Introduction
Prostate cancer is one of the most common cancers in
men (1). Conventional therapy,
including surgery, radiotherapy and chemotherapy may be useful for
early stage prostate cancer. However, once the cancer has
metastasized to distant organs, conventional therapy may achieve
small improvements in the overall survival of patients with
prostate cancer (2). Therefore, a
better understanding of the molecular mechanisms involved in
prostate cancer progression is essential for the diagnosis and
treatment of prostate cancer.
There are 38 HOX genes that have been identified in
human cells, and can be divided into 4 groups (HOXA-HOXD). The HOXA
gene family has been reported to play a pivotal role in the
regulation of several important cellular processes. Overexpression
of the HOXA gene family has been observed in several malignant
tissues, and is closely associated with the progression of cancer
(3–5). Thus, the HOXA gene family may serve as
potential targets for antitumor therapy (6). As one important member of HOXA, HOXA1
has been found to be highly expressed in many types of cancer
including breast and ovarian cancer, and overexpression of HOXA1 is
correlated with tumor progression and poor prognosis (7,8).
However, little is known regarding to the biological function of
HOXA1 in prostate cancer.
In the present study, we detected the expression of
HOXA1 in different prostate cancer cell lines by western blot
analysis. We further stably silenced HOXA1 expression in prostate
cancer DU-145 and PC-3 cells by short hairpin RNA (shRNA) to
evaluate the effect of HOXA1 knockdown on the growth, invasion and
metastasis of prostate cancer cells in vitro and in
vivo.
Materials and methods
Antibodies
Mouse monoclonal anti- HOXA1 and anti-β-actin
antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). Rabbit polyclonal anti-E-cadherin, p-ERK1/2, ERK1/2,
p-AKT and AKT, and mouse monoclonal anti-Snail antibodies were
purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse
monoclonal anti-MMP-3 antibody was purchased from Abcam (Cambridge,
MA, USA).
Cell lines and culture conditions
Human prostate cancer cell lines LNCap, PC-3, and
DU-145 and normal prostate cell line RPWE-1 were all obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
Prostate cancer cell lines were maintained in RPMI-1640 medium
containing 10% fetal bovine serum (FBS) in a 5% CO2
atmosphere at 37°C. RPWE-1 cells were maintained in K-SFM medium
containing 10% FBS in a 5% CO2 atmosphere at 37°C.
Western blot analysis
Cells were washed with cold PBS and then lysed in
RIPA lysis buffer containing protease inhibitor. Total protein
concentration was determined using the BCA protein assay kit
(Pierce Company, Rockford, IL, USA). Equal amounts of total protein
(~50 µg) were separated by SDS-polyacrylamide separating
gels and then transferred onto PVDF membranes. Then the PVDF
membranes were blocked with TBS-T buffer containing 5% BSA. The
PVDF membranes were probed with the primary antibodies against
HOXA1 (1:500), E-cadherin (1:1,000), Snail (1:1,000), MMP-3
(1:500), p-ERK1/2 (1:1,000), ERK1/2 (1:1,000), p-AKT(1:1,000), AKT
(1:1,000) or β-actin (1:1,000) and incubated at 4°C overnight.
Next, the membranes were washed in TBS-T for 3 times and then
incubated with either the anti-mouse or anti-rabbit horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature. The membranes were further treated with enhanced
chemiluminescence (Pierce Company) and the bands were exposed to
X-ray film. Densitometric analysis of the bands was performed with
Quantity One software (Bio-Rad, Hercules, CA, USA).
Knockdown of HOXA1 expression by
shRNA
shRNAs were designed and obtained from Genchem
Biotechnology Company (Shanghai, China). Briefly, 4 different
shRNAs targeting the HOXA1 gene (ShRNA#1, ShRNA#2, ShRNA#3 and
ShRNA#4) were ligated into the pGPU6/Neo vector for expressing
HOXA1 shRNA. A scrambled sequence was ligated into the pGPU6/Neo
vector and used as the negative control (NC) shRNA. DU-145 and PC-3
cells were transfected with NC, ShRNA#1, ShRNA#2, ShRNA#3 or
ShRNA#4, respectively. G418 selection was used to isolate the
stable cell clones (Gibco, Grand Island, NY, USA).
Cell count assay
Cells were seeded into a 24-well plate at a density
of 1×104 cells/well and then incubated in medium for the
indicated times. Then the medium was removed and cells were
trypsinized. After staining with trypan blue, the cell number was
counted in a hemocytometer. Each sample was measured in triplicate
and repeated 3 times.
MTT assay
Cells were seeded in a 96-well plate in triplicate
at a concentration of 1×103 cells/well. On the indicated
day, the medium was removed and MTT solution was added to the
wells. Four hours later, DMSO was added and the cells were further
incubated for 10 min with gentle shaking at 37°C. Finally, the
optical density of each well was measured using a microplate reader
(Bio-Rad) at 490 nm.
Invasion and migration assays
A 24-well Transwell plate with 8.0-µm pore
size polycarbonate membranes was purchased from Costar (Corning
Incorporated, Corning, NY, USA) and used in the invasion and
migration assays. In the in vitro invasion assay, the upper
chambers were coated with 50 µl Matrigel diluted with
serum-free medium. Cells were resuspended in RPMI-1640 medium
containing 0.5% FBS at a density of 5×105 cells/ml. Then
200 µl of cell suspension was loaded into the upper chambers
and the lower chambers were filled with 500 µl of RPMI-1640
medium containing 20% FBS. After 24 h of incubation, the cells that
invaded through the Matrigel-coated filters were fixed and stained
with crystal violet. The number of invaded cells was counted under
a microscope at ×50 magnification. In the in vitro migration
assay, the upper chambers were not coated with Matrigel. Cells
(1×105 cells in 200 µl) were seeded into the
upper chambers and 500 µl of RPMI-1640 medium containing 20%
FBS was added into the lower chambers. Twenty-four hours later, the
migrated cells were stained with crystal violet and counted by
light microscope at ×50 magnification. Three independent
experiments were performed for the invasion and migration
assays.
In vivo growth and metastasis assay
Male BABL/c nude mice (6-weeks old) were maintained
in pathogen-free conditions. All procedures were conducted
following the Animal Care and Use Committee guidelines of the
Second Military Medical University. The mice were randomly
separated into 3 groups (n=8). DU-145 cells at a density of
2.0×105 cells/100 µl were subcutaneously injected
in the back of mice. The length (L) and width (W) of the tumors in
the mice were measured with calipers on week 1, 3, 5 and 7. The
tumor volume (V) was calculated with the formula: V = (length ×
width2)/2. Eight weeks later, the mice were sacrificed
and tumor weights were measured. Next, the lungs and livers were
fixed in 4% paraformaldehyde and then were sectioned into slices.
Each slice was further stained with haematoxylin and eosin
(H&E) and observed under a light microscope to examine the
micrometastasis in the lungs and livers.
Statistical analysis
All experimental data are expressed as mean ± SD.
Statistical significance was determined by Student's t-test between
two groups or one-way analysis of variance (ANOVA) among multiple
groups. Fisher's exact test was performed to compare proportions
between groups. P<0.05 was considered to indicate a
statistically significant result.
Results
Expression of HOXA1 in prostate cancer
cells and knockdown of HOXA1 expression by shRNA
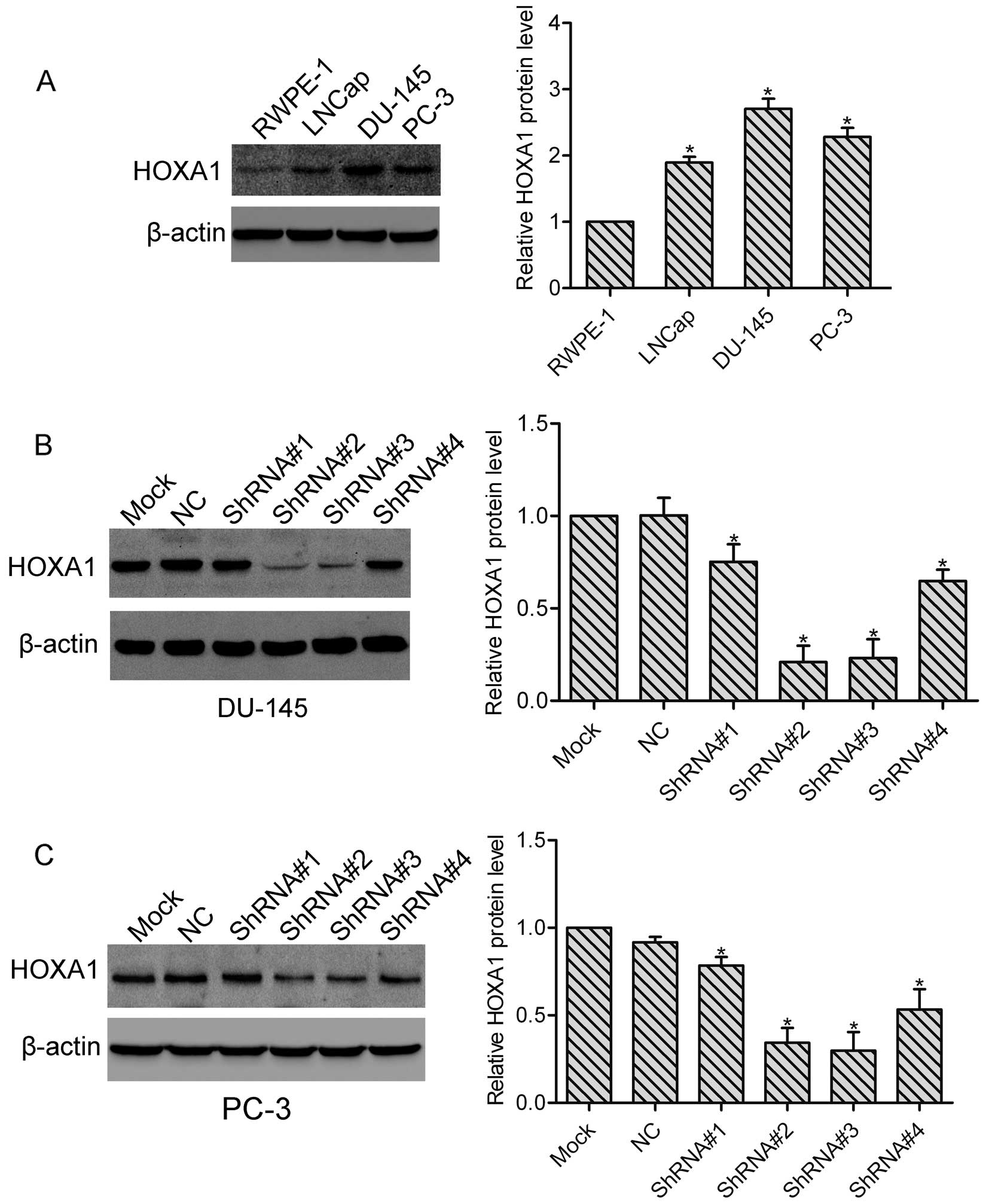
We first examined the expression of HOXA1 in the
prostate cancer cells. Using western blot analysis, we found that
compared with normal prostate RPWE-1 cells, the protein level of
HOXA1 was highly expressed in the LNCap, PC-3 and DU-145 cells
(Fig. 1A), indicating that HOXA1
may be involved in the progression of prostate cancer. In addition,
the highest expression of HOXA1 was observed in the DU-145 cells.
To investigate the role of HOXA1 in prostate cancer cells, we
transfected DU-145 and PC-3 cells with either a control shRNA
vector (NC) or 4 different HOXA1 shRNAs (ShRNA#1, ShRNA#2, ShRNA#3
and ShRNA#4), respectively. Stable cell clones were isolated, and
the knockdown efficiency was tested by western blotting. We found
that all HOXA1 shRNAs suppressed the expression of HOXA1 in the
DU-145 and PC-3 cells. Relative expression of HOXA1 relative to the
Mock cells was: for DU-145: NC, 1.0±0.09; ShRNA#1, 0.75±0.10;
ShRNA#2, 0.21±0.09; ShRNA#3, 0.23±0.10 and ShRNA#4, 0.65±0.06; for
PC-3: NC, 0.93±0.02; ShRNA#1, 0.78±0.03; ShRNA#2, 0.31±0.05;
ShRNA#3, 0.30±0.06 and ShRNA#4, 0.57±0.07 (Fig. 1B and C). As RNAi efficiency was much
higher in the ShRNA#2 and ShRNA#3 transfected cells, we then used
ShRNA#2 and ShRNA#3 transfected cells in the subsequent
experiments.
Role of HOXA1 in prostate cancer cell
growth in vitro
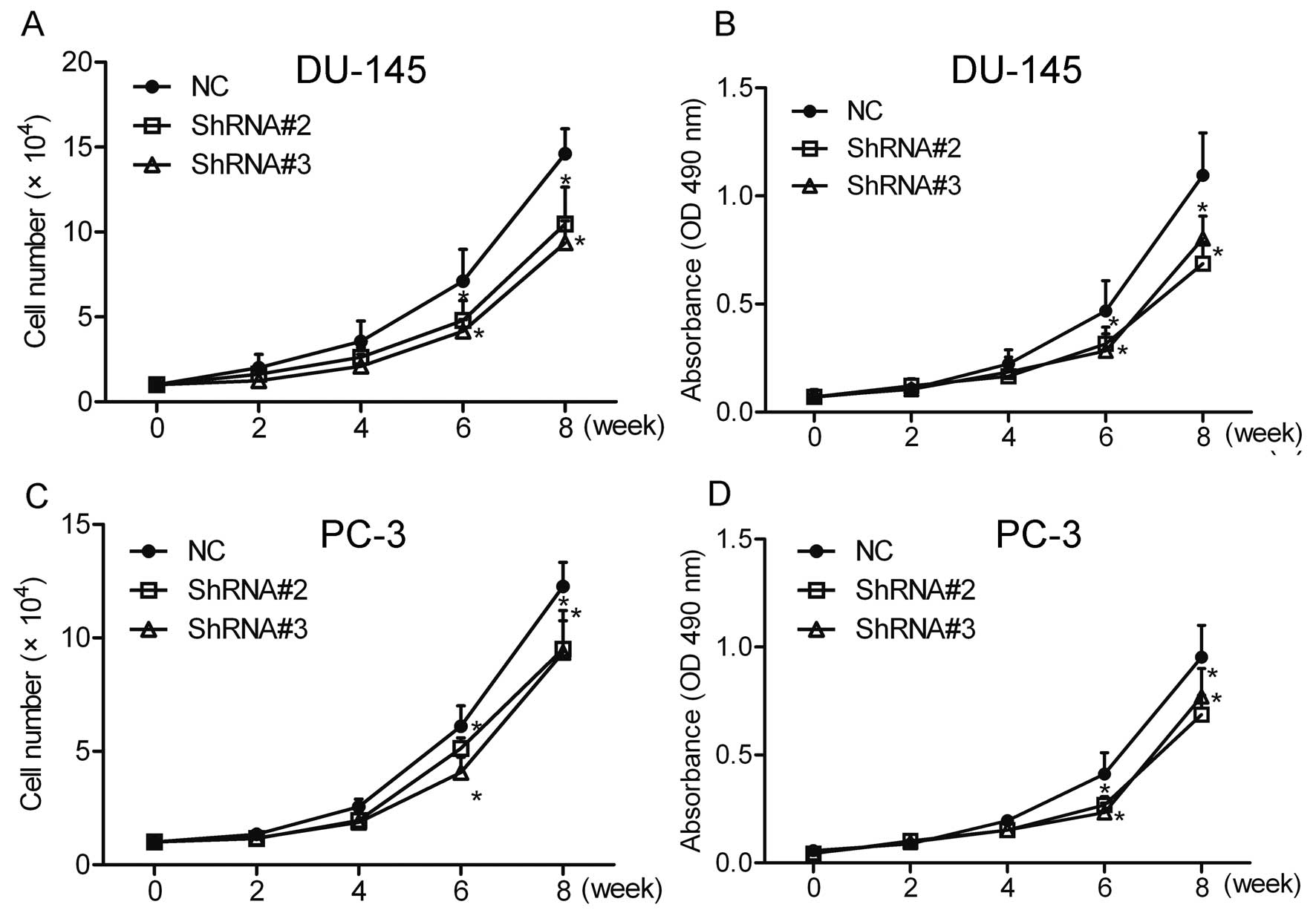
Cell count and MTT assays were performed to
investigate the effect of HOXA1 knockdown on prostate cancer cell
growth. The results showed that compared with the NC cells,
knockdown of HOXA1 by shRNA greatly inhibited the growth of the
DU-145 and PC-3 cells (Fig. 2).
These data suggest that HOXA1 is involved in the growth of prostate
cancer cells.
HOXA1 participates in the invasion and
migration of prostate cancer cells in vitro
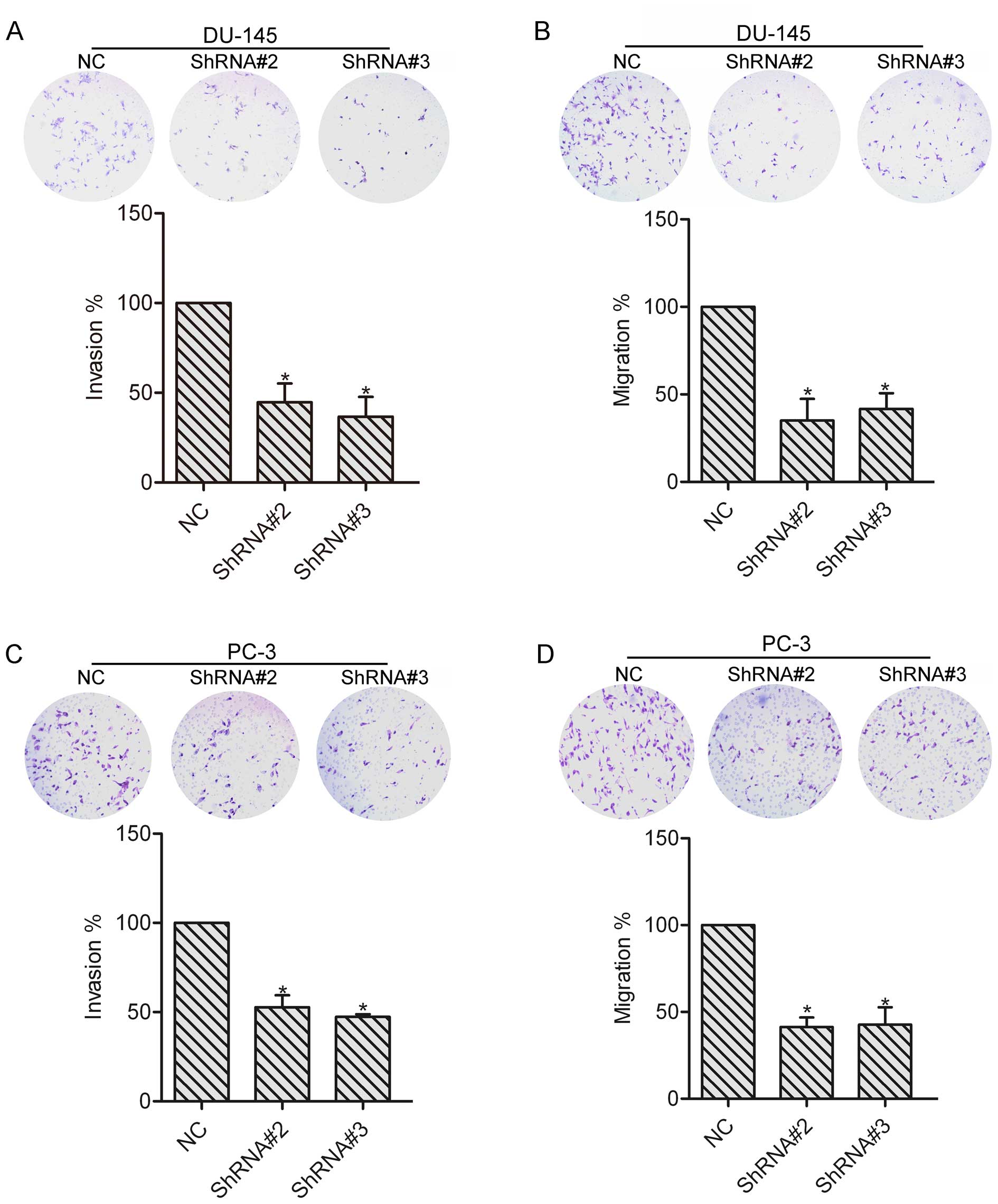
To explore the role of HOXA1 in prostate cancer cell
invasion and migration, invasion and migration assays were
performed in the NC, ShRNA#2 and ShRNA#3 transfected cells. DU-145
and PC-3 cells were allowed to invade or migrate for 24 h, and
images were captured to count the number of cells. The results
showed that knockdown of HOXA1 by shRNA markedly reduced the
invasion and migration abilities as compared to the NC cells
(Fig. 3), suggesting the
involvement of HOXA1 in prostate cancer cell invasion and
migration. The following experiments were performed with DU-145
cells.
HOXA1 regulates the expression of
E-cadherin, Snail and MMP-3
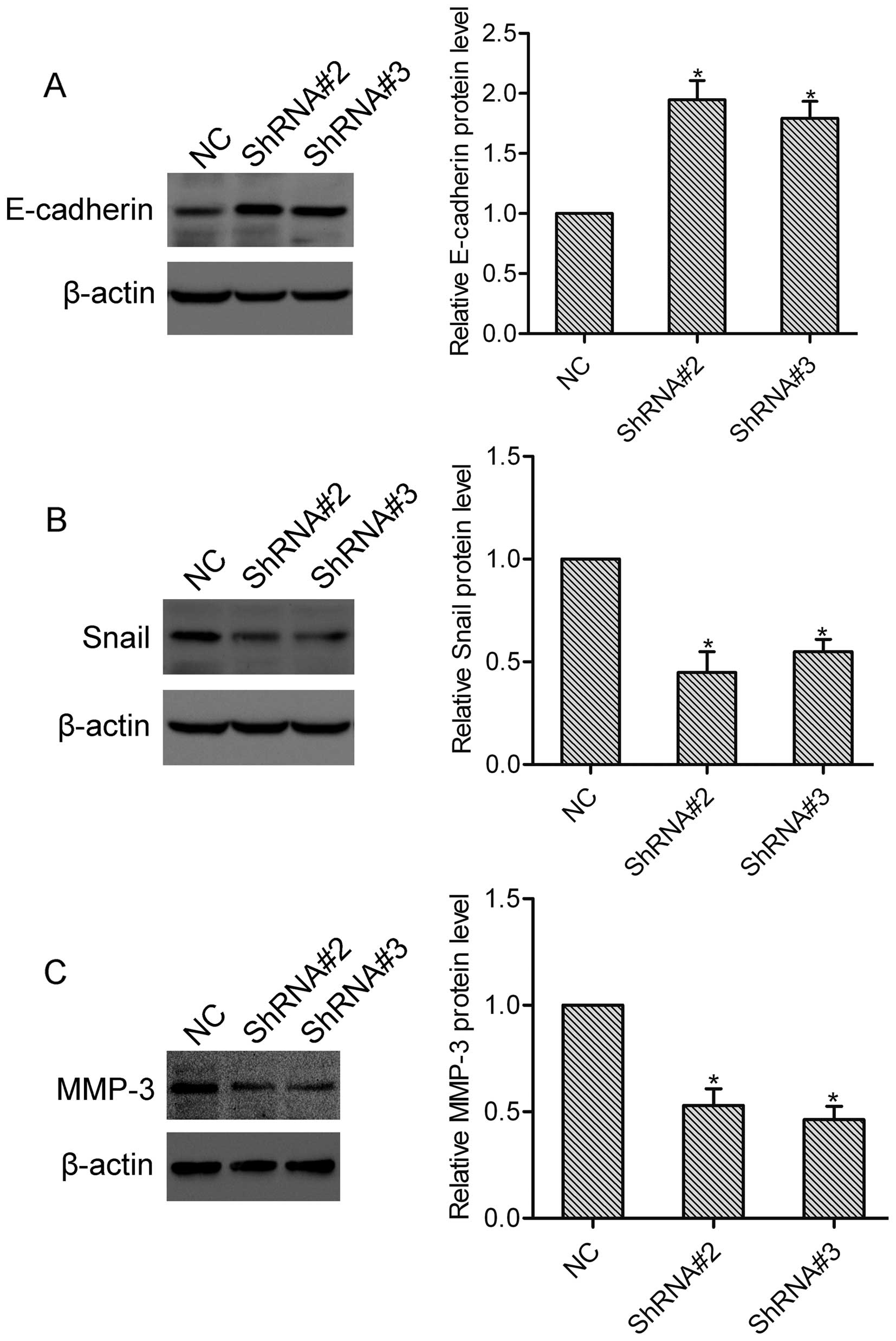
E-cadherin and Snail are well-established
epithelial-mesenchymal transition (EMT) markers in cancer, and
participate in the regulation of tumor invasion and metastasis
(9). To investigate the effect of
HOXA1 on E-cadherin and Snail expression, DU-145 cells transfected
with or without HOXA1 shRNA were collected and subjected to western
blotting. Here, western blot analysis showed that compared with the
NC cells, the expression of E-cadherin was upregulated, while the
expression of Snail was downregulated in both the ShRNA#2 and
ShRNA#3 transfected cells (Fig. 4A and
B). These data suggest that HOXA1 regulates the expression of
E-cadherin and Snail in prostate cancer cells. MMP-3 is a crucial
modulator in tumor invasion and metastasis (10). Using western blotting, we found that
knockdown of HOXA1 significantly suppressed the expression of MMP-3
in the DU-145 cells (Fig. 4C),
indicating that HOXA1 affects the expression of MMP-3 in prostate
cancer cells.
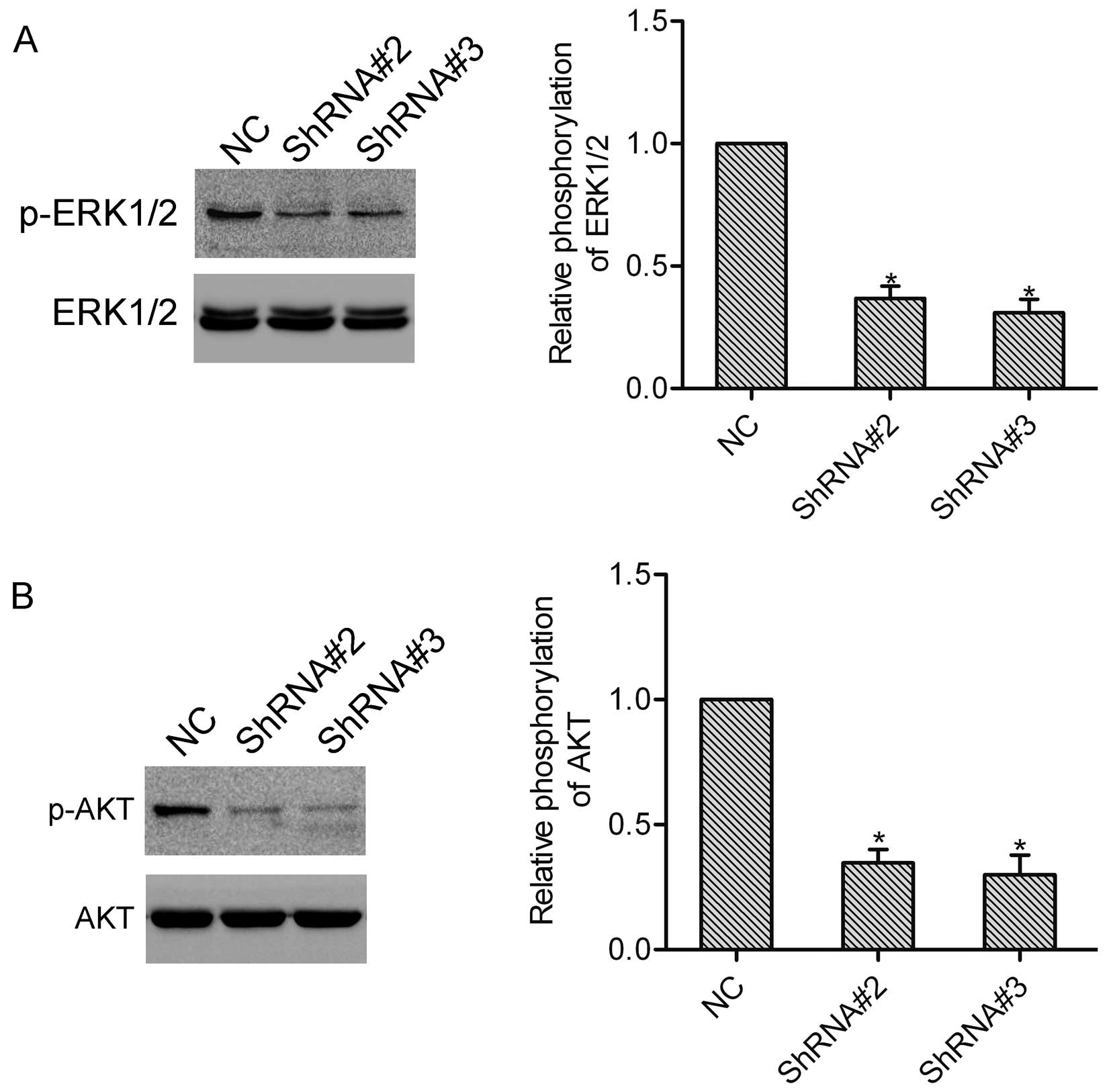
HOXA1 induces activation of ERK1/2 and
AKT
We further detected the function of HOXA1 in the
activation of ERK1/2 and AKT, which both play vital roles in the
progression of prostate cancer. Notably, western blot analysis
showed that knockdown of HOXA1 in the DU-145 cells markedly
inhibited activation of ERK1/2 and AKT (Fig. 5A and B).
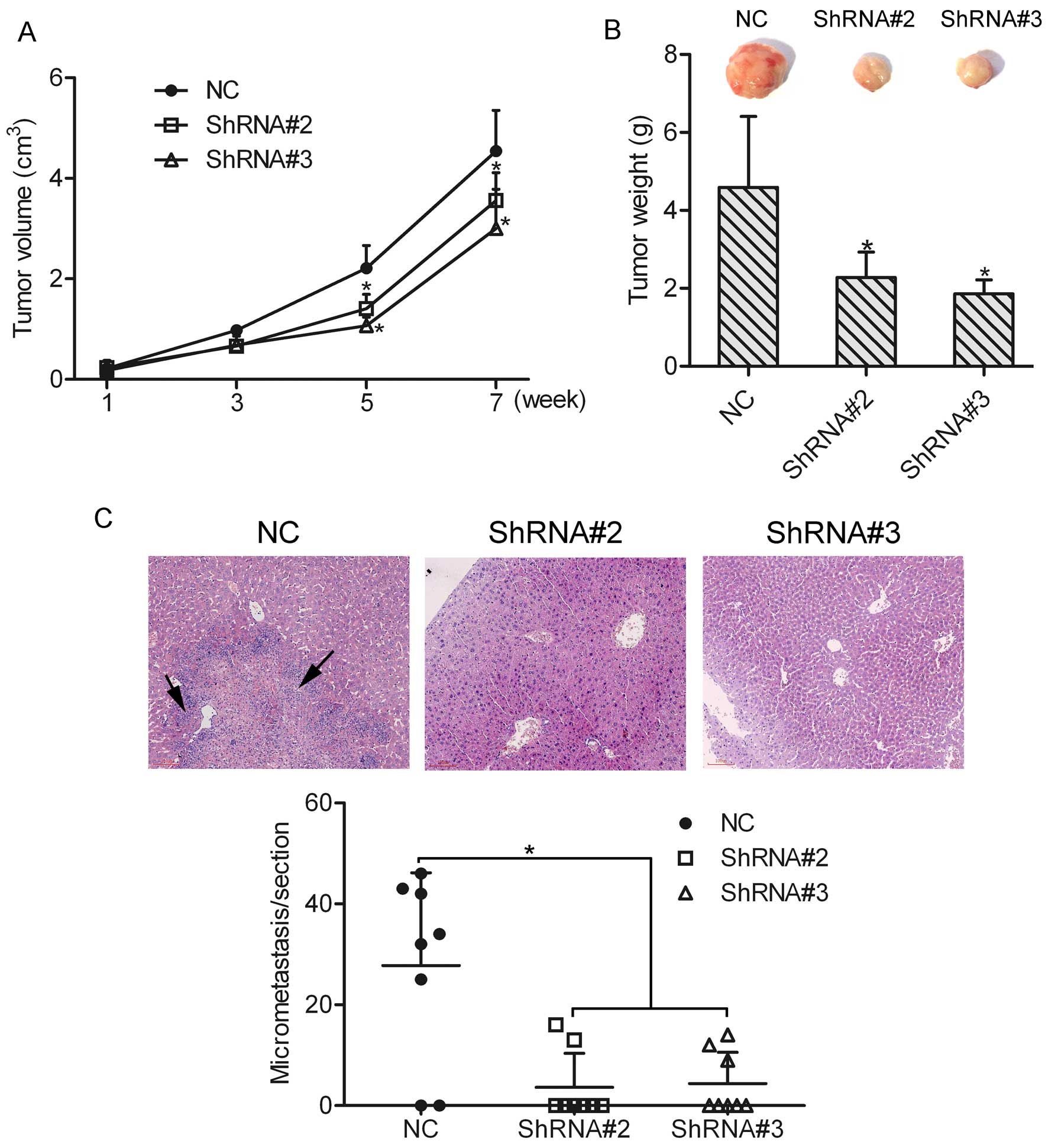
Knockdown of HOXA1 suppresses prostate
cancer cell growth and metastasis in vivo
To determine the effect of HOXA1 knockdown on cell
growth and metastasis in vivo, an in vivo tumor
xenograft model was used, and DU-145 cells transfected with or
without HOXA1 shRNA were injected subcutaneously into the back of
the mice, respectively. An in vivo growth curve showed that
the tumor size in the ShRNA#2 or ShRNA#3 group was much smaller
than that in the NC group (Fig.
6A). Eight weeks later, the mice were sacrificed and the mean
tumor weight was calculated. The mean tumor weights in the NC,
ShRNA#2 and ShRNA#3 groups were 4.59±1.82, 2.28±0.37 and 1.86±0.21
g, respectively (Fig. 6B). In
agreement with the tumor volume results, the mean tumor weights in
the ShRNA#2 and ShRNA#3 groups were decreased as compared to that
in the NC group (P<0.05). These data suggest that knockdown of
HOXA1 suppressed the growth of prostate cancer cells. Furthermore,
we found that liver metastasis was 75% (6/8) in mice injected with
the NC cells. However, liver metastasis in mice injected with the
ShRNA#2 and ShRNA#3 cells was 25% (2/8) and 37.5% (3/8),
respectively. In addition, micrometastasis in the liver section was
counted under a microscope, and the results showed that the number
of micrometastases was greatly decreased in the ShRNA#2 and ShRNA#3
groups as compared to the NC group (Fig. 6C). However, lung metastasis was not
observed in either the NC group or the ShRNA#2 and ShRNA#3 groups.
Together, these findings support the notion that HOXA1 participates
in the growth and metastasis of prostate cancer cells in
vivo.
Discussion
Overexpression of HOXA1 has been observed in breast
cancer and oral squamous cell cancer, and a high HOXA1 expression
level correlates with poor prognosis in patients with cancer
(8,11). In the present study, we found that
the protein level of HOXA1 was highly expressed in prostate cancer
cells, suggesting a possible role of HOXA1 in prostate cancer
progression.
It has been reported that HOXA1 can alter cell
proliferation and the cell cycle in non-small cell lung cancer
cells (12,13). However, a recent study demonstrated
that enforced expression of HOXA1 in lung H69AR cells increased
cell apoptosis and cell cycle arrest (14). The effect of HOXA1 on prostate
cancer cell growth is largely unknown. Here, we found that
knockdown of HOXA1 suppressed the growth of prostate cancer DU-145
and PC-3 cells in vitro. Moreover, Brock et al
demonstrated that knockdown of HOXA1 reduced tumor growth in a
mouse xenograft model of mammary tumor progression (15). Here, we found that knockdown of
HOXA1 suppressed the tumor growth of human prostate cancer DU-145
cells in vivo. These data suggest that HOXA1 contributes to
cell proliferation in prostate cancer cells. It has been reported
that HOXA1 promotes the invasion and migration of hepatocellular
carcinoma cells (7), and is
required for the metastasis and tumorigenesis of melanoma cancer
cells (16). However, the function
of HOXA1 in prostate cancer cell invasion and metastasis remains
unclear. Here, for the first time, our results showed that
knockdown of HOXA1 in prostate cancer cells suppressed the invasion
and migration in vitro, and inhibited the metastasis in
vivo, supporting the conclusion that HOXA1 is involved in the
regulation of invasion and metastasis in prostate cancer cells.
Epithelial-mesenchymal transition (EMT) is regarded
as a critical step in tumor metastasis, and E-acdherin and Snail
are both EMT markers in cancer. It is well known that E-cadherin,
which can be transcriptionally repressed by Snail, mediates
cell-cell adhesion and is essential for the invasion and metastasis
of prostate cancer (17). Studies
have found that knockdown of HOXD3, a member of the HOX gene
family, increased the expression of E-cadherin in lung cancer cells
(18,19). Zhang et al showed that HOXA1
was required for the E-cadherin-dependent anchorage-independent
survival of human mammary carcinoma cells (20). However, whether HOXA1 knockdown can
influence the expression level of E-cadherin and Snail in prostate
cancer cells is still unclear. Our study showed that knockdown of
HOXA1 upregulated the protein level of E-cadherin, and
downregulated the protein level of Snail in the DU-145 cells,
suggesting that HOXA1 may regulate EMT in prostate cancer cells.
Belonging to the matrix metalloproteinase (MMP) family, MMP-3 can
degrade various components of the extracellular matrix (ECM), and
plays a crucial role in tumor invasion and metastasis (21). Using shRNA technology, we found that
HOXA1 regulated the expression of MMP-3 in prostate cancer cells.
As we proved that downregulation of HOXA1 can suppress prostate
cancer cell invasion and metastasis, these data indicate that HOXA1
may promote the invasion and metastasis of prostate cancer cells
via the regulation of E-cadherin, Snail and MMP-3 expression.
It is well known that the ERK1/2 and AKT signaling
pathways both are pivotal players in the progression of cancer
(22,23). Many studies have confirmed that
activation of ERK1/2 as well as AKT regulates tumor cell processes,
including growth, migration, apotosis, invasion and metastasis
(24,25). Hou et al showed that
increased expression of HOXA1 enhanced the phosphorylation of
ERK1/2 in breast cancer MCF-7 cells (26). In the present study, we found that
knockdown of HOXA1 inhibited activation of ERK1/2 and AKT in DU-145
cells. These data indicate that HOXA1 may affect the progression of
prostate cancer cells via the ERK1/2 and AKT pathways.
In summary, our findings showed that HOXA1 was
highly expressed in prostate cancer cells. Knockdown of HOXA1 by
shRNA inhibited the growth, invasion and migration of prostate
cancer cells. Furthermore, knockdown of HOXA1 affected the
expression level of E-cadherin, Snail and MMP-3, and induced the
activation of ERK1/2 and AKT in vitro. In addition,
knockdown of HOXA1 suppressed the growth and metastasis of prostate
cancer cells in vivo. Together, the present study indicates
that downregulation of HOXA1 can inhibit the progression of
prostate cancer cells. Thus, HOXA1 may be a promising target for
the treatment of prostate cancer.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 81202036 and 81272818) and the
Postdoctoral Funds of China (2013M532143 and 2013M542435).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung LW, Baseman A, Assikis V and Zhau
HE: Molecular insights into prostate cancer progression: The
missing link of tumor microenvironment. J Urol. 173:10–20. 2005.
View Article : Google Scholar
|
|
3
|
Zhang ML, Nie FQ, Sun M, Xia R, Xie M, Lu
KH and Li W: HOXA5 indicates poor prognosis and suppresses cell
proliferation by regulating p21 expression in non small cell lung
cancer. Tumour Biol. Dec 31–2014.Epub ahead of print.
|
|
4
|
Ma RL, Shen LY and Chen KN: Coexpression
of ANXA2, SOD2 and HOXA13 predicts poor prognosis of esophageal
squamous cell carcinoma. Oncol Rep. 31:2157–2164. 2014.PubMed/NCBI
|
|
5
|
Yamatoji M, Kasamatsu A, Yamano Y, Sakuma
K, Ogoshi K, Iyoda M, Shinozuka K, Ogawara K, Takiguchi Y, Shiiba
M, et al: State of homeobox A10 expression as a putative prognostic
marker for oral squamous cell carcinoma. Oncol Rep. 23:61–67.
2010.
|
|
6
|
Liu XH, Lu KH, Wang KM, Sun M, Zhang EB,
Yang JS, Yin DD, Liu ZL, Zhou J, Liu ZJ, et al: MicroRNA-196a
promotes non-small cell lung cancer cell proliferation and invasion
through targeting HOXA5. BMC Cancer. 12:3482012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zha TZ, Hu BS, Yu HF, Tan YF, Zhang Y and
Zhang K: Overexpression of HOXA1 correlates with poor prognosis in
patients with hepatocellular carcinoma. Tumour Biol. 33:2125–2134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bitu CC, Destro MF, Carrera M, da Silva
SD, Graner E, Kowalski LP, Soares FA and Coletta RD: HOXA1 is
overexpressed in oral squamous cell carcinomas and its expression
is correlated with poor prognosis. BMC Cancer. 12:1462012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al Saleh S, Sharaf LH and Luqmani YA:
Signalling pathways involved in endocrine resistance in breast
cancer and associations with epithelial to mesenchymal transition
(Review). Int J Oncol. 38:1197–1217. 2011.PubMed/NCBI
|
|
10
|
McDonnell S and Matrisian LM: Stromelysin
in tumor progression and metastasis. Cancer Metastasis Rev.
9:305–319. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chariot A and Castronovo V: Detection of
HOXA1 expression in human breast cancer. Biochem Biophys Res
Commun. 222:292–297. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho HS, Toyokawa G, Daigo Y, Hayami S,
Masuda K, Ikawa N, Yamane Y, Maejima K, Tsunoda T, Field HI, et al:
The JmjC domain-containing histone demethylase KDM3A is a positive
regulator of the G1/S transition in cancer cells via
transcriptional regulation of the HOXA1 gene. Int J Cancer.
131:E179–E189. 2012. View Article : Google Scholar
|
|
13
|
Zhan M, Qu Q, Wang G, Liu YZ, Tan SL, Lou
XY, Yu J and Zhou HH: Let-7c inhibits NSCLC cell proliferation by
targeting HOXA1. Asian Pac J Cancer Prev. 14:387–392. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao F, Bai Y, Chen Z, Li Y, Luo L, Huang
J, Yang J, Liao H and Guo L: Downregulation of HOXA1 gene affects
small cell lung cancer cell survival and chemoresistance under the
regulation of miR-100. Eur J Cancer. 50:1541–1554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brock A, Krause S, Li H, Kowalski M,
Goldberg MS, Collins JJ and Ingber DE: Silencing HoxA1 by
intraductal injection of siRNA lipidoid nanoparticles prevents
mammary tumor progression in mice. Sci Transl Med. 6:217ra22014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wardwell-Ozgo J, Dogruluk T, Gifford A,
Zhang Y, Heffernan TP, van Doorn R, Creighton CJ, Chin L and Scott
KL: HOXA1 drives melanoma tumor growth and metastasis and elicits
an invasion gene expression signature that prognosticates clinical
outcome. Oncogene. 33:1017–1026. 2014. View Article : Google Scholar :
|
|
17
|
Lee MY and Shen MR: Epithelial-mesenchymal
transition in cervical carcinoma. Am J Transl Res. 4:1–13.
2012.PubMed/NCBI
|
|
18
|
Ohta H, Hamada J, Tada M, Aoyama T,
Furuuchi K, Takahashi Y, Totsuka Y and Moriuchi T:
HOXD3-overexpression increases integrin alpha v beta 3 expression
and deprives E-cadherin while it enhances cell motility in A549
cells. Clin Exp Metastasis. 23:381–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamada Ji, Omatsu T, Okada F, Furuuchi K,
Okubo Y, Takahashi Y, Tada M, Miyazaki YJ, Taniguchi Y, Shirato H,
et al: Overexpression of homeobox gene HOXD3 induces coordinate
expression of metastasis-related genes in human lung cancer cells.
Int J Cancer. 93:516–25. 2001. View
Article : Google Scholar
|
|
20
|
Zhang X, Emerald BS, Mukhina S, Mohankumar
KM, Kraemer A, Yap AS, Gluckman PD, Lee KO and Lobie PE: HOXA1 is
required for E-cadherin-dependent anchorage-independent survival of
human mammary carcinoma cells. J Biol Chem. 281:6471–6481. 2006.
View Article : Google Scholar
|
|
21
|
Johansson N, Ahonen M and Kähäri VM:
Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci.
57:5–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edlind MP and Hsieh AC: PI3K-AKT-mTOR
signaling in prostate cancer progression and androgen deprivation
therapy resistance. Asian J Androl. 16:378–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Torii S, Yamamoto T, Tsuchiya Y and
Nishida E: ERK MAP kinase in G cell cycle progression and cancer.
Cancer Sci. 97:697–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar A, Rajendran V, Sethumadhavan R and
Purohit R: AKT kinase pathway: A leading target in cancer research.
Sci World J. 2013:7561342013. View Article : Google Scholar
|
|
26
|
Hou L, Xu B, Mohankumar KM, Goffin V,
Perry JK, Lobie PE and Liu DX: The prolactin receptor mediates
HOXA1-stimulated oncogenicity in mammary carcinoma cells. Int J
Oncol. 41:2285–2295. 2012.PubMed/NCBI
|