Introduction
Osteosarcoma (OS) is the most common form of
malignant bone tumor and occurs most frequently in children and
adolescents (1). It is highly
aggressive, expands into the cortex of the bone, later erupts
through the cortex into the soft tissues, and frequently leads to
the development of micrometastases in the lung (1,2). The
primary treatment of osteosarcoma is the complete removal of tumor
by wide excision with neo-adjuvant and adjuvant chemotherapy
(2). Despite progress in
chemotherapy, the prognosis remains particularly poor for patients
with recurrence and metastasis. This is largely attributed to a
lack of complete understanding of the exact mechanisms for this
malignancy. Therefore, further understanding of the molecular
mechanisms of cancer progression and the development of new
therapeutic tools based on these mechanisms are required.
The Wnt-β-catenin signaling pathway regulates a
variety of genes that in turn orchestrate diverse cell functions
such as morphogenesis, differentiation and proliferation (3). It also plays an important role in
tumorigenesis and its aberrant activation has been associated with
the pathogenesis of various tumors in human (4–6). It
has been reported that salinomycin selectively targets OS stem
cells possibly by inhibiting the Wnt/β-catenin signaling pathway
(7), which suggests that
Wnt/β-catenin may be important in OS.
Apigenin (4′,5,7-trihydroxyflavone), a type of
flavonoid, is widely contained in many fruits and vegetables such
as oranges, tea, chamomile, onions and wheat sprouts (8). Findings of previous studies have
demonstrated that apigenin inhibits the growth, invasion, and
metastasis of tumors in vitro and in vivo (9–11).
Results of a recent study showed that apigenin possesses anticancer
properties for the induction of apoptosis in U2OS cells and
inhibits the xenograft tumor growth (12). However, the precise molecular
mechanisms of the anticancer effect of apigenin remain to be
clarified.
In the present study, we investigated the effects of
apigenin on OS cell proliferation and invasion. Furthermore, we
investigated the molecular mechanisms of the anticancer effect of
apigenin.
Materials and methods
Cell culture
The U2OS and MG63 human OS cell lines were obtained
from the American Type Culture Collection. U2OS cells were
maintained in McCoy’s 5A medium, which was supplemented with 10%
fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin
(100 μg/ml) at 37μC with 5% CO2. MG-63
cells were maintained in Eagle’s minimum essential medium in 5%
CO2 at 37μC.
MTT assay
Cell viability was determined by the MTT assay. In
brief, cells were plated in 96-well plates at 3×104
cells per well. At 24, 48, 72, and 96 h post plating, the cells
were treated with various concentrations of apigenin, and ~20
μl MTT reagent (5 mg/ml) was added into each well and then
incubated at 37μC for 30 min. To dissolve formazan crystals,
culture medium was replaced with an equal volume of DMSO. After the
mixture was agitated at room temperature for 10 min, the absorbance
of each well was determined at 490 nm using a microplate reader.
Experiments were repeated in triplicate and the results are
presented as the percentage of growth inhibition.
Cell cycle assay
The cells were seeded in 6-well plates and treated
with various doses of apigenin or DMSO for 24 h. The cells were
harvested and resuspended in 200 μl ice-cold
phosphate-buffered saline (PBS), added to 4 ml ice-cold ethanol and
incubated on ice for 45 min. After an additional washing, the cells
were incubated with RNase A (20 μg/ml) at 37μC for 30
min, stained with propidium iodide (100 μg/ml;
Sigma-Aldrich, St. Louis, MO, USA) for 10 min, and analyzed with
flow cytometry.
Cell invasion assay
Cell invasion was measured using a modified Matrigel
Boyden chamber (BD Bioscience, Bedford, MA, USA) (13). The cells were treated with various
concentrations of apigenin or pretreatment of cells with apigenin
was treated with overexpression-β-catenin or siRNA-β-catenin for 4
h, and then seeded in the upper compartment. The medium including
10% FBS was added into the lower compartment. After 48 h, the cells
that filtered through the lower side of the membrane were stained
with hematoxylin and eosin and quantified by counting five
high-power fields in the center of each well.
Quantitative PCR
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Quantitative PCR (qPCR) reactions were
performed on the Bio-Rad iQ5 real-time thermal cyclers using
SYBR® Premix Ex Taq™ II kit (Takara, Dalian, China). The
specific primers used were: β-catenin sense: 5′-TGAGGACAAGC
CACAAGATTAC-3′ and antisense: 5′-TCCACCAGAGTGAA AAGAACG-3′; β-actin
sense: 5′-GATCATTGCTCCTCCTG AGC-3′ and antisense:
5′-ACTCCTGCTTGCTGATCCAC-3′. These primers were synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China). The PCR procedure was
as follows: polymerase activation for 30 sec at 95μC, 40
cycles of amplification each consisting of 95μC for 5 sec,
60μC for 20 sec, and 1 cycle of dissociation consisting of
95μC for 15 sec, 60μC for 30 sec and 95μC for
15 sec. Relative quantification of gene expression was performed
using the 2−ΔΔCt method and with β-actin mRNA as an
internal control. The reactions were performed in triplicate.
Western blot analysis
The cells were sonicated with lysis buffer (PBS with
1% Triton X-100 and protease inhibitors). The cell lysate
supernatants were harvested by centrifugation at 10,000 rpm for 10
min at 4μC. Protein concentrations of the cell supernatants
were evaluated and measured by BCA Protein Assay kit. An equal
amount of the proteins from each extract was separated on a
SDS-PAGE, and transferred electrophoretically using PVDF membranes.
The membranes were then blocked by 5% non-fat dry milk in PBST [PBS
with 0.1% Tween-20, (pH 7.6)] for 1 h at room temperature and
probed overnight with appropriate primary antibodies
(anti-β-catenin or anti-β-actin) diluted in PBST at 4μC. The
membranes were rinsed three times with PBST and incubated with
appropriate secondary antibodies diluted in PBST for 1 h at room
temperature. The membranes were then rinsed three times with PBST
at room temperature for 10 min, and the blots were visualized by
enhanced chemiluminescence using Kodak X-omat LS film (Eastman
Kodak, Rochester, NY, USA). The protein level quantification was
carried out by ImageJ (Molecular Dynamics, Sunnyvale, CA, USA).
Plasmid construction and
transfection
Total RNA from U2OS cells was extracted using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA), according to the
manufacturer’s instructions. The cDNA was synthesized by reverse
transcription of total RNA, using the Prime Script® RT
reagent kit (Takara, Dalian, China) with oligo-dT primers,
according to the manufacturer’s instructions. The open reading
frame of β-catenin cDNA was subsequently cloned into the pcDNA3.1
vector (Invitrogen) to construct the recombinant pcDNA3.1-β-catenin
expression vector. In addition, lentivirus-mediated siRNA
constructs were designed by Shanghai Genechem (Shanghai, China).
The sequences corresponding to the siRNA of β-catenin were: sense:
5′-UGGUUGCCUUGCUCAAdTdT-3′ and antisense:
5′-UUGUUGAGCAAGGCAACCAdTdT-3′. For the in vitro
transfection, cells (5×104) were seeded in each of the
24-well microplates, grown for 24 h to reach 50% confluency, and
then incubated with a mixture of overexpression-β-catenin or
siRNA-β-catenin and Lipofectamine 2000 reagent (Invitrogen) in 100
μl serum-free Opti-Mem according to the manufacturer’s
instructions. The transfection efficiency was examined by qPCR and
western blotting.
Statistical analysis
Data were presented as the mean ± standard error of
the mean (SEM). The differences between the control and
apigenin-treated groups were compared by Dunnett’s test subsequent
to ANOVA. P<0.05 was considered to indicate a statistically
significant difference. All the experiments were repeated at least
three times.
Results
Cytotoxicity of apigenin on OS cells
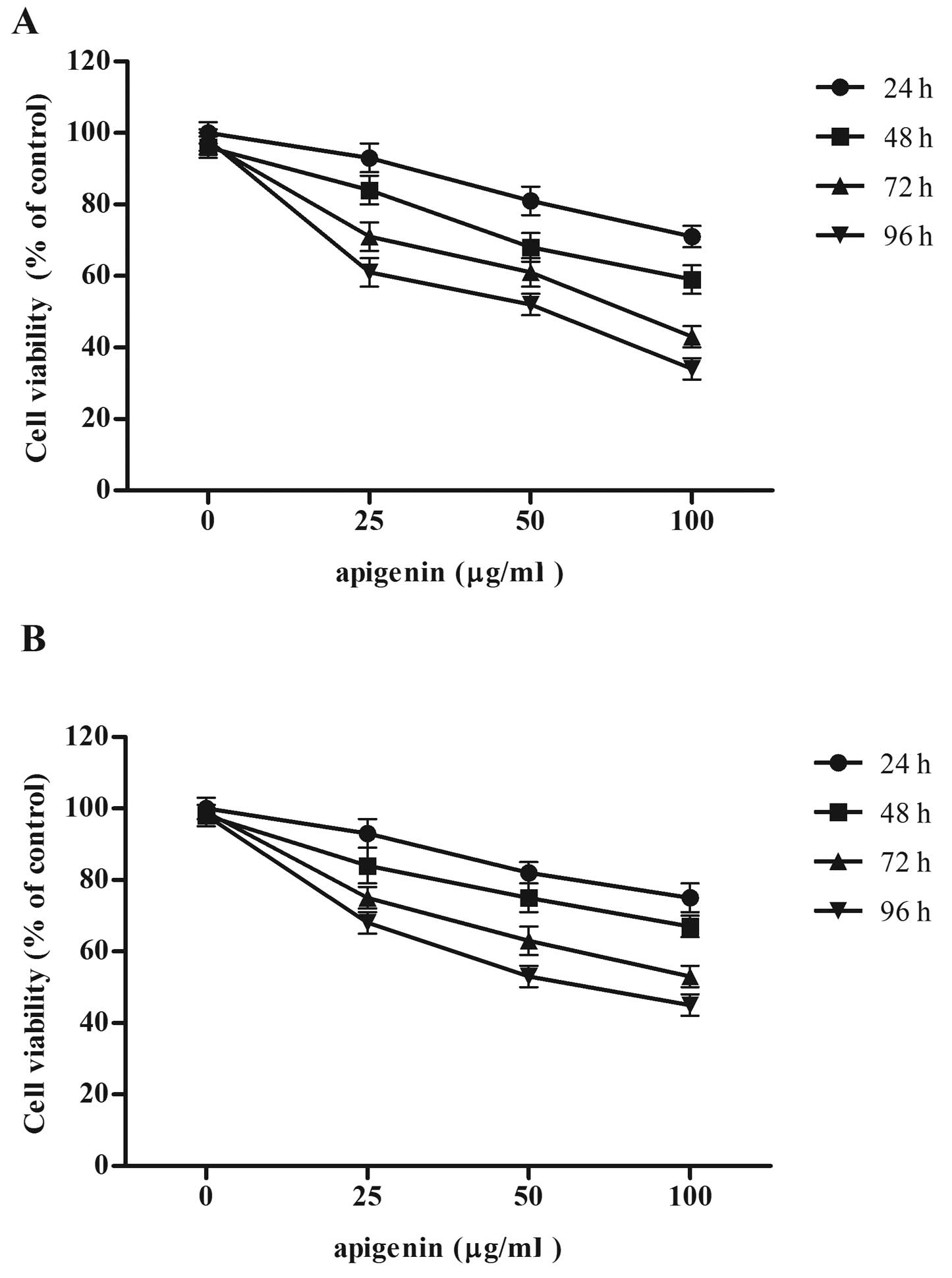
We measured the effect of apigenin on OS cell
proliferation by MTT assay. As shown in Fig. 1, there was a marked decrease in the
proliferation of cells with increasing doses of apigenin. Apigenin
inhibited cell proliferation in a time- and dose-dependent manner
in the U2OS and MG-63 cells. These findings suggested that apigenin
was able to inhibit the proliferation and survival of U2OS and
MG-63 cells.
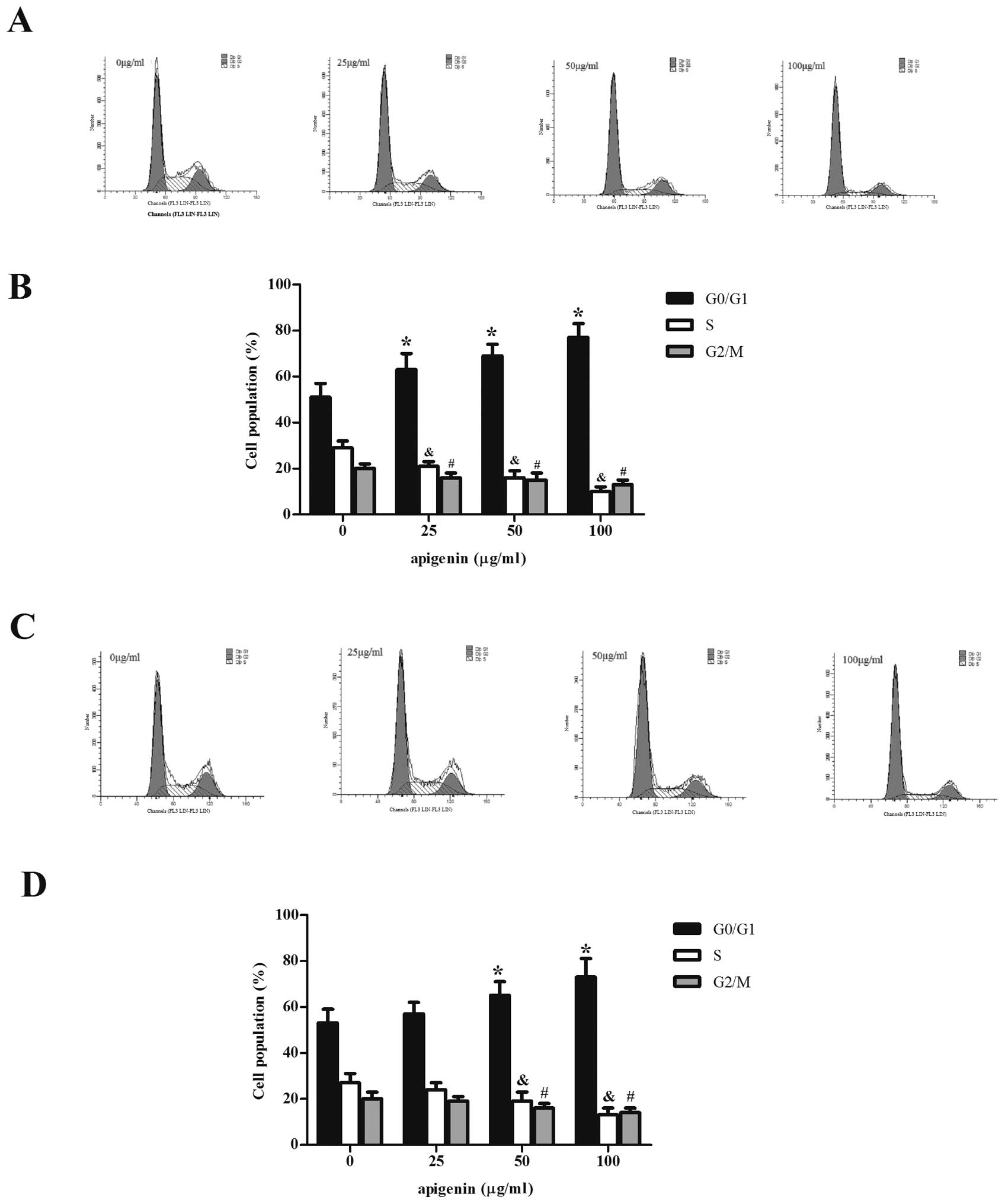
Effect of apigenin on OS cell cycle
We investigated the effect of apigenin on the OS
cell cycle. As shown in Fig. 2,
compared to the control group, apigenin-treated cells exhibited
obvious cell arrest in the G0/G1 phase after 24 h. The increase in
the G0/G1 cell population was accompanied by a concomitant decrease
in the population in the S and G2/M phases of the cell cycle. These
results indicated that the decreased proliferation in
apigenin-treated OS cells is at least partially a result of the
cell cycle arrest by apigenin.
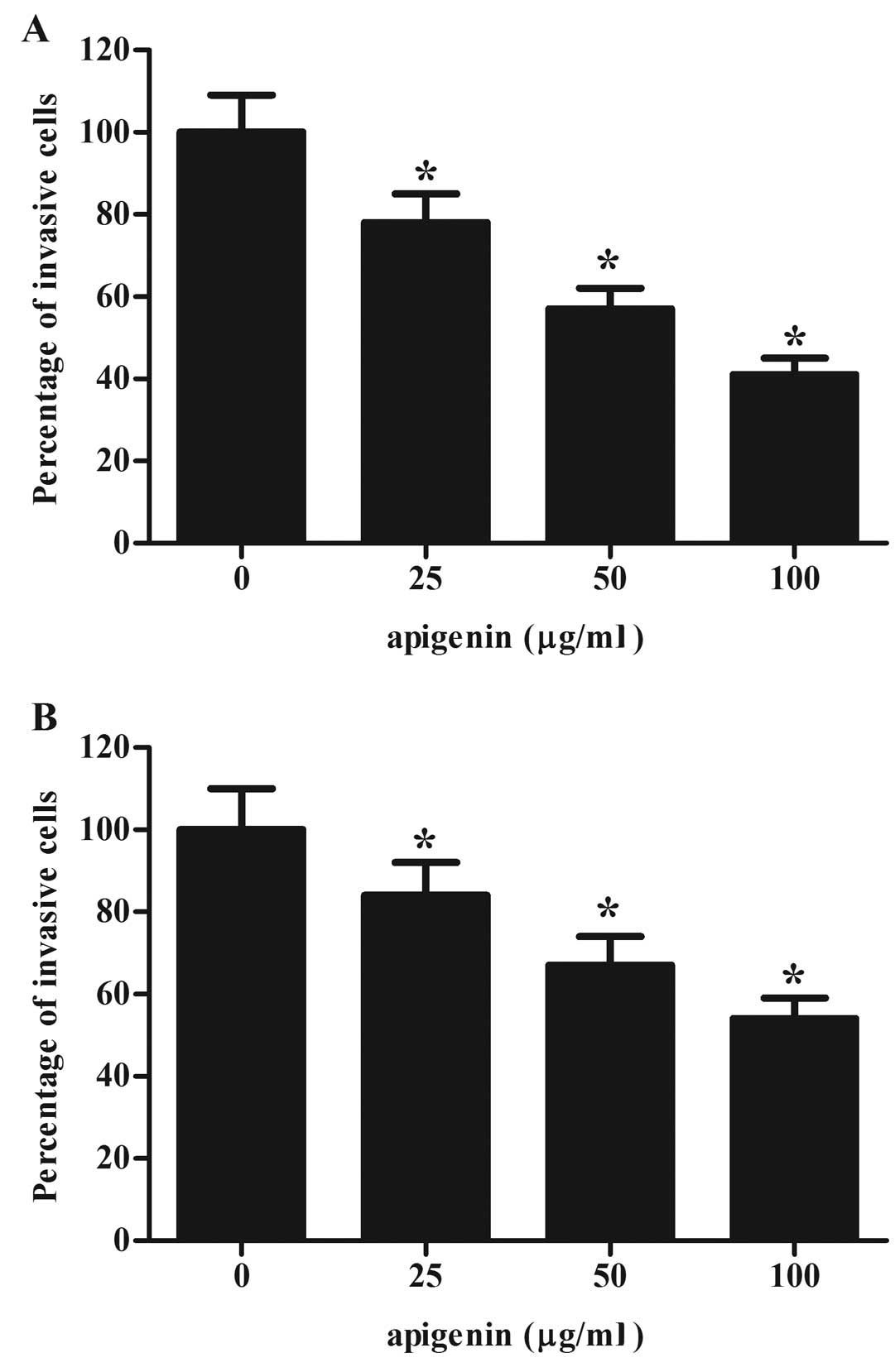
Effect of apigenin on OS cell
invasion
Provided the development of metastasis is highly
dependent on cell migration and invasion (14), we investigated the impact of
apigenin on OS cell invasion by a modified Matrigel Boyden chamber.
As shown in Fig. 3, after treatment
with apigenin, the number of invaded cells was significantly
decreased, as compared with the control group. These findings
indicate that apigenin inhibited the invasion of U2OS and MG-63
cells. However, U2OS is more sensitive for apigenin treatment,
which was selected for the subsequent experiments.
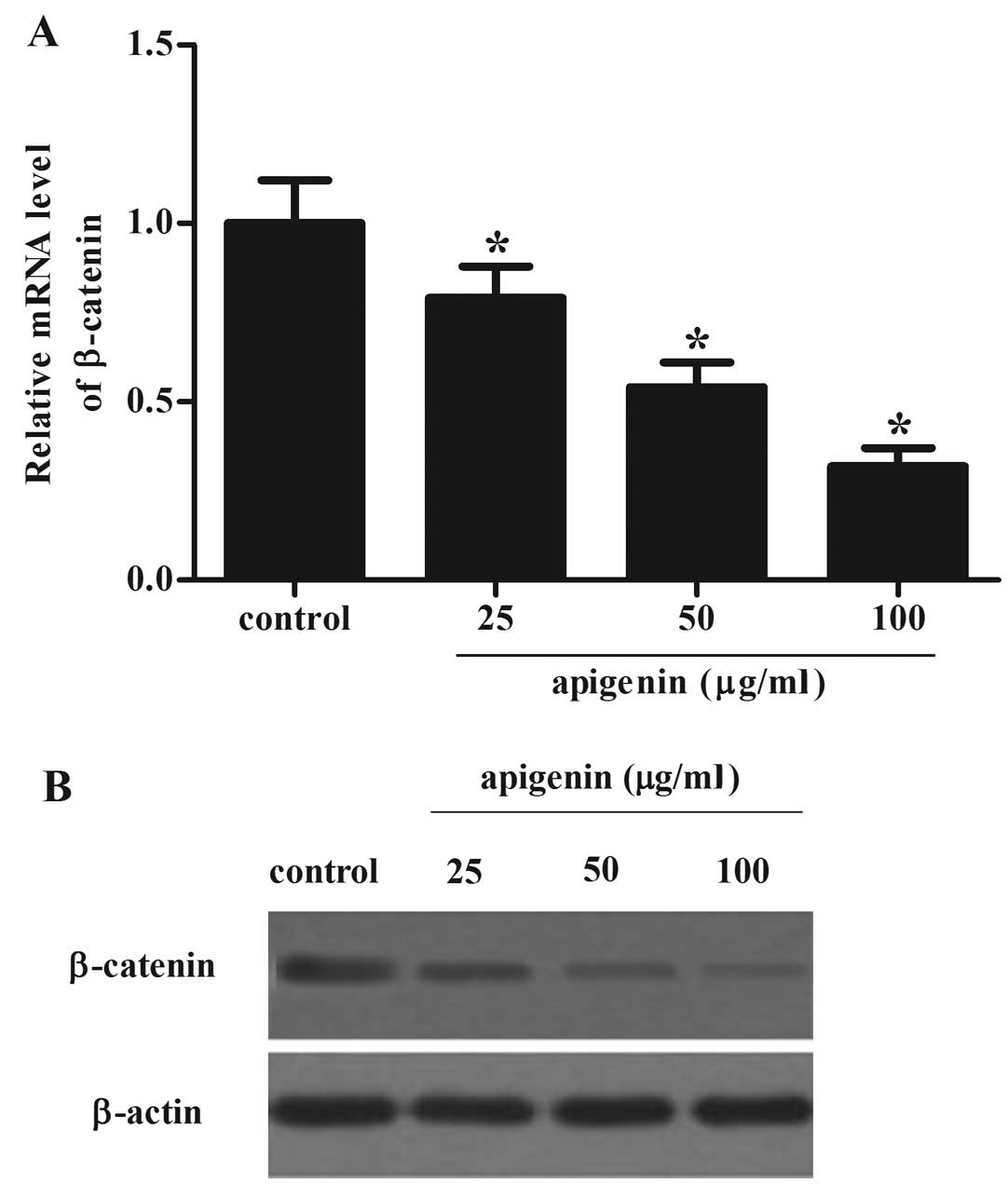
Apigenin inhibited the expression of
β-catenin in OS cells
We investigated a potential mechanism for
apigenin-mediated OS cell proliferation and invasion. It is well
known that the Wnt/β-catenin signaling pathway modulates cancer
cell proliferation, apoptosis and metastasis, therefore, we
examined whether apigenin was able to inhibit the expression of
β-catenin. As shown in Fig. 4A, as
compared with the control group, obvious downregulation of
β-catenin mRNA was identified in OS cells when treated with
apigenin. Simultaneously, the corresponding decrease in β-catenin
protein levels was also confirmed by western blotting (Fig. 4B). These data suggested that
apigenin inhibited the expression of β-catenin in OS cells.
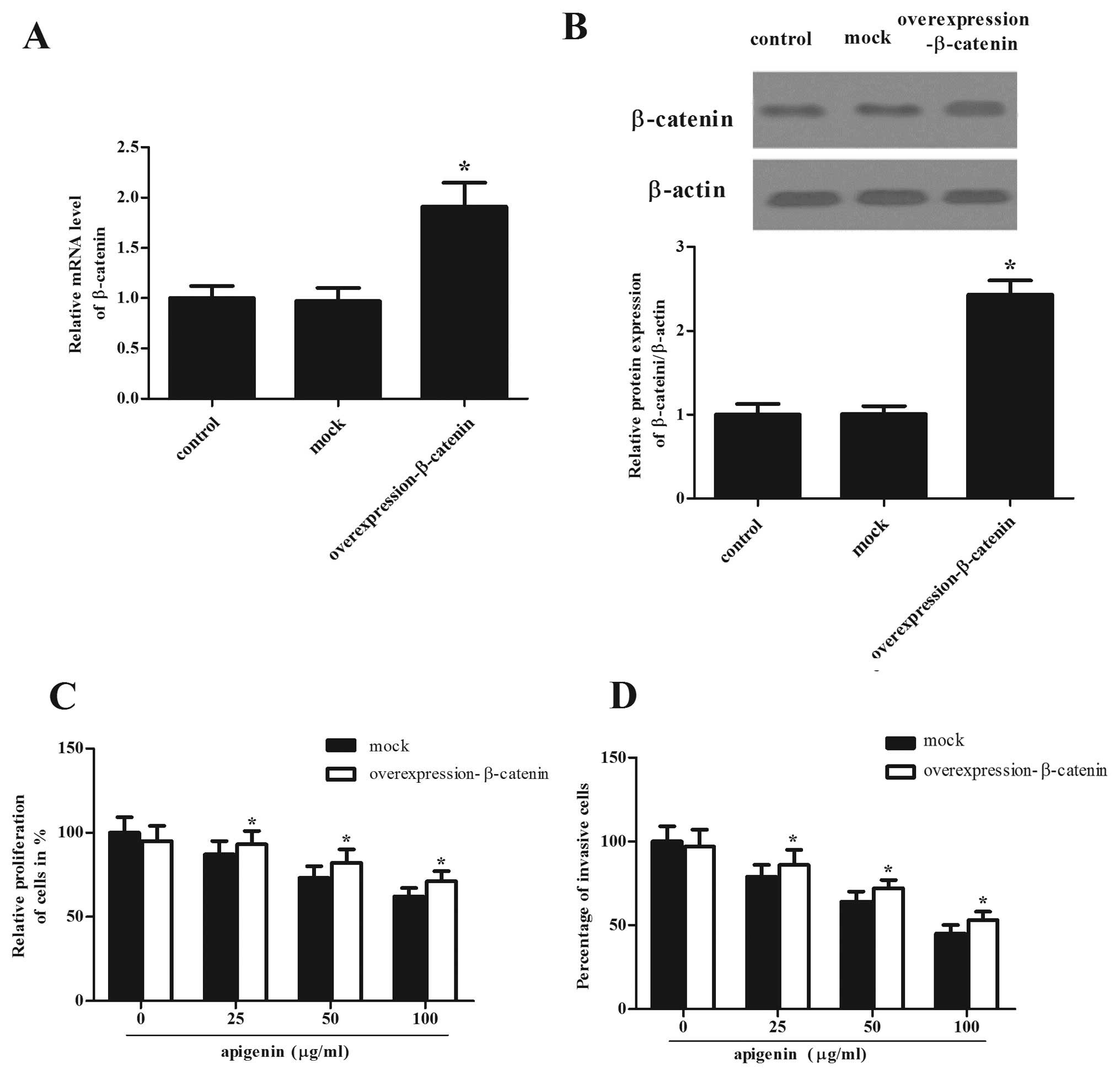
Overexpression of β-catenin reversed the
inhibitory effect of apigenin on OS cells
The role of β-catenin in apigenin-mediated
phenotypes was then evaluated. In this study, we found that the
mRNA and protein levels of β-catenin in the
overexpression-β-catenin-transfected group were significantly
higher than those in the mock group (Fig. 5A and B). We also examined whether
overexpression of β-catenin reversed the inhibitory effect of
apigenin on OS cells. As expected, following treatment with
β-catenin overexpression, the inhibitory effect of apigenin on
proliferation and invasion was significantly reversed in OS cells,
as compared with the mock group (Fig.
5C and D).
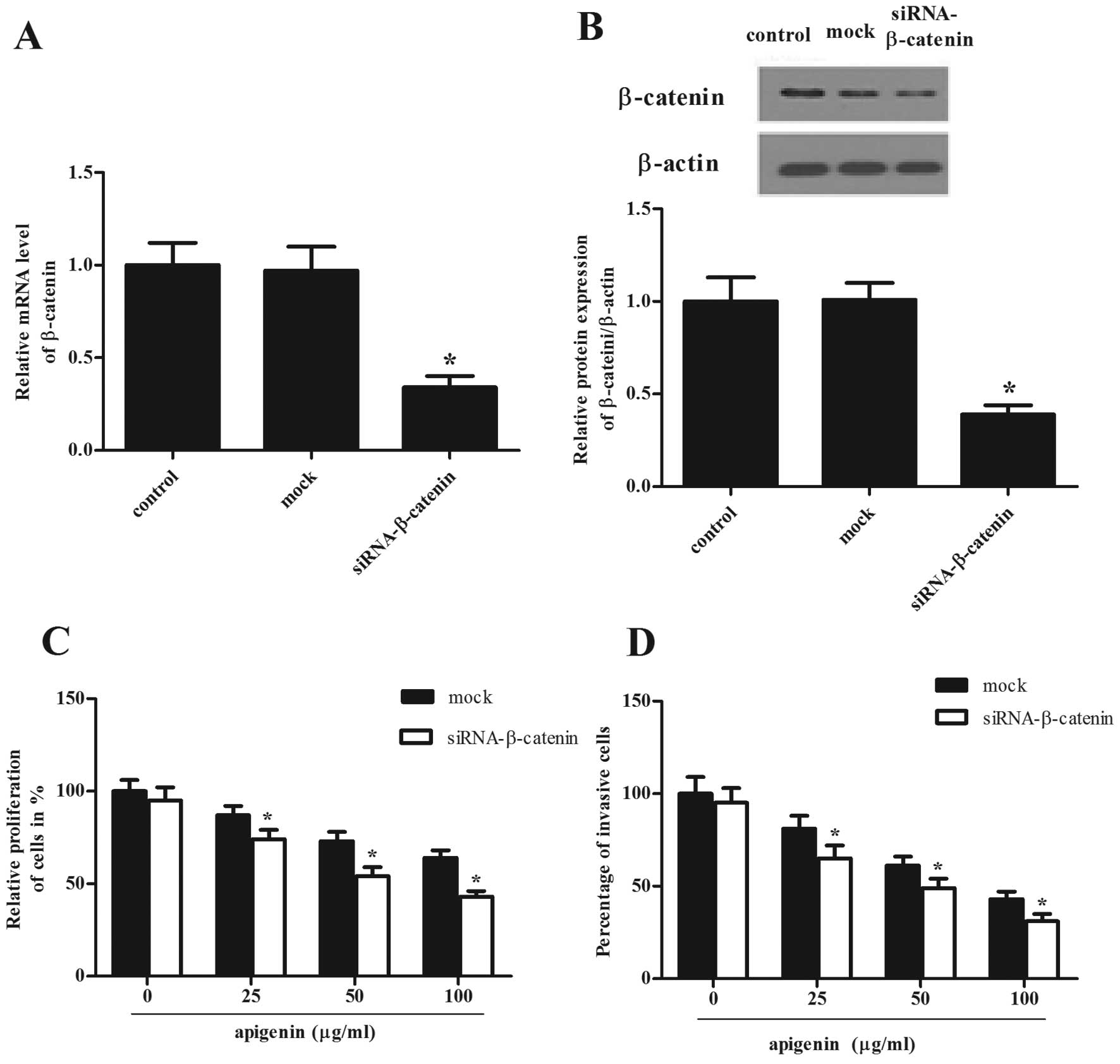
Knockdown of β-catenin enhanced
apigenin-inhibited proliferation and invasion in OS cells
To confirm the role of β-catenin in OS cell
proliferation and invasion, a siRNA experiment was performed in OS
cells. The mRNA and protein levels of β-catenin in the
siRNA-β-catenin-transfected group were significantly lower than
those in the mock group (Fig. 6A and
B). We also examined the effect of knockdown of β-catenin on
the activity of apigenin, including the inhibition of cell
proliferation and invasion. The results showed that siRNA-β-
catenin obviously potentiated apigenin-induced proliferation
inhibition (Fig. 6C). Moreover,
siRNA-β-catenin significantly promoted apigenin-induced invasion
inhibition (Fig. 6D). The results
indicated that knockdown of β-catenin enhanced apigenin-inhibited
proliferation and invasion in OS cells.
Discussion
Apigenin, a naturally occurring plant flavone that
is abundantly present in common fruits and vegetables, is a
bioactive flavonoid shown to possess anticancer properties.
However, the molecular mechanism involved in the anticancer effect
of apigenin in OS has yet to be elucidated. In the present study,
we found that apigenin exhibits anti-proliferative and
anti-invasive activity in OS cells. In addition, apigenin was able
to downregulate the expression of β-catenin. Overexpression of
β-catenin reversed the inhibitory effect of apigenin on OS cells,
and knockdown of β-catenin enhanced apigenin-inhibited
proliferation and invasion in OS cells.
Findings of previous studies have demonstrated that
apigenin inhibited pancreatic cancer cell proliferation in a
dose-dependent manner (11), and it
also inhibited the migration and invasion of A2780 human ovarian
cancer cells (15). Furthermore, OS
tumorigenesis is often associated with tumor cell proliferation and
invasion (16). Therefore, we
investigated the effects of apigenin on OS cell proliferation and
invasion. Consistent with previous studies, results of the present
study demonstrate that apigenin inhibited the proliferation and
invasion of OS cells in vitro.
The Wnt pathway consists of highly conserved
secreted ligands that bind cell-surface receptors known as frizzled
proteins and lipoprotein receptor-related proteins (LRPs). In the
presence of Wnt signaling, β-catenin is accumulated in the cytosol,
translocated into the nucleus, and forms a complex with the
lymphocyte enhancer factor (LEF)/T-cell factor (TCF) family of
transcription factors to activate target genes (17). β-catenin was shown to promote
tumorigenesis, progression, and invasion in cancers (6,18,19).
For example, it is overexpressed in human glioblastoma, and
knockdown of β-catenin inhibits glioblastoma cell proliferation and
invasive ability, and induces apoptotic cell death (20). Furthermore, the intratumoral
introduction of siRNA targeting β-catenin into established
subcutaneous gliomas also delayed tumor growth. In OS, several
secreted protein families modulate the Wnt/β-catenin signaling,
including secreted Frizzled-related proteins (sFRPs), Wnt
inhibitory protein (WIF), Dickkopf proteins, sclerostin, and small
molecules. It was shown that the Wnt inhibitory factor 1 is
epigenetically silenced in human osteosarcoma, and its disruption
accelerates osteosarcoma development in mice (21). In a recent study, it was shown that
a high β-catenin level in OS samples is positively correlated with
lung metastasis (22). Therefore,
we hypothesize that apigenin downregulates the expression of
β-catenin. In this study, we found that apigenin downregulates the
expression of β-catenin in OS cells.
The canonical Wnt-β-catenin signaling pathway is a
key component of normal skeletal development and disease. Aberrant
activation of the Wnt-β-catenin signaling pathway plays a critical
role in OS pathogenesis (23). It
has been reported that a decreased β-catenin expression can
suppress matrix metalloproteinase 14 (MMP14) expression, thereby
resulting in suppression of the invasion and motility of MG-63
cells (24). In this study, we
found that overexpression of β-catenin reversed the inhibitory
effect of apigenin on OS cells, and knockdown of β-catenin enhanced
apigenin-inhibited proliferation and invasion in OS cells. These
results support the hypothesis that β-catenin is involved in OS
cell proliferation and invasion in response to apigenin.
In conclusion, the present study suggests that
apigenin is particularly effective in inhibiting proliferation and
invasion of OS cells by suppressing the Wnt/β-catenin signaling
pathway. Therefore, apigenin may be a chemopreventive and/or
therapeutic agent in the prevention of OS cancer.
References
|
1
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci P: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polakis P: Wnt signaling and cancer. Gene
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
5
|
Uematsu K, He B, You L, Xu Z, McCormick F
and Jablons DM: Activation of the Wnt pathway in non small cell
lung cancer: evidence of dishevelled overexpression. Oncogene.
22:7218–7221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hatsell S, Rowlands T, Hiremath M and
Cowin P: Beta-catenin and Tcfs in mammary development and cancer. J
Mammary Gland Biol Neoplasia. 8:145–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ,
Zou CY, Xie XB, Zeng YX, Shen JN and Kang T: Salinomycin inhibits
osteosarcoma by targeting its tumor stem cells. Cancer Lett.
311:113–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel D, Shukla S and Gupta S: Apigenin
and cancer chemo-prevention: Progress, potential and promise
(Review). Int J Oncol. 30:233–245. 2007.
|
|
9
|
Wang W, Heideman L, Chung CS, Pelling JC,
Koehler KJ and Birt DF: Cell-cycle arrest at G2/M and growth
inhibition by apigenin in human colon carcinoma cell lines. Mol
Carcinog. 28:102–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Way TD, Kao MC and Lin JK: Apigenin
induces apoptosis through proteasomal degradation of HER2/neu in
HER2/neuoverexpressing breast cancer cells via the
phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem.
279:4479–4489. 2004. View Article : Google Scholar
|
|
11
|
Ujiki MB, Ding X-Z, Salabat MR, Bentrem
DJ, Golkar L, Milam B, Talamonti MS, Bell RH, Iwamura T and Adrian
TE: Apigenin inhibits pancreatic cancer cell proliferation through
G2/M cell cycle arrest. Mol Cancer. 5:762006. View Article : Google Scholar
|
|
12
|
Lin CC, Chuang YJ, Yu CC, Yang JS, Lu CC,
Chiang JH, Lin JP, Tang NY, Huang AC and Chung JG: Apigenin induces
apoptosis through mitochondrial dysfunction in U-2 OS human
osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth
in vivo. J Agric Food Chem. 60:11395–11402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu X, Zhang X, Xiang J, Lv Y and Shi J:
miR-451: Potential role as tumor suppressor of human hepatoma cell
growth and invasion. Int J Oncol. 45:739–745. 2014.PubMed/NCBI
|
|
14
|
Jaffe N: Osteosarcoma: review of the past,
impact on the future. The American experience. Cancer Treat Res.
152:239–262. 2009. View Article : Google Scholar
|
|
15
|
Hu XW, Meng D and Fang J: Apigenin
inhibited migration and invasion of human ovarian cancer A2780
cells through focal adhesion kinase. Carcinogenesis. 29:2369–2376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q
and Ma B: MicroRNA-34a inhibits the proliferation and metastasis of
osteosarcoma cells both in vitro and in vivo. PLoS One.
7:e337782012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brabletz T, Jung A, Dag S, Hlubek F and
Kirchner T: Beta-catenin regulates the expression of the matrix
metalloproteinase-7 in human colorectal cancer. Am J Pathol.
155:1033–1038. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li YJ, Wei ZM, Meng YX and Ji XR:
Beta-catenin up-regulates the expression of cyclinD1, c-myc and
MMP-7 in human pancreatic cancer: relationships with carcinogenesis
and metastasis. World J Gastroenterol. 11:2117–2123. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pu P, Zhang Z, Kang C, Jiang R, Jia Z,
Wang G and Jiang H: Downregulation of Wnt2 and beta-catenin by
siRNA suppresses malignant glioma cell growth. Cancer Gene Ther.
16:351–361. 2009. View Article : Google Scholar
|
|
21
|
Kansara M, Tsang M, Kodjabachian L, Sims
NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons
PJ, Dawid IB and Thomas DM: Wnt inhibitory factor 1 is
epigenetically silenced in human osteosarcoma, and targeted
disruption accelerates osteosarcomagenesis in mice. J Clin Invest.
119:837–851. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kidani T, Nakamura A, Kamei S, Norimatsu
Y, Miura H and Masuno H: Overexpression of cytoplasmic β-catenin
inhibits the metastasis of the murine osteosarcoma cell line LM8.
Cancer Cell Int. 14:312014. View Article : Google Scholar
|
|
23
|
Lin CH, Ji T, Chen CF and Hoang BH: Wnt
signaling in osteosarcoma. Adv Exp Med Biol. 804:33–45. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang F, Chen A, Chen J, Yu T and Guo F:
SiRNA-mediated silencing of beta-catenin suppresses invasion and
chemosensitivity to doxorubicin in MG-63 osteosarcoma cells. Asian
Pac J Cancer Prev. 12:239–245. 2011.PubMed/NCBI
|