Introduction
Glioma is the most common malignant tumor and
accounts for ~10% of tumors in the central nervous system (CNS)
(1,2). Glioma accounts for 80% of malignant
brain tumors (3). It is alarming
that the prognosis of glioma, particularly high-grade (III–IV)
glioma, is generally poor with standard chemotherapy and radiation
therapy, and patients can only survive for an average of 1 year
after diagnosis as a result of chemotherapy resistance (4). It is regrettable that the etiology of
this disease still remains largely elusive. Elucidation of the
molecular mechanisms of glioma is expected to lead to the
identification of new therapeutic targets and to contribute to
better clinical treatment and management of this devastating
disease.
Mitogen-activated protein kinase (MAPK), which is a
serine based protein kinase, and a highly conserved group of
protein kinases that responds to miscellaneous changes of the
cellular, intracellular or/and extracellular environment (DNA
damage, hyperosmosis, oxidative stress, potentially harmful
stimuli) (5–9), playing significant roles in signal
transduction and taking part in a diverse range of physiological
and pathological processes by modulating gene transcription in the
nucleus, including cell proliferation, mitosis, differentiation,
apoptosis, cell survival and gene expression (6). In mammals, MAPK consists of at least
11 members. The most studied MAPKs are the p38 MAPK, extracellular
signal-regulated protein kinases 1/2 (ERK1/2) and c-Jun N-terminal
kinases (JNK) (6). It has been
reported that p38 MAPK and ERK1/2 pathways are important for glioma
development (10–12). Previous studies have demonstrated
that MAPKs (p38 MAPK and ERK1/2) also activate other signaling
cascades, such as cyclooxygenase-2 (COX-2) (13,14),
in a variety of cancer cell types. COX-2 expression is closely
associated with tumor cell growth and is a crucial molecule in the
development of malignant tumors, and angiogenesis (15,16),
anti-apoptosis (17), invasiveness
(18) and proliferation (19). In addition, a previous study
demonstrated that COX-2 was activated dependent on the ERK1/2
pathway in glioma (20). However,
evidence that the p38 MAPK/ERK1/2-mediated activation of the COX-2
pathway may be involved in growth of C6 glioma is lacking.
Hydrogen sulfide (H2S) has been qualified
as the third gasotransmitter following nitric oxide (NO) and carbon
monoxide (CO) (21–23). It can be endogenously produced
mainly by cystathionine β-synthase (CBS) in the CNS. In recent
years, more and more attention is paid to H2S for its
extensive physiological and pathophysiological properties on cancer
progresses. Some findings from in vivo and in vitro
studies showed that H2S is beneficial for cancer cell
growth, proliferation, migration and invasion (24–30),
owing to its vascular relaxant and angiogenesis effects.
H2S promotes the supply of nutrients and blood to the
tumor cells and tissues (24). Our
latest research also demonstrated that exogenous H2S
promoted cancer cell
proliferation/anti-apoptosis/angiogenesis/migration effects via
amplifying the activation of NF-κB pathway (31). Furthermore, to the best of our
knowledge, no study exists focused on the effect of exogenous
H2S on C6 glioma cells and its potential mechanisms.
Based on recent studies (24–31),
we investigated whether exogenous H2S contributes to
cancer progress and explored these potential effects via
amplification of p38 MAPK/ERK1/2-COX-2 pathways in C6 glioma
cells.
Materials and methods
Materials
NaHS, a donor of H2S, was obtained from
Sigma Chemicals Co. (St. Louis, MO, USA), stored at 2–4°C and
protected from sunlight. Hoechst 33258, AOAA (an inhibitor of CBS),
SB203580 (an inhibitor of p38 MAPK), PD-98059 (an inhibitor of
ERK1/2) and NS-398 (an inhibitor of COX-2) were also purchased from
Sigma Chemicals Co. The Cell Counting Kit-8 (CCK-8) was supplied by
Dojindo Laboratories (Kumamoto, Japan). Fetal bovine serum (FBS)
and 1640 medium were obtained from Gibco-BRL (Grand Island, NY,
USA). Anti-p38 MAPK, anti-ERK1/2, anti-p-p38 MAPK, anti-p-ERK1/2,
anti-caspase-3 and anti-COX-2 antibodies were supplied by Cell
Signaling Technology (Boston, MA, USA). Horseradish peroxidase
(HRP)-conjugated secondary antibody and BCA protein assay kit were
obtained from KangChen Biotech, Inc. (Shanghai, China). Enhanced
chemiluminescence (ECL) solution was purchased from KeyGen
Biotech.
Cell culture and treatments
The C6 glioma cells were supplied by Sun Yat-sen
University Experimental Animal Center (Guangzhou, Guangdong,
China). The cells were grown in 1640 medium supplemented with 10%
FBS under an atmosphere of 5% CO2 and at 37°C with 95%
air. The cells were treatment with 400 µmol/l NaHS for 24 h
or co-treatment with 400 µmol/l NaHS and 30 µmol/l SB
203580 or 20 µmol/l PD-98059 or 10 µmol/l NS-398 for
24 h.
Hoechst 33258 nuclear staining for
evaluation of apoptosis
Apoptotic cell death was tested by the Hoechst 33258
staining followed by photofluorography. Firstly, C6 glioma cells
were plated in 35 mm dishes at a density of 1×106
cells/well. Following the above indicated treatments, the C6 glioma
cells were fixed with 4% paraformaldehyde in 0.1 mol/l
phosphate-buffered saline (PBS; pH 7.4) for 10 min at 4°C. Then,
the slides were washed three times with PBS. After staining
followed by 5 mg/ml Hoechst 33258 for 15 min, the PLC cells were
washed three times with PBS. Lastly, the cells were visualized
under a fluorescence microscope (B×50-FLA; Olympus, Tokyo, Japan).
Viable C6 glioma cells displayed a uniform blue fluorescence
throughout the nucleus and normal nuclear size. However apoptotic
C6 glioma cells showed condensed, distorted or fractured nuclei.
The experiment was carried out three times.
Western blot analysis
The cells were harvested and lysed with cell lysis
solution at 4°C for 30 min. The total proteins were quantified
through using the BCA protein assay kit. Loading buffer was added
to cytosolic extracts, and then boiling for 6 min, the same amounts
of supernatant from each sample were fractionated by 10% sodium
dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and
the total proteins were transferred into polyvinylidene difluoride
(PVDF) membranes. The membranes were blocked with 5% fat-free milk
for 60 min in fresh blocking buffer [0.1% Tween-20 in Tris-buffered
saline (TBS-T)] at room temperature, and incubated with either
anti-p38 MAPK (1:1,000 dilution), anti-ERK1/2 (1:1,000 dilution),
anti-p-p38 MAPK (1:1,000 dilution), anti-p-ERK1/2 (1:1,000
dilution), anti-caspase-3 (1:1,000 dilution) and anti-COX-2
antibodies (1:1,000 dilution) in freshly prepared TBS-T with 3%
free-fat milk throughout the night with gentle agitation at 4°C.
Membranes were washed for 5 min with TBS-T three times and
incubated with HRP-conjugated goat anti-rabbit secondary antibody
at a concentration of 1:3,000 dilution; (KangChen Biotech, Inc.),
in TBS-T with 3% fat-free milk for 1.5 h at room temperature. Then
membranes were washed three times with TBS-T for 5 min. The
immunoreactive signals were visualized using the ECL detection. In
order to quantify the protein expression, X-ray film was scanned
and analyzed with ImageJ 1.47i software. The experiment was carried
out three times.
Measurement of cell viability
The cells were seeded into 96-well plates at
concentration of 1×104/ml, and were incubated at 37°C,
the CCK-8 assay was employed to assess the cell viability of PLC
cells. After the indicated treatments, 10 µl CCK-8 solution
at a 1/10 dilution was added to each well and the plate was
incubated for 1.5 h in the incubator. Absorbance at 450 nm was
evaluated using a microplate reader (Molecular Devices, Sunnyvale,
CA, USA). The means of the optical density (OD) of three wells in
the indicated groups were used to calculate the percentage of cell
viability according to the formula: Cell viability (%) = (OD
treatment group/OD control group) × 100%. The experiment was
carried out three times.
Cell proliferation
The cells were seeded into 24-well plates at
concentration of 1×104/ml and were incubated at 37°C,
After the indicated treatments, the cell number were directly
detected under a fully automatic inverted microscope.
Statistical analysis
All data are presented as the mean ± SEM.
Differences between groups were analyzed by one-way analysis of
variance (ANOVA) using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA)
software, and followed by LSD post hoc comparison test. Statistical
significance was considered as P<0.05.
Results
NaHS promotes cell proliferation in C6
glioma cells
In order to test the effect of exogenous
H2S in C6 glioma cell viability, firstly, a
dose-response study with varying doses (100, 200, 400, 800 and
1,600 µmol/l) of NaHS (a donor of H2S) for 24 h
was performed to calculate the effective doses of NaHS. As shown in
Fig. 1A, the doses of NaHS from 100
to 1,600 µmol/l markedly promoted cells proliferation,
leading to an increase in cell viability and reaching a peaking at
400 µmol/l. Therefore, 400 µmol/l NaHS was used in
the subsequent time-response study with different treatment times
(12, 24, 36, 48 and 60 h). As shown in Fig. 1B, treatment of C6 glioma cells with
400 µmol/l NaHS for the indicated times all markedly
promoted cells viability, reaching the maximal effect at 24 h.
Based on the above results, C6 glioma cells were treated with 400
µmol/l NaHS for 24 h in all subsequent experiments.
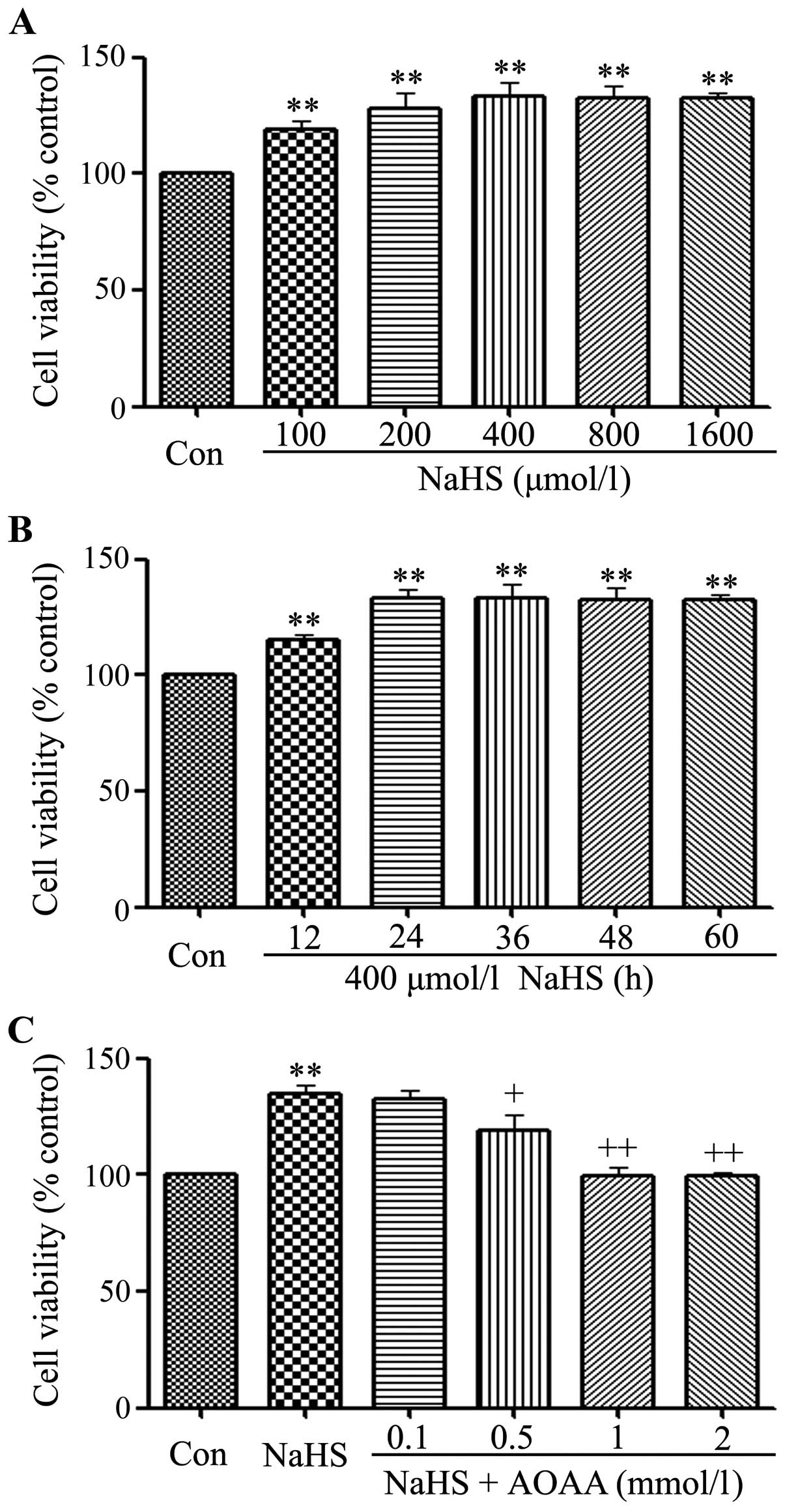 | Figure 1NaHS promotes cell proliferation in
C6 glioma cells. Cell viability was tested using the Cell Counting
Kit-8 (CCK-8). (A) C6 glioma cells were treated with different
doses of NaHS (100, 200, 400, 800 and 1,600 µmol/l) for 24
h. (B) Cells were treated with 400 µmol/l NaHS for the
indicated times (12, 24, 36, 48, 60 and 72 h). (C) Cells were
co-treated with 400 µmol/l NaHS and different doses of AOAA
(0.1, 0.5, 1 and 2 mmol/l) for 24 h. Data are the mean ± SEM (n=3).
**p<0.01 compared with the control group,
+p<0.05, ++p<0.01 compared with the
NaHS group. Con, the control group; NaHS, a donor of
H2S. |
Notably, the above increased cell viability was
repressed by co-treatment with 400 µmol/l NaHS and different
doses of AOAA (a specific inhibitor of CBS) for 24 h. As shown in
Fig. 1C, at the doses of AOAA from
0.1 to 2 mmol/l significantly suppressed cell proliferation,
leading to a decrease in cell viability and reaching the minimum at
1 mmol/l. According to the above results, C6 glioma cells were
co-treated with 400 µmol/l NaHS and 2 mmol/l AOAA for 24 h
in all the following experiment.
AOAA alleviates NaHS-induced cell
proliferation in C6 glioma cells
As shown in Fig. 1,
NaHS notably promotes cells proliferation in C6 glioma cells. In
order to validate this phenomenon, the number of the cells was
detected under the microscope. The cells were treated with
different doses of NaHS (100, 200, 400, 800 and 1,600
µmol/l). As shown in Fig. 2,
the doses of NaHS from 100 to 1,600 µmol/l markedly promoted
cell proliferation, leading to an increase in cell number and
reaching a peaking at 400 µmol/l. Thus, we showed that AOAA
alleviates NaHS-induced increased cell proliferation in C6 glioma
cells. As shown in Fig. 2G, H and
I, at the doses of AOAA from 0.1 to 1 mmol/l significantly
alleviated NaHS-induced increase of cell proliferation in C6 glioma
cells, leading to a decrease in cell number and reaching the
minimum at 1 mmol/l.
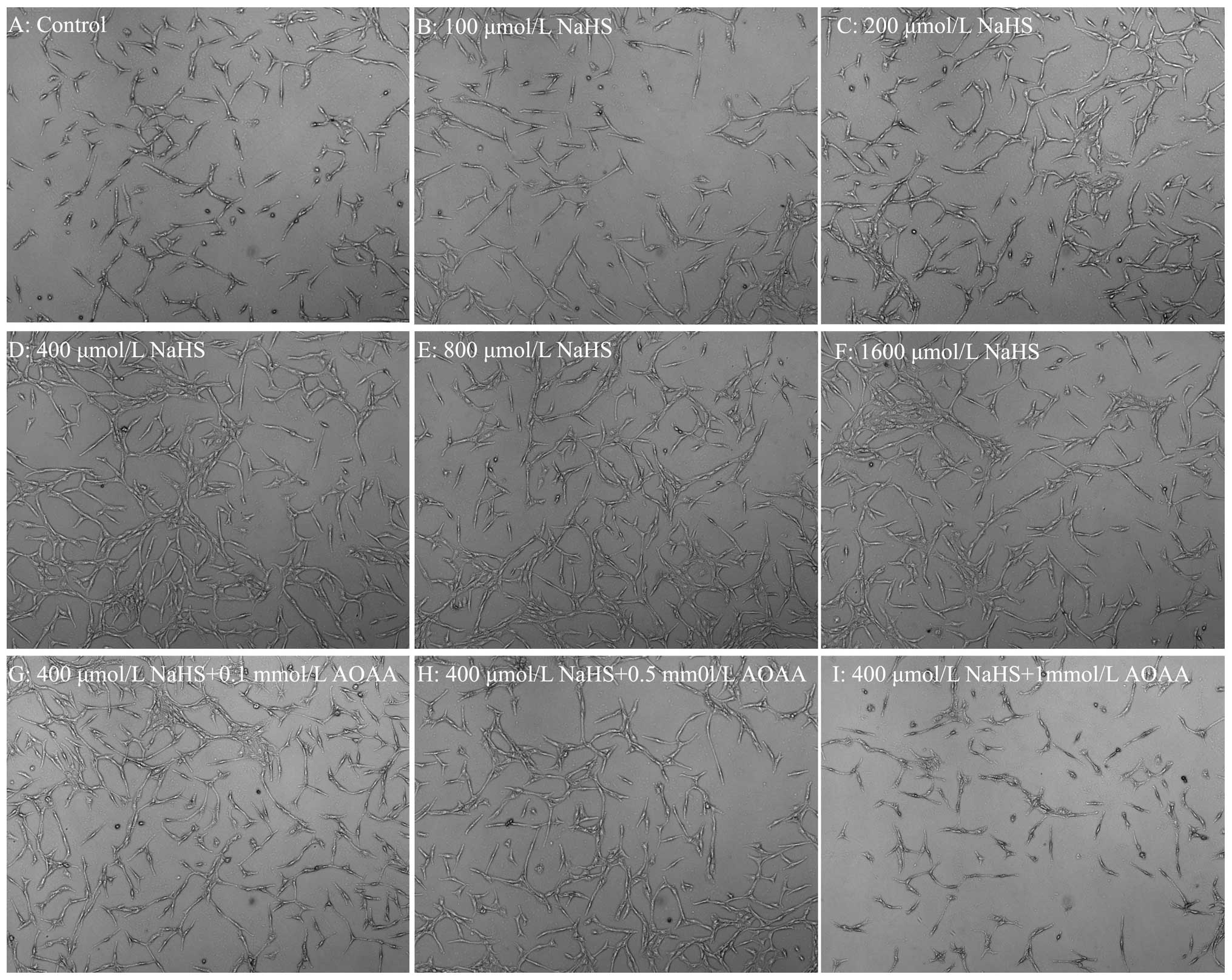 | Figure 2AOAA alleviates NaHS-induced cell
proliferation in C6 glioma cells. Cell number was detected using a
fully automatic inverted microscope. (A–F) C6 glioma cells were
treated with different doses of NaHS (0, 100, 200, 400, 800 and
1,600 µmol/l) for 24 h, respectively. (G–I) The cells were
co-treated with 400 µmol/l NaHS and different doses of AOAA
(0.1, 0.5 and 1 mmol/l) for 24 h, respectively. Con, the control
group; NaHS, a donor of H2S. |
AOAA ameliorates the phosphorylation of
p38 MAPK and ERK1/2 is induced by NaHS in C6 glioma cells
We observed the effects of NaHS on MAPK (including
p38 MAPK and ERK1/2) phosphorylation. First, we further performed
the time-study on the expression of p-p38 MAPK, after C6 glioma
cells were exposed to 400 µmol/l NaHS for the indicated
times (3, 6, 9, 12 and 24 h), the expression levels of p-p38 MAPK
were significantly upregulated, reaching a peak at 3 h, and the
t-p38 MAPK expression was unchanged.
Similarly, exposure of cells to 400 µmol/l
NaHS also increased the expression levels of p-ERK1/2, as shown in
the time-response study (Fig. 3E and
F).
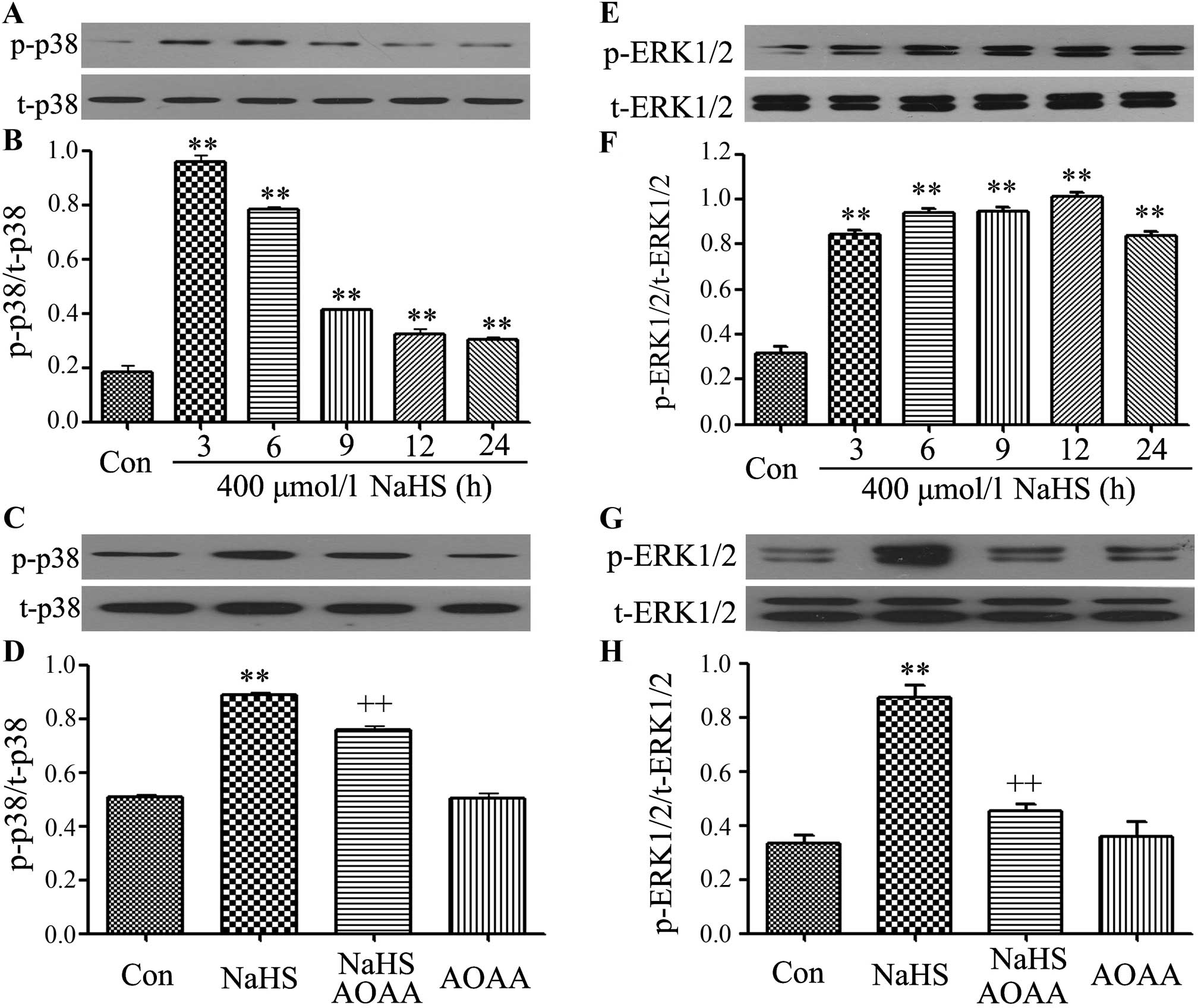 | Figure 3AOAA ameliorates the phosphorylation
of p38 MAPK and ERK1/2 induced by NaHS in C6 glioma cells. (A, B, E
and F) C6 glioma cells were treated with 400 µmol/l NaHS for
the indicated times (3, 6, 9, 12 and 24 h). (C, D, G and F) C6
glioma cells were co-treated with 400 µmol/l NaHS and 2
mmol/l AOAA for 24 h. The expression levels of p38 MAPK, and ERK1/2
were detected by western blotting. (A, C, E and G) The
representative image shows the changes in the expression levels of
p38 MAPK (A and C) and ERK1/2 (E and G) in the indicated groups.
(B, D, F and H) Densitometric analysis for the results in A, C, E
and G, respectively. Data are presented as mean ± SEM (n=3).
**p<0.01 compared with the control group;
++p<0.01 compared with the NaHS-treated group. |
To observe effects of AOAA on the activation of MAPK
induced by NaHS, C6 glioma cells were co-treated with 400
µmol/l NaHS and 2 mmol/l AOAA for 24 h. As shown in Fig. 3C, D, G and F, the increased
phosphorylation of p38 MAPK and ERK1/2 was reduced.
SB203580 and PD98059 alleviates the
NaHS-induced increase of cell viability in C6 glioma cells
As shown in Fig. 4,
exposure of C6 glioma cells to 400 µmol/l NaHS for 24 h
obviously induced cell proliferation, leading to an increase in
cell viability. However, the increased cell viability was repressed
by co-treatment with 400 µmol/l NaHS and different doses of
SB203580 (a specific inhibitor of p38 MAPK pathway) or PD-98059 (a
specific inhibitor of ERK1/2 pathway) for 24 h.
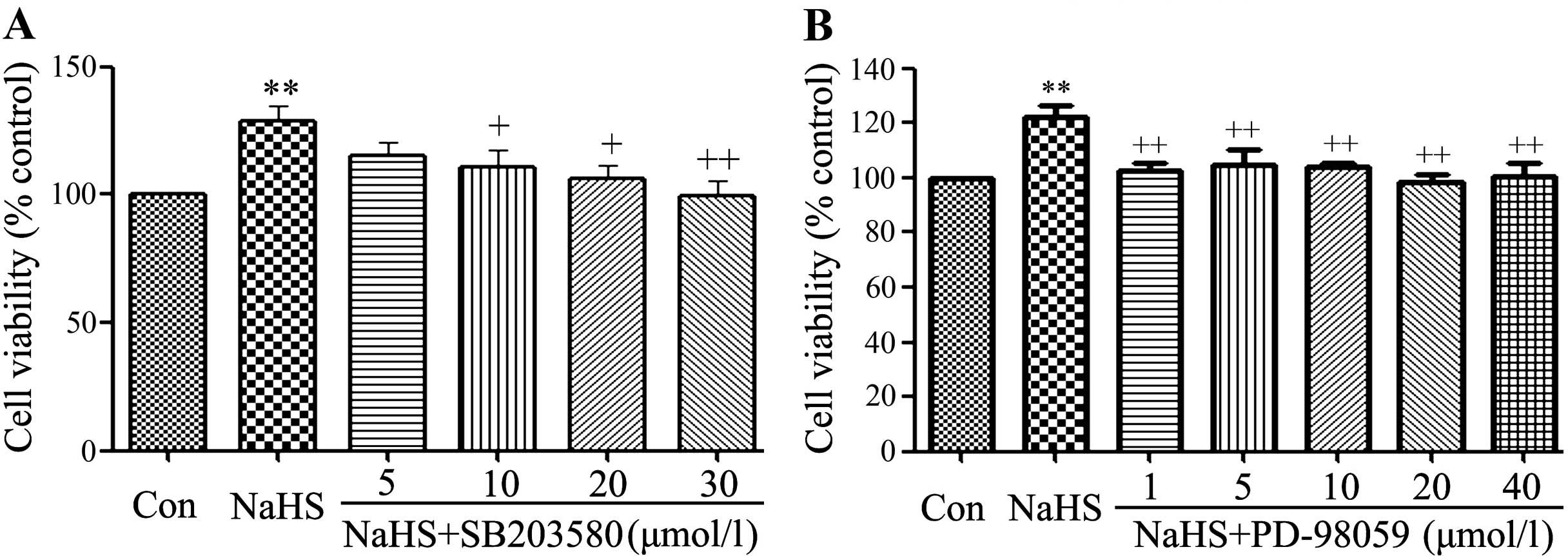 | Figure 4SB203580 and PD98059 alleviate
NaHS-induced cell proliferation in C6 glioma cells. C6 glioma cells
were co-conditioned with 400 µmol/l NaHS and different doses
of SB203580 (5, 10, 20 and 30 µmol/l) or PD-98059 (1, 5, 10,
20 and 40 µmol/l) for 24 h. Data are the mean ± SEM (n=3).
**p<0.01 compared with the control group.
+p<0.05, ++p<0.01 compared with the
NaHS group. Con, the control group; NaHS, a donor of
H2S; SB203580, a specific inhibitor of p38 MAPK pathway;
PD-98059, a specific inhibitor of ERK1/2 pathway. |
As shown in Fig. 4A,
at 5 µmol/l SB203580 did not alter the cell viability. On
the contrary, the dose of PDTC (10, 20 and 30 µmol/l)
significantly suppressed the cell proliferation, leading to a
decrease in cell viability and reaching the minimum at 30
µmol/l. According to the above results, C6 glioma cells were
co-treated with 400 µmol/l NaHS and 30 µmol/l
SB203580 for 24 h in all the following experiments.
Similarly, exposure of cells to 400 µmol/l
NaHS and different doses of PD-98059 (1, 5, 10, 20 and 40
µmol/l) also suppressed cell proliferation, leading to a
decrease in cell viability and reaching the minimum at 20
µmol/l, as shown in Fig. 4B.
According to the above results, C6 glioma cells were co-treated
with 400 µmol/l NaHS and 20 µmol/l PD-98059 for 24 h
in all the following experiments.
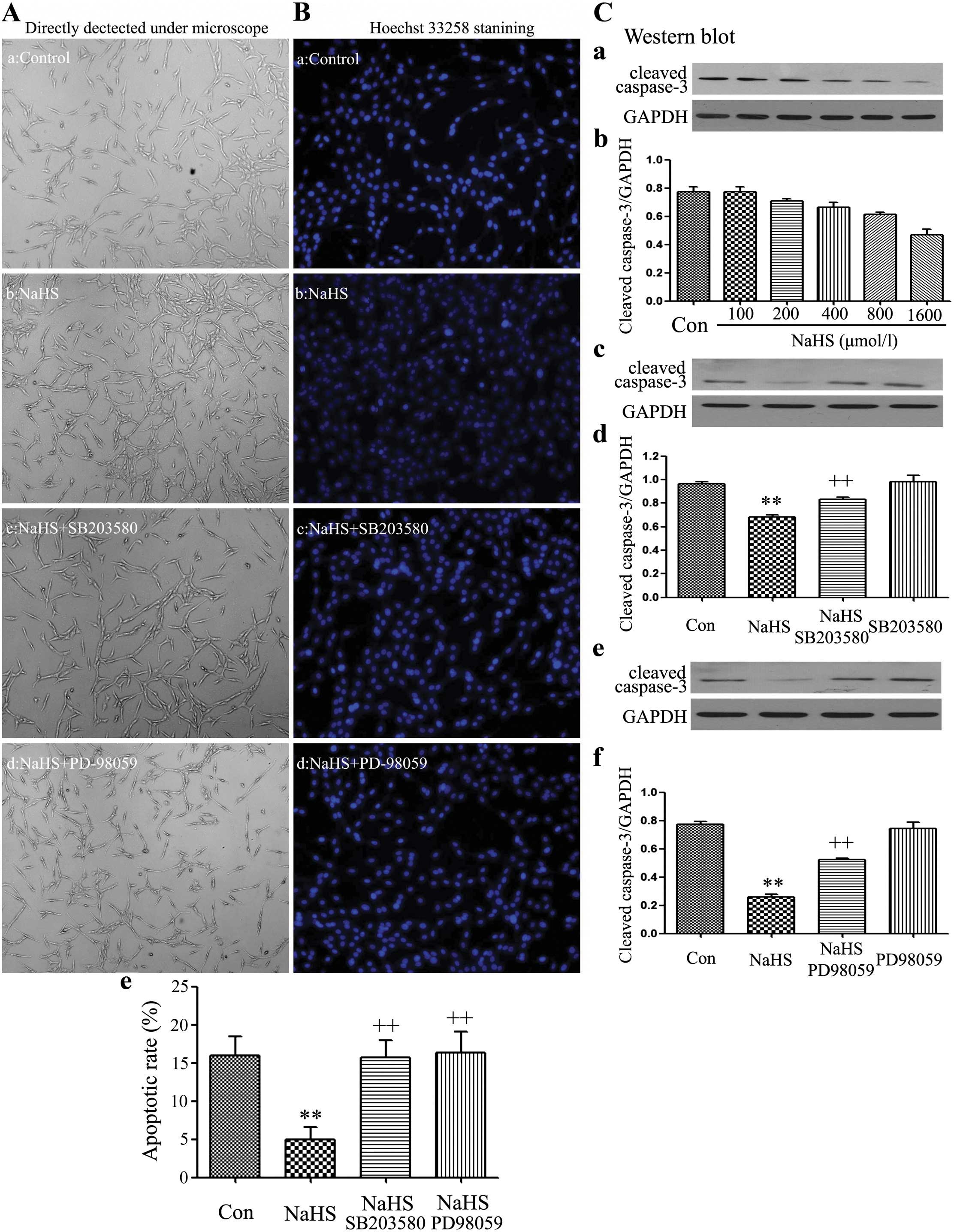
SB203580 and PD98059 increase the
NaHS-induced cell proliferation in C6 glioma cells
The cell number was detected using a fully automatic
inverted microscope (Fig. 5-a–d).
C6 glioma cells were co-treated with 400 µmol/l NaHS and 30
µmol/l SB203580 or 20 µmol/l PD-98059 for 24 h,
respectively. As observed in Fig.
2, 400 µmol/l NaHS markedly promoted cells
proliferation, leading to an increase in cell number. However,
exposure of cells to 400 µmol/l NaHS and 30 µmol/l
SB203580 or 20 µmol/l PD-98059 both significantly alleviated
NaHS-induced cell proliferation in C6 glioma cells.
Apoptotic cell death was tested by Hoechst 33258
staining followed by photofluorography. As shown in the Fig. 5B-a–e, exposure of C6 glioma cells to
400 µmol/l NaHS for 24 h reduced the typical characteristics
of apoptosis, as evidenced by the condensation of chromatin, the
shrinkage of nuclei and the formation of apoptotic bodies. On the
contrary, exposure of cells to 400 µmol/l NaHS and 30
µmol/l SB203580 or 20 µmol/l PD-98059 significantly
induced the typical characteristics of apoptosis.
On the contrary, NaHS alone markedly upregulated the
expression level of cleaved caspase-3. As illustrated in Fig. 5F and G, exposure of cells to
indicated doses of NaHS (100, 200, 400, 800 and 1,600
µmol/l) for 24 h markedly downregulated the expression level
of cleaved caspase-3. Whereas, exposure of cells to 400
µmol/l NaHS and 30 µmol/l SB203580 (Fig. 5C-c and -d) or 20 µmol/l
PD-98059 (Fig. 5C-e and -f)
significantly upregulated the expression level of cleaved
caspase-3.
SB203580, PD98059 and AOAA inhibit the
NaHS-induced increase of expression of COX-2 in C6 glioma
cells
In order to observe the effects of NaHS on the
expression levels of COX-2 and MMP-2 in C6 glioma cells, cells were
exposured to 400 µmol/l NaHS for different times (3, 6, 9,
12 and 24 h). As shown in Fig. 6A and
B, NaHS significantly enhanced the expression levels of COX-2
reaching a peak at 12 h. Notably, co-treatment of C6 glioma cells
with 400 µmol/l NaHS and 30 µmol/l SB203580 (Fig. 6E and F) or 20 µmol/l PD-98059
(Fig. 6G and H) or 2 mmol/l AOAA
(Fig. 6C and D) for 24 h
considerably depressed the NaHS-induced increase of expression of
COX-2. Treatment of cells with 30 µmol/l, SB203580 alone, or
single treatment of 20 µmol/l PD-98059 or 2 mmol/l AOAA,
respectively, for 24 h did not alter the basal expression level of
COX-2.
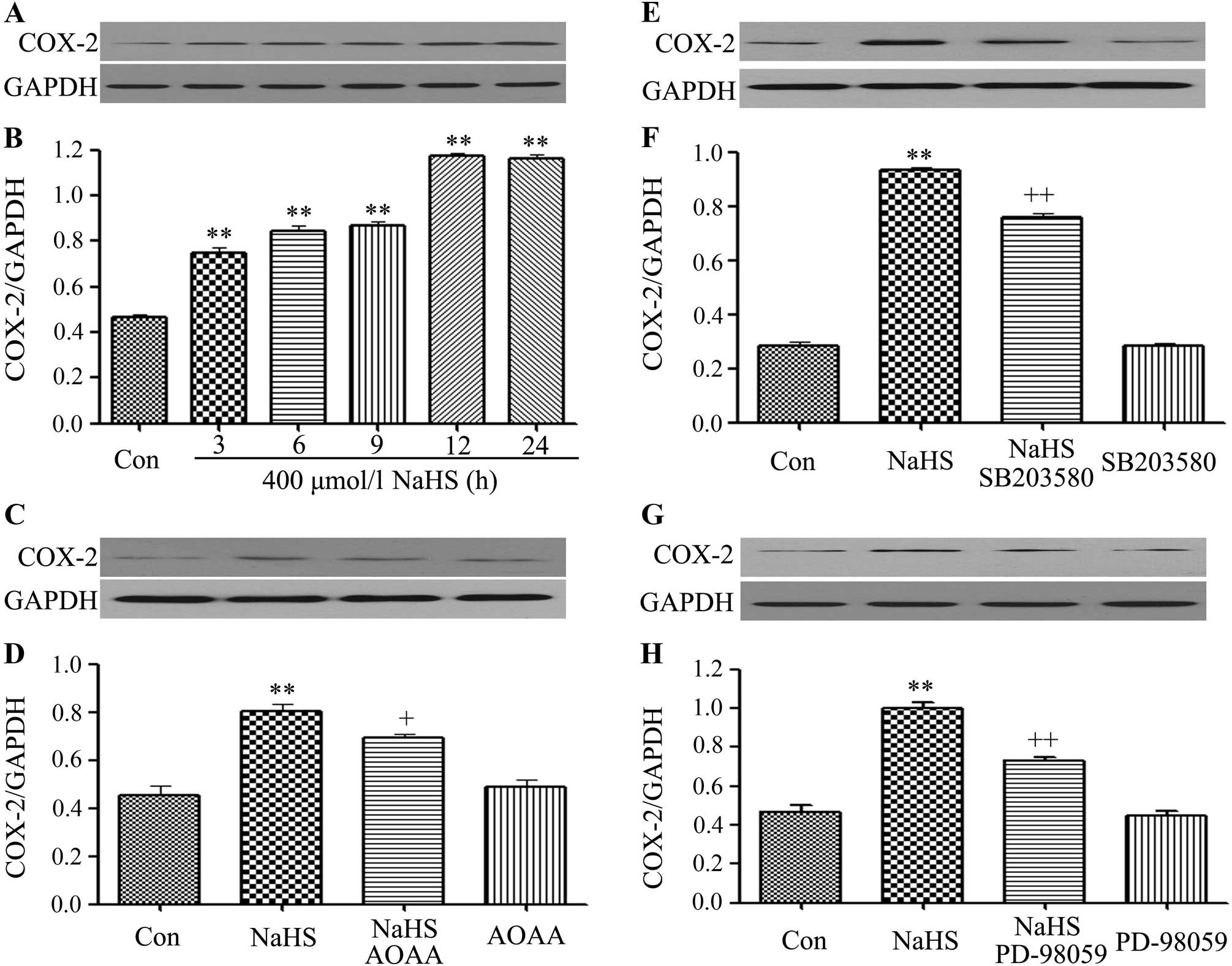 | Figure 6SB203580, PD98059 and AOAA inhibit
the NaHS-induced increase of COX-2 expression in C6 glioma cells.
(A and B) C6 glioma cells were exposed to 400 µmol/l NaHS
for different times (3, 6, 9, 12 and 24 h). (C and D) Cells were
co-treated with 400 µmol/l NaHS and 2 mmol/l AOAA for 24 h.
(E and F) Cells were co-treated with 400 µmol/l NaHS and 30
µmol/l SB203580 for 24 h. (G and H) Cells were co-treated
with 400 µmol/l NaHS and 20 µmol/l PD-98059 for 24 h.
The expression level of COX-2 was measured by western blot
analysis. (B, D, F and H) The data in (A, C, E and G) was
quantified by densitometric analysis with ImageJ 1.47i software.
Data are shown as the mean ± SEM (n=3). **p<0.01 vs.
with the control group; +p<0.05,
++p<0.01 vs. the NaHS group. Con, the control group;
NaHS, a donor of H2S; SB203580, a specific inhibitor of
p38 MAPK pathway; PD-98059, a specific inhibitor of ERK1/2
pathway. |
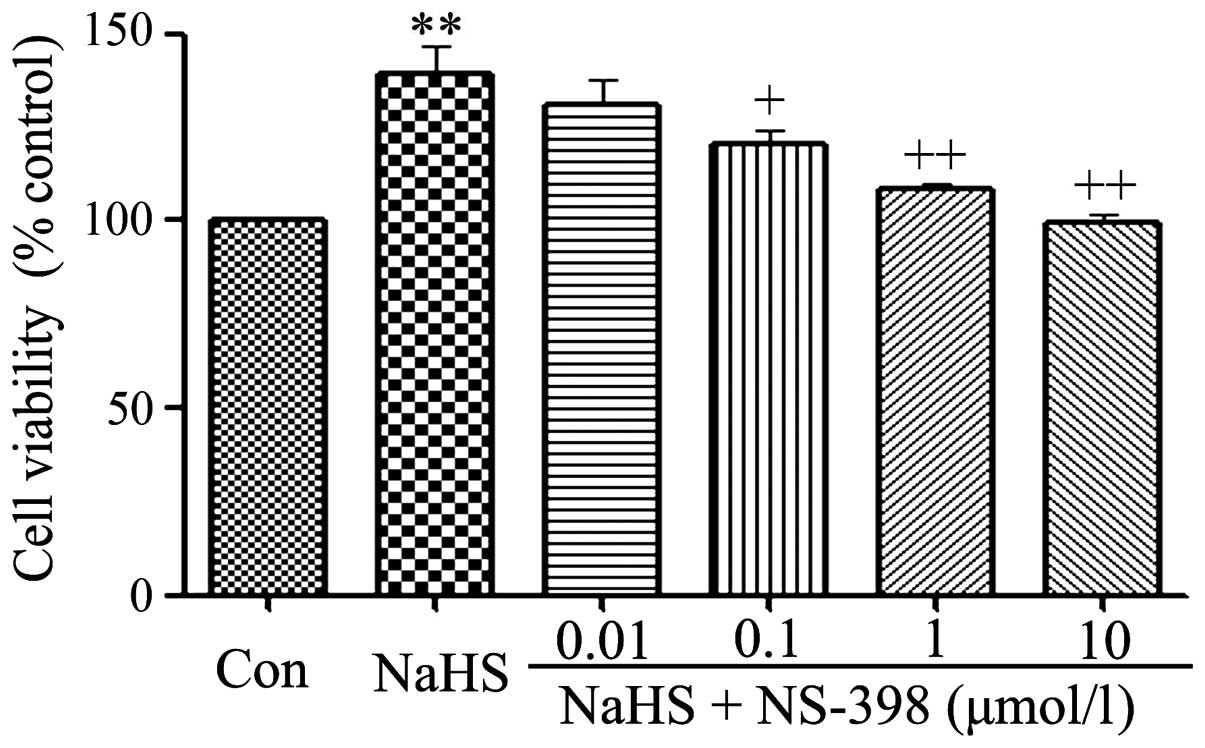
NS-398 alleviates the NaHS-induced
increase of cell viability in C6 glioma cells
As shown in Fig. 7,
exposure of C6 glioma cells to 400 µmol/l NaHS for 24 h
obviously induced cell proliferation, leading to an increase in
cell viability. However, the increased cell viability was repressed
by co-treatment with different doses of NS-398 (a specific
inhibitor of COX-2 pathway) for 24 h.
As shown in Fig. 4,
0.01 µmol/l NS-398 did not alter cell viability. On the
contrary, the dose of NS-398 from 0.1 to 10 µmol/l
significantly suppressed the cell proliferation, leading to a
decrease in cell viability and reaching the minimum at 10
µmol/l. According to the above results, C6 glioma cells were
co-treated with 400 µmol/l NaHS and 10 µmol/l NS-398
for 24 h in the following experiments.
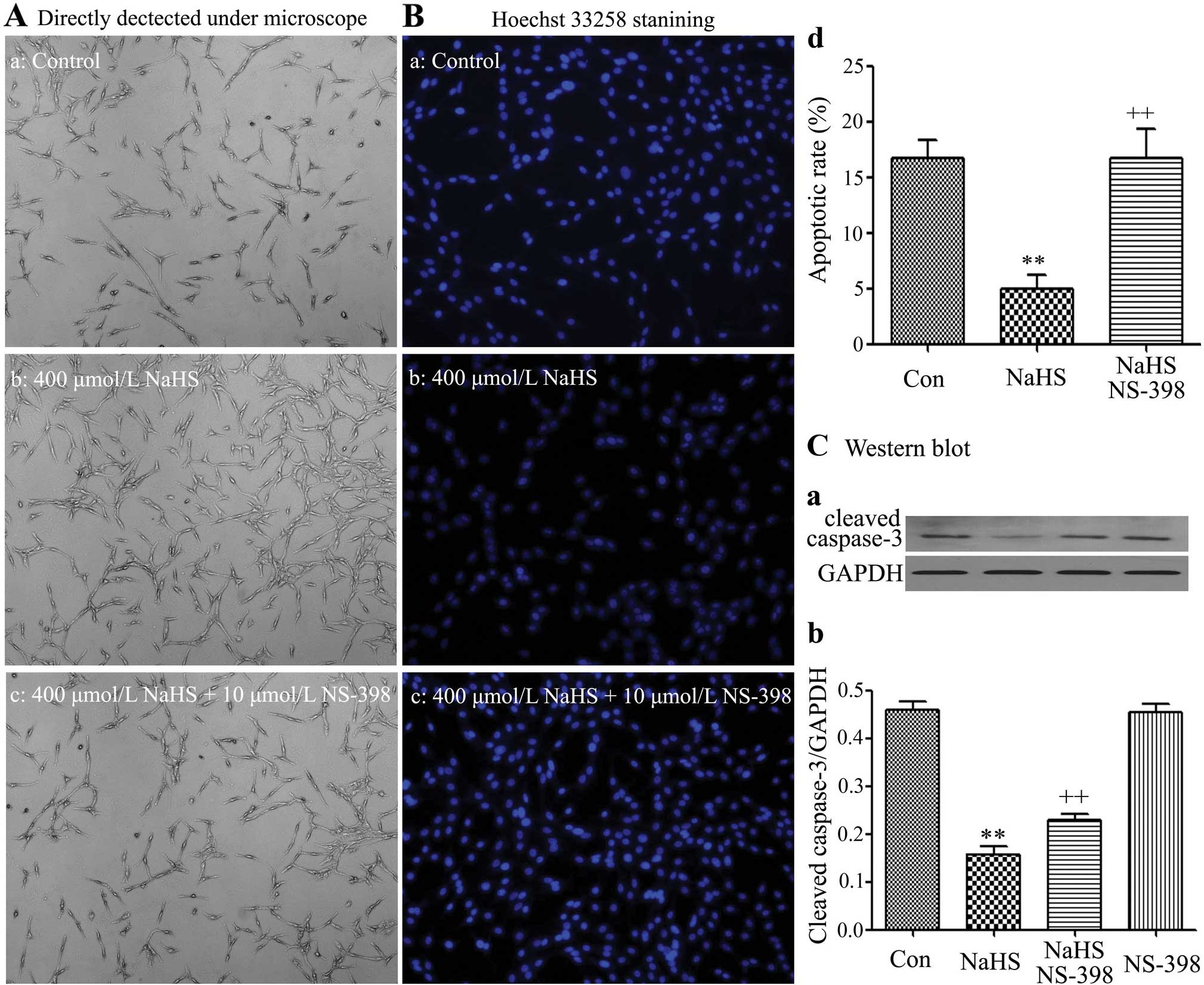
NS-398 increases the NaHS-induced cell
proliferation in C6 glioma cells
Cell number was detected using a fully automatic
inverted microscope (Fig. 5-a–d).
C6 glioma cells were co-treated with 400 µmol/l NaHS and 10
µmol/l NS-398 for 24 h. As observed in Fig. 8A–b, alone 400 µmol/l NaHS
markedly promoted cell proliferation, leading to an increase in the
cell number. However, exposure of cells to 400 µmol/l NaHS
and 10 µmol/l NS-398 significantly alleviated the
NaHS-induced cell proliferation in C6 glioma cells.
Apoptotic cell death was evaluated by the Hoechst
33258 staining followed by photofluorography. As shown in the
Fig. 8B-a–d, exposure of C6 glioma
cells to 400 µmol/l NaHS for 24 h reduced the typical
characteristics of apoptosis, as evidenced by the condensation of
chromatin, the shrinkage of nuclei and the formation of apoptotic
bodies. On the contrary, exposure of cells to 400 µmol/l
NaHS and 10 µmol/l NS-398 significantly induced the typical
characteristics of apoptosis.
On the contrary, NaHS alone markedly downregulated
the expression level of cleaved caspase-3. As illustrated in
Fig. 8C-a and -b, exposure of cells
to 400 µmol/l NaHS for 24 h markedly downregulated the
expression level of cleaved caspase-3. Whereas, exposure of cells
to 400 µmol/l NaHS and 10 µmol/l NS-398 significantly
upregulated the expression level of cleaved caspase-3.
Discussion
In the present study, we demonstrated a novel
finding of tumor development by hydrogen sulfide (H2S)
on C6 glioma cells and also provided data to revel its potential
mechanisms. This conclusion is supported by several lines of
evidence as follows: i) addition of NaHS (a donor of
H2S; 100–1,600 µmol/l) promoted cell
proliferation, leading to an increase in cell viability and an
increase in cell number; ii) NaHS-induced a decrease in cell
apoptosis, by decreasing expression level of caspase-3 and cell
apoptosis; iii) AOAA inhibited the NaHS-induced proliferation and
anti-apoptosis; iv) NaHS activated the p38 MAPK/ERK1/2 pathways and
promoted the expression level of COX-2, and those effects were
abolished by AOAA; v) SB203580 and PD98059 downregulated the
NaHS-induced increased expression level of COX-2, respectively; vi)
co-treatment of C6 glioma cells with NaHS SB203580, PD98059 or
NS-398 suppressed the NaHS-induced proliferation and
anti-apoptosis.
H2S is produced in the body mainly by
three pyridoxal-59-phosphate-dependent enzymes, 3-mercaptopyruvate
sulfurtransferase (3-MST), cystathionine-β-synthase (CBS) and
cystathionine-γ-lyase (CSE). However, CBS is mainly found in the
central nervous system (CNS) (32,33). A
recent study suggested that H2S plays an important role
in various physiological and pathological processes of the nervous
system as a neuromodulator and neuroprotectant (34). Jiang et al found that
H2S protected the brain against injuries and exerted
neuroprotection by modulating the underlying signaling pathways
(35–37). In addition, H2S exerted
protection to nerve cancer cells, such as PC12 cells (38). It can be concluded that
H2S may be involved in development of the nervous system
cancer cells. In order to confirm this hypothesis, C6 glioma cells
were exposed to NaHS (a donor of H2S). Unexpectedly, we
found two interesting results. Firstly, different doses of NaHS
(100–1,600 µmol/l) promoted cell proliferation in the range
of physiological doses of H2S (0.2–1 mmol/l) the optimal
concentration of NaHS that induced maximal effect of proliferation
was 400 µmol/l, leading to an increase in cell viability and
an increase in cell number. Secondly, treatment of cells with 400
µmol/l NaHS for 24 h markedly diminished cell apoptosis, and
decreased the expression of caspase-3, one of the apoptotic
factors, and the above NaHS-induced effects were inhibited by AOAA.
These results demonstrate that H2S induces C6 glioma
cell proliferation via exerting its dual cytoprotective and
anti-apoptosis effects, and H2S may be involved in
glioma growth under physiological conditions. Along with a previous
study that H2S-protected PC12 cells from formaldehyde
induced apoptosis (38), the
results suggest that H2S exerts a cytoprotective effect
for C6 glioma cells. The effects of H2S on cell
proliferation and anti-apoptosis are complicated and unclear. In
the present study, we investigated whether H2S promoted
the proliferation ability and anti-apoptosis of C6 glioma cells
in vitro by activation of the p38 MAPK/ERK1/2-COX-2
signaling pathways.
It has been reported that COX-2 pathway is closely
associated with tumor cell growth and is a crucial molecule in the
development of malignant tumors, and angiogenesis (15,16),
anti-apoptosis (17), invasiveness
(18) and proliferation (19). In the present study, we found that
NS-398 (an inhibitor of COX-2) increased caspase-3 expression,
which is consistent with previous research results (17,31).
In addition, treatment of cells with 400 µmol/l NaHS for 24
h promoted the expression level of COX-2, along with the
NaHS-induced pro-proliferative effect and anti-apoptosis. Combined
treatment with a COX-2 inhibitor (NS-398) and 400 µmol/l
NaHS resulted in a decrease in cell viability, a decrease in cell
number, an increase in cell apoptosis and in increased expression
level of cleaved caspase-3, indicating that NS-398 increases
NaHS-induced cell proliferation in C6 glioma cells. Those results
suggest that COX-2 pathway is necessary in NaHS-induced C6 glioma
cell proliferation and anti-apoptosis.
The COX-2 pathway and its inhibitors are regulated
by multiple signaling cascades, including the p38 signaling pathway
as well as ERK1/2 and NF-κB-mediated pathways (15,20,31).
To confirm whether NaHS-mediated activation of COX-2 was regulated
by p38 MAPK or ERK1/2, C6 glioma cells were co-treated with 400
µmol/l NaHS and SB203580 (an inhibitor of p38 MAPK) or
PD98059 (an inhibitor of ERK1/2), the expression level of COX-2 was
downregulated. This result suggested that COX-2 was downstream of
p38 MAPK or ERK1/2 pathways. In addition, treatment C6 glioma cells
with 400 µmol/l NaHS activated p38 MAPK and ERK1/2 pathway,
which is supported by NaHS-induced upregulation of p-p38 MAPK and
ERK1/2 expression levels. Co-treatment of C6 glioma cells with NaHS
and SB203580, PD98059 or NS-398 suppressed NaHS-induced
proliferation and anti-apoptosis. Therefore, we concluded that p38
MAPK/ERK1/2-COX-2 pathways are involved in NaHS-induced cancer cell
proliferation and anti-apoptotic in C6 glioma cells.
NaHS exert its proliferation and anti-apoptosis via
activating p38 MAPK/ERK1/2-COX-2 pathways in C6 glioma cells,
however, whether NaHS exerts its migration via activating p38
MAPK/ERK1/2-COX-2 pathways is still unclear, and needs further
research in vivo and in vitro. The effects of
H2S on the cancer are not completely coincident. Our
research (31) and other studies
(24–30) suggest that H2S is
beneficial for cancer cells growth, proliferation, migration and
invasion. On the contrary, some other researchers found that
H2S exerted potential anticancer efficiency in SGC-7901
gastric cancer cells (40), oral
cancer cell lines (41), colon
cancer cells (42), and in several
different human cancer cell lines (HeLa, HCT-116, Hep G2, HL-60,
MCF-7, MV4-11 and U2OS) (43). As
the above is contradictory further research must be carried out to
clarify these issues.
In summary, H2S-induced cells
proliferation and anti-apoptosis in C6 glioma cells. This effect
may be mediated by the activation of p38 MAPK/ERK1/2-COX-2
pathways, leading to downregulation of caspase-3, increased cell
viability, increased in cell number and decreased number of
apoptotic cells. In C6 glioma, the findings provide a novel insight
into a unified concept and identify CBS-derived H2S as
an endogenous tumor-promoting factor and anticancer drug target.
Furthermore, the migration and invasion of H2S in C6
glioma cells is still unclear and needs to be further
investigated.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei J, Gabrusiewicz K and Heimberger A:
The controversial role of microglia in malignant gliomas. Clin Dev
Immunol. 2013:2852462013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jansen M, Yip S and Louis DN: Molecular
pathology in adult gliomas: Diagnostic, prognostic, and predictive
markers. Lancet Neurol. 9:717–726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schaeffer HJ and Weber MJ:
Mitogen-activated protein kinases: Specific messages from
ubiquitous messengers. Mol Cell Biol. 19:2435–2444. 1999.PubMed/NCBI
|
|
6
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
7
|
Turjanski AG, Vaqué JP and Gutkind JS: MAP
kinases and the control of nuclear events. Oncogene. 26:3240–3253.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding D, Wei S, Song Y, Li L, Du G, Zhan H
and Cao Y: Osthole exhibits anti-cancer property in rat glioma
cells through inhibiting PI3K/Akt and MAPK signaling pathways. Cell
Physiol Biochem. 32:1751–1760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang B, Wu T, Wang Z, Zhang Y, Wang J,
Yang B, Zhao Y, Rao Z and Gao J: p38MAPK activation mediates tumor
necrosis factor-α-induced apoptosis in glioma cells. Mol Med Rep.
11:3101–3107. 2015.
|
|
12
|
Li F, Chen T, Hu S, Lin J, Hu R and Feng
H: Superoxide mediates direct current electric field-induced
directional migration of glioma cells through the activation of AKT
and ERK. PLoS One. 8:e611952013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Subbaramaiah K, Chung WJ and Dannenberg
AJ: Ceramide regulates the transcription of cyclooxygenase-2.
Evidence for involvement of extracellular signal-regulated
kinase/c-Jun N-terminal kinase and p38 mitogen-activated protein
kinase pathways. J Biol Chem. 273:32943–32949. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Limami Y, Pinon A, Leger DY, Pinault E,
Delage C, Beneytout JL, Simon A and Liagre B: The
P2Y2/Src/p38/COX-2 pathway is involved in the resistance
to ursolic acid-induced apoptosis in colorectal and prostate cancer
cells. Biochimie. 94:1754–1763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JY, Chung SW, Kim SY and Byun Y:
Enhanced anti-angiogenic effect of low molecular weight heparinbile
acid conjugates by co-administration of a selective COX-2
inhibitor. Pharm Res. 32:2318–2327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tegeder I, Niederberger E, Israr E,
Gühring H, Brune K, Euchenhofer C, Grösch S and Geisslinger G:
Inhibition of NF-kappaB and AP-1 activation by R- and
S-flurbiprofen. FASEB J. 15:2–4. 2001.
|
|
17
|
Seo KW, Coh YR, Rebhun RB, Ahn JO, Han SM,
Lee HW and Youn HY: Antitumor effects of celecoxib in COX-2
expressing and non-expressing canine melanoma cell lines. Res Vet
Sci. 96:482–486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu X, Cai M, Ji F and Lou LM: The impact
of COX-2 on invasion of osteosarcoma cell and its mechanism of
regulation. Cancer Cell Int. 14:272014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu T, Han C, Lunz JG III, Michalopoulos G,
Shelhamer JH and Demetris AJ: Involvement of 85-kd cytosolic
phospholipase A and cyclooxygenase-2 in the proliferation of human
cholangio-2 carcinoma cells. Hepatology. 36:363–373. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chiu WT, Shen SC, Chow JM, Lin CW, Shia LT
and Chen YC: Contribution of reactive oxygen species to
migration/invasion of human glioblastoma cells U87 via
ERK-dependent COX-2/PGE2 activation. Neurobiol Dis.
37:118–129. 2010. View Article : Google Scholar
|
|
21
|
Wang R: Two's company, three's a crowd:
Can H2S be the third endogenous gaseous transmitter?
FASEB J. 16:1792–1798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guidotti TL: Hydrogen sulfide: Advances in
understanding human toxicity. Int J Toxicol. 29:569–581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kilburn KH, Thrasher JD and Gray MR:
Low-level hydrogen sulfide and central nervous system dysfunction.
Toxicol Ind Health. 26:387–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szabo C, Coletta C, Chao C, Módis K,
Szczesny B, Papapetropoulos A and Hellmich MR: Tumor-derived
hydrogen sulfide, produced by cystathionine-β-synthase, stimulates
bioenergetics, cell proliferation, and angiogenesis in colon
cancer. Proc Natl Acad Sci USA. 110:12474–12479. 2013. View Article : Google Scholar
|
|
25
|
Pupo E, Pla AF, Avanzato D, Moccia F, Cruz
JE, Tanzi F, Merlino A, Mancardi D and Munaron L: Hydrogen sulfide
promotes calcium signals and migration in tumor-derived endothelial
cells. Free Radic Biol Med. 51:1765–1773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du SX, Xiao J, Guan F, Sun LM, Wu WS, Tang
H, Du JB, Tang CS and Jin HF: Predictive role of cerebrospinal
fluid hydrogen sulfide in central nervous system leukemia. Chin Med
J. 124:3450–3454. 2011.
|
|
27
|
Levine J, Ellis CJ, Furne JK, Springfield
J and Levitt MD: Fecal hydrogen sulfide production in ulcerative
colitis. Am J Gastroenterol. 93:83–87. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rose P, Moore PK, Ming SH, Nam OC,
Armstrong JS and Whiteman M: Hydrogen sulfide protects colon cancer
cells from chemopreventative agent beta-phenylethyl isothiocyanate
induced apoptosis. World J Gastroenterol. 11:3990–3997.
2005.PubMed/NCBI
|
|
29
|
Cai WJ, Wang MJ, Ju LH, Wang C and Zhu YC:
Hydrogen sulfide induces human colon cancer cell proliferation:
Role of Akt, ERK and p21. Cell Biol Int. 34:565–572. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao Q, Zhang L, Yang G, Xu C and Wang R:
Butyrate-stimulated H2S production in colon cancer
cells. Antioxid Redox Signal. 12:1101–1109. 2010. View Article : Google Scholar
|
|
31
|
Zhen Y, Pan W, Hu F, Wu H, Feng J, Zhang Y
and Chen J: Exogenous hydrogen sulfide exerts
proliferation/anti-apoptosis/angiogenesis/migration effects via
amplifying the activation of NF-κB pathway in PLC/PRF/5 hepatoma
cells. Int J Oncol. 46:2194–2204. 2015.PubMed/NCBI
|
|
32
|
Tan BH, Wong PT and Bian JS: Hydrogen
sulfide: A novel signaling molecule in the central nervous system.
Neurochem Int. 56:3–10. 2010. View Article : Google Scholar
|
|
33
|
Rong W, Kimura H and Grundy D: The
neurophysiology of hydrogen sulfide. Inflamm Allergy Drug Targets.
10:109–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X and Bian JS: Hydrogen sulfide: A
neuromodulator and neuroprotectant in the central nervous system.
ACS Chem Neurosci. 5:876–883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chan SJ, Chai C, Lim TW, Yamamoto M, Lo
EH, Lai MK and Wong PT: Cystathionine β-synthase inhibition is a
potential therapeutic approach to treatment of ischemic injury. ASN
Neuro. 7:17590914155787112015. View Article : Google Scholar
|
|
36
|
Chen WL, Niu YY, Jiang WZ, Tang HL, Zhang
C, Xia QM and Tang XQ: Neuroprotective effects of hydrogen sulfide
and the underlying signaling pathways. Rev Neurosci. 26:129–142.
2015. View Article : Google Scholar
|
|
37
|
Donatti AF, Soriano RN, Sabino JP and
Branco LG: Involvement of endogenous hydrogen sulfide
(H2S) in the rostral ventrolateral medulla (RVLM) in
hypoxia-induced hypothermia. Brain Res Bull. 108:94–99. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang JM, Zhou CF, Gao SL, Tian Y, Wang
CY, Wang L, Gu HF and Tang XQ: BDNF-TrkB pathway mediates
neuroprotection of hydrogen sulfide against formaldehyde-induced
toxicity to PC12 cells. PLoS One. 10:e01194782015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu K, Wang L and Shu HK: COX-2
overexpression increases malignant potential of human glioma cells
through Id1. Oncotarget. 5:1241–1252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma K, Liu Y, Zhu Q, Liu CH, Duan JL, Tan
BK and Zhu YZ: H2S donor, S-propargylcysteine, increases
CSE in SGC-7901 and cancer-induced mice: Evidence for a novel
anti-cancer effect of endogenous H2S? PLoS One.
6:e205252011. View Article : Google Scholar
|
|
41
|
Murata T, Sato T, Kamoda T, Moriyama H,
Kumazawa Y and Hanada N: Differential susceptibility to hydrogen
sulfide-induced apoptosis between PHLDA1-overexpressing oral cancer
cell lines and oral keratinocytes: Role of PHLDA1 as an apoptosis
suppressor. Exp Cell Res. 320:247–257. 2014. View Article : Google Scholar
|
|
42
|
Chattopadhyay M, Kodela R, Olson KR and
Kashfi K: NOSH-aspirin (NBS-1120), a novel nitric oxide- and
hydrogen sulfide-releasing hybrid is a potent inhibitor of colon
cancer cell growth in vitro and in a xenograft mouse model. Biochem
Biophys Res Commun. 419:523–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH,
Li L, Moore PK and Deng LW: The slow-releasing hydrogen sulfide
donor, GYY4137, exhibits novel anti-cancer effects in vitro and in
vivo. PLoS One. 6:e210772011. View Article : Google Scholar : PubMed/NCBI
|