Introduction
Telomeres are protective structures that cap the
ends of eukaryotic chromosomes (1),
comprising multiple 5′-TTAGGG-3′ repeats and ending in a
single-stranded overhang of the G-rich sequence (2). Telomeres protect chromosome ends from
end-to-end fusion, nucleolytic decay, degradation and atypical
recombination (3). The telomere
repeat sequence becomes shortened by each cell division, DNA damage
due to oxidative stress or through changes in telomere-associated
proteins (4,5). It has been proposed that telomere
shortening is an important biological factor involved in
carcinogenesis, cell senescence, cell replication, cell immortality
and aging (6–8). Accumulating evidence has led to the
hypothesis that telo-mere dysfunction contributes to genetic
changes intrinsic to the development and progression of tumors
(9–11), and that telomere length can be
considered as a biological index of malignant potential (12). It has been reported that telomere
shortening occurs in breast cancer cells (13) and contributes to tumor progression
in numerous cancer types including breast cancer (14,15).
Breast cancer is the most frequent malignant disease
and the leading cause of cancer-related death among women
worldwide. Globally, 1.4 million new cases of breast cancer are
diagnosed annually of which approximately one third are fatal
(16). It is well established that
telomere shortening is present in the majority of in situ
and invasive carcinomas (14)
including breast cancers (17,18).
In addition to their role in tumor initiation, short dysfunctional
telomeres affect disease progression. Previous studies have shown
that telomeres are shorter in grade III tumors (19), that telomere shortening is
correlated with aneuploidy and lymph node metastasis (20), and that shorter telomeres are
associated with higher stage and histological grade (21). Moreover, it has been reported that
short telomeres are associated with tumor size, nodal involvement
and TNM stage (22). However,
another study found no correlation between telomere length and
tumor volume, grading, node status or expression of estrogen
receptor (ER) and progesterone receptor (PR) (23).
The aim of the present study was to clarify whether
the telomeres in three histological types of breast cancer
(scirrhous, papillotubular and solid-tubular carcinomas) are
shorter than those of normal epithelial cells, and whether telomere
length is correlated with TNM stage and several pathologic factors.
For this purpose, we measured telomere lengths in breast cancer
using quantitative fluorescence in situ hybridization
(Q-FISH), which allowed us to estimate the telomere lengths of
individual cells in each section.
Materials and methods
Tissue specimens
We examined a total of 44 breast cancers, including
17 scirrhous, 15 papillotubular and 12 solid-tubular carcinomas.
Both tumor and adjacent normal tissues were obtained from each
individual patient and embedded in paraffin using standard
processing procedures. Sections 4-µm thick were prepared for tissue
Q-FISH and immunohistochemistry (IHC) analysis. All samples were
collected at the Division of Surgical Endocrinology of Tokyo
Women’s Medical University, Tokyo, Japan, after obtaining informed
consent from all of the patients. Table
I summarizes the clinicopathological results. All pathologic
examinations were conducted by one of the authors who was a trained
pathologist (M.K.).
 | Table IClinicopathological characteristics
of the study patients. |
Table I
Clinicopathological characteristics
of the study patients.
| Histological
type | Scirrhous carcinoma
(n=17) | Papillotubular
carcinoma (n=15) | Solid-tubular
carcinoma (n=12) |
|---|
| Clinical
characteristics | | | |
| Age
(years)a | 55.5±11.0 | 52.0±17.0 | 56.1±14.3 |
| TNM stage | | | |
| 0 (DCIS) | 1 | 1 | 0 |
| I | 5 | 5 | 6 |
| II | 9 | 8 | 5 |
| III | 2 | 1 | 1 |
| Pathological
characteristics | | | |
| Tumor size
(mm)a | 22.2±12.0 | 23.3±15.9 | 21.4±12.1 |
| Lymph
nodemetastasesb | | | |
| pN0 | 9 | 13 | 9 |
| pN1 | 3 | 1 | 3 |
| pN3 | 2 | 0 | 0 |
| Vascular
invasionb | | | |
| + | 10 | 8 | 7 |
| − | 6 | 7 | 4 |
| ER statusb | | | |
| + | 14 | 11 | 6 |
| − | 1 | 3 | 6 |
| PR statusb | | | |
| + | 14 | 9 | 5 |
| − | 1 | 5 | 7 |
| HER2
statusb | | | |
| 0–1 | 11 | 12 | 8 |
| 2 | 3 | 2 | 2 |
| 3 | 1 | 1 | 2 |
| Ki67 LI
(%)a | 6.8±11.6 | 13.0±14.4 | 11.4±9.7 |
Tissue Q-FISH
Tissue Q-FISH was performed as previously described
(24–26). In brief, tissue sections were
deparaffinized and treated with 0.2 N HCl and 1 M sodium
thiocyanate at 80°C, 1% pepsin at 37°C and 10 mg/ml RNase at 37°C.
A peptide nucleic acid (PNA) telomere probe conjugated to Cy3 (telo
C Cy3 probe, 5′-CCCTAACCCTAACCCTAA-3′); and a PNA centromere probe
conjugated to fluorescein isothiocyanate (FITC) (Cenp 1 probe,
5′-CTTCGTTGGAAACGGGGT-3′) (both from Fasmac, Kanagawa, Japan) were
applied to each section. The nuclei were stained with
4′,6-diamidino-2-phenyl-indole (DAPI) (Sigma-Aldrich, St, Louis,
MO, USA).
FISH images were captured by a CCD camera attached
to an epifluorescence microscope (Eclipse 90i; Nikon, Tokyo, Japan)
equipped with a triple band-pass filter set for DAPI/FITC/Cy3
(61000v2m; Chroma Technology Corp., Rockingham, VT, USA) and a ×40
objective lens (Plan Fluor ×40/0.75; Nikon). Microscope control and
image recording were performed using Image-Pro Plus software
(version 6.3; Media Cybernetics, Bethesda, MD, USA). The recorded
images were analyzed as previously described using original
software prepared by our colleague (S.P.) (24–26),
‘Tissue Telo Version 3.2′, which allows manual identification of
nuclear regions from the composite color image: DAPI (blue
channel), FITC (green) and Cy3 (red). Fluorescence intensities of
telomere (Cy3) and centromere signals (FITC) for each nucleus were
measured, and then the telomere-centromere ratio (TCR) was
calculated, since there is no guarantee that all information on
telomere signals will be acquired within any given tissue
section.
IHC
The expression of ER, PR, human epidermal growth
factor receptor 2 (HER2) and Ki67 (Dako Japan, Tokyo, Japan)
protein was determined by IHC staining. For ER, PR and Ki67, the
tissue sections were pretreated with Tris-EDTA buffer solution (pH
9.0) at 95°C, and for HER2 with citrate buffer solution (pH 6.0) at
95°C. After incubation with the primary antibody for 2 h,
visualization was performed using a polymer IHC detection system
(EnVision kit; Dako Japan). The Ki67 labeling index (LI) was
calculated by counting at least 500 cells in each specimen.
Statistical analysis
The significance of differences in mean TCR was
examined by Welch’s t-test. When three groups were compared, we
used one-way ANOVA and the Tukey-Kramer post hoc test. For
analyzing correlations between continuous variables (pathological
tumor size and Ki67 LI) and TCR, Pearson’s correlation coefficient
was calculated. Differences at P<0.05 were considered to
indicate a statistically significant result.
Results
Telomere length (TCR distribution) in
cancer and adjacent normal breast tissues
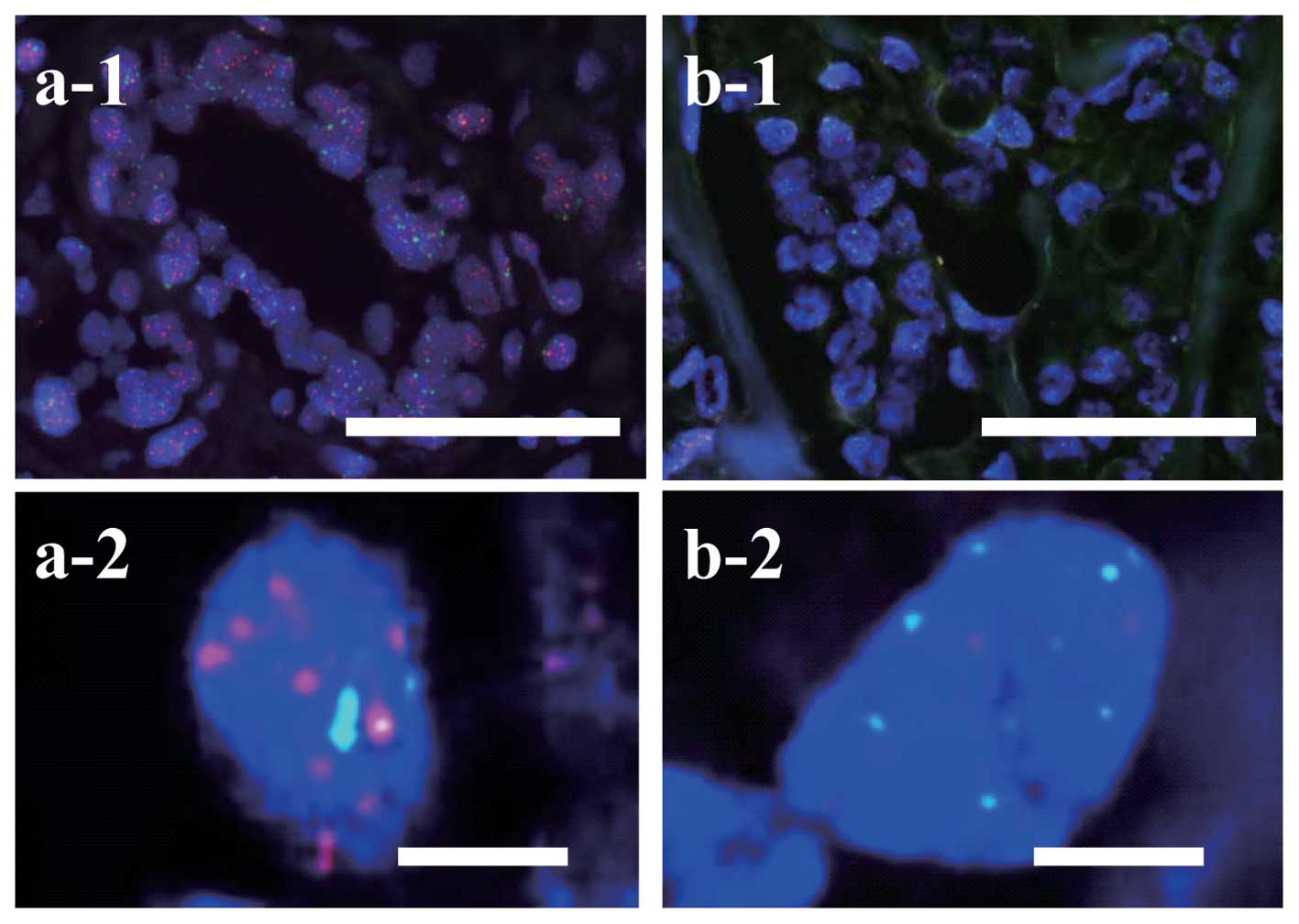
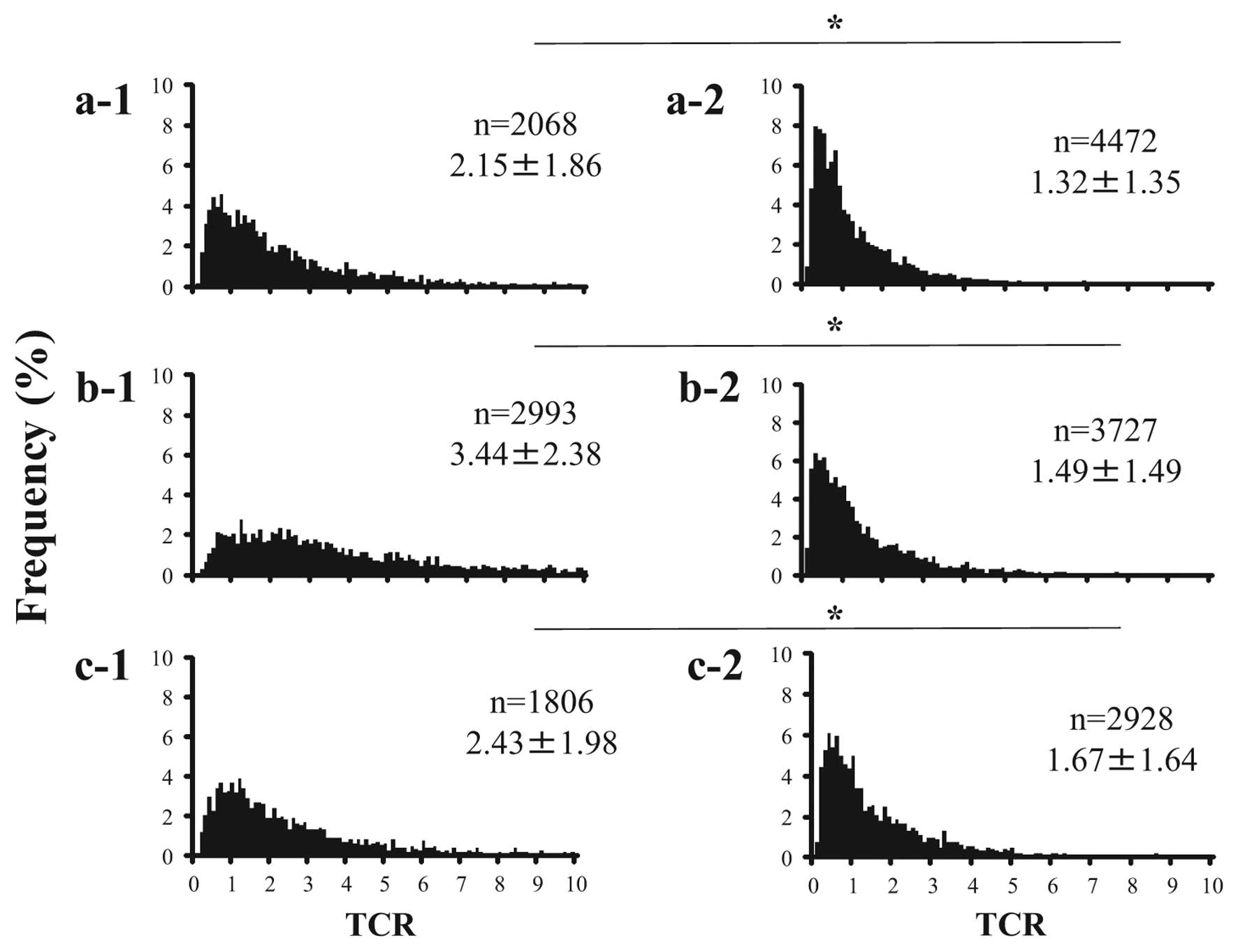
Telomere signals were evident within nuclei as small
red spots in normal epithelial cells (Fig. 1a-1). Telomere signals of tumor cells
in scirrhous carcinomas (Fig. 1b-1 and
b-2) were weaker than those in adjacent n ormal epithelial
cells (Fig. 1a-1 and a-2). Cancer
cells most frequently had very short telomeres, yet a few cells had
long telomeres such as TCR >4, and the peak frequency of TCR was
in <1 (Fig. 2a-2, b-2 and c-2).
On the other hand, normal epithelial cells had relatively long
telomeres (TCR >4) and the TCR had a very wide distribution
(Fig. 2a-1, b-1 and c-1). The mean
TCR of cancer cells was significantly lower than that of normal
epithelial cells in all histological types (Fig. 2, P<0.05).
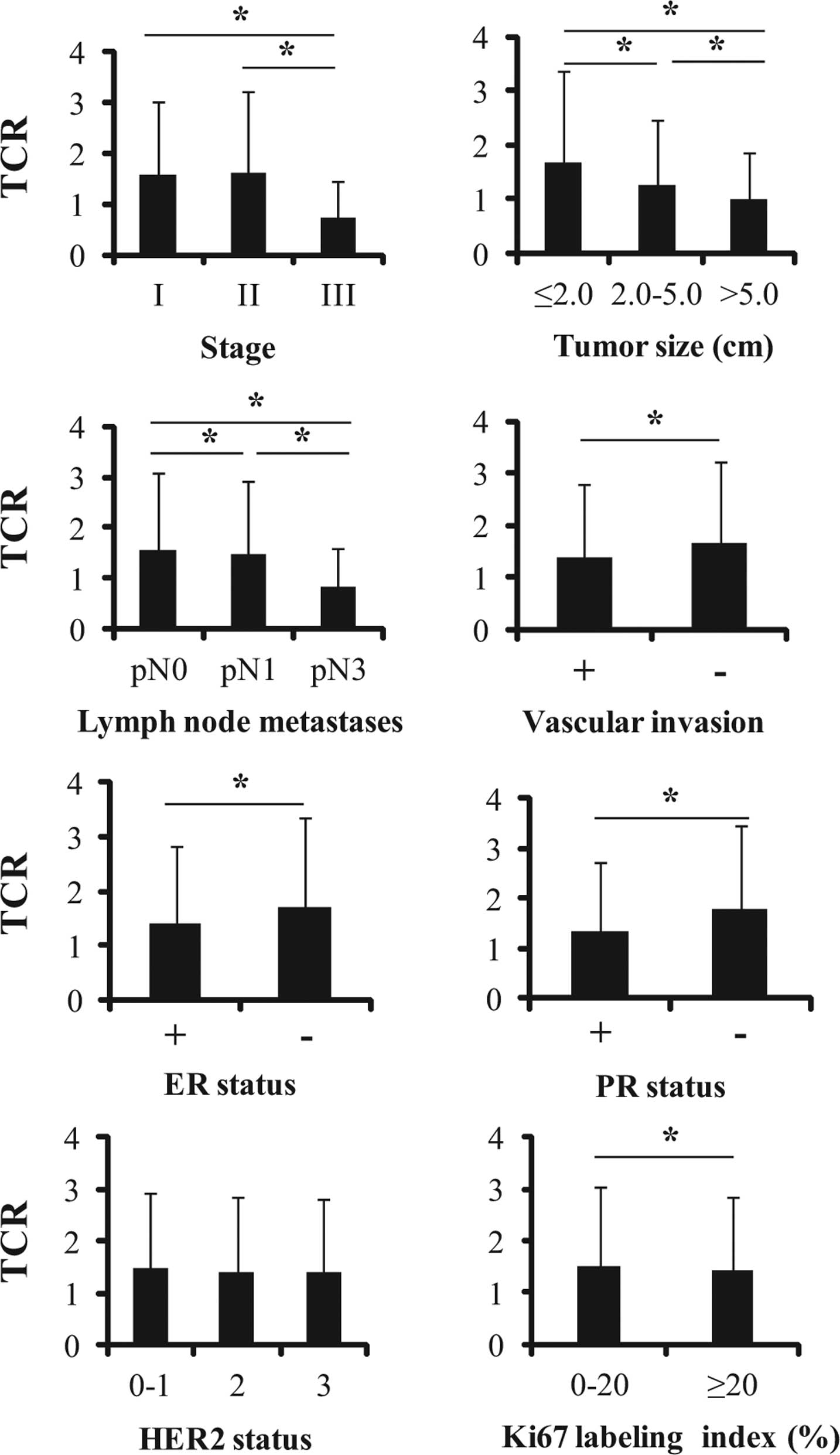
Relationship between telomere length and
several pathological and immunohistochemical factors
In the present study, associations between mean TCR
and clinical TNM stage, histological tumor size, pathologically
proven lymph node metastasis, vascular invasion, ER, PR, HER2
status and Ki67 LI were analyzed (Fig.
3). Tumor size was divided into three groups (≤20, 20–50 and
>50 mm) in accordance with the UICC criteria. For Ki67 LI,
though cut-off values have varied among previous studies (27–30), a
nuclear LI of ≥20% was considered high and one of <20% was
considered low for the purposes of the present study. Telomere
shortening was associated with TNM stage III, a large tumor size,
presence of a large number of lymph node metastases, presence of
vascular invasion, ER positivity and PR positivity (Fig. 3). However, HER2 status did not
correlate with telomere length (Fig.
3). Telomeres lengths of Ki67 LI ≥20% patients were shorter
than those of LI <20% patients (Fig.
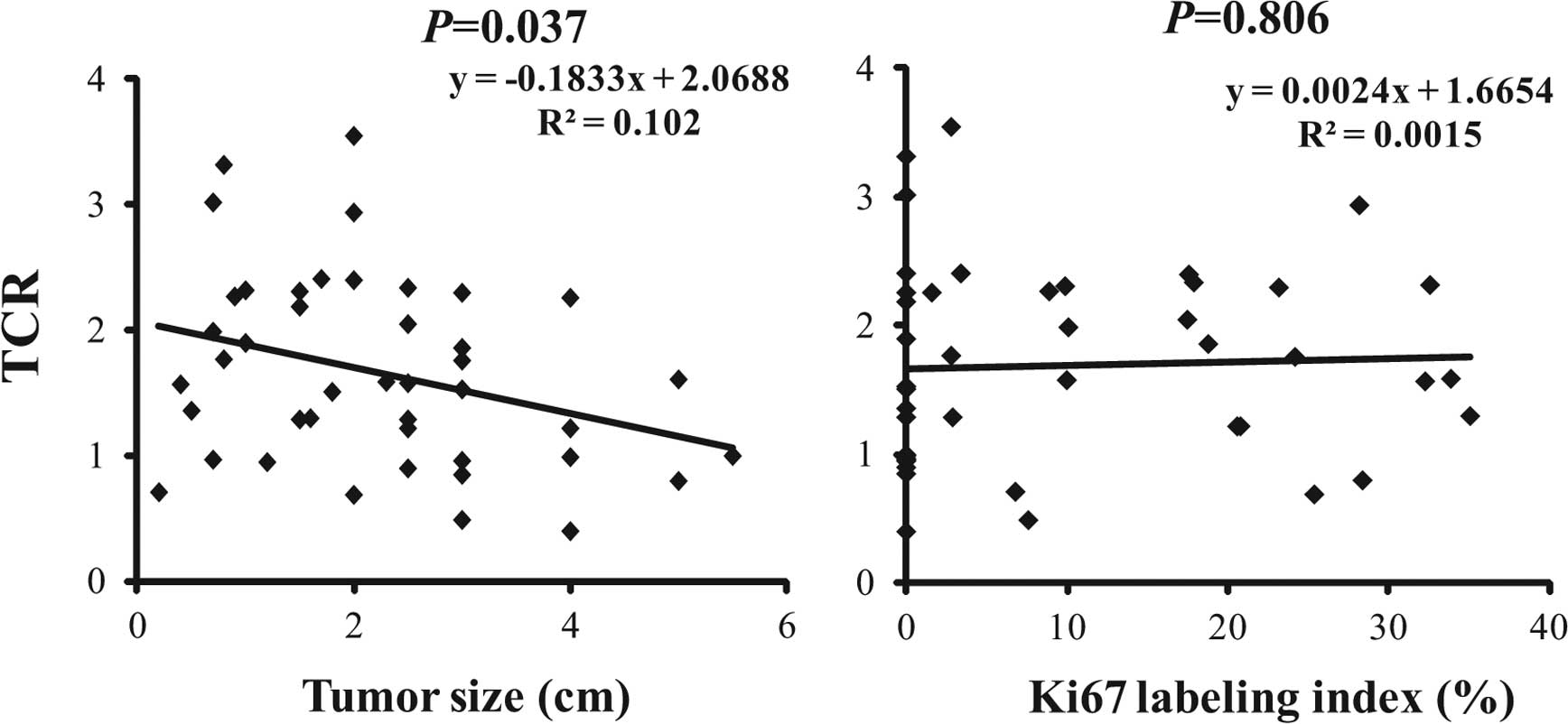
3). However, there was no significant correlation between Ki67
LI and mean TCR when we analyzed the Pearson’s correlation
coefficient (P=0.806, Fig. 4).
We analyzed the correlation between histological
tumor size and mean TCR by calculation of Pearson’s correlation
coefficient. Although correlation coefficient was not so strong
(R2=0.102), statistically significant inverse
correlation was observed (P=0.037, Fig.
4).
Discussion
Generally, the cells of malignant tumors including
breast cancer have shorter telomeres than the corresponding cells
in normal tissue (13,21), and telomere dysfunction or
shortening has been considered as a negative prognostic indicator
in patients with solid tumors (31,32),
including breast cancer (33,34).
Furthermore, it has been reported that telomere shortening
contributes to tumor progression (14,15).
In the present study, using tissue Q-FISH technique, we evaluated
telomere length in breast cancer tissues and also confirmed that
cancer cells had shorter telomeres than the adjacent normal
epithelial cells in three histological types of carcinoma
(scirrhous, papillotubular and solid-tubular carcinomas). Tissue
Q-FISH effectively estimates telomere length in different cell
types using separate PNA probes for telomeres and centromeres, thus
allowing specific evaluation for cancer cells. We obtained the
telomere-centromere ratio (TCR) as a parameter representative of
telomere length, and many previous studies have verified its
accuracy for this purpose (24–26).
Moreover, we evaluated whether telomere length was correlated with
several pathological features, indicating tumor progression.
The principal conclusion emerging from the present
study was that telomere shortening is associated with some
parameters of cancer progression. Mean telomere length was
significantly less in patients with TNM stage III disease, a large
tumor size, a large number of lymph node metastases and vascular
invasion. As previously described, several studies of breast cancer
have revealed that telomere length is associated with TNM stage,
tumor size, nodal involvement and prognosis (21,22,35).
On the other hand, some studies have found no correlation between
telomere length and tumor volume, grading, nodal or ER and PR
status (20,23). Our present findings are in agreement
with the results of some of these studies (21,22,35),
and suggest that telomere length may be a useful index of tumor
aggressiveness in breast cancer. Although tumor size has been the
traditional prognostic factor, it has been regarded as one of the
most powerful predictors of tumor behavior in breast cancer
(36–38). Since the present study demonstrated
an inverse relationship between mean TCR and histologically evident
tumor size, our results reinforce the assumption that telomere
length reflects the prognosis.
Recently, breast cancer has been classified into
different molecular subtypes with different biological features,
clinical outcome and response to therapy (39–42).
These subtypes can be distinguished on the basis of ER, PR and HER2
status: luminal A (ER+ and/or PR+,
HER2™), luminal B (ER+ and/or PR+,
HER2+), HER2 (ER™ and PR™,
HER2+) and basal-like (ER™, PR™,
HER2™). Various studies have indicated the importance of
using the proliferation index (Ki67 LI) to distinguish between the
luminal A and B subtypes (27,43).
In addition, telomere shortening is reportedly associated with
specific breast cancer subtypes; some studies have indicated that
ER- and/or PR-negative cancers have shorter telomeres than ER-
and/or PR-positive cases (44,45).
In the present study, both ER- and PR-positive tumors had shorter
telomeres than ER- and PR-negative tumors, respectively, being
contradictory to results obtained in previous studies. This may
have been due to the fact that both the ER- and PR-positive groups
in the present study included pN3 cases with very short telomeres.
However, due to the limited number of patients with ER- and/or
PR-negative tumors, we considered that these results should be
viewed with caution, and that no clear conclusion can yet to be
drawn. Furthermore, telomere length did not differ significantly
according to the HER2 status, and no correlation between Ki67 LI
and telomere length was evident. Accordingly, the data obtained in
the present study were considered insufficient for discussing their
relationship with molecular subtypes such as the luminal
classification. However, as previously described, it is thought
that telomere metabolism is a very important factor impacting on
tumor behavior, and that tissue Q-FISH provides an accurate
estimation of telomere length. We considered that further studies
will be needed to evaluate telomere length using the tissue Q-FISH
method in relation to luminal molecular subtypes.
In summary, we have demonstrated a significant
correlation between telomere length and TNM stage, tumor size and
lymph node metastasis in breast cancer. Our present results suggest
that the telomere length of cancer cells is strongly correlated
with cancer progression.
Acknowledgments
This study was partly supported by a Grant-in-Aid
for Scientific Research from the Japanese Ministry of Education,
Culture, Sports, Science and Technology (#20591555). We would like
to thank Dr Steven S.S. Poon (Terry Fox Laboratory, British
Columbia Cancer Research Center) for preparation of the software
for measurement of telomere length. We would also like to thank
Miyoko Matsumoto (Kanaji Thyroid Hospital) for assistance with the
manuscript preparation.
References
|
1
|
Deng Y, Chan SS and Chang S: Telomere
dysfunction and tumour suppression: The senescence connection. Nat
Rev Cancer. 8:450–458. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palm W and de Lange T: How shelterin
protects mammalian telomeres. Annu Rev Genet. 42:301–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart SA and Weinberg RA: Telomeres:
Cancer to human aging. Annu Rev Cell Dev Biol. 22:531–557. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Zglinicki T: Oxidative stress shortens
telomeres. Trends Biochem Sci. 27:339–344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harley CB and Villeponteau B: Telomeres
and telomerase in aging and cancer. Curr Opin Genet Dev. 5:249–255.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Lange T: Telomeres and senescence:
Ending the debate. Science. 279:334–335. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DePinho RA: The age of cancer. Nature.
408:248–254. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Artandi SE and DePinho RA: Telomeres and
telomerase in cancer. Carcinogenesis. 31:9–18. 2010. View Article : Google Scholar :
|
|
10
|
Blasco MA: Telomeres and human disease:
Ageing, cancer and beyond. Nat Rev Genet. 6:611–622. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prescott J, Wentzensen IM, Savage SA and
De Vivo I: Epidemiologic evidence for a role of telomere
dysfunction in cancer etiology. Mutat Res. 730:75–84. 2012.
View Article : Google Scholar
|
|
12
|
Svenson U and Roos G: Telomere length as a
biological marker in malignancy. Biochim Biophys Acta.
1792:317–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurabayashi R, Takubo K, Aida J, Honma N,
Poon SS, Kammori M, Izumiyama-Shimomura N, Nakamura K, Tsuji E,
Matsuura M, et al: Luminal and cancer cells in the breast show more
rapid telomere shortening than myoepithelial cells and fibroblasts.
Hum Pathol. 39:1647–1655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meeker AK, Hicks JL, Iacobuzio-Donahue CA,
Montgomery EA, Westra WH, Chan TY, Ronnett BM and De Marzo AM:
Telomere length abnormalities occur early in the initiation of
epithelial carcinogenesis. Clin Cancer Res. 10:3317–3326. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meeker AK and Argani P: Telomere
shortening occurs early during breast tumorigenesis: A cause of
chromosome destabilization underlying malignant transformation? J
Mammary Gland Biol Neoplasia. 9:285–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meeker AK, Hicks JL, Gabrielson E, Strauss
WM, De Marzo AM and Argani P: Telomere shortening occurs in subsets
of normal breast epithelium as well as in situ and invasive
carcinoma. Am J Pathol. 164:925–935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chin K, de Solorzano CO, Knowles D, Jones
A, Chou W, Rodriguez EG, Kuo WL, Ljung BM, Chew K, Myambo K, et al:
In situ analyses of genome instability in breast cancer. Nat Genet.
36:984–988. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Odagiri E, Kanada N, Jibiki K, Demura R,
Aikawa E and Demura H: Reduction of telomeric length and c-erbB-2
gene amplification in human breast cancer, fibroadenoma, and
gynecomastia. Relationship to histologic grade and clinical
parameters. Cancer. 73:2978–2984. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Griffith JK, Bryant JE, Fordyce CA,
Gilliland FD, Joste NE and Moyzis RK: Reduced telomere DNA content
is correlated with genomic instability and metastasis in invasive
human breast carcinoma. Breast Cancer Res Treat. 54:59–64. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radpour R, Barekati Z, Haghighi MM, Kohler
C, Asadollahi R, Torbati PM, Holzgreve W and Zhong XY: Correlation
of telomere length shortening with promoter methylation profile of
p16/Rb and p53/p21 pathways in breast cancer. Mod Pathol.
23:763–772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fordyce CA, Heaphy CM, Bisoffi M, Wyaco
JL, Joste NE, Mangalik A, Baumgartner KB, Baumgartner RN, Hunt WC
and Griffith JK: Telomere content correlates with stage and
prognosis in breast cancer. Breast Cancer Res Treat. 99:193–202.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rogalla P, Rohen C, Bonk U and Bullerdiek
J: Telomeric repeat fragment lengths are not correlated to
histological grading in 85 breast cancers. Cancer Lett.
106:155–161. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugishita Y, Kammori M, Yamada O, Yamazaki
K, Ito K, Fukumori T, Yoshikawa K and Yamada T: Biological
differential diagnosis of follicular thyroid tumor and Hürthle cell
tumor on the basis of telomere length and hTERT expression. Ann
Surg Oncol. 21:2318–2325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aida J, Izumiyama-Shimomura N, Nakamura K,
Ishii A, Ishikawa N, Honma N, Kurabayashi R, Kammori M, Poon SS,
Arai T, et al: Telomere length variations in 6 mucosal cell types
of gastric tissue observed using a novel quantitative fluorescence
in situ hybridization method. Hum Pathol. 38:1192–1200. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kammori M, Izumiyama N, Nakamura K,
Kurabayashi R, Kashio M, Aida J, Poon SS and Kaminishi M: Telomere
metabolism and diagnostic demonstration of telomere measurement in
the human esophagus for distinguishing benign from malignant tissue
by tissue quantitative fluorescence in situ hybridization.
Oncology. 71:430–436. 2006. View Article : Google Scholar
|
|
27
|
Ahlin C, Aaltonen K, Amini RM, Nevanlinna
H, Fjällskog ML and Blomqvist C: Ki67 and cyclin A as prognostic
factors in early breast cancer. What are the optimal cut-off
values? Histopathology. 51:491–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members: Strategies for
subtypes - dealing with the diversity of breast cancer: Highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knutsvik G, Stefansson IM, Aziz S, Arnes
J, Eide J, Collett K and Akslen LA: Evaluation of Ki67 expression
across distinct categories of breast cancer specimens: A
population-based study of matched surgical specimens, core needle
biopsies and tissue microarrays. PLoS One. 9:e1121212014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Donaldson L, Fordyce C, Gilliland F, Smith
A, Feddersen R, Joste N, Moyzis R and Griffith J: Association
between outcome and telomere DNA content in prostate cancer. J
Urol. 162:1788–1792. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frías C, García-Aranda C, De Juan C, Morán
A, Ortega P, Gómez A, Hernando F, López-Asenjo JA, Torres AJ,
Benito M, et al: Telomere shortening is associated with poor
prognosis and telomerase activity correlates with DNA repair
impairment in non-small cell lung cancer. Lung Cancer. 60:416–425.
2008. View Article : Google Scholar
|
|
33
|
Bisoffi M, Heaphy CM and Griffith JK:
Telomeres: Prognostic markers for solid tumors. Int J Cancer.
119:2255–2260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heaphy CM, Baumgartner KB, Bisoffi M,
Baumgartner RN and Griffith JK: Telomere DNA content predicts
breast cancer-free survival interval. Clin Cancer Res.
13:7037–7043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Diehl MC, Idowu MO, Kimmelshue KN, York
TP, Jackson-Cook CK, Turner KC, Holt SE and Elmore LW: Elevated
TRF2 in advanced breast cancers with short telomeres. Breast Cancer
Res Treat. 127:623–630. 2011. View Article : Google Scholar
|
|
36
|
Carter CL, Allen C and Henson DE: Relation
of tumor size, lymph node status, and survival in 24,740 breast
cancer cases. Cancer. 63:181–187. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leitner SP, Swern AS, Weinberger D, Duncan
LJ and Hutter RV: Predictors of recurrence for patients with small
(one centimeter or less) localized breast cancer (T1a,b N0 M0).
Cancer. 76:2266–2274. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gebauer G, Fehm T, Lang N and Jäger W:
Tumor size, axillary lymph node status and steroid receptor
expression in breast cancer: Prognostic relevance 5 years after
surgery. Breast Cancer Res Treat. 75:167–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sotiriou C and Pusztai L: Gene-expression
signatures in breast cancer. N Engl J Med. 360:790–800. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Voduc KD, Cheang MC, Tyldesley S, Gelmon
K, Nielsen TO and Kennecke H: Breast cancer subtypes and the risk
of local and regional relapse. J Clin Oncol. 28:1684–1691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Heaphy CM, Subhawong AP, Gross AL, Konishi
Y, Kouprina N, Argani P, Visvanathan K and Meeker AK: Shorter
telomeres in luminal B, HER-2 and triple-negative breast cancer
subtypes. Mod Pathol. 24:194–200. 2011. View Article : Google Scholar
|
|
45
|
Martinez-Delgado B, Gallardo M, Tanic M,
Yanowsky K, Inglada-Perez L, Barroso A, Rodriguez-Pinilla M,
Cañamero M, Blasco MA and Benitez J: Short telomeres are frequent
in hereditary breast tumors and are associated with high tumor
grade. Breast Cancer Res Treat. 141:231–242. 2013. View Article : Google Scholar : PubMed/NCBI
|