Introduction
Colorectal cancer (CRC) is one of the most common
types of cancers and is the leading cause of cancer-related death
worldwide (1). When the tumor is
limited to the mucosa or submucosa, CRC can be completely cured by
endoscopic or surgical therapy; however, many patients are already
at an advanced stage at diagnosis. A chemotherapy regimen of
fluoropyrimidines plus either oxaliplatin or irinotecan is
considered the standard treatment for advanced CRC (2–5).
Recently, interest in the use of phytomedicines, such as botanical
extracts, for cancer treatment is increasing (6). These natural therapies using
plant-derived extracts may reduce adverse side effects compared
with traditional cancer treatments (7).
Maple syrup is a natural sweetener consumed by men
and women of all ages throughout the world. Maple syrup contains
not only abundant amounts of sucrose and glucose, but also various
other components such as oligosaccharides, organic acids, amino
acids, vitamins, and minerals including manganese and zinc
(8–12). Moreover, recent studies have shown
that maple syrup contains various phenolic compounds such as
lignans and coumarin (13,14), quebecol (15), and ginnalin (16,17).
These phenolic compounds in maple syrup may possess various types
of activity. An in vitro study of a butanol extract from
maple syrup demonstrated inhibitory activity toward α-glucosidase
(18). Ethyl acetate extracts of
maple syrup showed antioxidant activity and anti-proliferative
effects against cancer cell lines (19). Ginnalin-A inhibited the cell growth
of colon cancer cell lines (16).
In addition, an effect of maple syrup has also been reported. The
increase in plasma glucose was found to be lower after the oral
administration of maple syrup than after the administration of
sucrose in the Otsuka Long-Evans Tokushima fatty rat, a model of
type II diabetes mellitus (20).
Although maple syrup is made from boiling down sap,
its color, aroma and taste change due to differences in the growth
conditions and season when the sap is collected. Thus, based on
Canadian standards, maple syrup is classified into five grades as
follows: AA (extra light), grade A (light), grade B (medium), grade
C (amber), and grade D (dark) (19). The syrup typically becomes darker in
color as the season progresses, and antioxidant activity is
proportional to the darkening color of the maple syrup (19). This suggests that different grades
of maple syrup may have different effects against cancer cells.
However, there are almost no reports or scientific evidence on the
effects of the various maple syrup grades on the behavior of cancer
cells. Therefore, there is a need to evaluate differences in maple
syrup grade on the functions of cancer cells to evaluate the use of
maple syrup as a phytomedicine for cancer treatment. In this study,
we examined the effect of three types of maple syrup, classified by
color, on the proliferation, migration, and invasion of CRC cells
in order to investigate whether maple syrup is suitable as a
phytomedicine for cancer treatment.
Materials and methods
Materials
The following chemicals and reagents of the highest
grade available were purchased as follows: urea from GE Healthcare,
UK, Ltd. (Buckinghamshire, UK);
3-[(3-chol-amidepropyl)dimethylammonio]-1-propanesulphonate (CHAPS)
from Wako Pure Chemical Industries (Osaka, Japan); and thiourea and
Triton X-100 from Nacalai Tesque, Inc. (Kyoto, Japan). All other
chemicals and reagents were purchased from Sigma Chemical Corp.
(St. Louis, MO, USA).
Maple syrup samples
Maple syrups were purchased at a local grocery
store. We chose three maple syrups of different colors, and
classified these syrups as three types based on their increasingly
darker color. Maple syrup I was slightly golden, maple syrup II was
amber; and maple syrup III was very dark brown.
High-performance liquid chromatography
(HPLC) analysis
We determined the sucrose concentration in each type
of maple syrup using an LC-10Advp HPLC system equipped with Shodex
RI-71 and an Asahipak NH2P-50 4E (all from Shimadzu Kyoto, Japan)
column at room temperature. The mobile phase used was
acetonitrile/milliQ water, 3:1 (v/v), at a flow rate of 1 ml/min,
and a 20-μl sample solution was injected. The sample solution was
prepared by diluting the syrup in water (1;100).
CRC cell lines
The DLD-1 and SW480 colorectal cancer cell lines and
CCD 841 CoN normal human colon epithelial cells were purchased from
the American Type Culture Collection (ATTC; Manassas, VA, USA). All
cells were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (Gibco, Carlsbad, CA, USA) in an atmosphere containing
5% CO2.
Cell proliferation assays
Cells were grown in 96-well plates at a density of
5×103 cells per well and grown in culture medium. The
next day, the medium was changed and cells were either grown in
culture medium containing sucrose or maple syrup. After 24, 48, 72
and 96 h, the cells were incubated with WST-8 cell counting reagent
(Wako) for 4 h at 37°C, and the optical density of the culture
solution in the plate was measured using an ELISA plate reader. In
addition to the WST-8 assay, the cell number was counted using a
Countess Automated Cell Counter (Life Technologies Japan, Tokyo,
Japan). The cells were cultured in a 6-well plate at a density of
5×104 cells per well and grown in culture medium. On the
next day, the medium was changed, and the cells were grown in
culture medium containing either sucrose or maple syrup. The number
of cells was counted after 24, 48, 72 and 96 h.
Cell migration and invasion assays
The in vitro migration assay was carried out
using a modified Boyden chamber technique (BD Bioscience, Franklin
Lakes, NJ, USA). Cells were plated on the inner surface of the
inserts at a density of 1×105 cells/insert, followed by
incubation at 37°C in a humidified 5% CO2 atmosphere.
Culture medium containing sucrose or maple syrup was placed in each
lower chamber as a chemoattractant. Using a previously reported
method, the DLD-1 and SW480 cells on the outer surface of the
inserts were counted after 48 (21)
and 20 h (22), respectively. All
assays were performed in triplicate, and 5 fields (x200) were
counted on each membrane in a blinded manner. The cell invasion
assay was performed using a modified Boyden chamber method in which
the inner surfaces of the inserts were coated with Matrigel.
Protein preparation
DLD-1 and SW480 cells were plated at a density of
5×105 cells per 100-mm dish and grown in culture medium.
On the following day, the medium was changed and cells were grown
in culture medium containing either sucrose or maple syrup. After
72 h, the cells were solubilized in urea lysis buffer (7 M urea, 2
M thiourea, 5% CHAPS, 1% Triton X-100). The protein concentration
was measured using the Bradford method.
Western blot analysis
The cell extract was subjected to SDS-PAGE under
reducing conditions, and the separated proteins were transferred to
polyvinylidene fluoride transfer membranes. The membranes were
incubated with an anti-phospho-p44/42 MAPK antibody or
anti-phospho-AKT antibody (Cell Signaling Technology Inc., Beverly,
MA, USA) at 4°C overnight. The membranes were washed and incubated
with HRP-conjugated anti-rabbit IgG antibody or HRP-conjugated
anti-mouse IgG antibody (American Qualex, San Clemente, CA, USA).
After washing, the blots were visualized by enhanced
chemiluminescence and detected using an ImageQuant LAS 500 system
(GE Healthcare). The same membranes were re-probed with the
anti-β-actin antibody (Sigma Chemical Corp., St. Louis, MO, USA),
anti-p44/42 MAPK (Erk1/2) antibody, or anti-AKT antibody (Cell
Signaling Technology) to confirm equal loading of the proteins. All
western blot analyses were performed in triplicate.
Statistical analysis
All data are presented as the mean ± standard error
of measurement (SEM). The data were analyzed using one-way analysis
of variance followed by Dunnett’s test. A P-value ≤0.05 was
considered to indicate a statistically significant difference.
Computations were performed using the GraphPad Prism version 5
(GraphPad Software, La Jolla, CA, USA).
Results
The sucrose concentration in the three
types of maple syrup
First, we examined the concentration of sucrose, the
main component of maple syrup (23), in the three selected maple syrups
(Table I). The sucrose
concentration was well controlled and the concentrations did not
differ significantly among the three types of maple syrup. We
accurately adjusted the concentration of sucrose in maple syrups II
and III to those of maple syrup I to ensure that the cells were
affected by an equivalent amount of sucrose in each dose of maple
syrup. We also prepared a sucrose solution containing an equivalent
amount of sucrose as in maple syrup I as a control solution.
 | Table IRelative color and sucrose
concentrations of the different maple syrup samples. |
Table I
Relative color and sucrose
concentrations of the different maple syrup samples.
| Color | Sucrose (g/100
ml) |
|---|
| Maple syrup I | Slightly golden | 60.01 |
| Maple syrup II | Amber | 62.89 |
| Maple syrup III | Very dark brown | 54.72 |
Cytotoxicity of sucrose against the CRC
cells
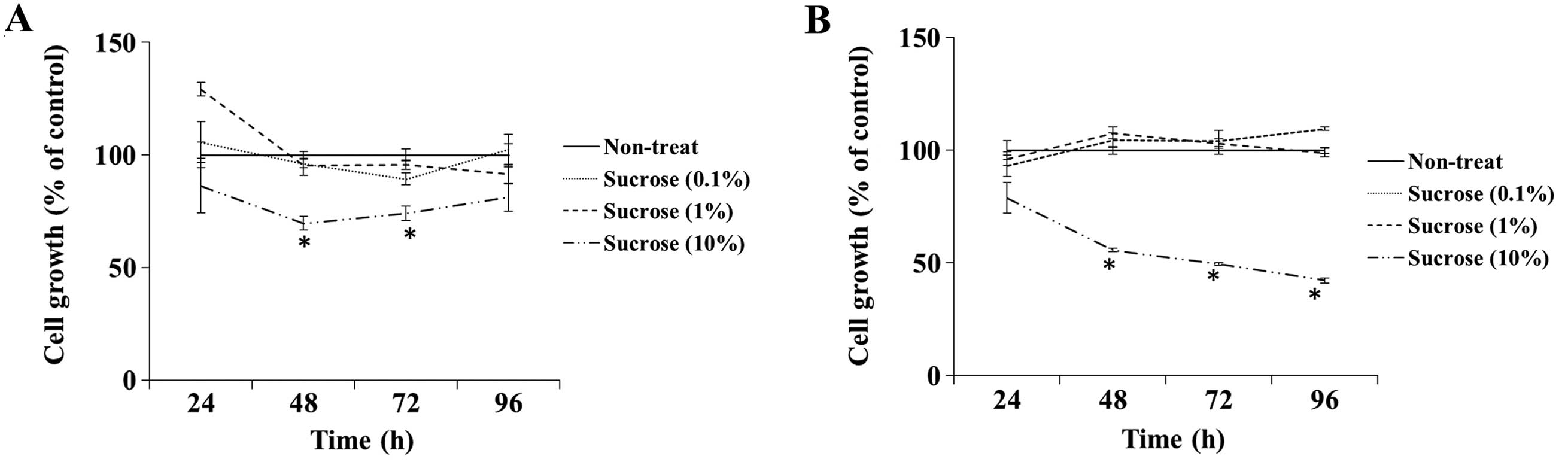
To examine the cytotoxic effect of high
concentrations of sucrose on DLD-1 and SW480 cells, we assessed the
cell growth rate when cells were grown in culture medium containing
the control sucrose solution at a concentration of 0.1–10% (v/v).
The growth rate of the DLD-1 cells cultured in the medium
containing 10% sucrose solution was significantly inhibited at 48
and 72 h compared with the rate in the non-treated cells, whereas
other concentrations of sucrose did not affect cell growth
(Fig. 1A). The 10% sucrose solution
also significantly inhibited the cell growth rate of SW480 cells at
48, 72 and 96 h (Fig. 1B). Thus, we
determined the dose of maple syrup and sucrose solution as 1% since
sucrose did not show cytotoxicity in the following experiments.
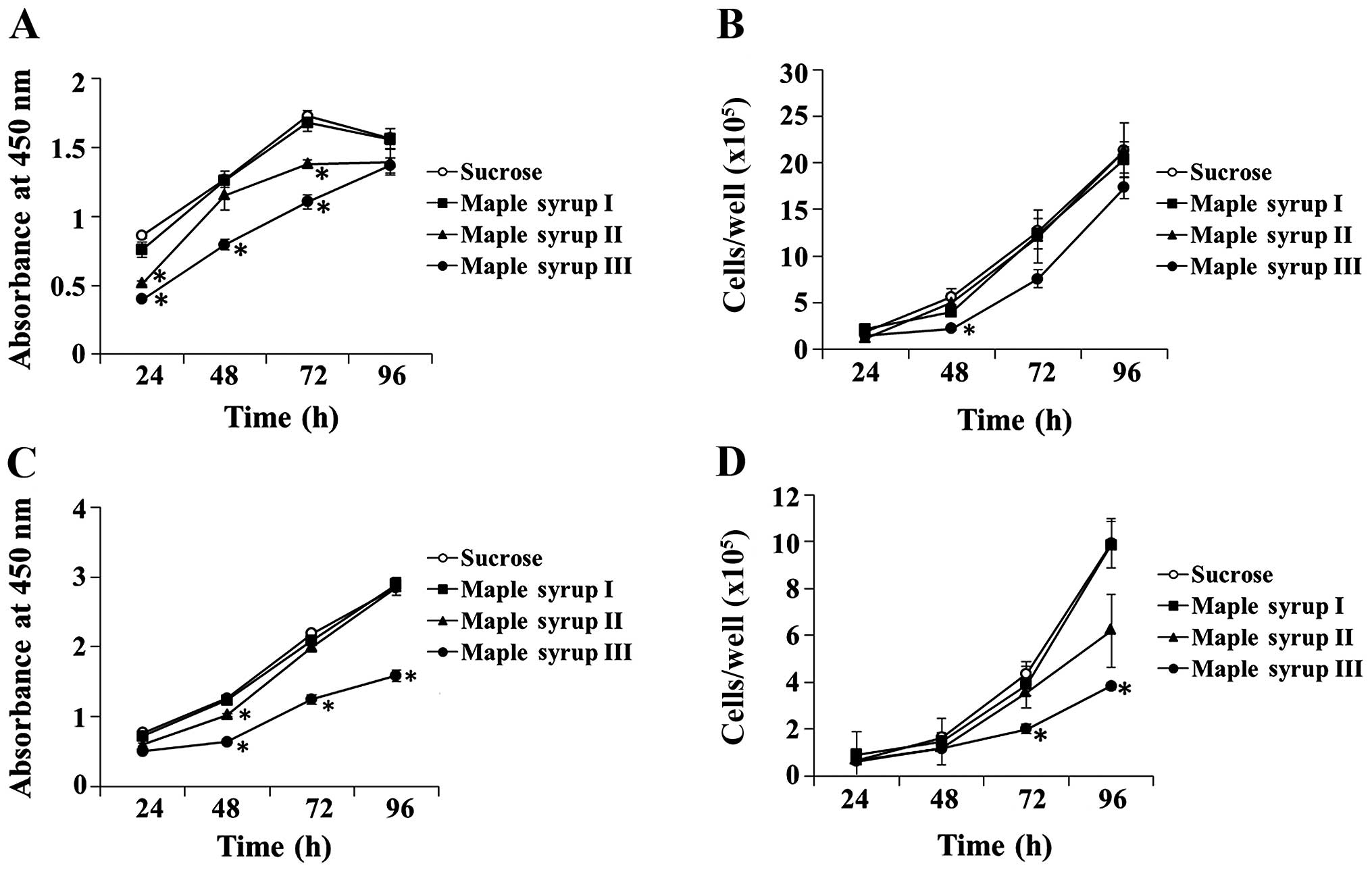
Effect of maple syrup on the cell growth
of CRC cells
We determined whether maple syrup had any effects on
the growth of CRC cells. Administration of maple syrup
significantly inhibited the cell growth of the DLD-1 cells at 24 h
(maple syrup II and maple syrup III), 48 h (maple syrup III) and 72
h (maple syrup II and maple syrup III) using the WST-8 assay
(P<0.01, Fig. 2A), and at 48 h
(maple syrup III) using the cell counting method (P<0.01,
Fig. 2B). Administration of maple
syrup also inhibited the cell growth of the SW480 cells at 48 h
(maple syrup II and maple syrup III), 72 h (maple syrup III), and
96 h (maple syrup III) using the WST-8 assay (P<0.01, Fig. 2C), and at 72 h (maple syrup III) and
96 h (maple syrup III) using the cell counting method (P<0.01,
Fig. 2D).
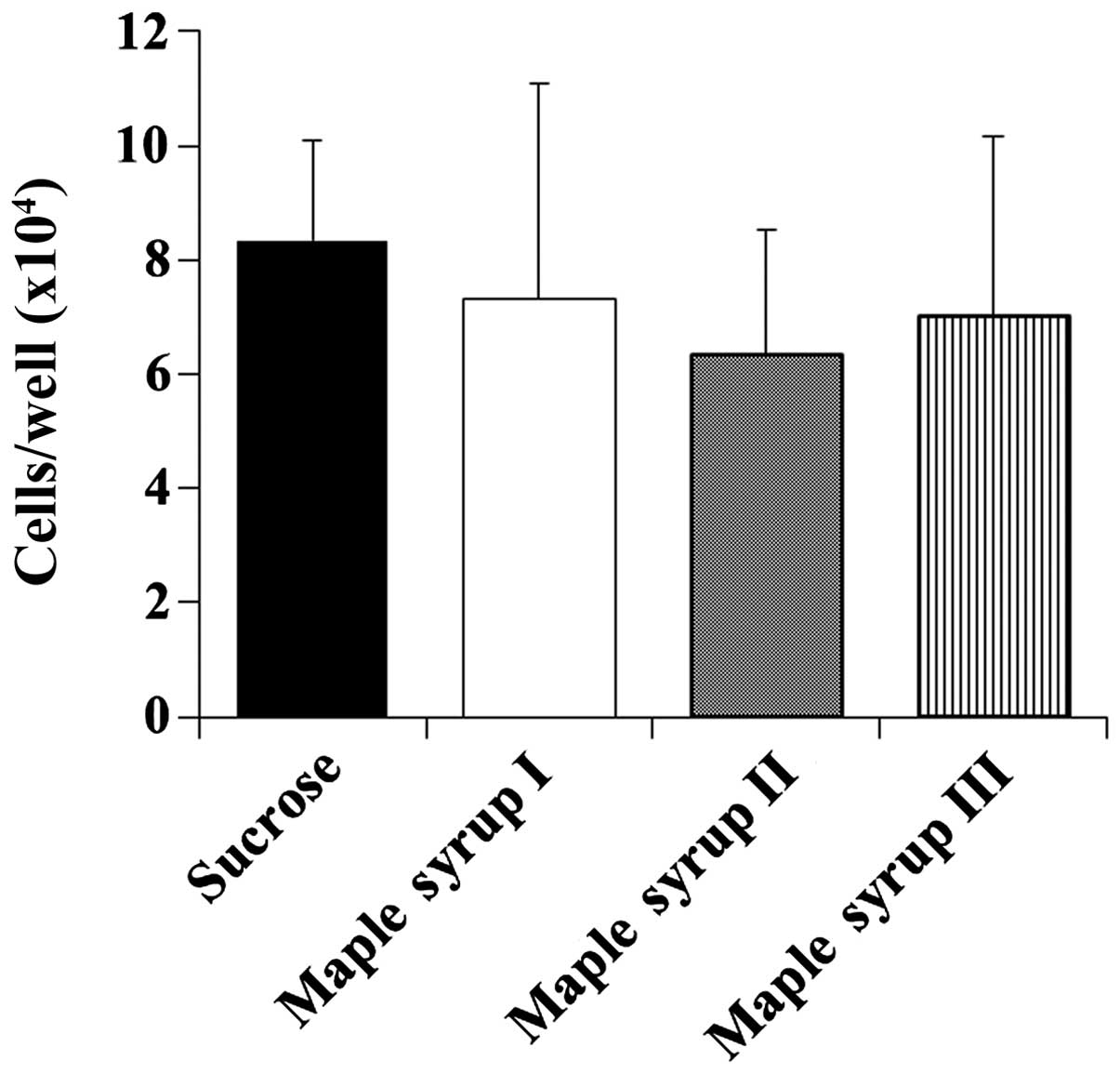
Effect of maple syrup on the cell growth
of normal human colon epithelium cells
Next, we determined the effect of maple syrup on the
cell growth of CCD 841 CoN cells to determine whether maple syrup
has any effects on the growth of normal colon cells. Maple syrup
administration had no effect on the growth of the CCD 841 CoN cells
at 72 h despite that the growth of the CRC cells was inhibited
(Fig. 3).
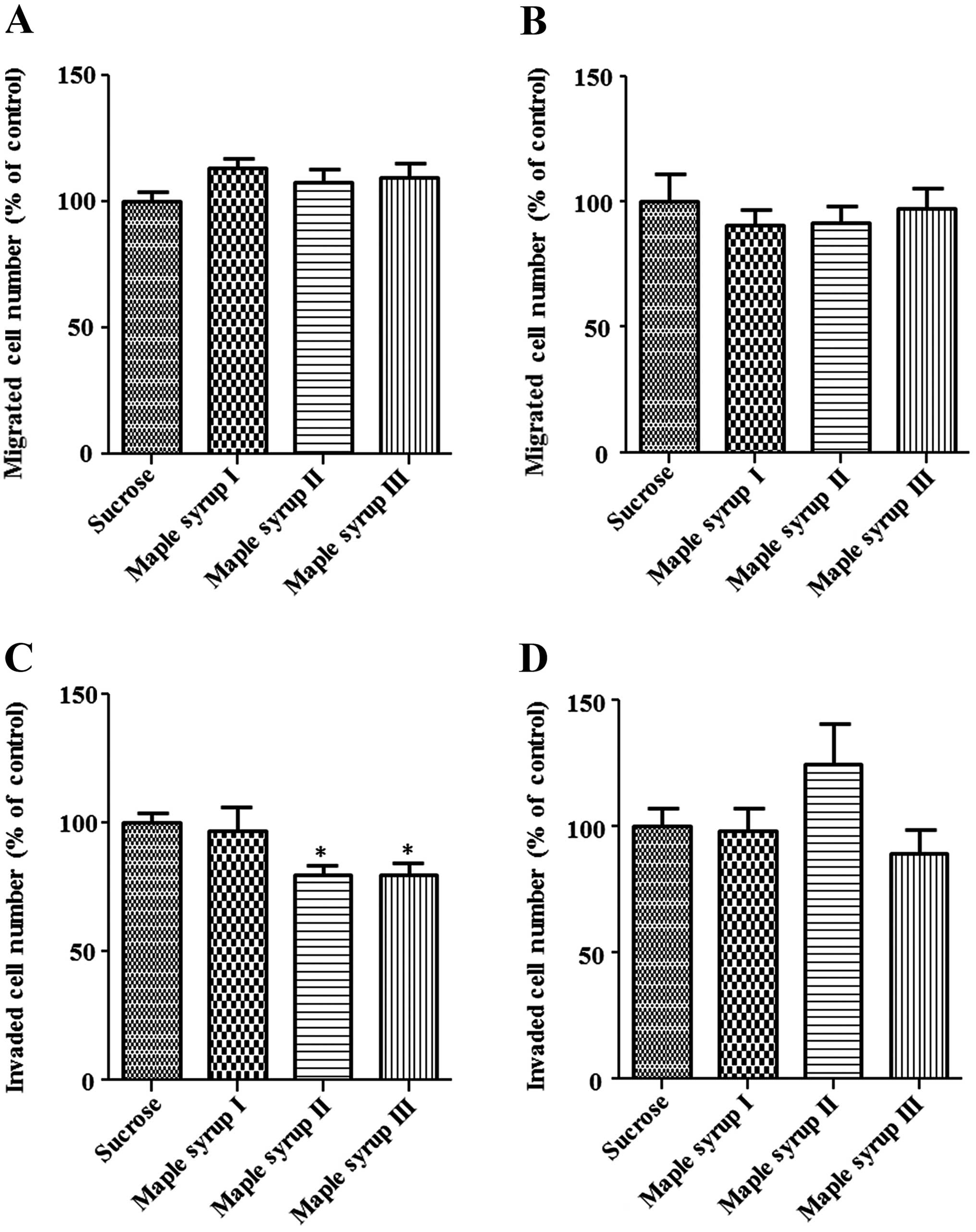
Effect of maple syrup on cell migration
and invasion
Next, we examined the migration and invasion of CRC
cells using the modified Boyden chamber method. The number of cells
that migrated from the inside of the chamber to the outside was not
significantly affected by maple syrup in the DLD-1 and SW480 cells
(Fig. 4A and B). However, an
invasion assay using a Matrigel-coated chamber showed that the
number of DLD-1 cells invading the Matrigel was significantly
inhibited by maple syrup II and maple syrup III (P<0.01,
Fig. 4C), and the number of SW480
cells invading the Matrigel tended to be inhibited by maple syrup
III (P=0.0779, Fig. 4D)
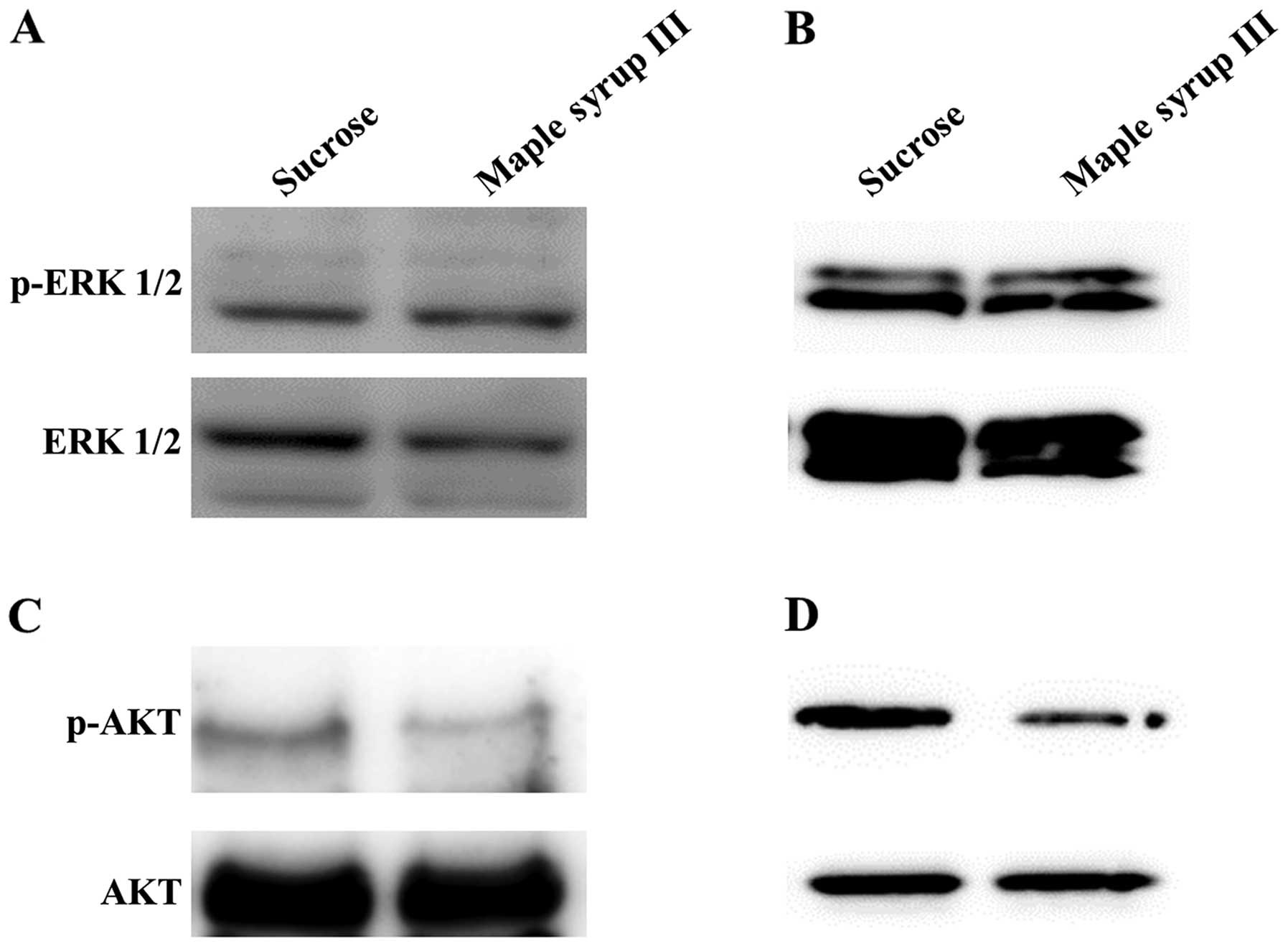
Effect of maple syrup on signaling
pathways in CRC cells
To determine the signaling pathway that is affected
by maple syrup, we examined the phosphorylation of ERK and AKT,
which play important roles in cell proliferation and invasion in
CRC cells, after administration of sucrose solution or maple syrup
III. Administration of maple syrup III did not affect the
phosphorylation of ERK (Fig. 5A and
B). In contrast, we found that administration of maple syrup
III clearly inhibited AKT phosphorylation (Fig. 5C and D).
Discussion
In the present study, we examined the effect of
different types of maple syrup on two different CRC cell lines.
First, we examined whether the high concentration of sucrose in
maple syrup affects CRC cell growth in order to clarify the
anti-proliferative effect of maple syrup against CRC cells. High
concentrations of sucrose significantly inhibited the growth of the
CRC cell lines (Fig. 1). This
result suggests that the high concentration (10%) of sucrose in
maple syrup has cytotoxic effects due to high osmotic pressure.
Previously, maple syrup was reported to inhibit the growth of
prostate (74%), lung (63%), breast (45%) and colorectal (37%
inhibition) cancer cell lines (19). However, these cancer cell lines were
grown in culture medium containing 5% (v/v) maple syrup. Therefore,
we were concerned that the growth of the cells might have been
affected by high osmotic pressure due to the high concentration of
sucrose. Thus, we estimated the dose of maple syrup that would not
have cytotoxic effects for subsequent experiments.
Under these conditions, CRC cells grown in culture
medium containing maple syrup had a decreased growth rate compared
with those grown in culture medium containing sucrose, using both
the WST-8 assay and cell counting method to determine growth rates
(Fig. 2). Notably, the
anti-proliferative effect of maple syrup increased as the color of
the maple syrup became darker. We also examined the effect of maple
syrup on the cell growth of normal colonic epithelial cells;
however, none of the maple syrups tested affected the growth of
normal colonic epithelial cells (Fig.
3). These data suggest that maple syrup may effectively inhibit
rapidly growing cells such as cancer cells.
Moreover, cell invasive activity was significantly
inhibited by maple syrup II and maple syrup III in the DLD-1 cells,
and tended to be inhibited by maple syrup III in the SW480 cells,
whereas there was no effect on the cell migration of either DLD-1
or SW480 cells (Fig. 4). These data
suggest that maple syrup might affect the expression or activation
of enzymes, such as MMP-2 and MMP-9, which play an important role
in cell invasion of the extracellular matrix, including the
basement membrane. Although there is no report on the effect of
maple syrup on cell invasion, a recent study reported that
polyphenols such as epigallocatechin-3-gallate inhibit MMP-2 and
MMP-9 expression (24–28). Therefore, the expression levels of
MMP-2 and MMP-9 in CRC cells might be inhibited by polyphenols such
as quebecol and ginnalins that are present in maple syrup.
We examined the phosphorylation status of ERK and
AKT using maple syrup III, which was the most effective maple syrup
in our study. AKT activation was clearly inhibited by
administration of maple syrup III, whereas ERK activation was not
affected (Fig. 5). These data
suggest that the anti-proliferative effect of maple syrup might be
due to apoptosis induction. In addition, inhibition of AKT
phosphorylation has been correlated with the inhibition of cancer
cell invasion by reducing MMP-2 and MMP-9 expression (29–31). A
recent study reported that ginnalins A-C, which are polyphenols
present in maple syrup, inhibited cell growth through cell cycle
arrest that did not induce apoptosis (17). Therefore, there is a possibility
that maple syrup contains other effective compounds in addition to
polyphenols. Further studies are needed to identify the compounds
responsible for the inhibition of cell growth and invasion observed
through the suppression of the AKT signaling pathway after the
administration of maple syrup. In preparation for future research,
we have begun to identify compounds using the high molecular weight
fraction (MW >10,000) of maple syrup that is related to the
inhibition of cell growth by maple syrup III, which demonstrated
the strongest inhibitory effect among the maple syrup types we
tested.
In conclusion, maple syrup, which is a natural
sweetener used throughout the world, inhibits CRC cell growth and
invasion through suppression of the AKT signaling pathway. These
findings suggest that maple syrup, particularly dark colored ones,
might be suitable as phytomedicines, which have fewer adverse
effects than traditional chemotherapy for CRC treatment.
Acknowledgements
This study was supported by the MEXT (Ministry of
Education, Culture, Sports. Science and Technology)-supported
Program for the Strategic Research Foundation at Private
Universities, 2014–2018, a grant from Maple Farms Japan, and a
Grant-in-Aid for Scientific Research from the Japan Society for the
Promotion of Science to T. Y. (C, No. 24591019).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.PubMed/NCBI
|
|
3
|
Douillard JY, Cunningham D, Roth AD, et
al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: a multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hochster HS, Hart LL, Ramanathan RK, et
al: Safety and efficacy of oxaliplatin and fluoropyrimidine
regimens with or without bevacizumab as first-line treatment of
metastatic colorectal cancer: results of the TREE Study. J Clin
Oncol. 26:3523–3529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Rivera F, Berry S, et al:
Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX,
FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the
BEAT study. Ann Oncol. 20:1842–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazzio EA and Soliman KF: In vitro
screening for the tumoricidal properties of international medicinal
herbs. Phytother Res. 23:385–398. 2009. View Article : Google Scholar :
|
|
7
|
Desai AG, Qazi GN, Ganju RK, et al:
Medicinal plants and cancer chemoprevention. Curr Drug Metab.
9:581–591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ball DW: The chemical composition of maple
syrup. J Chem Educ. 84:U1647–U1648. 2007. View Article : Google Scholar
|
|
9
|
Davison RM and Young H: Abscisic acid
content of xylem sap. Planta. 109:95–98. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perkins TD and van den Berg AK: Maple
syrup - production, composition, chemistry, and sensory
characteristics. Adv Food Nutr Res. 56:101–143. 2009. View Article : Google Scholar
|
|
11
|
Taga A, Sato A, Suzuki K, Takeda M and
Kodama S: Simple determination of a strongly aromatic compound,
sotolon, by capillary electrophoresis. J Oleo Sci. 61:45–48. 2012.
View Article : Google Scholar
|
|
12
|
Taga A and Kodama S: Analysis of reducing
carbohydrates and fructosyl saccharides in maple syrup and maple
sugar. Chromatographia. 75:1009–1016. 2012. View Article : Google Scholar
|
|
13
|
Li L and Seeram NP: Maple syrup
phytochemicals include lignans, coumarins, a stilbene, and other
previously unreported antioxidant phenolic compounds. J Agric Food
Chem. 58:11673–11679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L and Seeram NP: Further investigation
into maple syrup yields 3 new lignans, a new phenylpropanoid, and
26 other phytochemicals. J Agric Food Chem. 59:7708–7716. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li LY and Seeram NP: Quebecol, a novel
phenolic compound isolated from Canadian maple syrup. J Funct
Foods. 3:125–128. 2011. View Article : Google Scholar
|
|
16
|
Gonzalez-Sarrias A, Li L and Seeram NP:
Effects of maple (Acer) plant part extracts on proliferation,
apoptosis and cell cycle arrest of human tumorigenic and
non-tumorigenic colon cells. Phytother Res. 26:995–1002. 2012.
View Article : Google Scholar
|
|
17
|
Gonzalez-Sarrias A, Ma H, Edmonds ME and
Seeram NP: Maple polyphenols, ginnalins A-C, induce S- and
G2/M-cell cycle arrest in colon and breast cancer cells mediated by
decreasing cyclins A and D1 levels. Food Chem. 136:636–642. 2013.
View Article : Google Scholar
|
|
18
|
Apostolidis E, Li LY, Lee C and Seeram NP:
In vitro evaluation of phenolic-enriched maple syrup extracts for
inhibition of carbohydrate hydrolyzing enzymes relevant to type 2
diabetes management. J Funct Foods. 3:100–106. 2011. View Article : Google Scholar
|
|
19
|
Legault J, Girard-Lalancette K, Grenon C,
Dussault C and Pichette A: Antioxidant activity, inhibition of
nitric oxide overproduction, and in vitro antiproliferative effect
of maple sap and syrup from Acer saccharum. J Med Food. 13:460–468.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagai N, Ito Y and Taga A: Comparison of
the enhancement of plasma glucose levels in type 2 diabetes Otsuka
Long-Evans Tokushima Fatty rats by oral administration of sucrose
or maple syrup. J Oleo Sci. 62:737–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siemens H, Jackstadt R, Hunten S, et al:
miR-34 and SNAIL form a double-negative feedback loop to regulate
epithelial-mesenchymal transitions. Cell Cycle. 10:4256–4271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Bavarva JH, Wang Z, et al: HEF1, a
novel target of Wnt signaling, promotes colonic cell migration and
cancer progression. Oncogene. 30:2633–2643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stuckel JG and Low NH: The chemical
composition of 80 pure maple syrup samples produced in North
America. Food Res Int. 29:373–379. 1996. View Article : Google Scholar
|
|
24
|
Kang JS, Park IH, Cho JS, et al:
Epigallocatechin-3-gallate inhibits collagen production of nasal
polyp-derived fibroblasts. Phytother Res. 28:98–103. 2014.
View Article : Google Scholar
|
|
25
|
Zhai X, Chi J, Tang W, et al: Yellow wine
polyphenolic compounds inhibit matrix metalloproteinase-2, -9
expression and improve atherosclerotic plaque in
LDL-receptor-knockout mice. J Pharmacol Sci. 125:132–141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh T and Katiyar SK: Green tea
polyphenol, (−)-epigallo-catechin-3-gallate, induces toxicity in
human skin cancer cells by targeting β-catenin signaling. Toxicol
Appl Pharmacol. 273:418–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao M, Tang SN, Marsh JL, Shankar S and
Srivastava RK: Ellagic acid inhibits human pancreatic cancer growth
in Balb c nude mice. Cancer Lett. 337:210–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsai CM, Sun FM, Chen YL, Hsu CL, Yen GC
and Weng CJ: Molecular mechanism depressing PMA-induced invasive
behaviors in human lung adenocarcinoma cells by cis- and
trans-cinnamic acid. Eur J Pharm Sci. 48:494–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Zheng L, Liu J, et al: Shikonin
inhibits prostate cancer cells metastasis by reducing matrix
metalloproteinase-2/-9 expression via AKT/mTOR and ROS/ERK1/2
pathways. Int Immunopharmacol. 21:447–455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuo CL, Lai KC, Ma YS, Weng SW, Lin JP and
Chung JG: Gallic acid inhibits migration and invasion of SCC4 human
oral cancer cells through actions of NFκB, Ras and matrix
metallo-proteinase-2 and -9. Oncol Rep. 32:355–361. 2014.PubMed/NCBI
|
|
31
|
Xu Q, Ma J, Lei J, et al: α-Mangostin
suppresses the viability and epithelial-mesenchymal transition of
pancreatic cancer cells by downregulating the PI3K/Akt pathway.
Biomed Res Int. 2014:5463532014. View Article : Google Scholar
|