Introduction
Colorectal cancers are the third most common types
of cancers worldwide (1–3). Surgical resection is unable to
eliminate tumors completely since metastasis occurs at the time of
diagnosis in approximately three-fifths of the patients. The 5-year
survival rate is also very low due to metastasis (4–6). At
present, there is no effective adjuvant chemotherapy, and
rationally designed new adjuvant therapeutic tools need to be
developed to manage the metastatic process in colorectal cancer
patients. In the past several decades, a large number of substances
derived from plants has been studied in antitumor research fields
and many have proven to exhibit chemopreventive properties
(7–10), which could be used as adjuvant
chemotherapy.
Cucurbitacin I, also known as elatericin B, is a
member of a family of natural occurring compounds with potent
antitumor activity in many human cancers, including glioblastoma,
adenocarcinoma of the lung and breast cancer cells (11–13).
The cucurbitacin family comprises a group of triterpenoid compounds
originally isolated from the plants of the Cucurbitaceae
family. Recently, these anticancer compounds have been observed in
many plant families, including Cruciferae,
Cucurbitaceae and Scrophulariaceae, and have been
used as traditional or folk medicines for centuries in China,
India, Brazil and Peru (13,14).
Previous studies have demonstrated that cucurbitacin I inhibits the
JAK/STAT3 pathway in a number of cancer cell lines and in
vivo tumor models (11,15). Upon inhibition of STAT3-dependent
gene transcription, cucurbitacin I elicits antiproliferative
effects in glioma, lung and breast cancer cells with activated
STAT3 (13,16).
Limited research has been carried out concerning the
effect of cucurbitacin I on colon cancer. A recently published
study demonstrated that cucurbitacin I induced cell death and G2/M
phase cell cycle arrest in SW480 cells (17). Our results in the present study
confirmed this effect of cucurbitacin I on colon cancer cells.
However, how cucurbitacin I influences colon cancer cell migration
and invasion is still elusive. In the present study, we
investigated the effect of cucurbitacin I on colon cancer cell
migration and invasion, and whether cucurbitacin I enhances the
chemosensitivity of the colon cancer cells. Furthermore, we also
investigated the molecular mechanisms of cucurbitacin I
function.
Materials and methods
Materials and cell culture
Cucurbitacin I was purchased from Calbiochem (Jersey
City, NJ, USA) and dissolved in dimethyl sulfoxide (DMSO) at the
concentration of 10 mM. The antibodies used in the study included:
anti-phospho-STAT3-Tyr705 (Cell Signaling Technology, Danvers, MA,
USA), anti-MMP-9 (Abcam, Cambridge, MA, USA) and anti-actin (Santa
Cruz Biotechnology, Dallas, TX, USA). Secondary antibodies were
purchased from Jackson ImmunoResearch (Baltimore, MD, USA).
The COLO205 colon cancer cell line was purchased
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and cultured in Dulbecco’s modified Eagle’s medium. All media were
supplemented with 10% fetal bovine serum (FBS) (Invitrogen,
Madison, WI, USA), and 100 U/ml penicillin and 100 μg/ml
streptomycin (Sigma, St. Louis, MO, USA).
Cell viability assays
Cell viability was measured by the CCK-8 Kit
(Dojindo, Kumamoto, Japan). Briefly, cells were plated at the
density of 1×104 cells/well in a 94-well plate. The
following day, the cells were incubated with either DMSO or
increasing concentrations of cucurbitacin I for 24 h. The 1/10
volume of CCK-8 solution of cultured medium was added to the cells.
Then, the cells were further incubated at 37°C for 2 h. The optical
density (OD) at 450 nm was measured by using a VICTOR™ X plate
reader (PerkinElmer, Waltham, MA, USA). The percentage of cell
viability was calculated as ODdrug/ODcontrol
× 100%.
Cell migration and invasion assays
The in vitro migration assays were performed
as previously described with some modifications (18,19)
using Transwells (8-μm pore size; BD Corporation, Franklin Lakes,
NJ, USA). The COLO205 cells were added to the upper inserts of the
chamber (200 μl serum-free medium containing 2×104
cells), and 600 μl medium with 1% FBS was added to the lower well.
After 6 h of incubation, the cells were removed from the upper
surface of the filter with a cotton swab and the cells that
migrated through the inserts were fixed with methanol and stained
with crystal violet. The migrated cells were counted under a
microscope (TS100; Nikon, Tokyo, Japan) and the migration ability
of the control group was set as 100%. The migration ability of the
treated group was calculated as the migrated cell number of the
drug-treated group/the migrated cell number of the control group ×
100%. Each experiment was performed in triplicate and this
experiment was repeated three times.
Cell invasion assays were performed as the migration
assays except that the Transwells used in the invasion assays were
Matrigel-coated, while in the migration assays the Transwells
remained uncoated.
Western blotting
The cell lysates were prepared as previously
described (20). After DMSO or
cucurbitacin I treatment for 24 h, the cells were washed with
ice-cold phosphate-buffered saline (PBS) three times and lysed in
RIPA lysis buffer supplemented with a proteinase inhibitor (both
from Beyotime, Nanjing, China) and 1 mM phenylmethylsulfonyl
fluoride (PMSF). Then, the cell lysates were harvested. Fifty grams
of whole cell lysates was electrophoresed by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE),
electro-transferred to polyvinylidene fluoride (PVDF) membranes and
probed with an appropriate primary antibody. Then the blots were
next probed with appropriate horseradish peroxidase
(HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) and were visualized by an enhanced
chemiluminescence assay kit (ECL kit; Applygen Technology, Beijing,
China). Membranes were also probed with an anti-actin antibody to
monitor the sampling difference.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as mean
± standard deviation (SD) and were analyzed by the Student’s
t-test. A difference was considered to be statistically significant
at P<0.05.
Results
Cucurbitacin I suppresses colon cancer
cell proliferation, migration and invasion in vitro
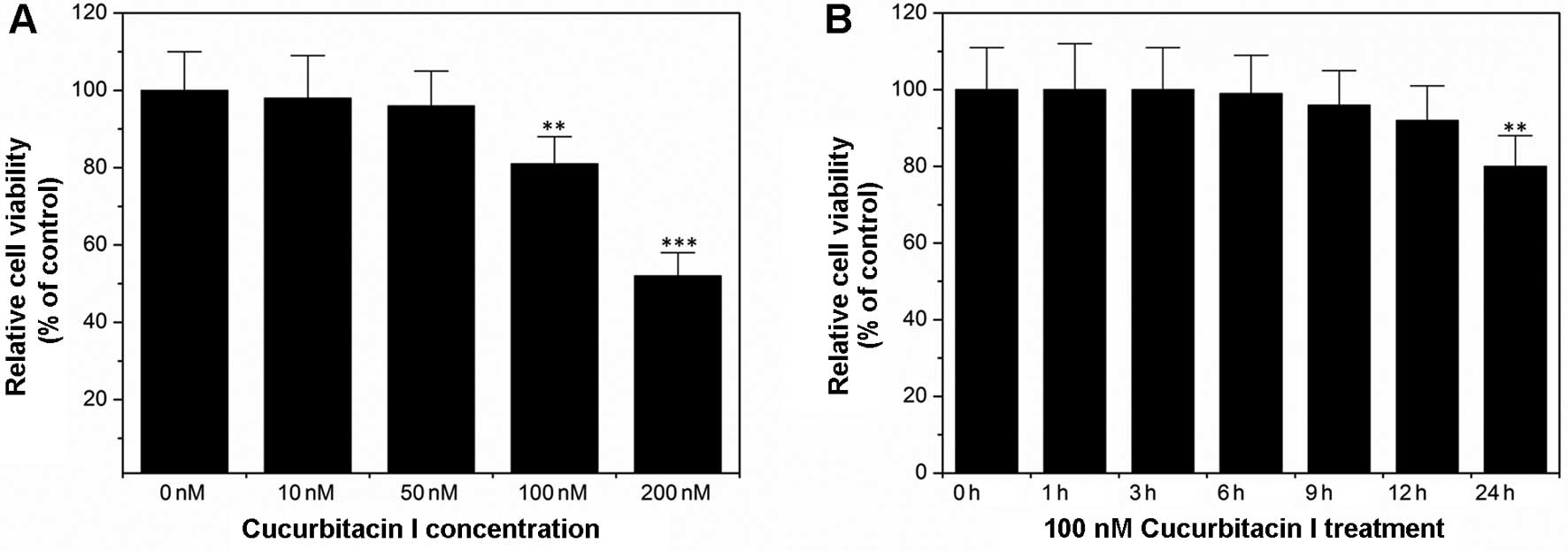
As shown in Fig. 1A,
cucurbitacin I inhibited colon cancer cell COLO205 proliferation in
a dose-dependent manner. These results confirmed a previous report
by other researchers (17).
However, to date it is not clear how cucurbitacin I affects colon
cancer cell migration and invasion. In order to answer this
question, Transwell assays were performed. Since Transwell assays
take ~6 h, we firstly determined the cell viability after treatment
with 100 nM cucurbitacin I for 6 h. As indicated in Fig. 1B, treatment with 100 nM cucurbitacin
I for 6 h had no statistically significant influence on the cell
viability as compared with the control. Thus, the parameters of 100
nM cucurbitacin I and the 6 h treatment were chosen for the
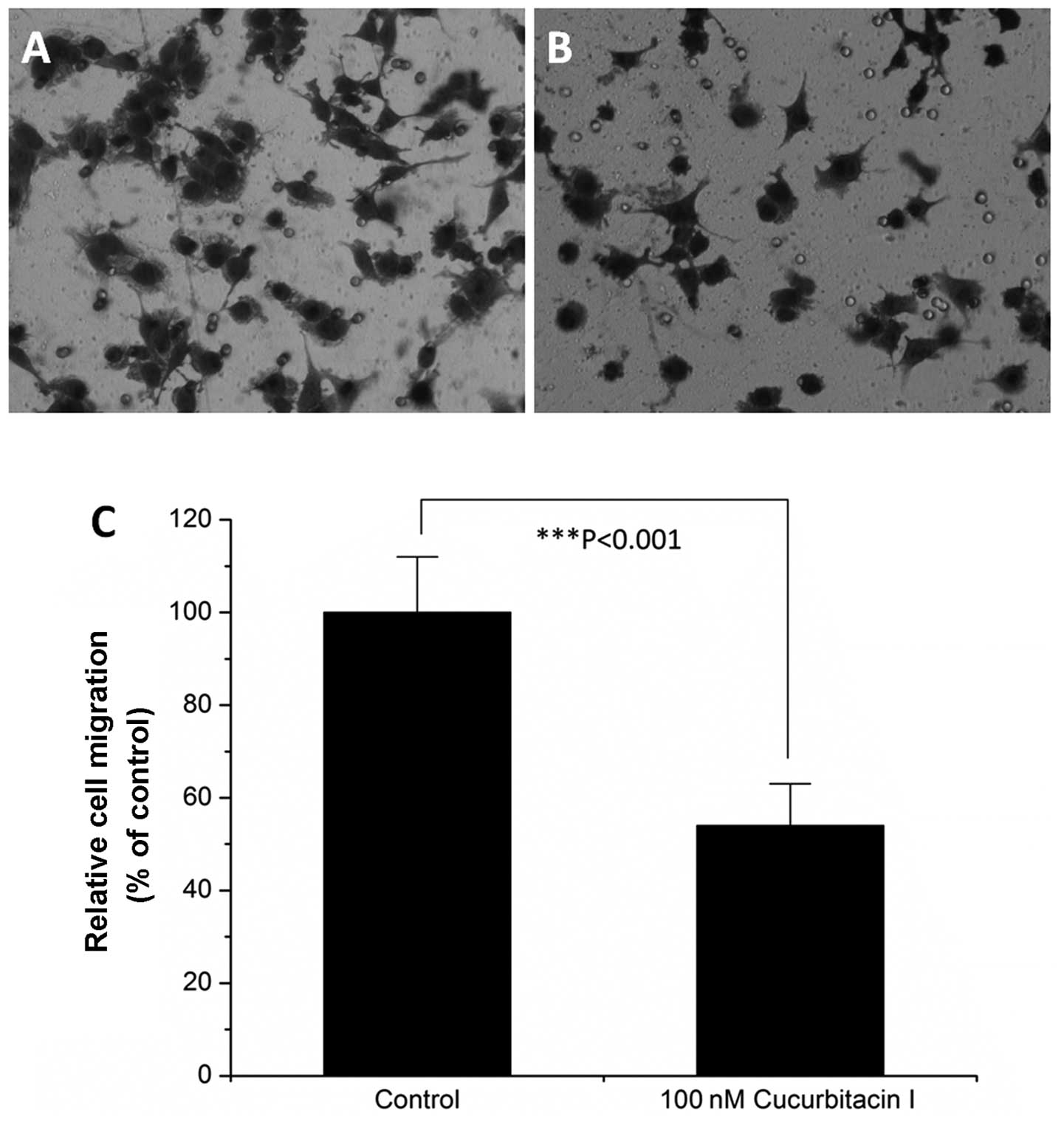
Transwell assays. As illustrated in Fig. 2, 100 nM cucurbitacin I treatment for
6 h reduced COLO205 colon cancer cell migration to ~50% as compared
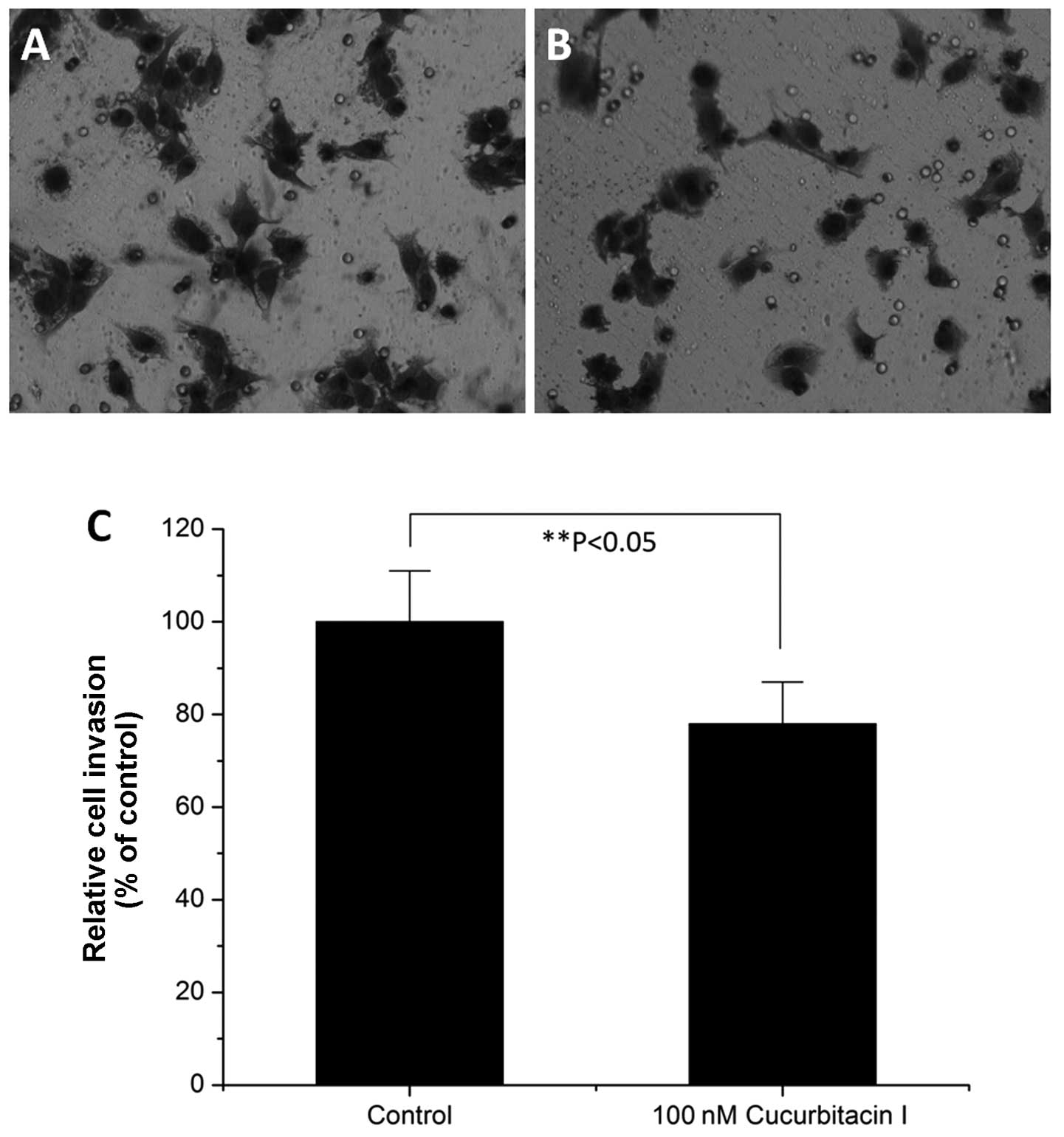
with the control (P<0.001). Cell invasion assays showed that 100
nM cucurbitacin I also inhibited COLO205 colon cancer cell invasion
in vitro (Fig. 3).
Cucurbitacin I sensitizes colon cancer
cell to chemotherapeutic agents
As confirmed above by our results and a number of
previous reports (11,13,17,21,22),
cucurbitacin I inhibits tumor cell proliferation, migration and
invasion, which makes cucurbitacin I a promising antitumor target
(23). However, to date it is not
known whether cucurbitacin I has a sensitizing effect on colon
cancer cells to chemotherapy. In the present study, we combined the
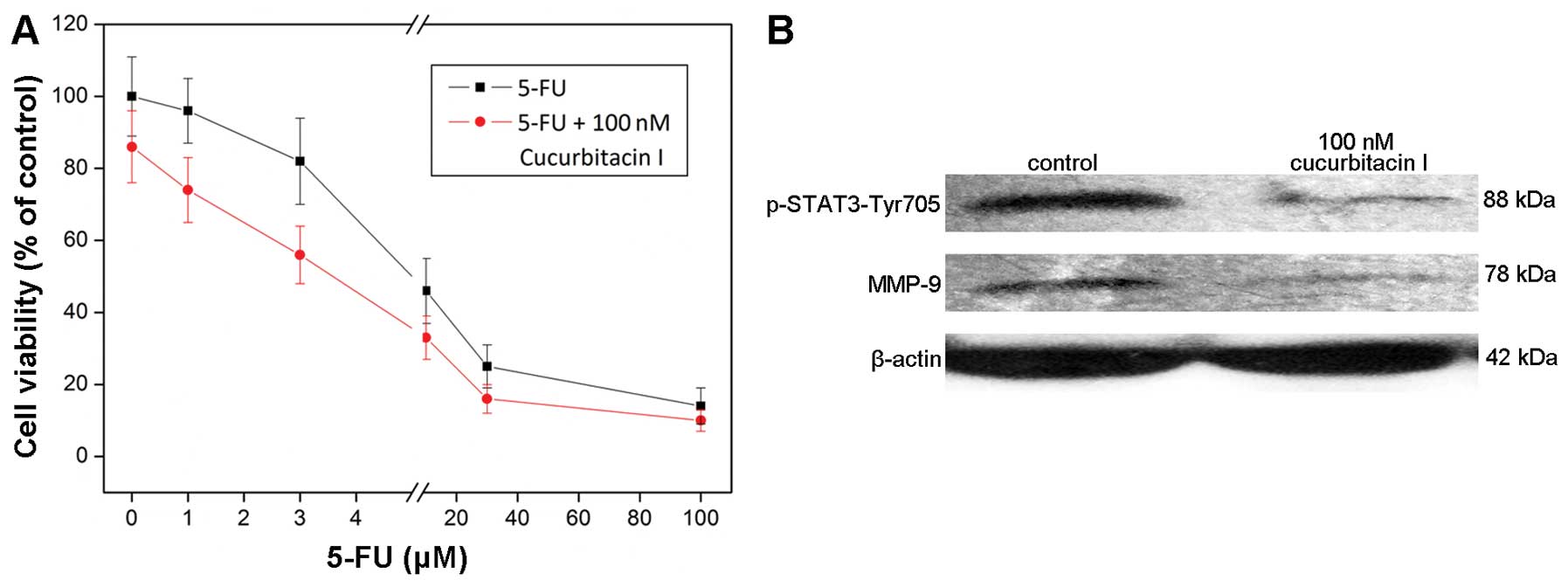
chemotherapy with the cucurbitacin I treatment. As shown in
Fig. 4A, the cell death was further
enhanced by the combination treatment of 5-fluorouracil (5-FU) and
100 nM cucurbitacin I, which proved that cucurbitacin I sensitized
the colon cancer cell line COLO205 to 5-FU treatment.
Cucurbitacin I suppresses STAT3
activation and decreases MMP-9 expression
Previous reports have shown that cucurbitacin I
suppresses STAT3 activation in other cancer cell lines, such as
breast cancer (24) and leukemia
cells (25). In the present study,
we determined the effect of cucurbitacin I on STAT3 in colon cancer
cells. As indicated in Fig. 4B, 100
nM cucurbitacin decreased the protein level of phospho-STAT3, which
proved the inhibitory effect of cucurbitacin I on STAT3
activation.
MMP-9 is an important enzyme for tumor cell invasion
(26). As cucurbitacin inhibited
colon cancer cell invasion, we determined whether cucurbitacin I
treatment could change the MMP-9 expression level. Western blotting
results showed that the MMP-9 expression level was decreased by 100
nM cucurbitacin treatment (Fig.
4B).
Discussion
At present, natural chemical compounds are important
targets in anticancer research due to the drug resistance and toxic
side-effects of current chemotherapy. Herbal medicine has attracted
increased attention of medical scientists (27). Cucurbitacin I is a natural component
extracted from plants of the Cucurbitaceae family, and
exerts anticancer activities in glioblastoma, adenocarcinoma of the
lung and breast cancer cells (11–13).
The molecular mechanisms of cucurbitacin I function involve the
inhibition of STAT3 activation (13,24),
and interference with actin dynamics (28,29). A
recently published study (17)
demonstrated that in SW480 cells cucurbitacin I decreased the cell
viability and cell proliferation by cleavage of caspase-3, -7, -8
and -9 and polyADP ribose polymerase and induced G2/M cell cycle
arrest by downregulation of the cell cycle proteins including
cyclin B1 and A, CDK1 and CDC25C. In the present study, we
confirmed the inhibitory effect of cucurbitacin I on colon cancer
cell growth as previously reported (17). Our results further showed that
cucurbitacin I inhibited colon cancer cell migration and invasion
in vitro, and sensitized colon cancer cells to 5-FU
treatment.
Transcription factor STAT3 has been implicated in
the promotion of growth and progression of many human cancers
including gastric cancers (30–37).
STAT3 is both a cytoplasmic signaling molecule and a nuclear
transcription factor, which belongs to the seven-member Stat gene
family of transcription factors (38). STAT3 becomes active by
phosphorylation of a specific tyrosine residue in the
carboxy-terminal domain by a tyrosine kinase (pTyr705). After
phosphorylation, STAT3 homodimerizes and translocates to the
nucleus where it binds to specific STAT3 response elements of
target gene promoters to regulate transcription (39). Transcription factor STAT3 is
constitutively active in many human cancers (30,40).
In the present study, we firstly observed that in colon cancer
cells cucurbitacin I suppressed phosphorylation of STAT3 (pTyr705).
As STAT3 activation was involved in tumor metastasis (31), inhibition of cucurbitacin I on STAT3
activation could explain its inhibitory effect on colon cancer cell
migration and invasion. Previous findings showed that, in
medulloblastoma-derived cancer stem cells, cucurbitacin I enhanced
chemoradiosensitivity by inhibiting STAT3 phosphorylation (40). In our results we observed that
cucurbitacin I sensitized colon cancer cells to chemotherapy, which
may be promoted by inhibiting STAT3 activation. Lastly, we also
observed that cucurbitacin I decreased the MMP-9 expression which
is an important enzyme for cell invasion (26).
In conclusion, the present study showed that
cucurbitacin I exhibited inhibitory effects on colon cancer cell
proliferation, migration and invasion, which may be accomplished by
downregulating phosphorylation of STAT3 and MMP-9 expression. Our
results also indicated that cucurbitacin I could sensitize colon
cancer cells to chemotherapy.
Acknowledgements
The present study was supported by the Joint Program
of the National Natural Science Foundation of China (NSFC), and the
Science and Technology Agency of Henan Province (no. U1204818).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Atkin W, Lenz HJ, et al:
Colorectal cancer. Lancet. 375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Labianca R, Beretta GD, Kildani B, et al:
Colon cancer. Crit Rev Oncol Hematol. 74:106–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pihl E, Hughes ES, McDermott FT, Milne BJ
and Price AB: Disease-free survival and recurrence after resection
of colorectal carcinoma. J Surg Oncol. 16:333–341. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cresanta JL: Epidemiology of cancer in the
United States. Prim Care. 19:419–441. 1992.PubMed/NCBI
|
|
6
|
Greenwald P: Colon cancer overview.
Cancer. 70(Suppl 5): S1206–S1215. 1992. View Article : Google Scholar
|
|
7
|
Zhang H, Qian Y, Liu Y, et al: Celastrus
orbiculatus extract induces mitochondrial-mediated apoptosis in
human hepatocellular carcinoma cells. J Tradit Chin Med.
32:621–626. 2012. View Article : Google Scholar
|
|
8
|
Liu J and Liu Y: Influence of Erbanxiao
solution on inhibiting angiogenesis in stasis toxin stagnation of
non-small cell lung cancer. J Tradit Chin Med. 33:303–306. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang J, Lee N, Ahn Y and Lee H: Study on
improving blood flow with Korean red ginseng substances using
digital infrared thermal imaging and Doppler sonography:
randomized, double blind, placebo-controlled clinical trial with
parallel design. J Tradit Chin Med. 33:39–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Surh YJ: Anti-tumor promoting potential of
selected spice ingredients with antioxidative and anti-inflammatory
activities: a short review. Food Chem Toxicol. 40:1091–1097. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su Y, Li G, Zhang X, et al: JSI-124
inhibits glioblastoma multiforme cell proliferation through G2/M
cell cycle arrest and apoptosis augment. Cancer Biol Ther.
7:1243–1249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Kester MS, Out-Luiting JJ, von dem
Borne PA, Willemze R, Tensen CP and Vermeer MH: Cucurbitacin I
inhibits Stat3 and induces apoptosis in Sézary cells. J Invest
Dermatol. 128:1691–1695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blaskovich MA, Sun J, Cantor A, Turkson J,
Jove R and Sebti SM: Discovery of JSI-124 (cucurbitacin I), a
selective Janus kinase/signal transducer and activator of
transcription 3 signaling pathway inhibitor with potent antitumor
activity against human and murine cancer cells in mice. Cancer Res.
63:1270–1279. 2003.PubMed/NCBI
|
|
14
|
Chen JC, Chiu MH, Nie RL, Cordell GA and
Qiu SX: Cucurbitacins and cucurbitane glycosides: structures and
biological activities. Nat Prod Rep. 22:386–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CL, Hsieh FC, Lieblein JC, et al:
Stat3 activation in human endometrial and cervical cancers. Br J
Cancer. 96:591–599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lo HW, Cao X, Zhu H and Ali-Osman F:
Constitutively activated STAT3 frequently coexpresses with
epidermal growth factor receptor in high-grade gliomas and
targeting STAT3 sensitizes them to Iressa and alkylators. Clin
Cancer Res. 14:6042–6054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HJ, Park JH and Kim JK:
Cucurbitacin-I, a natural cell-permeable triterpenoid isolated from
Cucurbitaceae, exerts potent anticancer effect in colon cancer.
Chem Biol Interact. 219:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu L, Feng J, Xu H, Luo M and Su D:
Polyphyllin I inhibits proliferation and metastasis of ovarian
cancer cell line HO-8910PM in vitro. J Tradit Chin Med. 33:325–333.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo XS, Wu XW and Gu Q: An experimental
study of a modified Dahuang Zhechong pill on the - angiogenesis of
RF/6A cells in vitro. J Tradit Chin Med. 32:75–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lui VW, Boehm AL, Koppikar P, et al:
Antiproliferative mechanisms of a transcription factor decoy
targeting signal transducer and activator of transcription (STAT)
3: the role of STAT1. Mol Pharmacol. 71:1435–1443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu HS, Huang PI, Chang YL, et al:
Cucurbitacin I inhibits tumorigenic ability and enhances
radiochemosensitivity in nonsmall cell lung cancer-derived
CD133-positive cells. Cancer. 117:2970–2985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YW, Chen KH, Huang PI, et al:
Cucurbitacin I suppressed stem-like property and enhanced
radiation-induced apoptosis in head and neck squamous
carcinoma-derived CD44+ALDH1+ cells. Mol
Cancer Ther. 9:2879–2892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alghasham AA: Cucurbitacins - a promising
target for cancer therapy. Int J Health Sci. 7:77–89. 2013.
View Article : Google Scholar
|
|
24
|
Ren Y, Yu K, Sun S, et al: JSI124 inhibits
breast cancer cell growth by suppressing the function of B cells
via the downregulation of signal transducer and activator of
transcription 3. Oncol Lett. 8:928–932. 2014.PubMed/NCBI
|
|
25
|
Ishdorj G, Johnston JB and Gibson SB:
Inhibition of constitutive activation of STAT3 by curcurbitacin-I
(JSI-124) sensitized human B-leukemia cells to apoptosis. Mol
Cancer Ther. 9:3302–3314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
27
|
Li X, Yang G, Li X, et al: Traditional
Chinese medicine in cancer care: a review of controlled clinical
studies published in Chinese. PLoS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Knecht DA, LaFleur RA, Kahsai AW, Argueta
CE, Beshir AB and Fenteany G: Cucurbitacin I inhibits cell motility
by indirectly interfering with actin dynamics. PLoS One.
5:e140392010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maloney KN, Fujita M, Eggert US, et al:
Actin-aggregating cucurbitacins from Physocarpus capitatus. J Nat
Prod. 71:1927–1929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abdulghani J, Gu L, Dagvadorj A, et al:
Stat3 promotes metastatic progression of prostate cancer. Am J
Pathol. 172:1717–1728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deng JY, Sun D, Liu XY, Pan Y and Liang H:
STAT-3 correlates with lymph node metastasis and cell survival in
gastric cancer. World J Gastroenterol. 16:5380–5387. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dauer DJ, Ferraro B, Song L, et al: Stat3
regulates genes common to both wound healing and cancer. Oncogene.
24:3397–3408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie TX, Huang FJ, Aldape KD, et al:
Activation of stat3 in human melanoma promotes brain metastasis.
Cancer Res. 66:3188–3196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li WC, Ye SL, Sun RX, et al: Inhibition of
growth and metastasis of human hepatocellular carcinoma by
antisense oligonucleotide targeting signal transducer and activator
of transcription 3. Clin Cancer Res. 12:7140–7148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Silver DL, Naora H, Liu J, Cheng W and
Montell DJ: Activated signal transducer and activator of
transcription (STAT) 3: localization in focal adhesions and
function in ovarian cancer cell motility. Cancer Res. 64:3550–3558.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Horiguchi A, Oya M, Shimada T, Uchida A,
Marumo K and Murai M: Activation of signal transducer and activator
of transcription 3 in renal cell carcinoma: a study of incidence
and its association with pathological features and clinical
outcome. J Urol. 168:762–765. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kusaba T, Nakayama T, Yamazumi K, et al:
Expression of p-STAT3 in human colorectal adenocarcinoma and
adenoma; correlation with clinicopathological factors. J Clin
Pathol. 58:833–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ihle JN: The Stat family in cytokine
signaling. Curr Opin Cell Biol. 13:211–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Levy DE and Darnell JE Jr: Stats:
transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang CJ, Chiang CH, Song WS, et al:
Inhibition of phosphorylated STAT3 by cucurbitacin I enhances
chemoradiosensitivity in medulloblastoma-derived cancer stem cells.
Childs Nerv Syst. 28:363–373. 2012. View Article : Google Scholar : PubMed/NCBI
|