Introduction
Uterine leiomyoma is the most widespread benign
neoplasm in women aged 30–50 years (1). Its prevalence based on clinical
examination is at 33%, on ultrasound scan at 50% and on
histological examination after hysterectomy at 77% (2). Due to health problems (pain, but more
importantly heavy uterine bleeding), leiomyomas are the most
frequent causes of hysterectomy (3).
At present, the interest for more conservative
treatment is on the increase as leiomyomas remain under-studied,
and the exact molecular pathways involved in their formation have
not been clearly understood. Their etiopathogenesis seems
multifactorial, and genetic factors and hormonal exposure are
relevant factors in the growth of leiomyomas (4).
Tumor growth is strongly dependent on estrogen and
progesterone, and both have a mitogenic effect on leiomyoma cells
(5). Estrogen and progesterone seem
to have a mitogenic effect on leiomyoma cells and to act by
influencing (directly and indirectly) other hormones and a large
number of growth factors, cytokines and apoptotic factors (6). Clinical and pharmacological studies
showed that anti-progestinic or selective progesterone receptor
modulators reduce the uterine volume of the leiomyoma (7). Preclinical tests with ulipristal
suggested that they inhibit cell proliferation only in leiomyoma
cells (8).
Myostatin and activin A can regulate myometrial cell
proliferation and are overexpressed in the leiomyoma when compared
to the normal myometrium (9).
Abnormal extracellular matrix (ECM) protein expression, and
increased growth factors, cytokines and chemokines leading to
disorganized ECM are involved in the development and growth of
uterine leiomyomas (10).
Modifications of the ECM, its viscosity and elasticity, may change
the intercellular communication by determining an increased mitotic
activity (11). Mechanical signals
are transmitted by the receptor from the ECM to the cytoskeleton,
in order to maintain an isometric state and structural organization
of a default (12). RhoA, a small
GTPase protein, appears to be part of a mechano-regulatory circuit
that could modify the interactions between proteins of the
cytoskeleton by inducing leiomyoma growth (13). A recent study focusing on different
cell lines indicated involvement of the ECM in tumorigenesis
(14). The tumor interstitial fluid
(TIF) consists of a liquid phase that accumulates inside the tumor
interstitium (15). In addition to
the group of blood soluble proteins, it contains a large subset of
abnormal proteins released by tumor cells through classical
secretion, non-classical secretion, membrane protein shedding and
secretion via exosomes (16). The
proteomic approach has been described for leiomyoma tissue
(17) and plasma (18). However, to the best of our
knowledge, no study has been conducted on the leiomyoma
interstitial fluid (IF). Examination of the IF proteins may be an
appropriate way to understand the active modification of the ECM
involved in leiomyoma growth.
The aim of the present study was to identify
proteins with altered expression in leiomyoma cells when compared
to the normal myometrium, possibly involved in the pathogenesis of
the leiomyoma. In order to identify proteins specifically regulated
in the leiomyoma IF, 10 pairs of normal and leiomyoma tissue
samples from individual patients were subjected to two-dimensional
gel electrophoresis (2-DE) analysis combined with protein
identification by MALDI-TOF/TOF mass spectrometry.
Materials and methods
Tissue samples
Tissue samples were obtained from 10 premenopausal
patients who underwent hysterectomy for symptomatic uterine
leiomyomas. The procedures complied with the Declaration of
Helsinki and were approved by the Review Board of the Institute for
Maternal and Child Health - IRCCS ‘Burlo Garofolo’ of Trieste,
Italy. All subjects signed a written informed consent. Two samples
were collected from 10 patients: one sample from the central area
of the leiomyoma and a second sample from the unaffected
myometrium. All the leiomyomas were confirmed histologically as
benign ordinary leiomyomas. The mean age of the patients was 45.6
years with a minimum of 40 years and a maximum of 53 years. Clean
fresh leiomyoma and myometrium (500 μg each) were washed
with sterile PBS at 4°C as previously described (16). Tissues were cut into sections of
~1–3 mm3, rinsed in sterile PBS at 4°C and placed in
sterile Petri dishes with 5 ml sterile PBS. The samples were
incubated for 24 h at 37°C in a humidified CO2
incubator. Thereafter, a protease inhibitor mix (2 mM PMSF, 1 mM
benzamidine, 1 mM EDTA, 1 mM iodoacetamide and 10 mM NaF) was added
and the samples were centrifuged at 2,000 × g for 30 min at 4°C.
Approximately 5 ml of supernatant was desalted and concentrated
with an Ultrafree-4 centrifugal filter unit with a cut-off
molecular weight of 10 kDa at 4,000 × g at 25°C until the remaining
volume reached 100–200 μl. The protein content of the
supernatant was determined by the Bradford assay.
Two-dimensional gel electrophoresis
Two hundred micrograms of proteins from each sample
were denatured in 150 μl of dissolution buffer [7 M urea, 2
M thiourea, 4% CHAPS, 40 mM Tris, 65 mM DTT and 0,24% Bio-Lyte
(3–10)] and a trace of bromophenol blue. The
solutions were vortexed at maximum speed several times and kept at
room temperature for 1 h.
Seven-centimeter pH 3–10 NL (IPG) Readystrips
(Bio-Rad, Hercules, CA, USA) were rehydrated at 50 V for 12 h at
20°C. Isoelectric focusing (IEF) was performed in a protein IEF
Cell (Bio-Rad) set to 65,000 Vh. After IEF, the IPG strips were
equilibrated by serial incubation (20 min) in equilibration buffer
(6 M urea, 2% SDS, 50 mM Tris-HCl pH 8.8, 30% glycerol, and 1% DTT)
and in equilibration buffer containing 4% iodoacetamide instead of
DTT. Equilibrated IPG strips were transferred onto a 12.5%
polyacrylamide gel for the second dimension. The second dimension
was run on Mini-Protean 3 Cell (200 V constant voltage) for ~40 min
until the bromophenol blue reached the bottom of the gel. After
protein fixation for 90 min in 40% methanol and 10% acetic acid,
the gels were washed twice for 20 min in distilled water. The gels
were stained for 48 h with colloidal Coomassie Blue, and excess dye
was removed with distilled water. On average, three experimental
replicates were performed per sample. Molecular masses were
determined by precision protein standard markers (Bio-Rad),
covering a range of 10–250 kDa. 2-DE gels were scanned with a
Molecular Imager PharosFX System and analyzed using the
Proteomeweaver 4 program (both from Bio-Rad).
Quantification of spot levels, prediction
of non-classical secreted protein candidates and biological
process
Spot normalization was automatically performed by
the software and was based on a normalization algorithm intended
for numerical analysis, which did not require any internal
standard. For each gel an intensity factor was computed to ensure
all normalization factors were as close to one as possible. The
matching produced a list of super spots, which represent a certain
protein species present in the gel. For the correct matching, each
super spot was manually controlled prior to normalization. For each
matched pair of gels, the quotient between the pair-matched spot
was calculated. The normalization factor was the median of these
quotients (ProteomeWeaver 4 program; Bio-Rad).
SecretomeP 2.0 (CBS, Technical University
Copenhagen, Denmark) was applied to classify proteins as
non-classical secreted in case they did not contain a signal
peptide and, at the same time, their NN-score exceeded the normal
threshold of 0.5. ExoCarta analyses (NHMRC Biomedical Research
Fellow, Bundoora, Australia) were performed to evaluate the number
of identified proteins known to be exosomal proteins. Differential
proteins distributed in the biological process data were obtained
from the Gene ontology (http://amigo.geneontology.org/rte).
In-gel digestion and MS analysis
Spots from 2-DE were digested with sequencing
grade-modified trypsin (Promega, Madison, WI, USA) and analyzed by
mass spectrometry, as described by Bertacco et al (19). Briefly, the spots were manually
excised from the gels, repeatedly washed with 50 mM
NH4HCO3, shrunk with acetonitrile (ACN) for
20 min and dried under vacuum. Eight to 10 μl of trypsin
(12.5 ng/μl in 50 mM NH4HCO3) were
added to each spot and the samples were kept in ice for 30 min
before being incubated overnight at 37°C. Peptides were then
extracted from the gel by three changes of 75% ACN/0.1%
trifluoroacetic acid (TFA). For MS analysis, the samples were dried
under vacuum and suspended in 10 μl of 20% ACN and 0.1% TFA.
One microliter of the sample was mixed with 1 μl of the
matrix solution (α-cyano-4-hydroxycinnamic acid, 5 mg/ml in 70%
ACN/0.1% TFA). An amount of 0.8 μl of the final
sample/matrix mixture was spotted onto a 386 wells stainless steel
MALDI plate. The samples were analyzed with a MALDI-TOF/TOF 4800
mass spectrometer (AB Sciex, Framingham, MA, USA) operating in a
data-dependent mode: a full MS scan (in the range of 900–3000 m/z)
was followed by MS/MS scans of the 10 most intense ions. A total of
1,500 and 3,500 laser shots were collected for MS and MS/MS
spectra, respectively. Data were converted into Mascot Generic
Format (MGF) files using the 4000 Series Explorer software (AB
Sciex) and searched using Mascot search engine (version 2.4; Matrix
Science, London, UK) against the human section of the Uniprot
database (version 20140416 88708 entries). Enzyme specificity was
set to trypsin with one missed cleavage. Carbamidomethyl cysteine
was set as fixed modification and methionine oxidation as variable
modification. Tolerance for precursor and fragment ions was set to
50 ppm and 0.3 Da, respectively. Proteins were considered
identified if ≥2 unique peptides with individual significant score
(P<0.05) were sequenced. A search under the same conditions but
against the corresponding randomized database did not return any
positive identification.
Immunohistochemistry
Immunohistochemistry was performed, using leiomyoma
and myometrial biopsies (n=10) from the same surgical specimen.
Fixed and paraffin-embedded leiomyoma and myometrial tissues were
deparaffinized and rehydrated using xylene and a graded alcohol, as
previously described (20–22). Tissue sections were treated with 3%
hydrogen peroxidase (15 min at room temperature) followed by
incubation for 30 min at room temperature with 1:100 diluted mouse
monoclonal anti-desmin (Thermo Fisher Scientific, Waltham, MA,
USA), pre-diluted rabbit polyclonal anti-α1-antitrypsin (Ventana
Medical Systems, Tucson, AZ, USA), 1:100 diluted rabbit monoclonal
anti-human estrogen receptor (Diagnostic Biosystems) and 1:50
diluted mouse monoclonal anti-human progesterone receptor (Dako).
Tissue sections were then incubated for 15 min at room temperature
with ready-to-use HRP-conjugated anti-mouse IgG (Thermo Scientific)
for desmin and human progesterone receptor, while HRP conjugated
anti-rabbit IgG (Thermo Fisher Scientific) was incubated for
α1-antitrypsin and human estrogen receptor. Chromogenic staining
was performed with a diaminobenzidine chromogen and counterstaining
was performed with hematoxylin. Immunohistochemical slides were
examined with a Leica DM 2500 and images were captured using a
D-Slight automated digital scanner (Menarini Diagnostics,
Grassina-Firenze, Italy) at a magnification of ×20 and ×40.
Positive immunostaining was scored quantitatively and qualitatively
by two blinded independent observers. The score ranged from 0 to 3,
where 0 was no staining; 1, minimal staining; 2, strong staining
and 3, intense staining.
Statistical analyses
Statistical analyses were carried out with the
non-parametric wilcoxon signed-rank test for paired samples.
P<0.05 was considered as being statistically significant.
Associations between values of desmin, α1-antitrypsin and
progesterone in the leiomyoma and the myometrium were performed
with a two-tailed Fisher’s exact test. The analyses were conducted
with Stata/IC 11.2 for windows (Stata Corp LP, College Station, TX,
USA).
Results
Two-dimensional electrophoresis map and
protein expression levels identified by MALDI-TOF/TOF mass
spectrometry
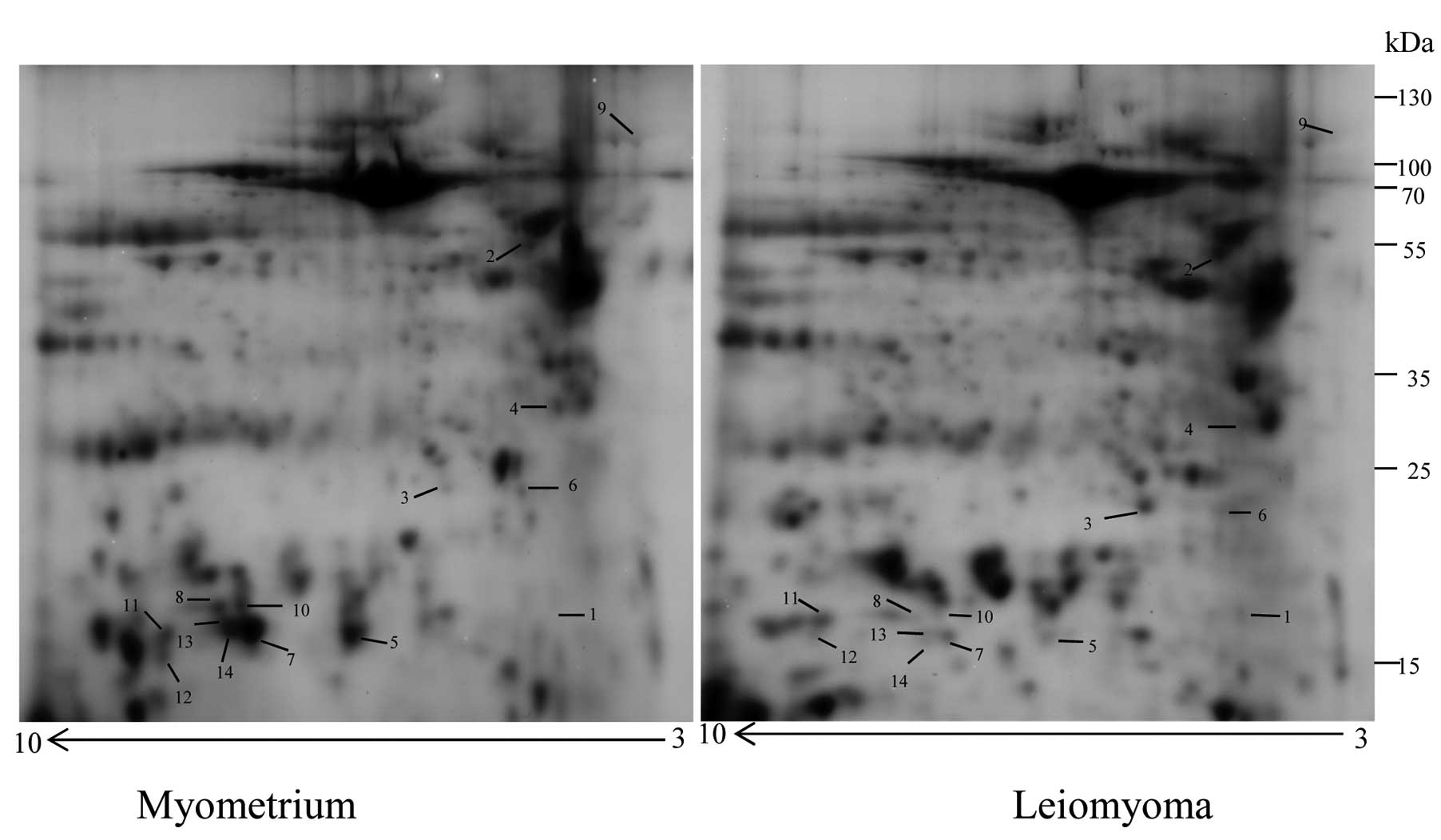
Approximately 600 protein spots were detected in the
2-DE gels for the two types of IF (Fig.
1). After image alignment, background removal, super spot
editing, normalization and statistical analysis, 14 protein spots
were found to be significantly different in leiomyoma samples as
compared to the myometrium. Three of the spots were upregulated
(>2-fold) and 11 downregulated (<0.5 fold), and corresponded
to seven unique proteins identified by MALDI-TOF/TOF and the
database search against the human section of the UniProt
database.
Differential proteins distributed in the biological
process data were obtained from the Gene ontology: cytoskeletal
organization proteins (desmin, prelamin-A/C, transgelin and
α-actinin-1), an inflammatory response (α1-antitrypsin), a response
to oxidative stress (peroxiredoxin-2), and a protein folding (heat
shock 70 kDa protein 1A/1B).
The SecretomeP 2.0 program revealed that
prelamin-A/C, α-actinin-1 and heat shock 70 kDa protein 1A/1B are
classically secreted proteins, while α1-antitrypsin, desmin,
peroxiredoxin-2 and transgelin are non-classically secreted
proteins. Additionally, the ExoCarta analysis confirmed that only
desmin, out of the seven identified proteins, could not be
classified as an exosomal protein. All the results are presented in
Table I. In particular, the three
upregulated proteins were desmin, α1-antitrypsin and the
antioxidant enzyme peroxire-doxin-2. Of the four downregulated
proteins, heat shock 70 kDa protein 1A/1B, α-actinin-1, and
prelamin-A/C were identified each in a single protein spot, while
transgelin was identified in eight different spots (Table I), suggesting the presence of
possible alternative splicing and/or isoforms of this protein.
 | Table IProtein expression levels measured in
the leiomyoma and myometrium interstitial fluid as identified by
the MALDI-TOF/TOF mass spectrometry. |
Table I
Protein expression levels measured in
the leiomyoma and myometrium interstitial fluid as identified by
the MALDI-TOF/TOF mass spectrometry.
| Accession no. | Spot no. | Protein
description | Sequence coverage
(%) | ExoCarta | Total score | NN-score | Mr/PI | Fold changea | P-value |
|---|
| Cytoskeletal
organization proteins |
| P17661 | 1 | Desmin | 15.96 | nR | 750.25 | 0.64 | 53.5/5.27 | 14 | 0.027 |
| Q01995 | 5 | Transgelin | 9.45 | yes | 87.64 | 0.56 | 22.6/8.84 | 0.3 | 0.004 |
| P02545 | 6 | Prelamin-A/C | 8.74 | yes | 294.65 | 0.07 | 74.1/6.84 | 0.28 | 0.018 |
| Q01995 | 7 | Transgelin | 26.37 | yes | 264.94 | 0.56 | 22.6/8.84 | 0.28 | 0.02 |
| Q01995 | 8 | Transgelin | 18.41 | yes | 221.51 | 0.56 | 22.6/8.84 | 0.23 | 0.025 |
| H9KV75 | 9 | α-actinin-1 | 10.34 | yes | 386.07 | 0.38 | 94.8/5.69 | 0.2 | 0.043 |
| Q01995 | 10 | Transgelin | 9.45 | yes | 87.64 | 0.56 | 22.6/8.84 | 0.18 | 0.001 |
| Q01995 | 11 | Transgelin | 9.45 | yes | 52.90 | 0.56 | 22.6/8.84 | 0.17 | 0.002 |
| Q01995 | 12 | Transgelin | 23.38 | yes | 135.97 | 0.56 | 22.6/8.84 | 0.08 | 0.01 |
| Q01995 | 13 | Transgelin | 18.41 | yes | 172.18 | 0.56 | 22.6/8.84 | 0.07 | 0.005 |
| Q01995 | 14 | Transgelin | 9.45 | yes | 52.90 | 0.56 | 22.6/8.84 | 0.07 | 0.005 |
| Inflammatory
response |
| P01009 | 2 | α1-antitrypsin | 13.65 | yes | 221.74 | 0.85 | 46.7/5.47 | 3 | 0.022 |
| Response to
oxidative stress |
| P32119 | 3 |
Peroxiredoxin-2 | 18.69 | yes | 314.71 | 0.52 | 21.9/5.97 | 2 | 0.014 |
| Protein
folding |
| E7EP94 | 4 | Heat shock 70 kDa
protein 1A/1B | 3.64 | yes | 66.49 | 0.28 | 59.8/5.6 | 0.47 | 0.009 |
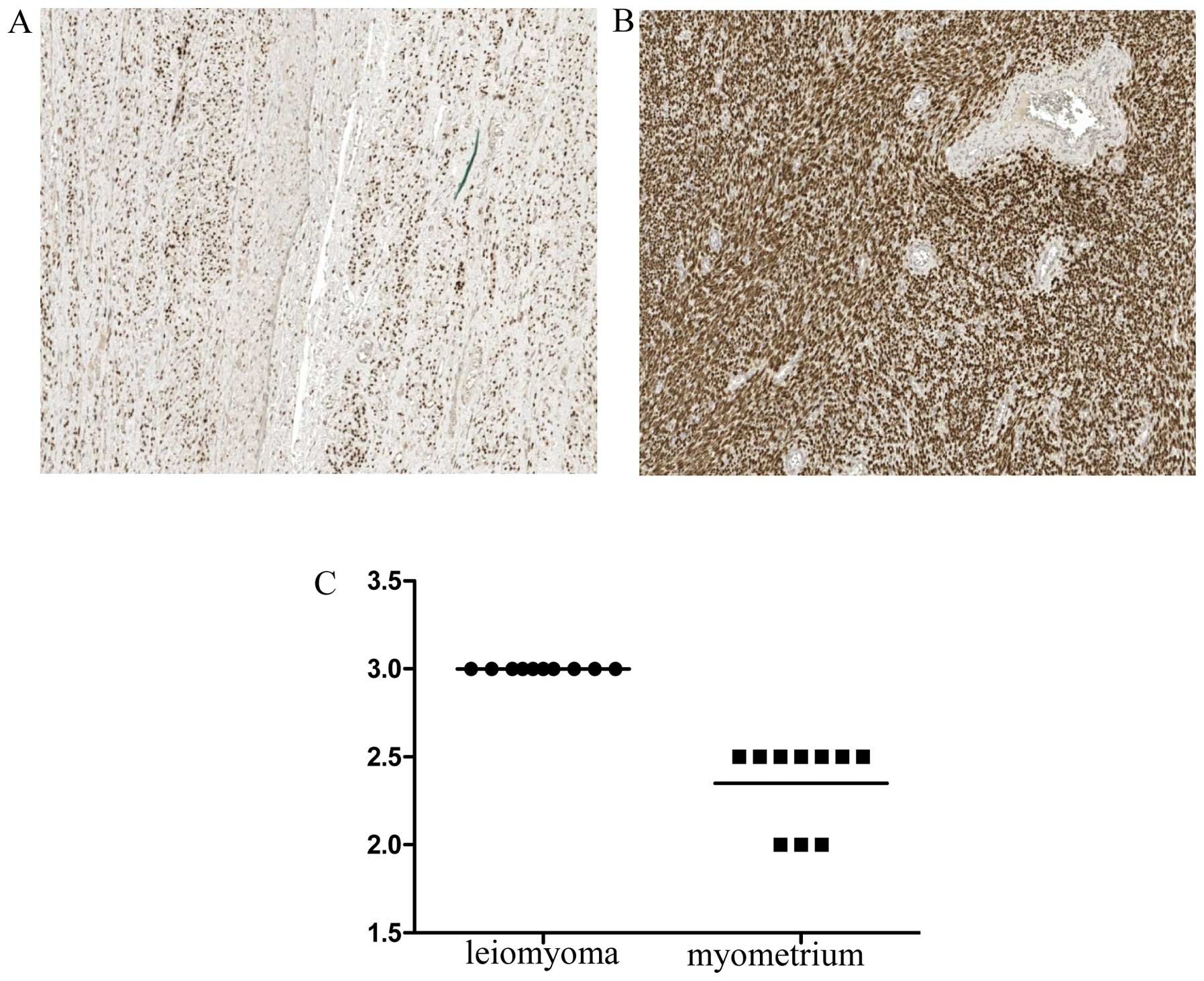
Immunohistochemical analysis of desmin,
α1-antitrypsin, progesterone and estrogen receptors
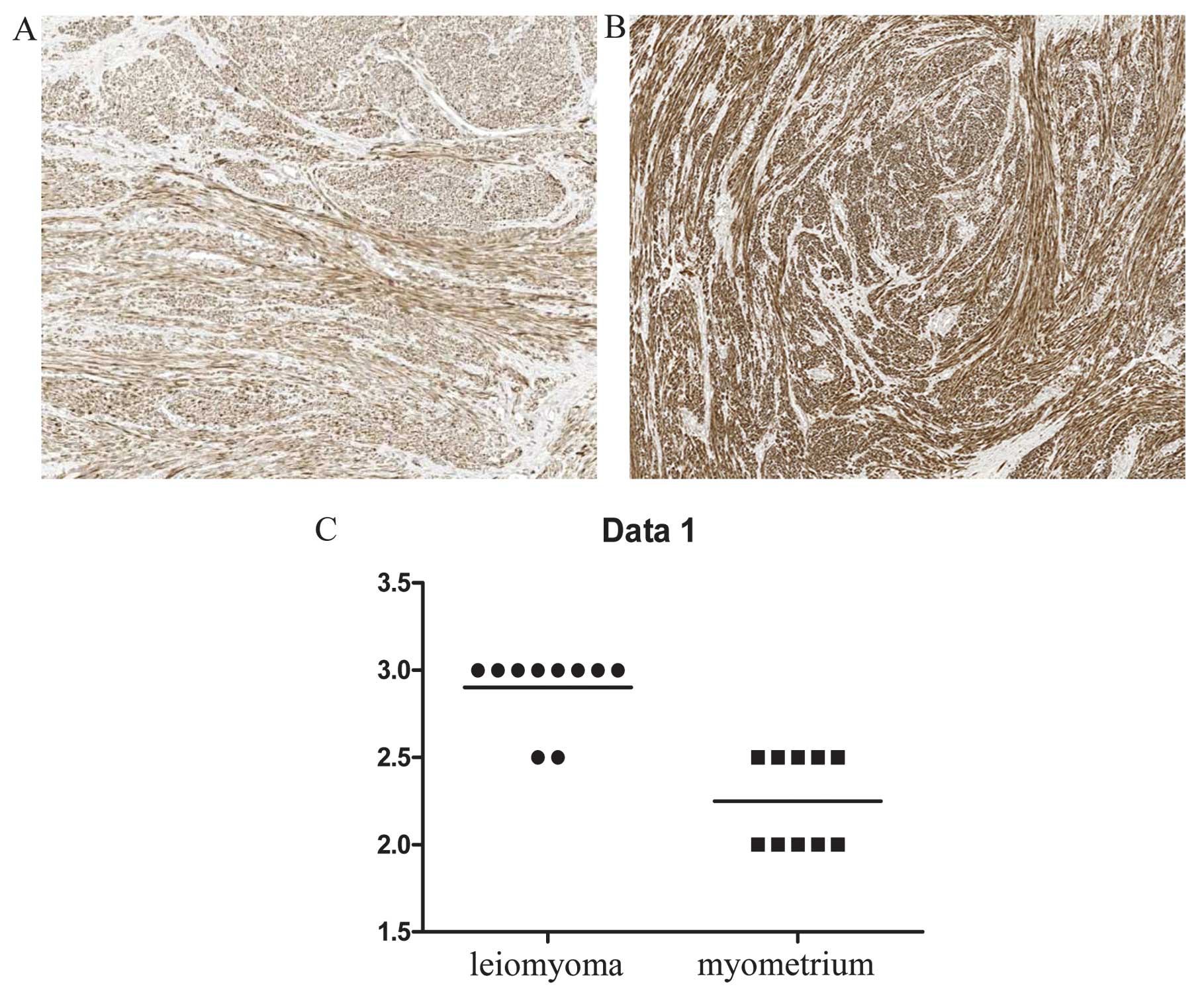
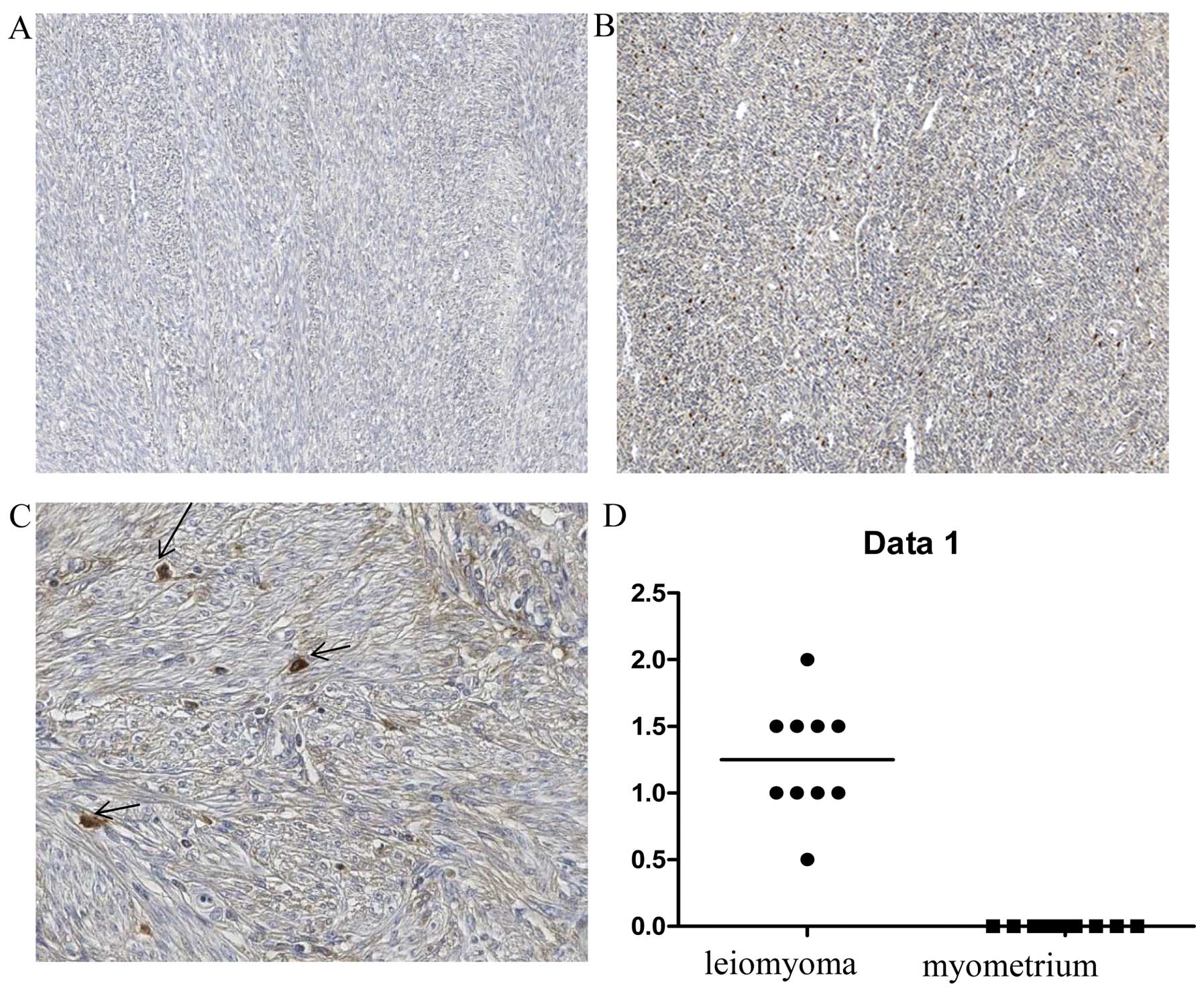
To determine the distribution of desmin- and
α1-antitrypsin-expressing cells in leiomyoma tissues, an
immunohistochemical analysis was performed of 10 individual-matched
leiomyomas and normal myometrium tissue specimens. Desmin showed a
stronger expression in the leiomyoma than in the myometrium
(P<0.05) (Fig. 2). Positive
staining for desmin was located in the cytoplasm of smooth muscle
leiomyoma and myometrium cells, while less intense staining of
desmin expression was observed in the sclerotic part of the
leiomyoma. Taken together, desmin immunohistochemical data
confirmed the result of 2-DE analysis. The positivity of
α1-antitrypsin in the leiomyoma was also confirmed by
immunohistochemistry (P<0.05). The leiomyomas were positive for
α1-antitrypsin, although at different levels of expression. By
contrast, the myometrium cells were negative for α1-antitrypsin
(Fig. 3). The intensity of
α1-antitrypsin staining in the leiomyoma was variable in different
areas but positive in all the tested samples, thereby supporting
the reliability of the data obtained by the proteomic approach.
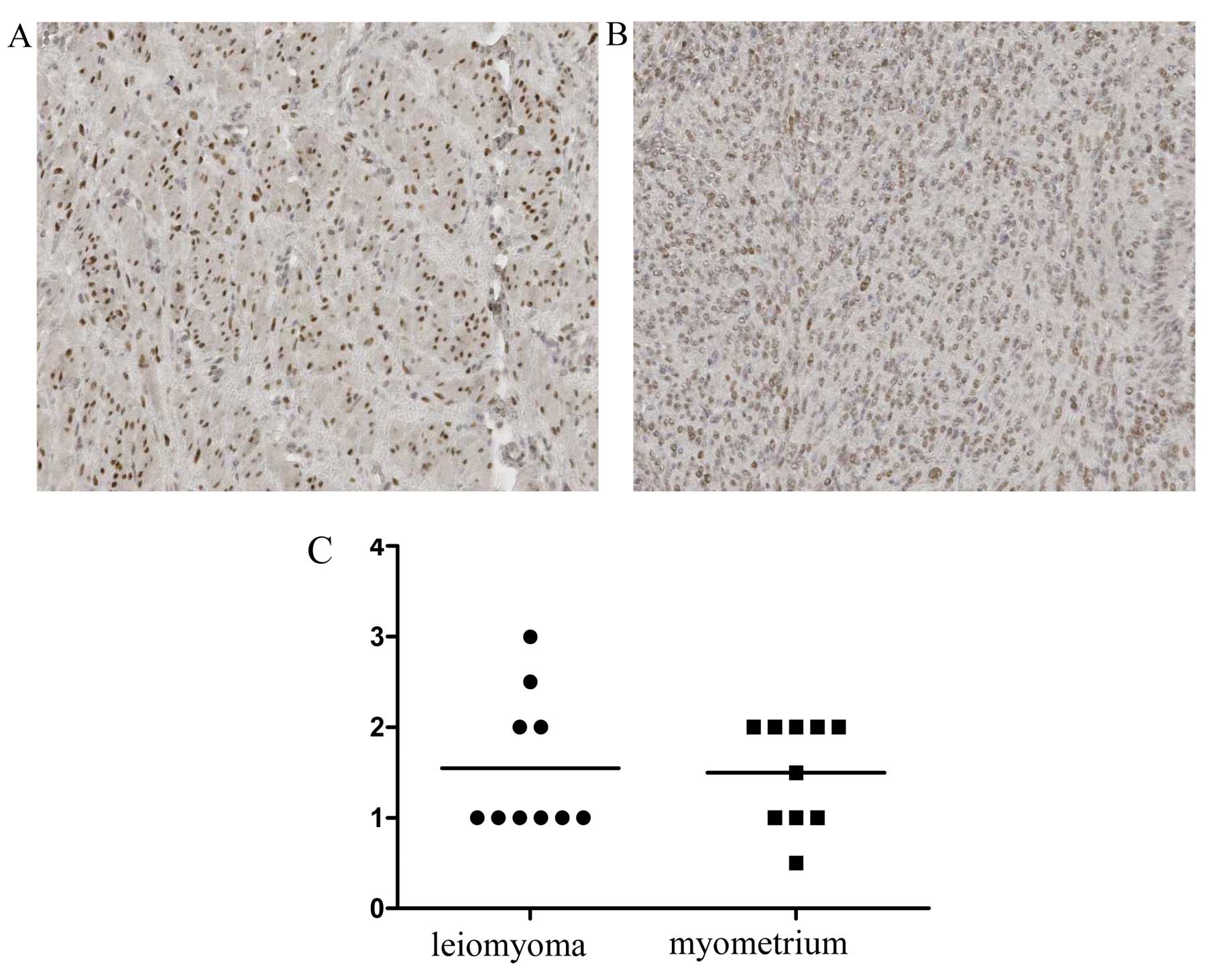
Immunohistochemical staining of estrogen
and progesterone receptors
The expression of estrogen and progesterone
receptors was also analyzed to evaluate any possible association
between desmin and α1-antitrypsin and progesterone expression.
While no statistically significant differences were observed with
respect to expression of nuclear estrogen receptors between the
leiomyoma and the normal myometrium (Fig. 4), progesterone receptor expression
was significantly stronger in the nuclei of leiomyoma cells than in
normal myometrium (Fig. 5)
(P<0.05). No significant association was found between desmin
and progesterone expression, and between α1-antitrypsin and
progesterone expression.
Discussion
In order to analyze the IF profile of leiomyomas in
comparison to normal myometrium, a classical proteomic approach
based on 2-DE, coupled with mass spectrometry was employed. In many
diseases, secreted proteins create conditions that are favorable to
the disorder, such as those that promote cancer metastasis
(23). Therefore, knowledge of the
qualitative and quantitative composition of the cell IF may be
crucial to understand the biology of cell interaction.
To the best of our knowledge, this is the first
proteomics study conducted to compare the IF profiles of the
leiomyoma and the normal myometrium. Using this approach we
identified seven proteins with a significantly different expression
between the leiomyoma and myometrium: α1-antitrypsin, desmin,
peroxiredoxin-2 (upregulated in leiomyomas), and prelamin-A/C,
transgelin, α-actinin-1, and heat shock 70 kDa protein 1A/1B
(downregulated in leiomyomas). Two of the upregulated proteins,
desmin and α1-antitrypsin (A1AT), found in the leiomyoma IF and
further validated by immunohistochemistry, had been previously
described at the tissue level of leiomyomas and leiomyosarcomas
(17–18,24).
Thus, we were able to confirm and expand previous
immunohistochemical data, indicating that these proteins were also
secreted. In particular, A1AT is a protease inhibitor belonging to
the serpin superfamily, which protects tissues from enzymes of
inflammatory cells, and whose expression has been associated with
survival in leiomyosarcomas (24).
We also demonstrated for the first time an increased
level of desmin in the leiomyoma IF. Desmin is a type III
intermediate filament expressed by skeletal, smooth, and cardiac
muscle tissue (25). The leiomyoma
is composed by an abnormal ECM (11). Thus, cells, exposed to different
viscoelastic forces, continue to grow and proliferate (26). Secreted desmin can induce an
alteration of the mechanotransduction signal from ECM via the
transmembrane receptor to the interior of the cell, intervening in
the growth of the leiomyoma. This observation is in agreement with
the models of wettschureck et al (27) and Ren et al (28) which demonstrated that ECM proteins
transduced signals to activate Rho GTPase and generated
cytoskeletal tension leading to possible cell growth.
The last upregulated protein in the IF of the
leiomyoma was peroxiredoxin-2 (PRDX2), a member of the antioxidant
peroxiredoxin 1–6 enzyme family, which reduced hydrogen peroxide
and alkyl hydroperoxides. It has been shown that PRDX2 may have a
proliferative effect and plays a role in the matrix production
(29). In a previous study, PRDX6,
but not PRDX2, was found to be upregulated in the leiomyoma
(30). A previous study suggested
that hypoxia induces cell damage, act through antioxidant enzymes
on quiescent cells inducing proliferation (31). Thus, our current observation of an
increased level of PRDX2 in the leiomyoma IF is unprecedented.
The levels of heat shock 70 kDa protein 1A/1B were
significantly lower in the leiomyoma IF. It is noteworthy that the
anticancer protein TNF-related apoptosis inducing ligand (TRAIl)
(32–33) upregulates the HSP70 protein in the
leiomyosarcoma cell line, thereby inducing apoptosis (34), which suggests that the increase of
HSP70 may play a role in the anticancer response to TRAIL.
Prelamin-A/C can accelerate smooth muscle cell
senescence. It disrupts mitosis and induces DNA damage in vascular
smooth muscle cells (VSMCs), leading to mitotic failure, genomic
instability, and premature senescence (35). Lamins constitute a class of
intermediate filaments with structural and functional roles,
closely associated with the inner nuclear membrane, nuclear pore
complexes and chromatine (36). A
decreased expression of lamin A/C in low-grade cancer compared with
benign epithelium was observed (37). Results of those studies are in
agreement with our findings for the leiomyoma and normal
myometrium. This protein may have a role in the regulation of cell
proliferation (37).
Our observation of a decreased expression of
transgelin in the leiomyoma compared to normal myometrium is in
agreement with that of other studies (38). Li et al found a reduction of
the protein in colorectal cancer cell lines, as transgelin
suppresses proliferation and invasion, and induces apoptosis. The
decrease of transgelin promotes cell proliferation, promoting the
growth of the leiomyoma (38).
Consistent with our present findings, an immunohistochemical study
on the myometrium and leiomyoma indicates a higher expression of
transgelin in the normal myometrium as compared to leiomyoma tissue
sections (39).
Another downregulated protein is α-actinin-1, an
actin-binding protein with multiple roles in different cell types
(40), including the connection
between the cytoskeleton and diverse signaling pathways (41). α-actinin-1 regulates gene
expression, thus accelerating ECM accumulation in glomerular
disease (40). α-actinin-1 has been
documented to be a prognostic factor in leiomyosarcoma survival
(24).
In conclusion, the 2-DE approach coupled with MS
provided a valuable tool for the study of leiomyoma IF. In the
present study, we have identified several proteins that were up- or
downregulated in the leiomyoma, with possible involvement in the
regulation of cell proliferation. Secreted proteins may be involved
in several pathogenic pathways, as suggested by other disease
models. All the proteins identified as being up- or downregulated
in the interstitial fluid may exert a proliferative action on the
cells, or even have a synergistic effect. A clear knowledge of
leiomyoma growth induction is required to identify new therapeutic
targets and to develop new pharmacological approaches for such a
widespread condition, which represents the major cause of
hysterectomy.
Acknowledgments
We greatly acknowledge the help of Ms. Tiziana and
Mr. Walter for obtaining the samples. Scientific input from Drs
Francesco Mangino and Francesco Fanfani is greatly appreciated. We
would like to thank Ms. Thomas Marcuzzo and Cristina Bottin for the
immunohistochemical analysis and data acquisition.
References
|
1
|
Jacobson GF, Shaber RE, Armstrong MA and
Hung YY: Hysterectomy rates for benign indications. Obstet Gynecol.
107:1278–1283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lepine LA, Hillis SD, Marchbanks PA,
Koonin LM, Morrow B, Kieke BA and Wilcox LS: Hysterectomy
surveillance - United States, 1980–1993. MMWR CDC Surveill Summ.
46:1–15. 1997.PubMed/NCBI
|
|
3
|
Bernstein SJ, McGlynn EA, Siu AL, Roth CP,
Sherwood MJ, Keesey JW, Kosecoff J, Hicks NR and Brook RH: The
appropriateness of hysterectomy. A comparison of care in seven
health plans Health Maintenance Organization Quality of Care
Consortium. JAMA. 269:2398–2402. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okolo S: Incidence, aetiology and
epidemiology of uterine fibroids. Best Pract Res Clin Obstet
Gynaecol. 22:571–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chegini N, Ma C, Tang XM and Williams RS:
Effects of GnRH analogues, ‘add-back’ steroid therapy, antiestrogen
and anti-progestins on leiomyoma and myometrial smooth muscle cell
growth and transforming growth factor-beta expression. Mol Hum
Reprod. 8:1071–1078. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei T, Geiser AG, Qian HR, Su C, Helvering
LM, Kulkarini NH, Shou J, N’Cho M, Bryant HU and Onyia JE: DNA
microarray data integration by ortholog gene analysis reveals
potential molecular mechanisms of estrogen-dependent growth of
human uterine fibroids. BMC Womens Health. 7:52007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JJ and Sefton EC: The role of
progesterone signaling in the pathogenesis of uterine leiomyoma.
Mol Cell Endocrinol. 358:223–231. 2012. View Article : Google Scholar
|
|
8
|
Maruo T, Ohara N, Matsuo H, Xu Q, Chen W,
Sitruk-Ware R and Johansson ED: Effects of levonorgestrel-releasing
IUS and progesterone receptor modulator PRM CDB-2914 on uterine
leiomyomas. Contraception. 75:S99–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ciarmela P, Bloise E, Gray PC, Carrarelli
P, Islam MS, De Pascalis F, Severi FM, Vale W, Castellucci M and
Petraglia F: Activin-A and myostatin response and steroid
regulation in human myometrium: disruption of their signalling in
uterine fibroid. J Clin Endocrinol Metab. 96:755–765. 2011.
View Article : Google Scholar :
|
|
10
|
Ciavattini A, Di Giuseppe J, Stortoni P,
Montik N, Giannubilo SR, Litta P, Islam MS, Tranquilli AL, Reis FM
and Ciarmela P: Uterine fibroids: pathogenesis and interactions
with endometrium and endomyometrial junction. Obstet Gynecol Int.
2013:1731842013.PubMed/NCBI
|
|
11
|
Norian JM, Owen CM, Taboas J, Korecki C,
Tuan R, Malik M, Catherino WH and Segars JH: Characterization of
tissue biomechanics and mechanical signaling in uterine leiomyoma.
Matrix Biol. 31:57–65. 2012. View Article : Google Scholar
|
|
12
|
Alenghat FJ and Ingber DE:
Mechanotransduction: all signals point to cytoskeleton, matrix, and
integrins. Sci STKE. 2002:pe62002.PubMed/NCBI
|
|
13
|
Paszek MJ, Zahir N, Johnson KR, Lakins JN,
Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M,
Boettiger D, Hammer DA and Weaver VM: Tensional homeostasis and the
malignant phenotype. Cancer Cell. 8:241–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Butcher DT, Alliston T and Weaver VM: A
tense situation: forcing tumour progression. Nat Rev Cancer.
9:108–122. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baronzio G, Schwartz L, Kiselevsky M,
Guais A, Sanders E, Milanesi G, Baronzio M and Freitas I: Tumor
interstitial fluid as modulator of cancer inflammation, thrombosis,
immunity and angiogenesis. Anticancer Res. 32:405–414.
2012.PubMed/NCBI
|
|
16
|
Gromov P, Gromova I, Bunkenborg J, Cabezon
T, Moreira JM, Timmermans-Wielenga V, Roepstorff P, Rank F and
Celis JE: Up-regulated proteins in the fluid bathing the tumour
cell micro-environment as potential serological markers for early
detection of cancer of the breast. Mol Oncol. 4:65–89. 2010.
View Article : Google Scholar
|
|
17
|
Lv J, Zhu X, Dong K, Lin Y, Hu Y and Zhu
C: Reduced expression of 14-3-3 gamma in uterine leiomyoma as
identified by proteomics. Fertil Steril. 90:1892–1898. 2008.
View Article : Google Scholar
|
|
18
|
Lin CP, Chen YW, Liu WH, Chou HC, Chang
YP, Lin ST, Li JM, Jian SF, Lee YR and Chan HL: Proteomic
identification of plasma biomarkers in uterine leiomyoma. Mol
Biosyst. 8:1136–1145. 2012. View Article : Google Scholar
|
|
19
|
Bertacco E, Millioni R, Arrigoni G, Faggin
E, Iop L, Puato M, Pinna LA, Tessari P, Pauletto P and Rattazzi M:
Proteomic analysis of clonal interstitial aortic valve cells
acquiring a pro-calcific profile. J Proteome Res. 9:5913–5921.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gugliotta L, Bagnara GP, Catani L,
Gaggioli L, Guarini A, Zauli G, Belmonte MM, Lauria F, Macchi S and
Tura S: In vivo and in vitro inhibitory effect of alpha-interferon
on mega-karyocyte colony growth in essential thrombocythaemia. Br J
Haematol. 71:177–181. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Secchiero P, Sun D, De Vico AL, Crowley
RW, Reitz MS Jr, Zauli G, Lusso P and Gallo RC: Role of the
extracellular domain of human herpesvirus 7 glycoprotein B in virus
binding to cell surface heparan sulfate proteoglycans. J Virol.
71:4571–4580. 1997.PubMed/NCBI
|
|
22
|
Zauli G, Secchiero P, Rodella L, Gibellini
D, Mirandola P, Mazzoni M, Milani D, Dowd DR, Capitani S and Vitale
M: HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene
expression in dopaminergic neuronal cells. J Biol Chem.
275:4159–4165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pavlou MP and Diamandis EP: The cancer
cell secretome: a good source for discovering biomarkers? J
Proteomics. 73:1896–1906. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suehara Y, Kondo T, Fujii K, Hasegawa T,
Kawai A, Seki K, Beppu Y, Nishimura T, Kurosawa H and Hirohashi S:
Proteomic signatures corresponding to histological classification
and grading of soft-tissue sarcomas. Proteomics. 6:4402–4409. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bär H, Strelkov SV, Sjöberg G, Aebi U and
Herrmann H: The biology of desmin filaments: how do mutations
affect their structure, assembly, and organisation? J Struct Biol.
148:137–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peddada SD, Laughlin SK, Miner K, Guyon
JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B
and Baird DD: Growth of uterine leiomyomata among premenopausal
black and white women. Proc Natl Acad Sci USA. 105:19887–19892.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wettschureck N and Offermanns S:
Rho/Rho-kinase mediated signaling in physiology and
pathophysiology. J Mol Med (Berl). 80:629–638. 2002. View Article : Google Scholar
|
|
28
|
Ren XD, Kiosses WB and Schwartz MA:
Regulation of the small GTP-binding protein Rho by cell adhesion
and the cytoskeleton. EMBO J. 18:578–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kubota D, Mukaihara K, Yoshida A, Tsuda H,
Kawai A and Kondo T: Proteomics study of open biopsy samples
identifies peroxiredoxin 2 as a predictive biomarker of response to
induction chemotherapy in osteosarcoma. J Proteomics. 91:393–404.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bae SM, Kim YW, Lee JM, Namkoong SE, Kim
CK and Ahn WS: Expression profiling of the cellular processes in
uterine leiomyomas: omic approaches and IGF-2 association with
leiomyosarcomas. Cancer Res Treat. 36:31–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mesquita FS, Dyer SN, Heinrich DA, Bulun
SE, Marsh EE and Nowak RA: Reactive oxygen species mediate
mitogenic growth factor signaling pathways in human leiomyoma
smooth muscle cells. Biol Reprod. 82:341–351. 2010. View Article : Google Scholar :
|
|
32
|
Secchiero P and Zauli G:
Tumor-necrosis-factor-related apoptosis-inducing ligand and the
regulation of hematopoiesis. Curr Opin Hematol. 15:42–48. 2008.
View Article : Google Scholar
|
|
33
|
Zauli G, Melloni E, Capitani S and
Secchiero P: Role of full-length osteoprotegerin in tumor cell
biology. Cell Mol Life Sci. 66:841–851. 2009. View Article : Google Scholar
|
|
34
|
Karlisch C, Harati K, Chromik AM, Bulut D,
Klein-Hitpass L, Goertz O, Hirsch T, Lehnhardt M, Uhl W and
Daigeler A: Effects of TRAIL and taurolidine on apoptosis and
proliferation in human rhabdomyosarcoma, leiomyosarcoma and
epithelioid cell sarcoma. Int J Oncol. 42:945–956. 2013.PubMed/NCBI
|
|
35
|
De Vos WH, Houben F, Hoebe RA, Hennekam R,
van Engelen B, Manders EM, Ramaekers FC, Broers JL and Van
Oostveldt P: Increased plasticity of the nuclear envelope and
hypermobility of telomeres due to the loss of A-type lamins.
Biochim Biophys Acta. 1800:448–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ragnauth CD, Warren DT, Liu Y, Mcnair R,
Tajsic T, Figg N, Shroff R, Skepper J and Shanahan CM: Prelamin A
acts to accelerate smooth muscle cell senescence and is a novel
biomarker of human vascular aging. Circulation. 121:2200–2210.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kong L, Schäfer G, Bu H, Zhang Y, Zhang Y
and Klocker H: Lamin A/C protein is overexpressed in
tissue-invading prostate cancer and promotes prostate cancer cell
growth, migration and invasion through the PI3K/AKT/PTEN pathway.
Carcinogenesis. 33:751–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Q, Shi R, Wang Y and Niu X: TAGLN
suppresses proliferation and invasion, and induces apoptosis of
colorectal carcinoma cells. Tumour Biol. 34:505–513. 2013.
View Article : Google Scholar
|
|
39
|
Robin YM1, Penel N, Pérot G, Neuville A,
Vélasco V, Ranchère-Vince D, Terrier P and Coindre JM: Transgelin
is a novel marker of smooth muscle differentiation that improves
diagnostic accuracy of leiomyosarcomas: a comparative
immunohistochemical reappraisal of myogenic markers in 900 soft
tissue tumors. Mod Pathol. 26:502–510. 2013. View Article : Google Scholar
|
|
40
|
Yang C and Glass WF II: Expression of
alpha-actinin-1 in human glomerular mesangial cells in vivo and in
vitro. Exp Biol Med (Maywood). 233:689–693. 2008. View Article : Google Scholar
|
|
41
|
Otey CA and Carpen O: Alpha-actinin
revisited: a fresh look at an old player. Cell Motil Cytoskeleton.
58:104–111. 2004. View
Article : Google Scholar : PubMed/NCBI
|