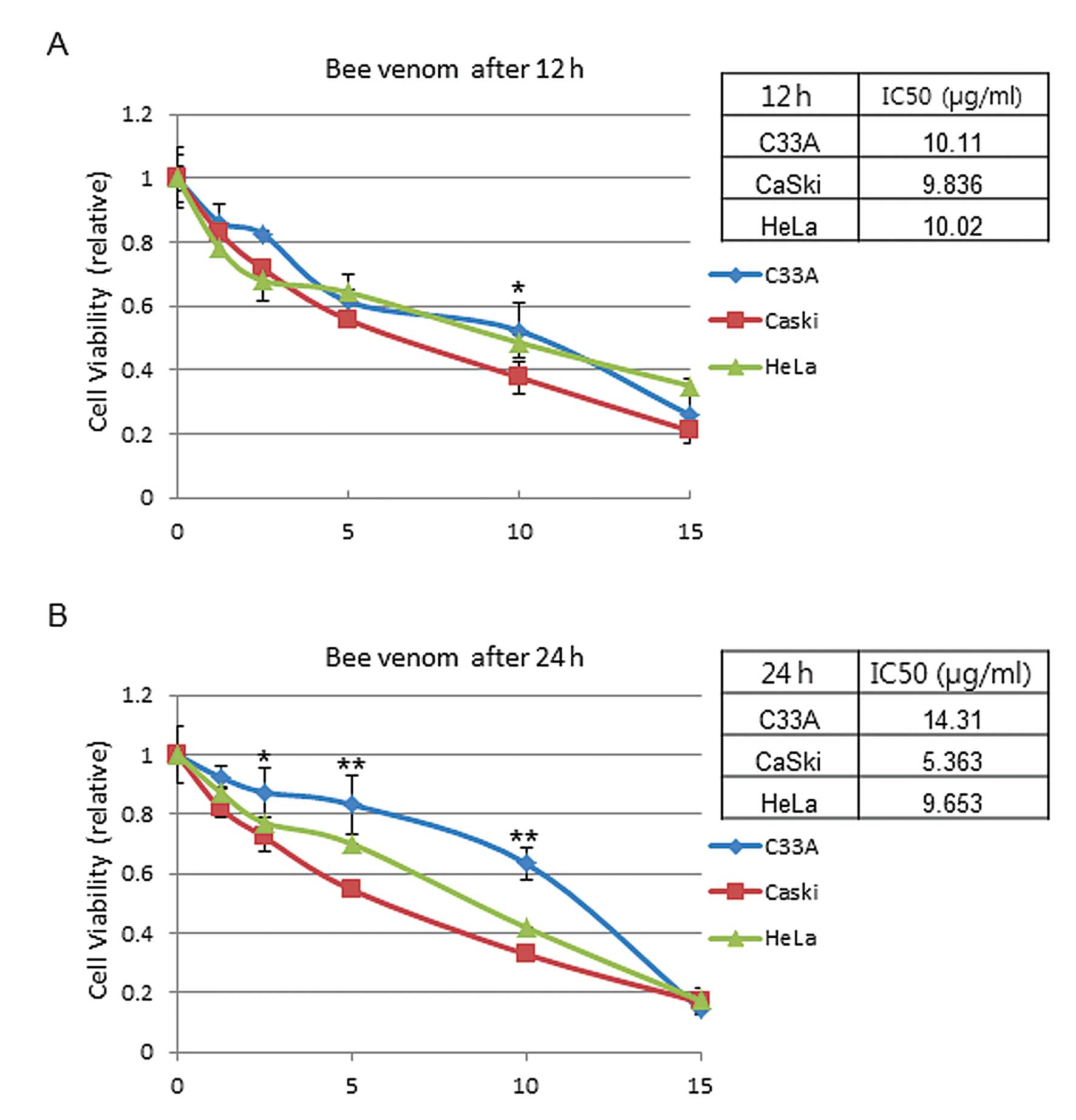
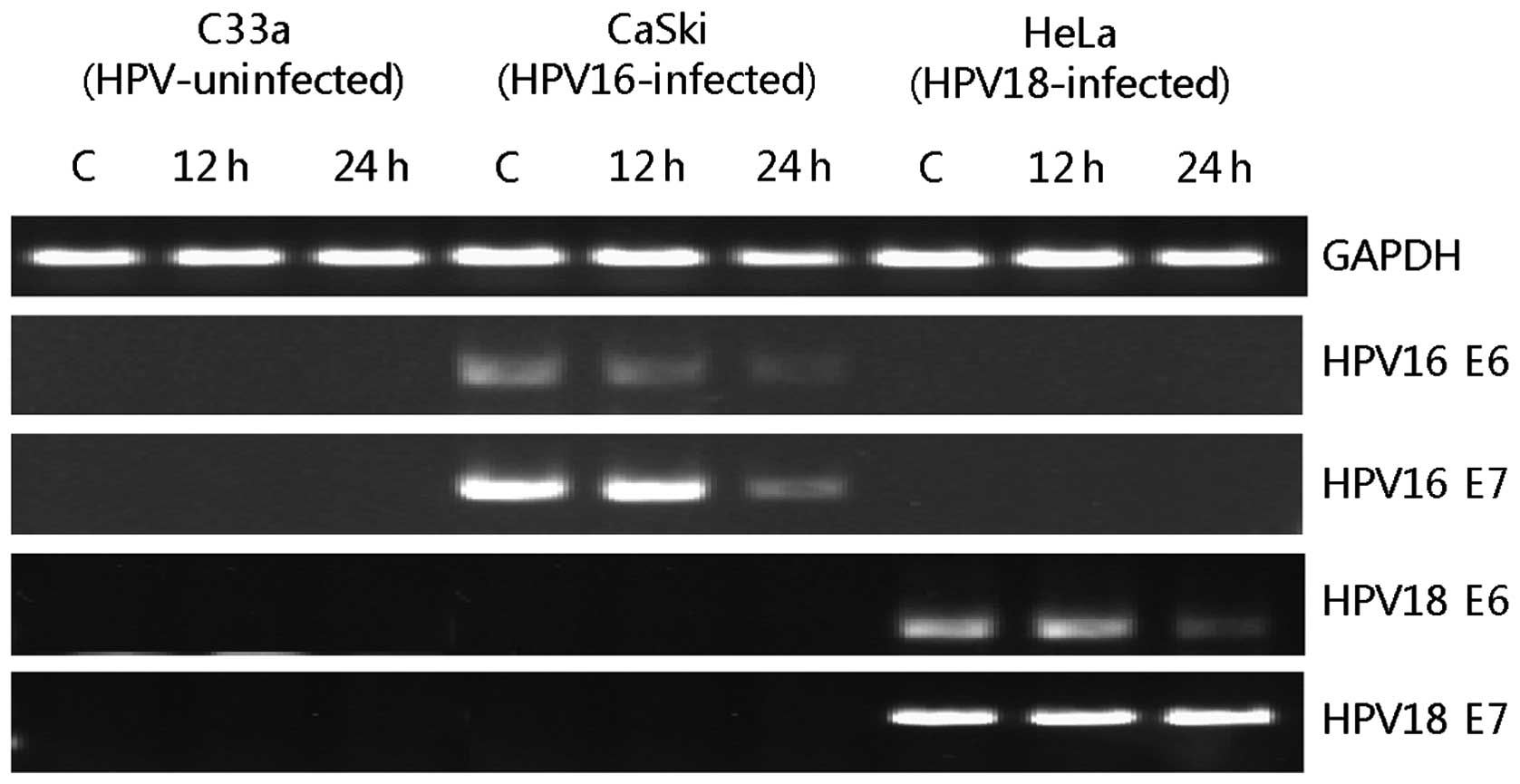
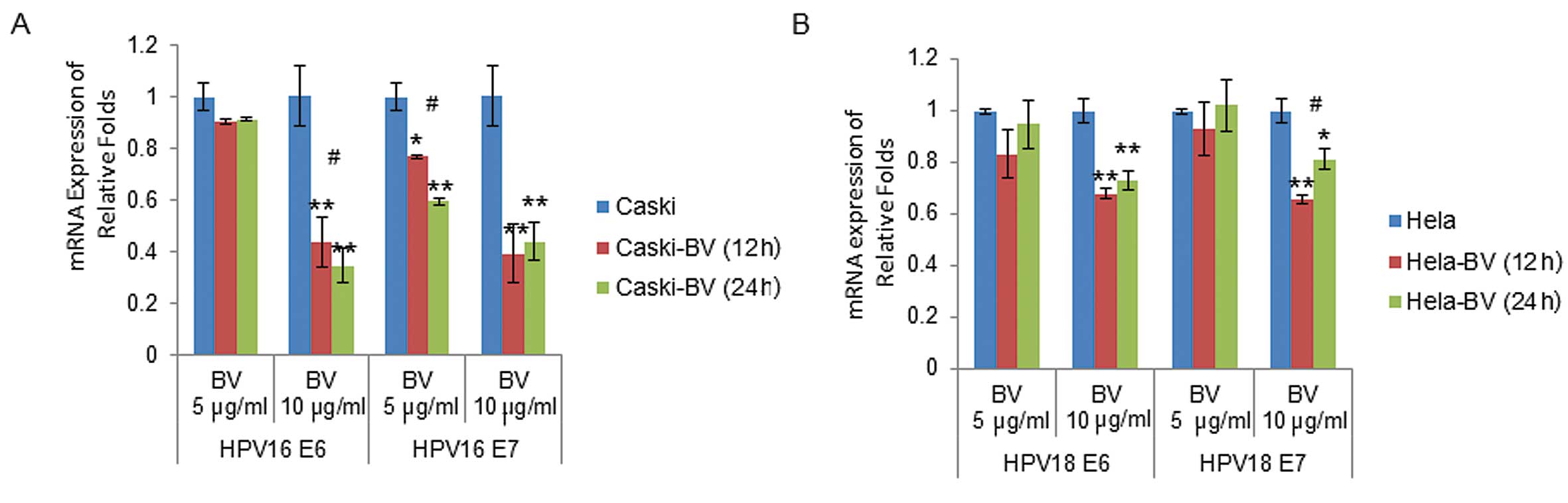
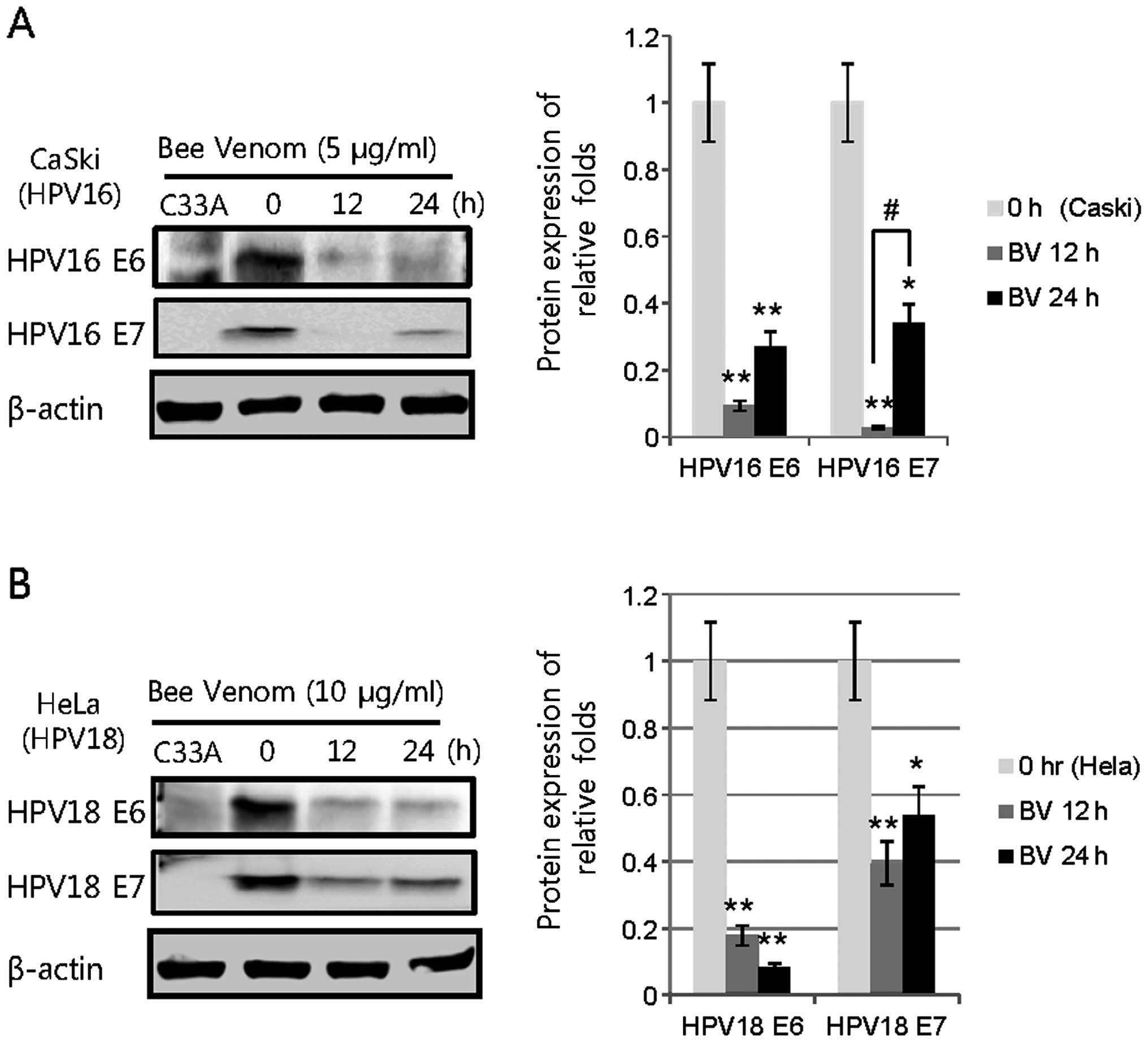
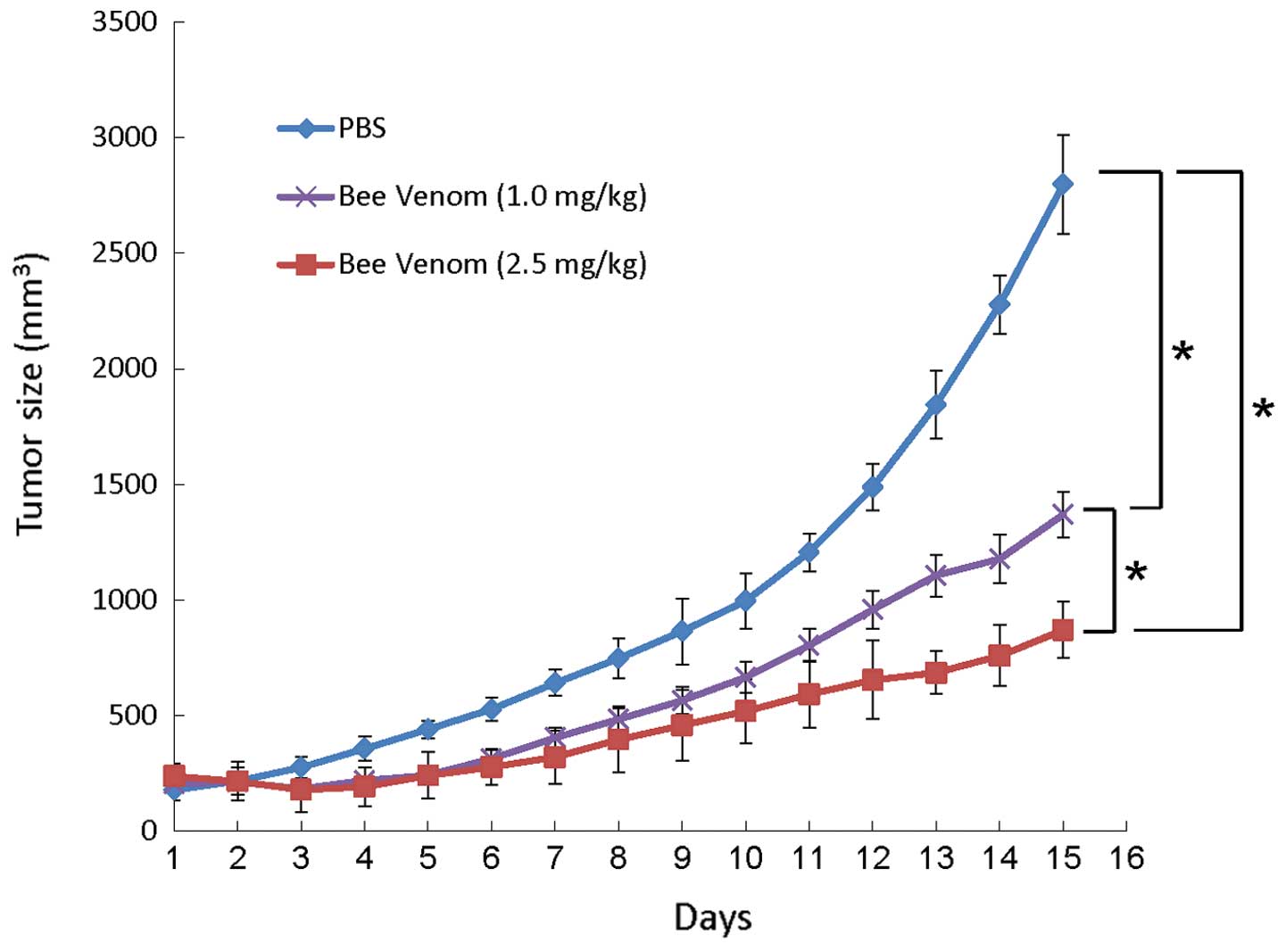
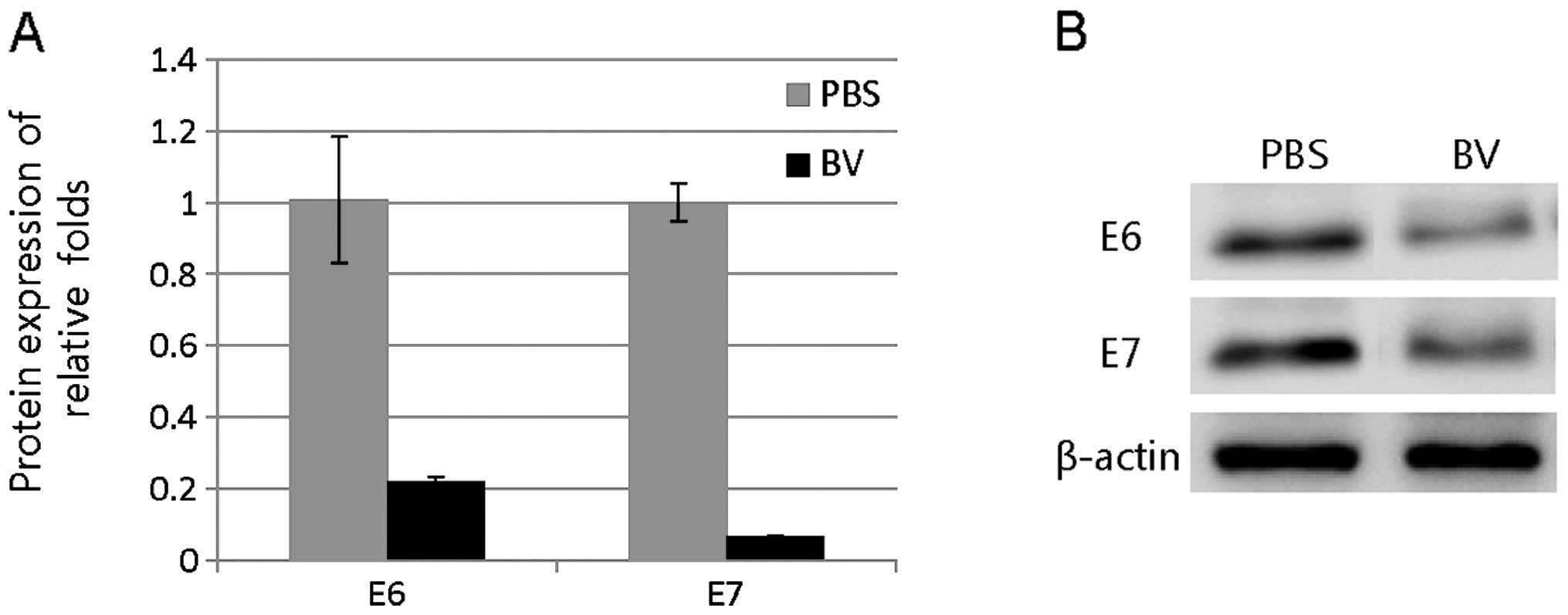
|
1
|
Billingham ME, Morley J, Hanson JM,
Shipolini RA and Vernon CA: Letter: An anti-inflammatory peptide
from bee venom. Nature. 245:163–164. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orlov BN, Omarov ShM, Gelashvili DB,
Korneva NV and Asafova NN: Chemistry and pharmacology of bee venom
(a review of the literature). Farmakol Toksikol. 41:358–369.
1978.(In Russian). PubMed/NCBI
|
|
3
|
Hockenhull J, Elremeli M, Cherry MG, et
al: A systematic review of the clinical effectiveness and
cost-effectiveness of Pharmalgen® for the treatment of
bee and wasp venom allergy. Health Technol Assess. 16:III–IV.
1–110. 2012.
|
|
4
|
Park HJ, Lee HJ, Choi MS, et al: JNK
pathway is involved in the inhibition of inflammatory target gene
expression and NF-kappaB activation by melittin. J Inflamm.
5:72008. View Article : Google Scholar
|
|
5
|
Habermann E: Bee and wasp venoms. Science.
177:314–322. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmidt JO: Biochemistry of insect venoms.
Annu Rev Entomol. 27:339–368. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Son DJ, Lee JW, Lee YH, Song HS, Lee CK
and Hong JT: Therapeutic application of anti-arthritis,
pain-releasing, and anti-cancer effects of bee venom and its
constituent compounds. Pharmacol Ther. 115:246–270. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Texier C, Pouvelle S, Busson M, et al:
HLA-DR restricted peptide candidates for bee venom immunotherapy. J
Immunol. 164:3177–3184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kleinschmidt JH, Mahaney JE, Thomas DD and
Marsh D: Interaction of bee venom melittin with zwitterionic and
negatively charged phospholipid bilayers: a spin-label electron
spin resonance study. Biophys J. 72:767–778. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Przybilla B: Insect venom allergy.
Removing the sting of killer bees! MMW Fortschr Med. 143:41–44.
2001.(In German). PubMed/NCBI
|
|
11
|
Hong SJ, Rim GS, Yang HI, et al: Bee venom
induces apoptosis through caspase-3 activation in synovial
fibroblasts of patients with rheumatoid arthritis. Toxicon.
46:39–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park MH, Choi MS, Kwak DH, et al:
Anti-cancer effect of bee venom in prostate cancer cells through
activation of caspase pathway via inactivation of NF-κB. Prostate.
71:801–812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JH, Kim KH, Kim SJ, Lee WR, Lee KG
and Park KK: Bee venom protects hepatocytes from tumor necrosis
factor-α and actinomycin D. Arch Pharm Res. 33:215–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XD, Zhang JL, Zheng HG, Liu FY and
Chen Y: Clinical randomized study of bee-sting therapy for
rheumatoid arthritis. Zhen Ci Yan Jiu. 33:197–200. 2008.(In
Chinese). PubMed/NCBI
|
|
15
|
Ip SW, Liao SS, Lin SY, et al: The role of
mitochondria in bee venom-induced apoptosis in human breast cancer
MCF7 cells. In Vivo. 22:237–245. 2008.PubMed/NCBI
|
|
16
|
Ip SW, Wei HC, Lin JP, et al: Bee venom
induced cell cycle arrest and apoptosis in human cervical
epidermoid carcinoma Ca Ski cells. Anticancer Res. 28:833–842.
2008.PubMed/NCBI
|
|
17
|
Han HJ, Lee JH, Park SH, et al: Effect of
bee venom and its melittin on apical transporters of renal proximal
tubule cells. Kidney Blood Press Res. 23:393–399. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hood JL, Jallouk AP, Campbell N, Ratner L
and Wickline SA: Cytolytic nanoparticles attenuate HIV-1
infectivity. Antivir Ther. 18:95–103. 2013. View Article : Google Scholar
|
|
19
|
Fenard D, Lambeau G, Maurin T, Lefebvre JC
and Doglio A: A peptide derived from bee venom-secreted
phospholipase A2 inhibits replication of T-cell tropic
HIV-1 strains via interaction with the CXCR4 chemokine receptor.
Mol Pharmacol. 60:341–347. 2001.PubMed/NCBI
|
|
20
|
Soman NR, Baldwin SL, Hu G, et al:
Molecularly targeted nanocarriers deliver the cytolytic peptide
melittin specifically to tumor cells in mice, reducing tumor
growth. J Clin Invest. 119:2830–2842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jemon K, Young V, Wilson M, et al: An
enhanced heterologous virus-like particle for human papillomavirus
type 16 tumour immunotherapy. PLoS One. 8:e668662013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YW, Bae SM, Battogtokh G, Bang HJ and
Ahn WS: Synergistic anti-tumor effects of combination of
photodynamic therapy and arsenic compound in cervical cancer cells:
in vivo and in vitro studies. PLoS One. 7:e385832012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SK, Park KY, Yoon WC, et al: Melittin
enhances apoptosis through suppression of IL-6/sIL-6R
complex-induced NF-κB and STAT3 activation and Bcl-2 expression for
human fibroblast-like synoviocytes in rheumatoid arthritis. Joint
Bone Spine. 78:471–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su PF and Wu FY: Differential suppression
of the tumorigenicity of HeLa and SiHa cells by adeno-associated
virus. Br J Cancer. 73:1533–1537. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon YB, Kim HW, Ham TW, et al: The
anti-inflammatory effect of bee venom stimulation in a mouse air
pouch model is mediated by adrenal medullary activity. J
Neuroendocrinol. 15:93–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ownby CL, Powell JR, Jiang MS and Fletcher
JE: Melittin and phospholipase A2 from bee (Apis
mellifera) venom cause necrosis of murine skeletal muscle in vivo.
Toxicon. 35:67–80. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Son DJ, Ha SJ, Song HS, et al: Melittin
inhibits vascular smooth muscle cell proliferation through
induction of apoptosis via suppression of nuclear factor-κB and Akt
activation and enhancement of apoptotic protein expression. J
Pharmacol Exp Ther. 317:627–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn WS, Han YJ, Bae SM, et al:
Differential suppression of human cervical cancer cell growth by
adenovirus delivery of p53 in vitro: arrest phase of cell cycle is
dependent on cell line. Jpn J Cancer Res. 93:1012–1019. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jang HS, Chung HS, Ko E, et al: Microarray
analysis of gene expression profiles in response to treatment with
bee venom in lipopolysaccharide activated RAW 264.7 cells. J
Ethnopharmacol. 121:213–220. 2009. View Article : Google Scholar
|
|
30
|
Han S, Lee K, Yeo J, et al: Effect of
honey bee venom on microglial cells nitric oxide and tumor necrosis
factor-α production stimulated by LPS. J Ethnopharmacol.
111:176–181. 2007. View Article : Google Scholar
|