
respectively. The staining score was evaluated independently by two
experienced pathologists. Concordance was achieved when the two
pathologists concurred on the same score for a patient. Discordant
patient cases were discussed by all the pathologists from the
Department of Pathology in Chongqing Medical University to reach a
consensus.
Cell lines and culture conditions
The FHC human normal colorectal mucosa cell line and
the human CRC HT-29, SW480, SW620 and LoVo cell lines were
purchased from the Shanghai Cell Bank at the Chinese Academy of
Sciences (Shanghai, China). The cell lines were cultured in
Leibovitz L-15 medium (Gibco, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (FBS) (Hyclone, Shanghai, China) and 2%
penicillin/streptomycin (Beyotime, Jiangsu, China). The cells were
then maintained at 37°C in a humidified atmosphere.
Western blot analysis
Total proteins extracted from human tissues and cell
lines were prepared in lysis buffer (Keygen Biotech, Nanjing,
China) consisting of 50 mM Tris (pH 7.4), 1% Triton X-100, and a
protease inhibitor mixture supplemented with 1 mM
phenylmethanesulfonyl fluoride (PMSF). The insoluble material was
centrifuged at 12,000 × g for 10 min at 4°C, and the supernatant
was obtained. The protein concentrations were quantified by
bicinchoninic acid (BCA) assay (Pierce, Rockford, IL, USA).
Electrophoresis was carried out using a Mini-Protean system
(Bio-Rad Laboratories, Hercules, CA, USA). Total proteins (50 μg)
were separated on 10% SDS-PAGE gel and transferred to the
polyvinylidene difluoride (PVDF) membranes (Millipore Corp.,
Billerica, MA, USA) by an electrophoretic transfer system (Bio-Rad
Laboratories). The PVDF membranes were blocked with 5% non-fat dry
milk in TBS with 0.1% Tween-20 for 1 h at 37°C, and then incubated
with primary antibodies, anti-sestrin 2 antibody (mouse monoclonal
antibody, 1:200; Santa Cruz Biotechnology, Inc.; cat. no.
sc-101249) and GAPDH antibody (mouse monoclonal antibody, 1:1,000;
Abcam Biotechnology, Cambridge, MA, USA; cat. no. ab125247)
overnight at 4°C. After washing, the membranes were incubated with
secondary antibodies (1:2,000 dilution, goat anti-mouse IgG-HRP;
Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. Proteins were
detected by enhanced chemiluminescence plus detection reagents
(Pierce). The membranes were scanned (Bio-Rad Laboratories), and
the pixel density of the images was quantified using Quantity One
software (Bio-Rad Laboratories). The band intensity ratio of
sestrin 2 relative to GAPDH (sestrin 2/GAPDH) was analyzed.
Immunofluorescence and confocal
microscopy
The cells were seeded and cultured on glass
coverslips the day prior to the analysis. Following incubation for
24 h, the cells were fixed with 4% paraformaldehyde at room
temperature for 15 min. After fixation, the cells were
permeabilized with 0.2 % Triton X-100 (Beyotime, Jiangsu, China)
and blocked with 10% normal goat serum for 1 h at room temperature,
as previously described (?). The cells were incubated with
anti-sestrin 2 antibody (mouse monoclonal antibody, 1:50; Santa
Cruz Biotechnology, Inc.; cat. no. sc-101249) overnight at 4°C.
After washing with PBS, the cells were incubated with DyLight
594-conjugated goat anti-mouse IgG (1:500, Zhongshan Golden Bridge,
Inc.) for 1 h at 37°C. The nuclei were counterstained with DAPI
(Keygen Biotech) for 10 min, and the images were captured with an
Olympus microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Continuous data are presented as mean ± standard
deviation (SD). Continuous variables were measured using an
independent Student’s t-test. The associations between sestrin 2
immunohistochemical staining and clinicopathological variables were
analyzed by the Mann-Whitney U test. The log-rank test and the
Kaplan-Meier analysis were used to the associations between sestrin
2 expression and the OS/DFS. Factors independently associated with
OS were identified using the Cox proportional hazards model for
univariate and multivariate analyses. Statistical analysis was
performed using SPSS Ver. 17.0 for Windows. P<0.05 was
considered to indicate a statistically significant result.
Results
Sestrin 2 is decreased in CRC and is
correlated with clinicopathological characteristics
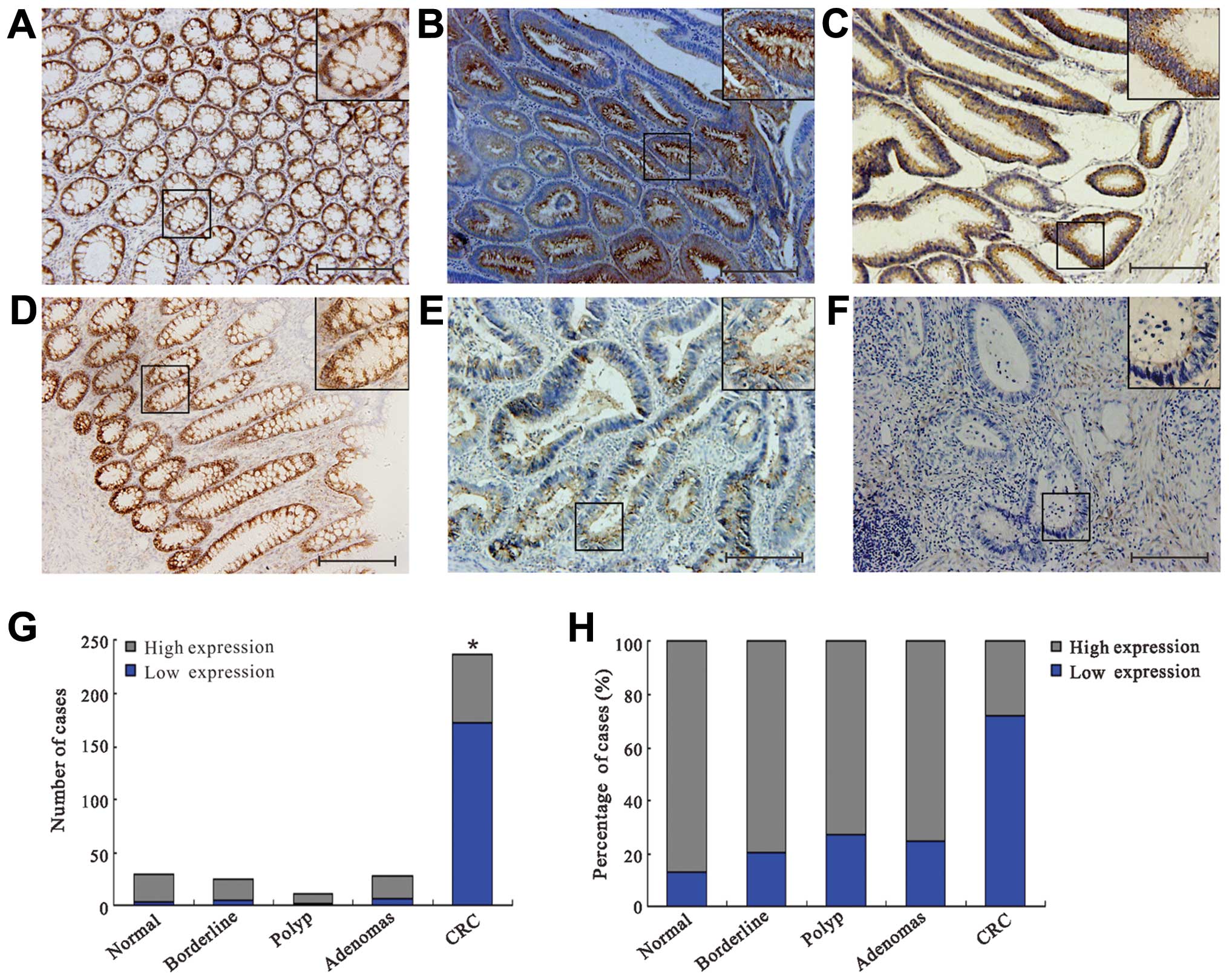
In the normal mucosa, polyp, adenomas and borderline
tissues, sestrin 2 was strongly and predominantly localized in
cytoplasm, whereas faint immunoreactivity for sestrin 2 was
observed in CRC patients (Fig.
1A–F). A significantly lower expression of sestrin 2 was
detected in the CRC group as compared to the normal mucosa, polyp,
adenomas and borderline groups respectively (P<0.05; Fig. 1G). No significant difference was
identified among the normal mucosa, polyp, adenomas and borderline
groups (P>0.05; Fig. 1G). In the
CRC group, 72.2% of cases exhibited a low expression of sestrin 2
and 27.8% a high expression (Fig.
1H). By contrast, sestrin 2 expression was high in the normal
mucosa (87.5%), polyp (71.8%), adenomas (80%) and borderline
(80.8%) samples (Fig. 1H). The
Mann-Whitney U test was used to evaluate whether a low expression
of sestrin 2 in CRC samples was associated with specific
clinicopathological variables (Table
I). A low expression of sestrin 2 was significantly associated
with TNM stage (P<0.001), lymphatic invasion (P=0.004), lymph
node metastasis (P=0.006), vascular invasion (P=0.012) and liver
metastasis (P=0.006). However, no significant associations were
found between sestrin 2 expression and age, gender, tumor site,
histology, peritoneal metastasis and serum carcinoembryonic antigen
(CEA) level, respectively (all P>0.05).
Correlation between the protein level of
sestrin 2 and clinicopathological characteristics
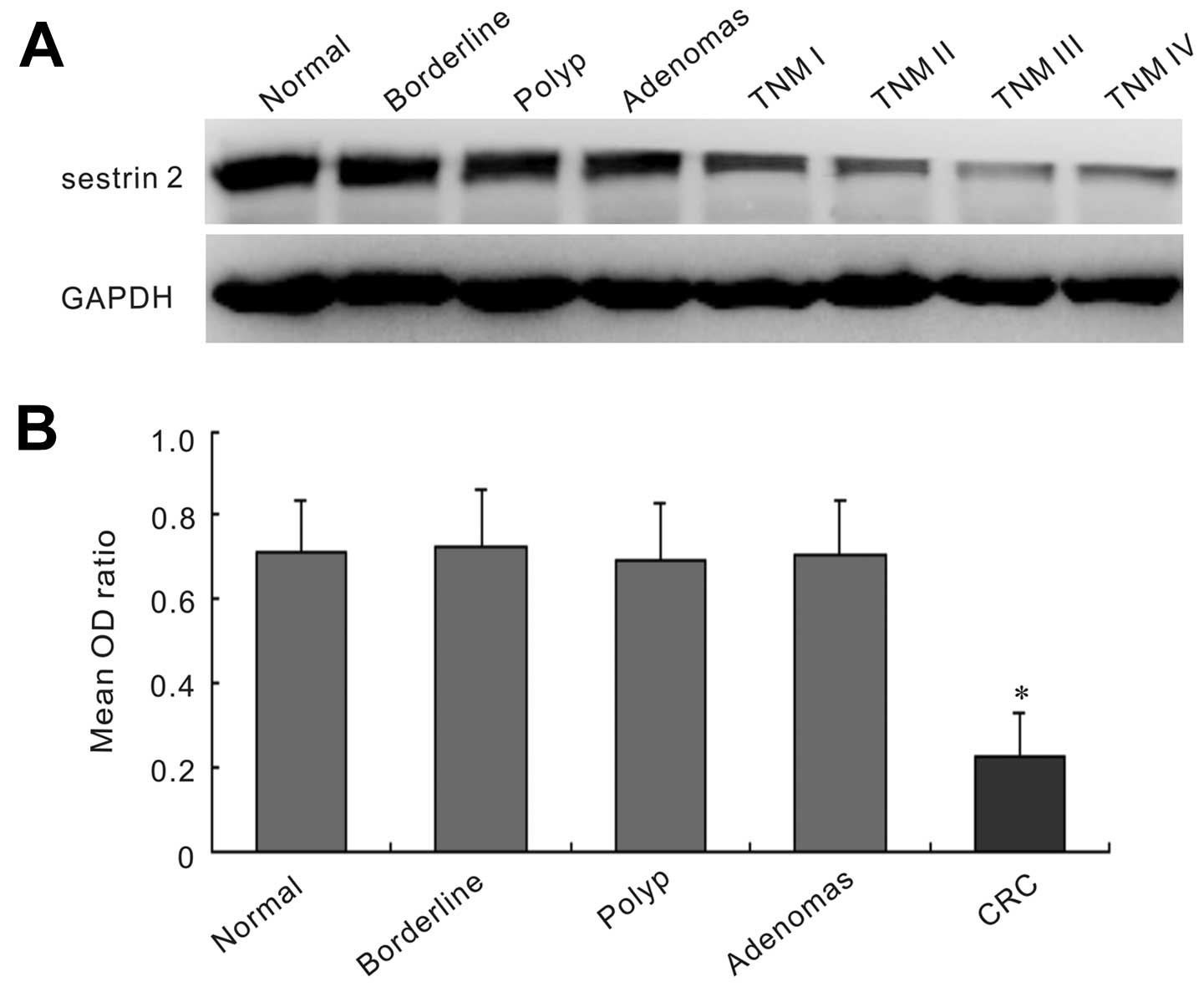
Western blot analysis was performed to evaluate the
sestrin 2 protein level from frozen tissues of 42 CRC patients and
controls including 19 normal mucosa, 20 polyp, 22 adenomas and 26
borderline (Fig. 2). The sestrin 2
expression was strong in the normal mucosa, polyp, adenomas and
borderline samples, while it was faint in CRC samples. The protein
expression of sestrin 2 in CRC tissues was significantly lower than
that in normal mucosa, polyp, adenomas and borderline groups
(P<0.05). No statistical significance was found among normal
mucosa, polyp, adenomas and borderline groups (P>0.05).
Furthermore, we analyzed the correlation between the protein level
of sestrin 2 and clinicopathological characteristics. The results
positively correlated with the immunohistochemical findings. A
significantly lower sestrin 2 protein level was associated with TNM
stage (P=0.005), lymphatic invasion (P=0.008), lymph node
metastasis (P=0.012), vascular invasion (P=0.037) and liver
metastasis (P=0.001) (Table
II).
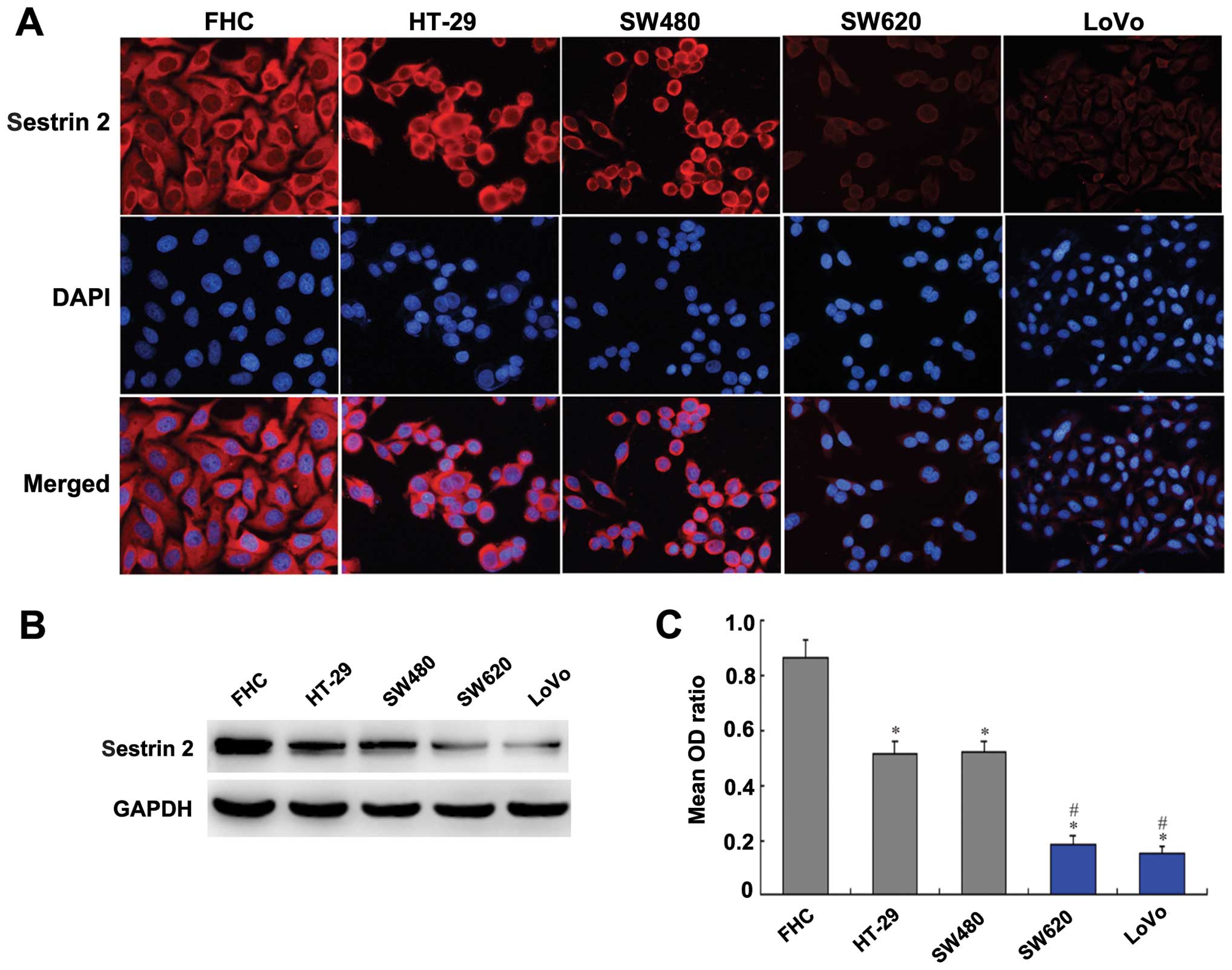
Sestrin 2 expression in colon normal
mucosa and CRC cell lines
The control cell line FHC and the HT-29, SW480,
SW620 and LoVo human CRC cell lines were selected to analyze the
expression of sestrin 2 at the protein level by immunofluorescence
and western blot analysis. Immunofluorescence showed the expression
of sestrin 2 was localized mainly in cytoplasm (Fig. 3A). Sestrin 2 expression was strong
in the FHC cells and moderate in HT-29 and SW480 cells, while it
was faint in the SW620 and LoVo cells (Fig. 3A). Western blot analysis also
revealed a markedly strong expression of sestrin 2 in FHC group and
a moderate expression in the HT-29 and SW480 groups, but a markedly
weak expression in the SW620 and LoVo groups (Fig. 3B). Compared with the FHC group, the
expression of sestrin 2 was significantly lower in the HT-29,
SW480, SW620 and LoVo groups (P<0.05; Fig. 3C). Furthermore, the expression of
sestrin 2 in the SW620 and LoVo cells was significantly lower than
that in the HT-29 and SW480 cells (P<0.05; Fig. 3C).
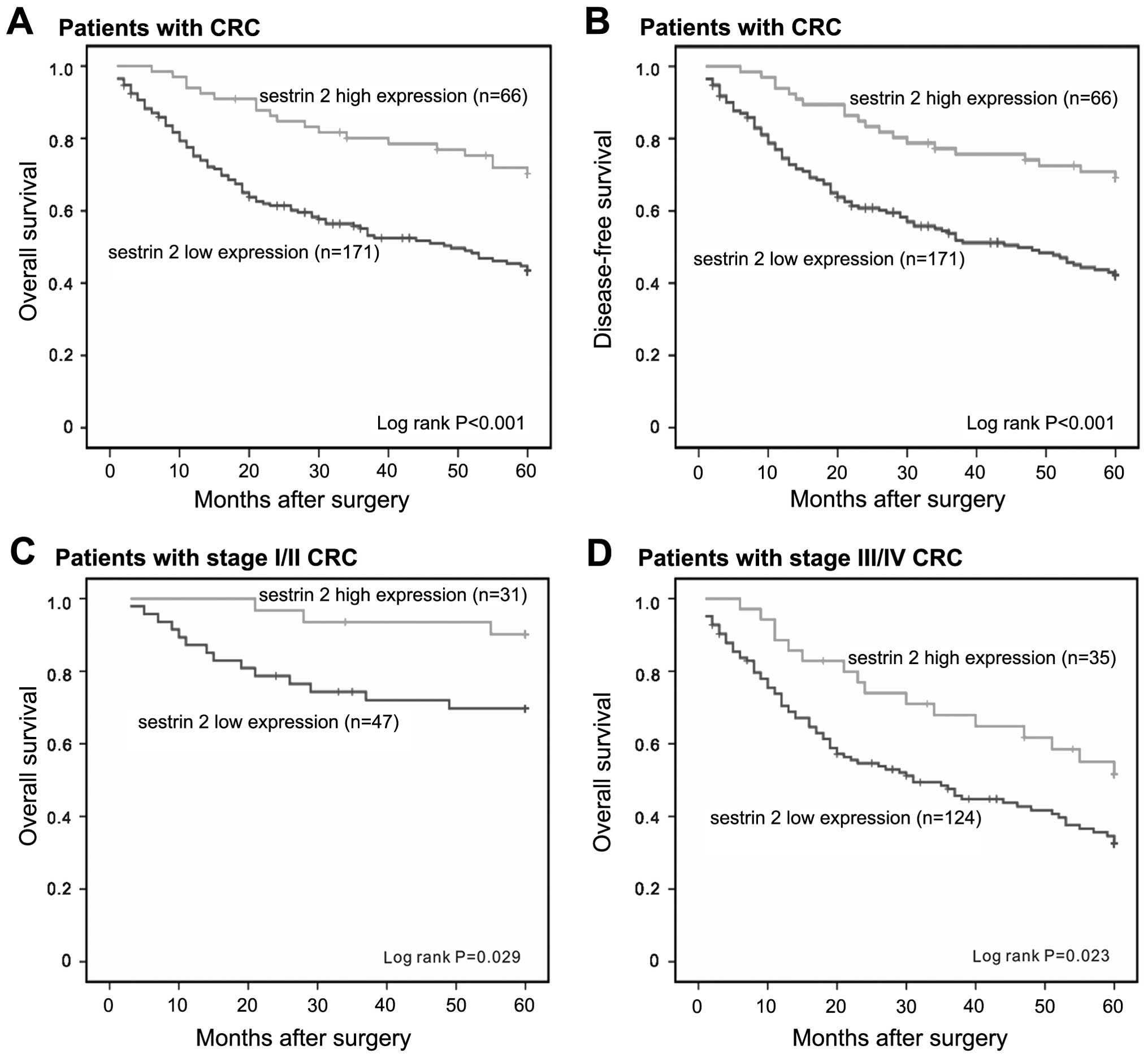
Low expression of sestrin 2 in CRC
predicts an unfavorable outcome
The correlation of sestrin 2 low expression and
clinical outcome was analyzed. Following 5-year follow-up, the mean
OS and DFS periods were 39.04±22.46 and 38.70±22.56 months,
respectively. To assess sestrin 2 as a predictor of survival, the
Kaplan-Meier analysis method was used to investigate the
correlation between sestrin 2 expression and survival. The log-rank
test showed that patients with low sestrin 2 staining had a
significantly worse OS and DFS than patients with high sestrin 2
staining (P<0.001 and P<0.001, respectively; Fig. 4A and B). Additionally, patients with
early or advanced stage CRC with low expression of sestrin 2 had a
shorter survival than patients with high expression (P=0.029 and
P=0.023, respectively; Fig. 4C and
Fig. 4D). At the 5-year follow-up,
50.99% of the patients with high sestrin 2 level survived. However,
only 37.67% of patients with low sestrin 2 staining survived.
The univariate analysis showed that the patients
with low sestrin 2 expression [DFS, hazard ratio (HR) =0.412,
P<0.001; OS, HR =0.398, P<0.001], advanced tumor stage (DFS,
HR =3.678, P<0.001; OS, HR =3.822, P<0.001), lymphatic
invasion (DFS, HR =2.773, P<0.001; OS, HR =2.825, P<0.001),
lymph node metastasis (DFS, HR =2.821, P<0.001; OS, HR =3.035,
P<0.001), vascular invasion (DFS, HR =2.538, P<0.001; OS, HR
=2.469, P<0.001), liver metastasis (DFS, HR =3.767, P<0.001;
OS, HR =3.809, P<0.001) and peritoneal metastasis (DFS, HR
=2.351, P<0.001; OS, HR =2.302, P=0.001) had shorter OS and DFS
(Table III). Furthermore, the
multivariate analysis showed that only low sestrin 2 expression
(DFS, HR =0.553, P=0.021; OS, HR =0.537, P=0.018), advanced TNM
stage (DFS, HR =3.043, P=0.026; OS, HR =3.352, P=0.016), lymphatic
node metastasis (DFS, HR =1.776, P=0.031; OS, HR =1.992, P=0.013),
vascular invasion (DFS, HR =2.012, P=0.006; OS, HR =1.929, P=0.012)
and liver metastasis (DFS, HR =2.469, P<0.001; OS, HR =2.516,
P<0.001) remained independent prognostic factors of poor OS and
DFS (Table III). However, no
significant correlation was detected between survival and other
clinicopathological variables including age, gender, tumor site,
histology, lymphatic invasion, peritoneal metastasis and serum CEA
level (all P>0.05; Table
III).
 | Table IIIUnivariate and multivariate analyses
of the associations between prognostic variables and DFS and OS in
237 CRC patients. |
Table III
Univariate and multivariate analyses
of the associations between prognostic variables and DFS and OS in
237 CRC patients.
| 5-year DFS HR (95%
CI), P-value | 5-year OS HR (95%
CI), P-value |
|---|
|
|
|
|---|
| Variables | Univariate | Multivariate | Univariate | Multivariate |
|---|
| Age (≥65 years vs.
<65 years) | 1.204
(0.830–1.746), P=0.328 | NA | 1.230
(0.843–1.794), P=0.283 | NA |
| Gender (male vs.
female) | 1.046
(0.724–1.512), P=0.810 | NA | 1.107
(0.762–1.606), P=0.594 | NA |
| Tumor site (distal
vs. proximal) | 0.987
(0.673–1.446), P=0.944 | NA | 1.032
(0.702–1.517), P=0.873 | NA |
| Histology
(poor/moderate vs. well) | 1.273
(0.870–1.862), P=0.215 | NA | 1.378
(0.933–2.035), P=0.107 | NA |
| TNM stage (III/IV
vs. I/II) | 3.678
(2.219–6.096), P<0.001 | 3.043
(1.143–8.103), P=0.026 | 3.822
(2.276–6.420), P<0.001 | 3.352
(1.249–8.999), P=0.016 |
| Lymphatic invasion
(yes vs. no) | 2.773
(1.777–4.327), P<0.001 | 0.556
(0.218–1.419), P=0.219 | 2.825
(1.796–4.446), P<0.001 | 0.479
(0.186–1.238), P=0.129 |
| Lymph node
metastasis (yes vs. no) | 2.821
(1.934–4.114), P<0.001 | 1.776
(1.054–2.993), P=0.031 | 3.035
(2.064–4.462), P<0.001 | 1.992
(1.158–3.425), P=0.013 |
| Vascular invasion
(yes vs. no) | 2.538
(1.616–3.986), P<0.001 | 2.012
(1.218–3.325), P=0.006 | 2.469
(1.559–3.912), P<0.001 | 1.929
(1.156–3.219), P=0.012 |
| Liver metastasis
(yes vs. no) | 3.767
(2.418–5.867), P<0.001 | 2.469
(1.512–4.031), P<0.001 | 3.809
(2.445–5.934), P<0.001 | 2.516
(1.540–4.112), P<0.001 |
| Peritoneal
metastasis (yes vs. no) | 2.351
(1.481–3.733), P<0.001 | 1.311
(0.799–2.152), P=0.284 | 2.302
(1.436–3.689), P=0.001 | 1.279
(0.772–2.119), P=0.339 |
| CEA level (≥5 μg/l
vs. <5 μg/l) | 0.972
(0.640–1.474), P=0.892 | NA | 0.962
(0.630–1.469), P=0.856 | NA |
| Sestrin 2 (high vs.
low) | 0.412
(0.254–0.668), P<0.001 | 0.553
(0.334–0.915), P=0.021 | 0.398
(0.249–0.654), P<0.001 | 0.537
(0.321–0.899), P=0.018 |
Discussion
ROS, which are thought to be a major source of
endogenous DNA damage, directly contribute to tumor progression and
metastasis (24–26). A great deal of evidence support the
view that oxidative stress and the accompanying ROS are genotoxic
and may contribute to the development of CRC (27). Furthermore, the genetic reduction of
mitochondrial oxidative stress reduces tumor grade and inhibits
metastasis (28). Therefore, tumors
occur when there is an imbalance between overproduction of ROS and
a decrease of antioxidant molecules in the body.
In the present study, we showed that the antioxidant
protein sestrin 2 was decreased in human CRC tissues. Similarly,
sestrin 2 was downregulated in human CRC cell lines. The expression
of sestrin 2 in SW620 and LoVo cells, which were derived from the
metastatic site of CRC, was significantly lower than that in the
HT-29 and SW480 cells, which were derived from the primary lesion
of CRC. In subsequent analysis of the association between the
sestrin 2 expression and clinicopathological variables, we found
that the low expression of sestrin 2 was correlated with lymph node
and liver metastasis. The findings reveal that there may be a
connection between the decreased expression of sestrin 2 and tumor
metastasis. Results of studies have shown that oxidative stress
directly contributes to tumor progression and metastasis (28,29).
In clinical findings, most current chemotherapy agents and
radiation therapy increase oxidative stress, leading to tumor
recurrence and metastasis (28).
Since sestrin 2 protect cells from oxidative stress, the
downregulation of sestrin 2 may increase oxidative stress, thus
aggravating tumor metastasis. However, the molecular pathway that
connects downregulation of sestrin 2 to the acquisition of
metastatic capacity during tumor progression remains to be
investigated. Besides the lymph node and liver metastasis, we found
that low expression of sestrin 2 was significantly correlated with
advanced tumor stage, lymphatic invasion and vascular invasion.
Previous studies reported that the abnormalities of
sestrin 2-related protein, p53, was associated with CRC patient
survival (30–33). However, the association between
sestrin 2 and cancer mortality has not been investigated in
clinical samples. Our results clearly demonstrate that a decreased
expression of sestrin 2 was an independent and significant
prognostic factor for 5-year DFS and OS. Additionally, early or
advanced stage CRC patients with a low expression of sestrin 2 had
a shorter survival than patients with a high expression. To the
least of our knowledg, this is the first study to show an
association between sestrin 2 expression and CRC patient survival.
These findings suggest that sestrin 2 and its associated proteins
may be crucial in CRC patient prognosis.
In conclusion, our study of patients with CRC
revealed the downregulation of sestrin 2 protein in human CRC
tissues compared with the normal mucosa, polyp, adenomas and
borderline tissues. The expression of sestrin 2 was decreased in
HT-29, SW480, SW620 and LoVo human CRC cell lines when compared
with the FHC control cell line. Additionally, decreased sestrin 2
was associated with an unfavorable prognosis and was an independent
prognostic factor for CRC, suggesting that sestrin 2 is a crucial
predictor for sestrin 2 metastasis. The results thus shed light on
the potential of sestrin 2 as a tumor-suppressor gene with a novel
antioxidant function in CRC, and that downregulation of sestrin 2
may aggravate tumor metastasis. However, future studies should be
conducted to examine the effects of changing sestrin 2 activity and
identify the possible mechanisms based on this novel protein
involved in CRC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81172295 and no. 81401070). The
authors sincerely thank the patients and their families for their
participation in this study.
References
|
1
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar :
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolpin BM and Mayer RJ: Systemic treatment
of colorectal cancer. Gastroenterology. 134:1296–1310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kovacic P and Jacintho JD: Mechanisms of
carcinogenesis: focus on oxidative stress and electron transfer.
Curr Med Chem. 8:773–796. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodrigues NR, Rowan A, Smith ME, et al:
p53 mutations in colorectal cancer. Proc Natl Acad Sci USA.
87:7555–7559. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sablina AA, Budanov AV, Ilyinskaya GV,
Agapova LS, Kravchenko JE and Chumakov PM: The antioxidant function
of the p53 tumor suppressor. Nat Med. 11:1306–1313. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vousden KH and Ryan KM: p53 and
metabolism. Nat Rev Cancer. 9:691–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Green DR and Kroemer G: Cytoplasmic
functions of the tumour suppressor p53. Nature. 458:1127–1130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Budanov AV and Karin M: p53 target genes
sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling.
Cell. 134:451–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Budanov AV, Sablina AA, Feinstein E,
Koonin EV and Chumakov PM: Regeneration of peroxiredoxins by
p53-regulated sestrins, homologs of bacterial AhpD. Science.
304:596–600. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Velasco-Miguel S, Buckbinder L, Jean P, et
al: PA26, a novel target of the p53 tumor suppressor and member of
the GADD family of DNA damage and growth arrest inducible genes.
Oncogene. 18:127–137. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Budanov AV, Shoshani T, Faerman A, et al:
Identification of a novel stress-responsive gene Hi95 involved in
regulation of cell viability. Oncogene. 21:6017–6031. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu W, Fu Z, Wang H, Feng J, Wei J and Guo
J: Peroxiredoxin 2 knockdown by RNA interference inhibits the
growth of colorectal cancer cells by downregulating Wnt/β-catenin
signaling. Cancer Lett. 343:190–199. 2014. View Article : Google Scholar
|
|
14
|
Lu W, Fu Z, Wang H, Feng J, Wei J and Guo
J: Peroxiredoxin 2 is upregulated in colorectal cancer and
contributes to colorectal cancer cells’ survival by protecting
cells from oxidative stress. Mol Cell Biochem. 387:261–270. 2014.
View Article : Google Scholar
|
|
15
|
Maiuri MC, Malik SA, Morselli E, et al:
Stimulation of autophagy by the p53 target gene Sestrin2. Cell
Cycle. 8:1571–1576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XY, Wu XQ, Deng R, Sun T, Feng GK
and Zhu XF: Upregulation of sestrin 2 expression via JNK pathway
activation contributes to autophagy induction in cancer cells. Cell
Signal. 25:150–158. 2013. View Article : Google Scholar
|
|
17
|
Wang N, Pan W, Zhu M, Zhang M, Hao X,
Liang G and Feng Y: Fangchinoline induces autophagic cell death via
p53/sentrin2/AMPK signaling in human hepatocellular carcinoma
cells. Br J Pharmacol. 164:731–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garber ME, Troyanskaya OG, Schluens K, et
al: Diversity of gene expression in adenocarcinoma of the lung.
Proc Natl Acad Sci USA. 98:13784–13789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wachi S, Yoneda K and Wu R:
Interactome-transcriptome analysis reveals the high centrality of
genes differentially expressed in lung cancer tissues.
Bioinformatics. 21:4205–4208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su LJ, Chang CW, Wu YC, et al: Selection
of DDX5 as a novel internal control for Q-RT-PCR from microarray
data using a block bootstrap re-sampling scheme. BMC Genomics.
8:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sanli T, Linher-Melville K, Tsakiridis T
and Singh G: Sestrin2 modulates AMPK subunit expression and its
response to ionizing radiation in breast cancer cells. PLoS One.
7:e320352012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim GT, Lee SH, Kim JI and Kim YM:
Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway
and induces apoptosis by increasing the generation of intracellular
ROS in a p53-independent manner. Int J Mol Med. 33:863–869.
2014.PubMed/NCBI
|
|
23
|
Au CW, Siu MK, Liao X, et al: Tyrosine
kinase B receptor and BDNF expression in ovarian cancers - Effect
on cell migration, angiogenesis and clinical outcome. Cancer Lett.
281:151–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee DJ and Kang SW: Reactive oxygen
species and tumor metastasis. Mol Cells. 35:93–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikawa M: Reactive oxygen species in
tumor metastasis. Cancer Lett. 266:53–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu WS: The signaling mechanism of ROS in
tumor progression. Cancer Metastasis Rev. 25:695–705. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Waris G and Ahsan H: Reactive oxygen
species: role in the development of cancer and various chronic
conditions. J Carcinog. 5:142006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sotgia F, Martinez-Outschoorn UE and
Lisanti MP: Mitochondrial oxidative stress drives tumor progression
and metastasis: should we use antioxidants as a key component of
cancer treatment and prevention? BMC Med. 9:622011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: how are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Elsaleh H, Powell B, McCaul K, et al: P53
alteration and microsatellite instability have predictive value for
survival benefit from chemotherapy in stage III colorectal
carcinoma. Clin Cancer Res. 7:1343–1349. 2001.PubMed/NCBI
|
|
31
|
Zeng ZS, Sarkis AS, Zhang ZF, et al: p53
nuclear overexpression: an independent predictor of survival in
lymph node-positive colorectal cancer patients. J Clin Oncol.
12:2043–2050. 1994.PubMed/NCBI
|
|
32
|
Houbiers JG, van der Burg SH, van de
Watering LM, et al: Antibodies against p53 are associated with poor
prognosis of colorectal cancer. Br J Cancer. 72:637–641. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu SJ, Yu JK, Ge WT, Hu HG, Yuan Y and
Zheng S: SPARCL1, Shp2, MSH2, E-cadherin, p53, ADCY-2 and MAPK are
prognosis-related in colorectal cancer. World J Gastroenterol.
17:2028–2036. 2011. View Article : Google Scholar : PubMed/NCBI
|