Introduction
Colorectal cancer is a leading cause of
cancer-related death worldwide (1).
Despite improved methods of early diagnosis and treatment, a large
proportion of patients with colorectal cancer die from cancer
progression including tumor invasion and metastasis (1–3).
Cancer progression is an extremely complex process including tumor
cell proliferation, invasion, apoptosis, angiogenesis,
lymphangiogenesis and metastasis into distant organs or tissues
(4–6). Therefore, understanding of the
biological and molecular mechanisms involved in colorectal cancer
progression is crucial to the development of more effective cancer
therapies for preventing cancer progression.
KAI1/CD82 is a member of the tetraspanin family,
which is involved in cell motility, and this molecule has been
identified as a suppressor of tumor spread (7,8).
Numerous previous studies revealed that downregulation or loss of
KAI1/CD82 is associated with tumor progression and poor prognosis
in many human cancers (9–13).
KAI1 COOH-terminal interacting tetraspanin (KITENIN)
was recently identified as a novel KAI1/CD82-binding protein. It
interacts specifically with the COOH-terminal region of KAI1/CD82,
which is crucial for the impact of KAI1/CD82 on cell motility
(14–18). In contrast to KAI1/CD82, previous
studies have demonstrated that KITENIN promotes the migration and
invasiveness of various types of human cancer cells, and gene
silencing of KITENIN via intravenous injection of short interfering
RNA inhibited tumor metastasis in a mouse colon cancer model and a
syngeneic mouse squamous cell tumor model (14–18).
Furthermore, KITENIN expression was found to be associated with
cancer progression and poor prognosis in human cancers including
colorectal cancer (19–24). These data indicate that KITENIN
plays an important role in cancer progression including tumor
invasion and metastasis.
Angiogenesis and lymphangiogenesis are crucial for
normal growth and development in addition to protective responses
such as wound healing and inflammation. However, aberrant
angiogenesis and lymphangiogenesis can occur in a variety of
pathological settings including tumor growth and dissemination
(25–29). Many lines of evidence indicate that
the progression of colorectal cancer depends on angiogenesis and
lymphangiogenesis (30–33). Therefore, the role of tumor
cell-derived factors in the promotion of angiogenesis and
lymphangiogenesis has been extensively studied, as these factors
are extremely important for understanding cancer progression.
However, the potential role of KITENIN in angiogenesis and
lymphangiogenesis in colorectal cancer progression has never been
investigated.
The aim of the present study was to evaluate whether
KITENIN affects tumor angiogenesis and lymphangiogenesis in
colorectal cancer.
Materials and methods
Cell culture and siRNA transfection
DLD1 and SW480 human colorectal cancer cell lines
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and were maintained in Dulbecco's modified
Eagle's medium (DMEM) (HyClone, Logan, UT, USA) supplemented with
10% fetal bovine serum and antibiotics. Synthesized human KITENIN
small interfering RNA (siRNA) (5′-GCUUGGACUUCAGCCUCGUAGUCAA-3′) and
scramble siRNA (Qiagen, Germantown, MD, USA) were transfected using
Lipofectamine™ RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer's instructions. After transfection for 5 h, the
medium was replaced with serum-free DMEM, and the cells were
incubated for 24 h. Cells were re-suspended in RIPA buffer, and the
supernatant was centrifuged to obtain the conditioned medium (CM),
which was used for migration and tube formation assays. Human
umbilical vein endothelial cells (HUVECs; Lonza, Walkersville, MD,
USA) and human lymphatic endothelial cells (HLECs; ScienCell, San
Diego, CA, USA) were grown in EBM™-2 medium supplemented with
EGM™-2 SingleQuots™ (Lonza).
Western blot analysis
Equal amounts of total cell lysates were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA), which
were incubated with 5% skim milk to block non-specific binding
followed by specific primary antibodies in 2% skim milk overnight
at 4°C. Specific proteins were detected using horseradish
peroxidase-conjugated secondary antibodies (Millipore, Billerica,
MA, USA) and visualized using the LAS-4000 luminescent image
analyzer (Fujifilm, Tokyo, Japan). The antibodies used in the
present study were as follows: anti-human KITENIN (Atlas,
Stockholm, Sweden); vascular endothelial growth factor (VEGF)-A,
VEGF-C, VEGF-D and β-tubulin (Santa Cruz Biotechnology, Santa Cruz,
CA, USA); and hypoxia-inducible factor-1α (HIF-1α) and angiostatin
(Abcam, Cambridge, UK).
Matrigel invasion assay
A Transwell filter chamber with 8-µm pores
was coated with Matrigel (1 mg/ml; BD Biosciences, San Diego, CA,
USA) and was dried at room temperature. HUVECs and HLECs were
plated in duplicate at a concentration of 3×104
cells/well with serum-free EGM-2 medium on the upper chamber. The
lower chambers were filled with prepared CM. After incubation for 3
h, cells that invaded through the Transwell membrane were stained
with Diff-Quik solution (Sysmex, Kobe, Japan) and were counted
under a microscope.
In vitro endothelial tube formation
assay
Forty-eight-well plates were coated with Matrigel
(10 mg/ml) and were incubated at 37°C to promote polymerization.
HUVECs and HLECs were re-suspended in CM and were added to each
well of the plate which were coated with Matrigel. After 18 h of
incubation, fields from each sample were photographed using an
inverted microscope, and the total tube length was analyzed using
the WimTube image analysis platform (Wimasis GmbH, Munich,
Germany).
Patients and tissue samples
For immunohistochemical staining, paraffin-embedded
tumor samples from 85 patients who underwent surgery for colorectal
cancer at the Chonnam National University Hwasun Hospital (Jeonnam,
Korea) between July 2007 and June 2008, were studied. No patient
had received preoperative radiotherapy or chemotherapy. Pathologic
studies and clinical histories at the time of surgery were reviewed
through the medical records. Tumor staging was performed in
accordance with the American Joint Committee on Cancer staging
system (34). Clinical outcomes
were determined from the time of surgery until follow-up on
December 31, 2014. The present study was approved by the
Institutional Review Board of Chonnam National University Hwasun
Hospital. All participants provided written consent for their
information to be stored in the hospital database and used for
research.
Immunohistochemistry
Paraffin-embedded tissue sections were rehydrated
and retrieved with pH 6.0 citrate buffer. Tissue sections were
immersed with peroxidase-blocking solution (Dako, Carpinteria, CA,
USA) to block endogenous peroxidase activity and incubated with
polyclonal rabbit ant-human KITENIN, CD34 (Abcam) and D2-40 (Dako,
Glostrup, Denmark) in primary diluent solution (Invitrogen)
overnight at 4°C. After washing in TBST, tissues were stained using
Dako Real™ EnVision HRP/DAB detection system (Dako). The slides
were counterstained with hematoxylin and were then mounted. Stained
tissues were viewed and photographed using a light microscope.
Evaluation of KITENIN expression
The immunoreactive score of KITENIN expression was
independently evaluated by two pathologists who were blinded to the
immunostaining pattern and clinical outcomes. Consensus scores were
assigned for each case by reviewing the slides with discrepancies
in scoring. The staining intensity was graded on a scale of 0–3 as
follows: 0 (no staining), 1 (weak staining), 2 (moderate staining)
and 3 (strong staining). The staining area was scored as 0 for no
positive staining of tumor cells, 1 for positive staining in
<10% of the tumor cells, 2 for positive staining in 10–50% of
the tumor cells, and 3 for positive staining in >50% of the
tumor cells. The staining index was calculated as the product of
the staining intensity and staining area. The tumors were
determined to have positive expression (staining index ≥4) or
negative expression (staining index <4).
Assessment of microvessel density (MVD)
and lymphatic vessel density (LVD)
All scores and interpretations of
immunohistochemical results were performed by one examiner without
knowledge of the clinical outcomes. MVD and LVD were measured on
anti-CD34 and anti-D2-40 antibody-immunoreactive specimens,
respectively. Vessel density was evaluated within neoplastic
tissues and within healthy tissues outside the tumor. The
immunostained sections were scanned at a low magnification of ×40
to identify the areas with the largest amount of vessels (hot
spots). In the present study, three hot spots were chosen for each
case with agreements of both observers, and five fields were
examined in each hot spot at a high magnification of ×200. The
average MVD and LVD were expressed as the mean value of
vessels.
Statistical analysis
Statistical Package for the Social Sciences (version
15.0; SPSS, Inc., Chicago, IL, USA) was used for statistical
analysis. The correlations of clinicopathological factors and
recurrence with KITENIN expression, MVD and LVD were assessed using
Chi-square and Fisher's exact tests. The survival rates of patients
were evaluated according to the Kaplan-Meier method, and the
differences were tested using a log-rank test. The subgroups of MVD
and LVD were analyzed using a t-test. P<0.05 was considered to
indicate a statistically significant result.
Results
Impact of KITENIN silencing on
angiogenesis in human colorectal cancer cells
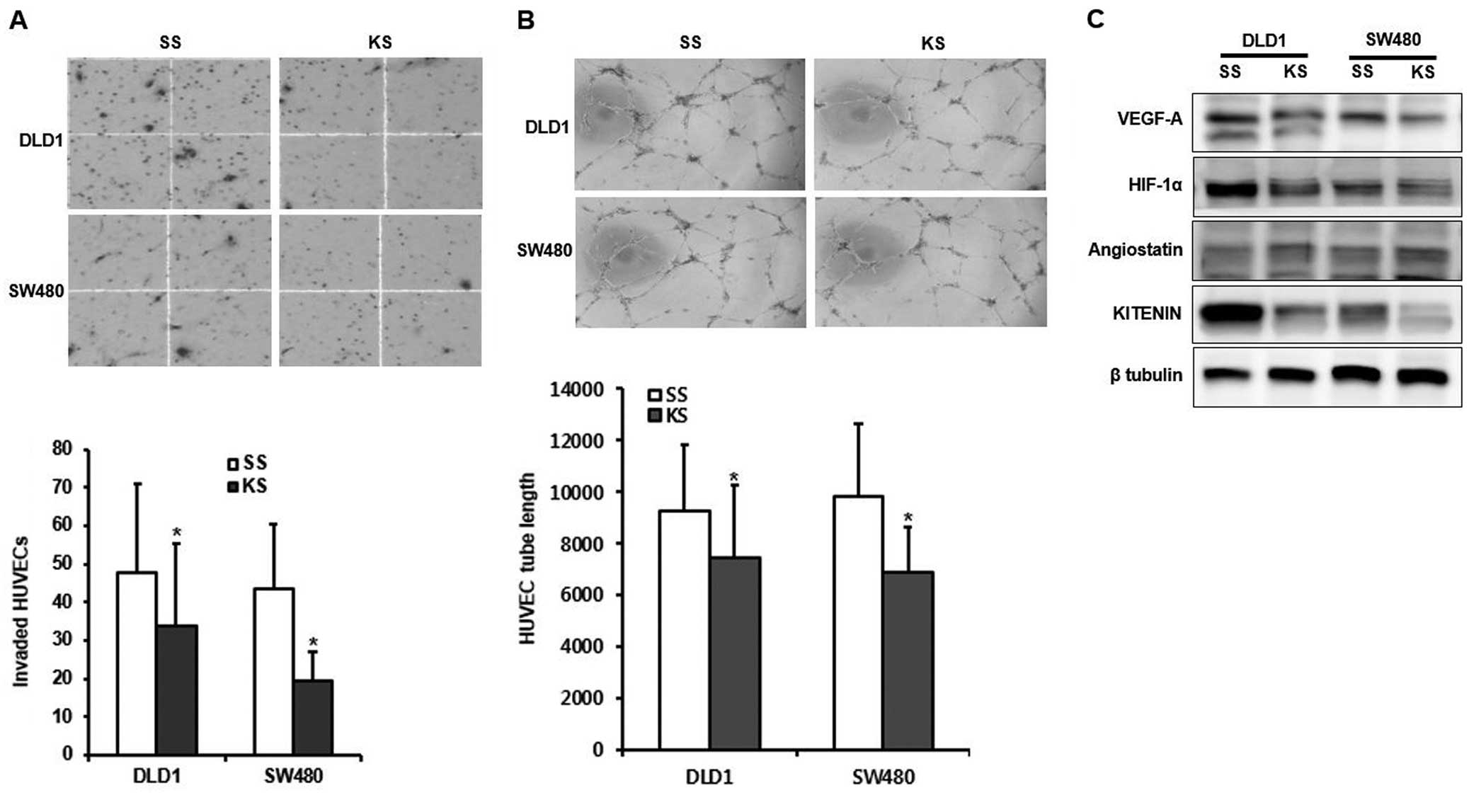
To determine whether CM from KITENIN and scrambled
siRNA-transfected colorectal cancer cells affects the invasiveness
of HUVECs, we performed a Matrigel invasion assay. The invasiveness
of HUVECs was significantly decreased in the CM of KITENIN
siRNA-transfected DLD1 and SW480 cells compared to the findings for
the scramble siRNA-transfected cells (P=0.031 and 0.015,
respectively) (Fig. 1A). Next, we
examined whether KITENIN silencing can stimulate endothelial tube
formation, which mimics in vivo angiogenesis. CM from
KITENIN siRNA-transfected DLD1 and SW480 cells less effectively
stimulated tube formation than that from the respective scrambled
siRNA-transfected cells (P=0.047 and 0.038, respectively) (Fig. 1B). KITENIN silencing led to
decreased expression of the angiogenic inducers VEGF-A and HIF-1α
and increased expression of the angiogenic inhibitor angiostatin in
both tested cells (Fig. 1C). These
results suggested that KITENIN is capable of increasing both
endothelial cell invasion and tube formation, key events in
angiogenesis and neovascularization.
Impact of KITENIN silencing on
lymphangiogenesis in human colorectal cancer cells
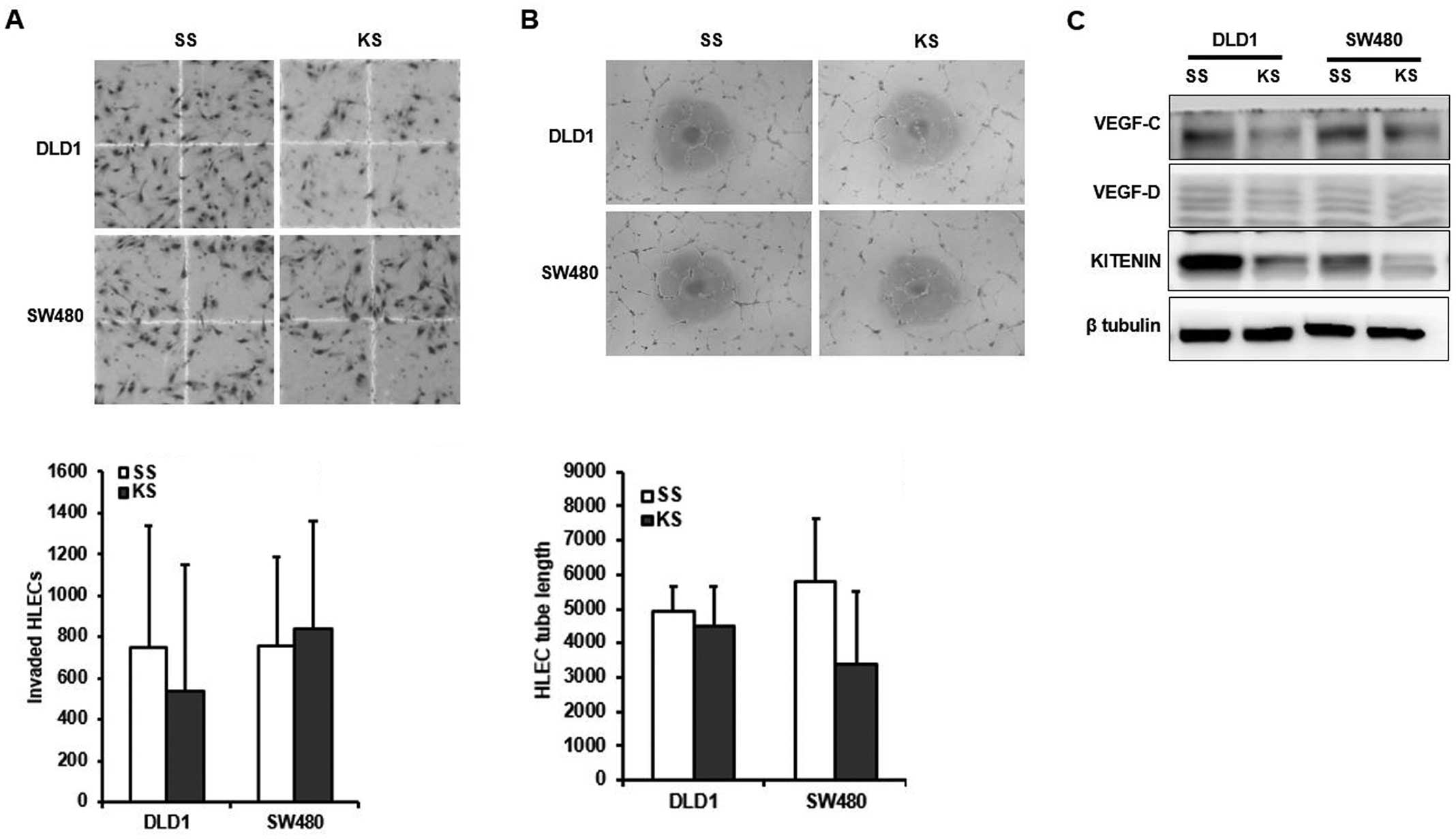
To evaluate the effects of KITENIN on
lymphangiogenesis in HLECs, we performed Matrigel invasion and tube
formation assays using CM from KITENIN and scrambled
siRNA-transfected DLD1 and SW480 cells. CM from KITENIN
siRNA-transfected DLD1 and SW480 cells did not inhibit invasion
(P=0.080 and 0.623, respectively) or tube formation (P=0.235 and
0.112, respectively) compared to that in the respective scrambled
siRNA-transfected cells (Fig. 2A and
B). KITENIN silencing resulted in decreased expression of the
lymphangiogenic inducer VEGF-C, but not VEGF-D in both tested cell
lines (Fig. 2C).
Correlations between KITENIN and
clinicopathological features in human colorectal cancers
To study the role of KITENIN in human colorectal
cancer progression, we investigated the protein expression of
KITENIN immunohistochemically in formalin-fixed, paraffin-embedded
tissue blocks obtained from 85 patients with colorectal cancer for
whom clinicopathological data were available. Survival and the
correlations between KITENIN immunostaining and clinicopathological
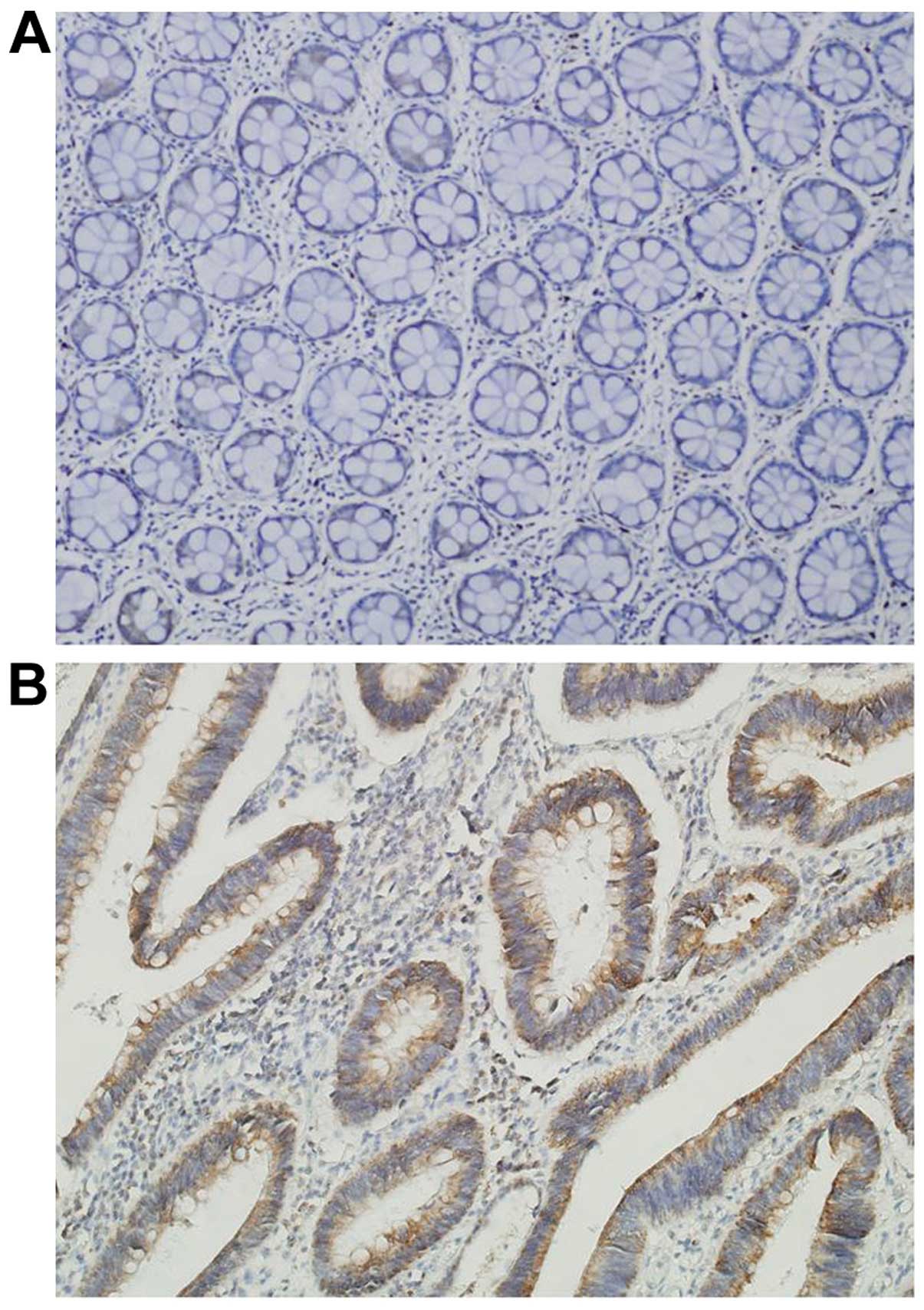
parameters were analyzed. KITENIN immunostaining was non-existent
or weak in the normal colorectal mucosa (Fig. 3A). KITENIN immunostaining was
predominantly identified in the cytoplasm of cancer cells, and it
was not detectable in the tumor stroma (Fig. 3B). The percentage of positive tumor
cells and the staining intensity for each sample were recorded. For
the 85 patient samples evaluated, positive-KITENIN expression was
observed in 39 (45.9%) colorectal cancer tissues (Table I). Immunostaining of KITENIN was
significantly associated with tumor stage, depth of invasion, lymph
node and distant metastasis (P<0.001, P= 0.004, P<0.001 and
P= 0.021, respectively) (Table I).
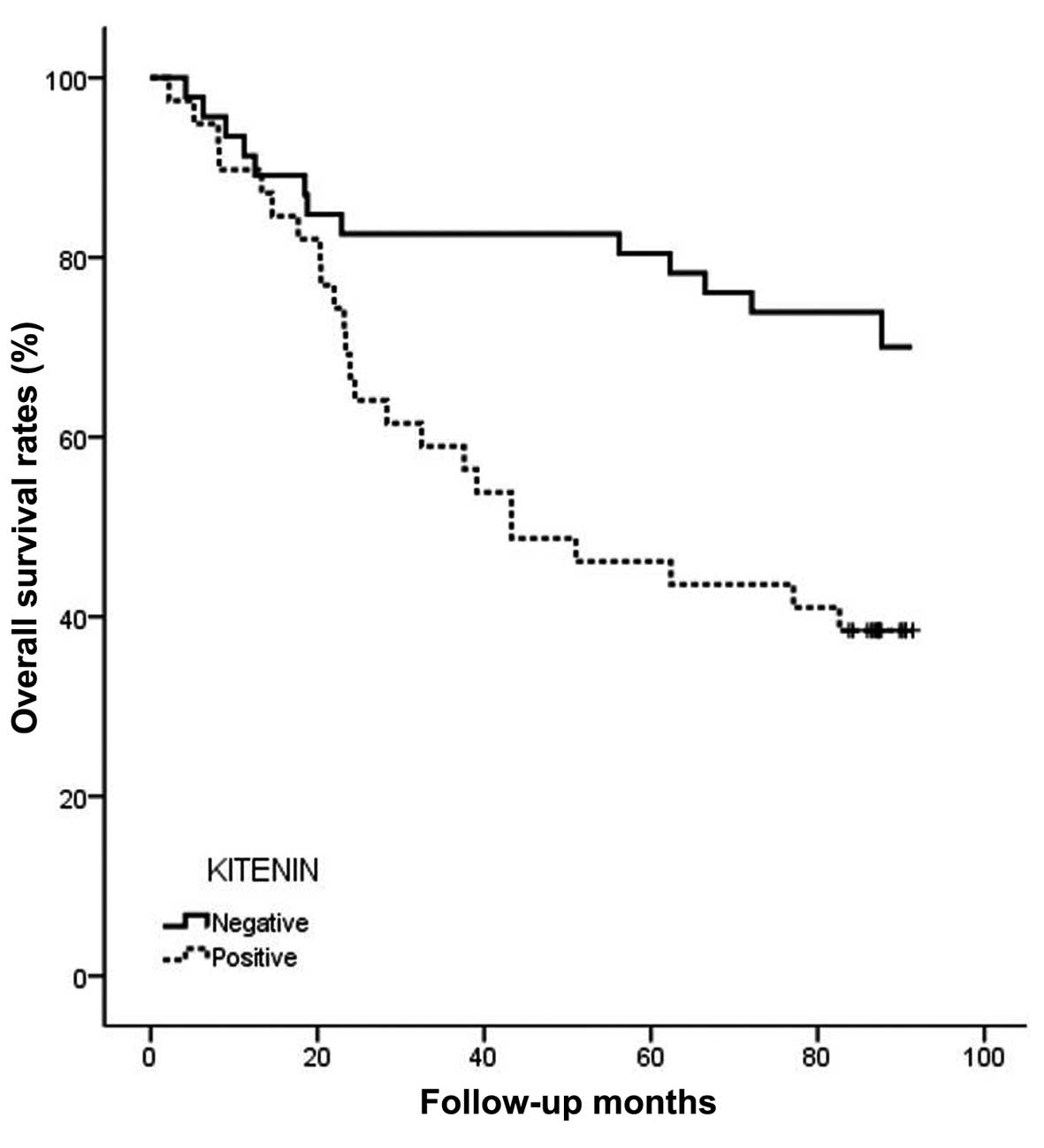
Moreover, overall survival for patients with positive KITENIN
immunostaining was significantly lower than the survival of the
patients with negative immunostaining (P=0.002) (Fig. 4).
 | Table ICorrelation between KITENIN
expression and the clinicopathological parameters of the colorectal
cancer cases. |
Table I
Correlation between KITENIN
expression and the clinicopathological parameters of the colorectal
cancer cases.
| Parameters | Total
(n=85) | KITENIN
| P-value |
|---|
Negative
(n=46) | Positive
(n=39) |
|---|
| Age (years) | | | | 0.073 |
| <66.1 | 39 | 17 | 22 | |
| ≥66.1 | 46 | 29 | 17 | |
| Gender | | | | 0.790 |
| Male | 51 | 27 | 24 | |
| Female | 34 | 19 | 15 | |
| Tumor size
(cm) | | | | 0.778 |
| <4.9 | 45 | 25 | 20 | |
| ≥4.9 | 40 | 21 | 19 | |
| Histological
type | | | | 0.497 |
|
Differentiated | 74 | 39 | 35 | |
|
Undifferentiated | 11 | 7 | 4 | |
| Stage | | | | <0.001 |
| I/II | 40 | 32 | 8 | |
| III/IV | 45 | 14 | 31 | |
| Depth of invasion
(T) | | | | 0.004 |
| T1/T2 | 21 | 17 | 4 | |
| T3/T4 | 64 | 29 | 35 | |
| Lymph node
metastasis (N) | | | | <0.001 |
| N0 | 43 | 33 | 10 | |
| N1-3 | 42 | 13 | 29 | |
| Distant metastasis
(M) | | | | 0.021 |
| M0 | 75 | 44 | 31 | |
| M1 | 10 | 2 | 8 | |
Correlation between KITENIN expression
and tumor cell angiogenesis or lymphangiogenesis in human
colorectal cancers
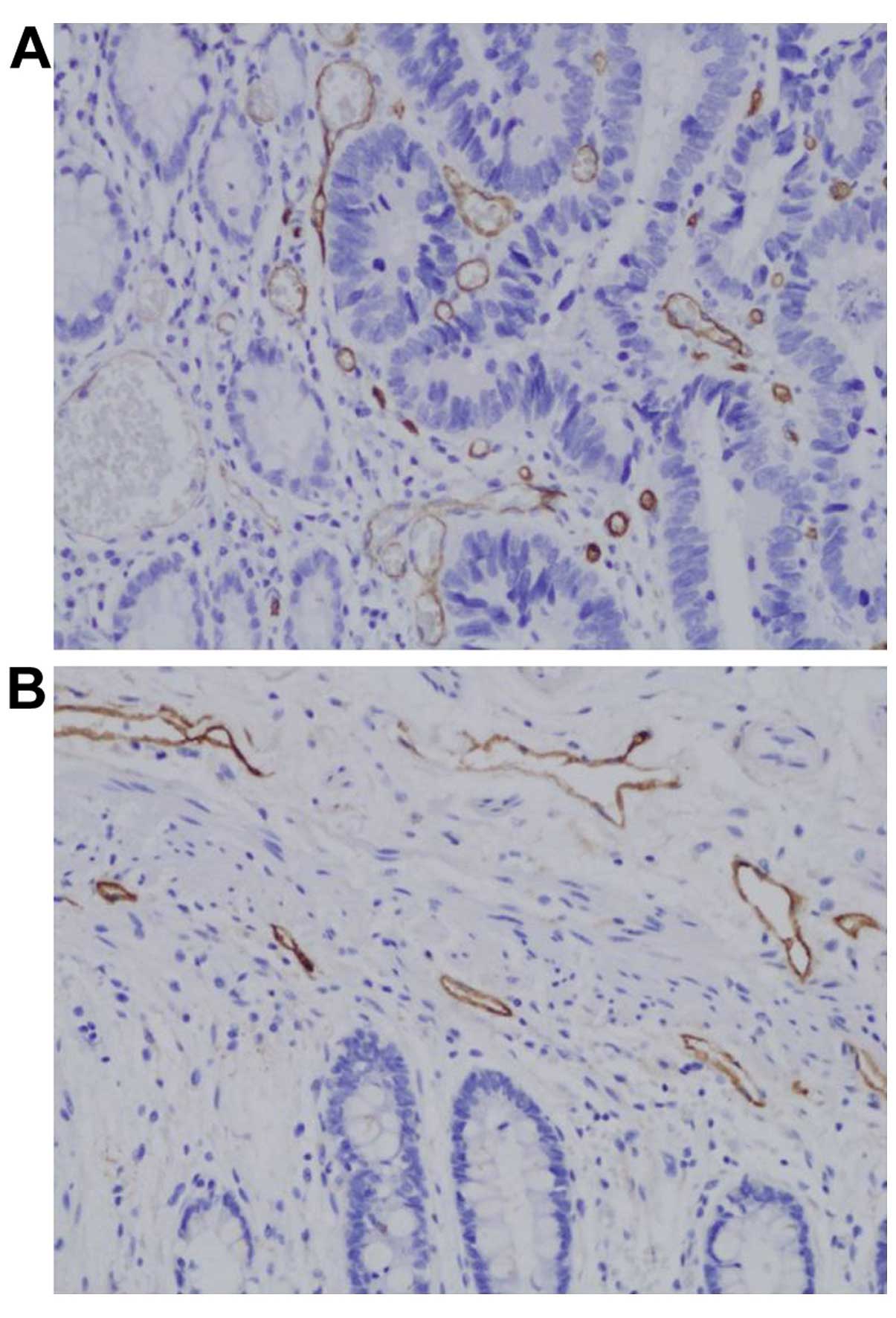
All tumor samples were subjected to immunostaining
for CD34 and D2-40 to identify tumor cell angiogenesis and
lymphangiogenesis (Fig. 5A and B).
The MVD for the 85 tumors ranged from 23.0 to 429.0 (mean,
115.2±74.7). The mean MVD of KITENIN-positive tumors was
133.4±85.0, which was significantly lower than the value for
KITENIN-negative tumors (P= 0.018) (Table II). The LVD for the 85 tumors
ranged from 4.0 to 31.3 (mean, 13.7±5.8). There was no significant
correlation between KITENIN expression and LVD (P=0.528) (Table II).
 | Table IICorrelation between KITENIN
expression and tumor cell angiogenesis or lymphangiogenesis in the
colorectal cancers. |
Table II
Correlation between KITENIN
expression and tumor cell angiogenesis or lymphangiogenesis in the
colorectal cancers.
| Indices | Total
(n=85) | KITENIN expression
| P-value |
|---|
Negative
(n=46) | Positive
(n=39) |
|---|
|
MVDa | 115.2±74.7 | 91.6±50.5 | 133.4±85.0 | 0.018 |
|
LVDa | 13.7±5.8 | 13.3±6.0 | 14.0±5.7 | 0.528 |
Correlation between MVD or LVD and
clinicopathological features in human colorectal cancers
The correlations between MVD or LVD and
clinicopathological parameters are summarized in Table III. When a mean MVD of 115.2 was
chosen as the cut-off for discrimination of the 85 patients into
two subgroups, 37 patients were determined to have low-MVD, whereas
48 patients had high-MVD. In addition, when a mean LVD of 13.7 was
chosen as the cut-off for discrimination of the 85 patients into
two subgroups, 39 patients were determined to have low-LVD and 46
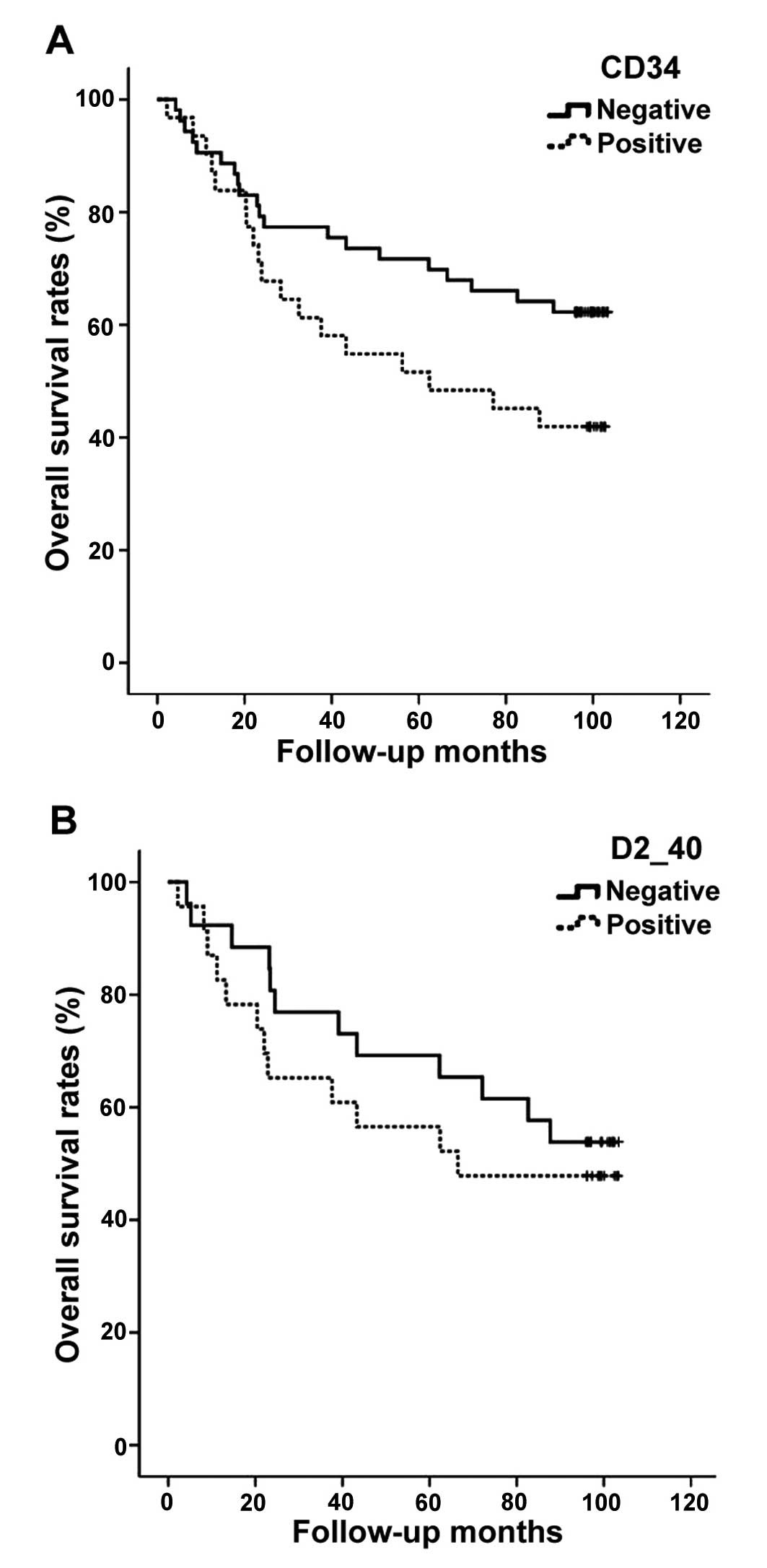
patients had high-LVD. No significant correlation was found between
MVD or LVD and various clinicopathological parameters (Table III). Moreover, overall survival
for patients with high-MVD or LVD was not significantly lower than
that for patients with low-MVD or LVD (P=0.073 and 0.492,
respectively) (Fig. 6A and B).
 | Table IIICorrelation between MVD or LVD and
clinicopathological parameters of the colorectal cancers. |
Table III
Correlation between MVD or LVD and
clinicopathological parameters of the colorectal cancers.
| Parameters | MVD
(mean ± SD) | P-value | LVD
(mean ± SD) | P-value |
|---|
| Stage | | 0.387 | | 0.091 |
| I/II | 104.9±64.9 | | 12.5±4.5 | |
| III/IV | 124.3±82.0 | | 14.7±6.5 | |
| Depth of invasion
(T) | | 0.179 | | 0.880 |
| T1/T2 | 137.4±85.4 | | 11.7±5.0 | |
| T3/T4 | 107.9±70.0 | | 14.3±5.9 | |
| Lymph node
metastasis (N) | | 0.376 | | 1.000 |
| N0 | 107.8±67.3 | | 13.3±5.5 | |
| N1-3 | 122.8±81.7 | | 14.0±5.9 | |
| Distant metastasis
(M) | | 0.795 | | 0.623 |
| M0 | 112.1±69.2 | | 13.6±6.0 | |
| M1 | 138.4±109.4 | | 14.4±0.6 | |
Discussion
Angiogenesis is an essential process needed by
primary tumors to grow and invade adjacent normal structures
(25–27). Angiogenesis is a complex process
controlled by a balance of angiogenic and angiostatic factors
involved in multiple pathways that result in endothelial cell
proliferation, differentiation and organization into a functional
network of vascular channels (25–27).
In the present study, we investigated the role and mechanisms of
KITENIN in promoting angiogenesis. In the present study, KITENIN
silencing decreased both endothelial cell migration and tube
formation for HUVECs, key events in angiogenesis and
neovascularization. In addition, KITENIN silencing resulted in
decreased expression of the angiogenic inducers VEGF-A and HIF-1α
and increased expression of the angiogenic inhibitor angiostatin in
human colorectal cancer cells. These results indicated that KITENIN
may play an important role in carcinogenesis by stimulating tumor
angiogenesis in concert with angiogenic and angiostatic factors in
human colorectal cancer.
Lymphangiogenesis is a dynamic process during
embryogenesis that does not occur in normal adult tissue. Indeed,
lymphangiogenesis is often activated in the tumor microenvironment
(27–29). Malignant tumors induce
lymphangiogenesis in primary tumors as well as draining sentinel
lymph nodes, thereby promoting lymph node metastasis. The
metastatic spread of tumor cells to lymph nodes is enhanced
following the induction of tumor lymphangiogenesis, which is driven
by lymphangiogenic factors such as VEGF-C and VEGF-D (27–29).
In the present study, KITENIN silencing did not decrease either
endothelial cell migration or tube formation in HLECs. In addition,
KITENIN silencing resulted in decreased expression of the
lymphangiogenic inducer VEGF-C, but not VEGF-D, in human colorectal
cancer cells. These results suggest that although the expression
VEGF-C, one of the central regulators of lymphangiogenesis, was
decreased by KITENIN silencing, KITENIN may not be a major factor
involved in lymphangiogenesis in colorectal cancer.
Next, we evaluated the expression of KITENIN in a
well-defined series of human colorectal cancers, with special
reference to patient prognosis. Previously, we reported that
KITENIN expression was significantly associated with advanced stage
and/or poor survival in various human cancers, including gastric,
colorectal, laryngeal and oral cavity cancer, and glioma (20–24).
In the present study, KITENIN expression was significantly
associated with stage, depth of invasion, lymph node and distant
metastasis, and poor survival. These results are in agreement with
our previous studies (20–24). Therefore, KITENIN expression plays
an important role in tumor progression, and this protein may serve
as a potential prognostic factor and target in human colorectal
cancer.
The intratumoral MVD as determined by
immunohistochemistry is considered to reflect the angiogenic
activity generated by neoplastic cells and the supporting stroma
(35). Numerous studies have
illustrated that increased angiogenesis as measured by MVD was
associated with the prognosis of patients with colorectal cancer
(36–38). In addition, previously,
immunostaining of podoplanin (D2-40) as a molecular marker specific
to the lymphatic endothelium has been used to assess
lymphangiogenesis in various human cancers (39,40).
In the present study, when a mean MVD (or LVD) value was chosen as
the cut-off for categorizing the study patients as having low or
high-MVD (or LVD), although the MVD and LVD were higher for
advanced tumors than for non-advanced tumors, no significant
correlation was found between MVD or LVD and various
clinicopathological parameters including patient survival. However,
many lines of evidence indicate that MVD and LVD are associated
with tumor progression and poor prognosis in colorectal cancer
(36–38,41–45).
These discrepancies may be attributable to the small size of in the
present study population, which may have influenced some results,
particularly the lack of association for various
clinicopathological parameters, and to the different scoring
systems and different antibodies used in immunohistochemistry as
quantitative markers.
Lastly, we evaluated the correlation between KITENIN
expression and tumor cell angiogenesis or lymphangiogenesis in
human colorectal cancer tissues to confirm the results of our human
colorectal cancer cell line studies. In the present study, the mean
MVD of the KITENIN-positive tumors was significantly higher than
that of KITENIN-negative tumors. However, the mean LVD of
KITENIN-positive tumors was not significantly different from that
of KITENIN-negative tumors. These results thus confirm the in
vitro findings that KITENIN silencing inhibits
angiogenesis.
Taken together, we found that KITENIN is associated
with tumor progression by enhancing angiogenesis in colorectal
cancer.
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar
|
|
2
|
Park SH, Song CW, Kim YB, Kim YS, Chun HR,
Lee JH, Seol WJ, Yoon HS, Lee MK, Lee JH, et al:
Clinicopathological characteristics of colon cancer diagnosed at
primary health care institutions. Intest Res. 12:131–138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee CK: Clinicopathological
characteristics of newly diagnosed colorectal cancers in community
gastroenterology practice. Intest Res. 12:87–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riethdorf S, Wikman H and Pantel K:
Review: Biological relevance of disseminated tumor cells in cancer
patients. Int J Cancer. 123:1991–2006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim ER and Kim YH: Clinical application of
genetics in management of colorectal cancer. Intest Res.
12:184–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu WM and Zhang XA: KAI1/CD82, a tumor
metastasis suppressor. Cancer Lett. 240:183–194. 2006. View Article : Google Scholar
|
|
8
|
Miranti CK: Controlling cell surface
dynamics and signaling: How CD82/KAI1 suppresses metastasis. Cell
Signal. 21:196–211. 2009. View Article : Google Scholar
|
|
9
|
Guo XZ, Friess H, Di Mola FF, Heinicke JM,
Abou-Shady M, Graber HU, Baer HU, Zimmermann A, Korc M and Büchler
MW: KAI1, a new metastasis suppressor gene, is reduced in
metastatic hepatocellular carcinoma. Hepatology. 28:1481–1488.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong JT, Suzuki H, Pin SS, Bova GS,
Schalken JA, Isaacs WB, Barrett JC and Isaacs JT: Down-regulation
of the KAI1 metastasis suppressor gene during the progression of
human prostatic cancer infrequently involves gene mutation or
allelic loss. Cancer Res. 56:4387–4390. 1996.PubMed/NCBI
|
|
11
|
Adachi M, Taki T, Ieki Y, Huang CL,
Higashiyama M and Miyake M: Correlation of KAI1/CD82 gene
expression with good prognosis in patients with non-small cell lung
cancer. Cancer Res. 56:1751–1755. 1996.PubMed/NCBI
|
|
12
|
Schindl M, Birner P, Breitenecker G and
Oberhuber G: Down-regulation of KAI1 metastasis suppressor protein
is associated with a dismal prognosis in epithelial ovarian cancer.
Gynecol Oncol. 83:244–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su JS, Arima K, Hasegawa M, Franco OE,
Umeda Y, Yanagawa M, Sugimura Y and Kawamura J: Decreased
expression of KAI1 metastasis suppressor gene is a recurrence
predictor in primary pTa and pT1 urothelial bladder carcinoma. Int
J Urol. 11:74–82. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JH, Seo YW, Park SR, Kim YJ and Kim
KK: Expression of a splice variant of KAI1, a tumor metastasis
suppressor gene, influences tumor invasion and progression. Cancer
Res. 63:7247–7255. 2003.PubMed/NCBI
|
|
15
|
Lee JH, Park SR, Chay KO, Seo YW, Kook H,
Ahn KY, Kim YJ and Kim KK: KAI1 COOH-terminal interacting
tetraspanin (KITENIN), a member of the tetraspanin family,
interacts with KAI1, a tumor metastasis suppressor, and enhances
metastasis of cancer. Cancer Res. 64:4235–4243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Cho ES, Kim MY, Seo YW, Kho DH,
Chung IJ, Kook H, Kim NS, Ahn KY and Kim KK: Suppression of
progression and metastasis of established colon tumors in mice by
intravenous delivery of short interfering RNA targeting KITENIN, a
metastasis-enhancing protein. Cancer Res. 65:8993–9003. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kho DH, Bae JA, Lee JH, Cho HJ, Cho SH,
Lee JH, Seo YW, Ahn KY, Chung IJ and Kim KK: KITENIN recruits
Dishevelled/PKC delta to form a functional complex and controls the
migration and invasiveness of colorectal cancer cells. Gut.
58:509–519. 2009. View Article : Google Scholar
|
|
18
|
Lee JK, Bae JA, Sun EG, Kim HD, Yoon TM,
Kim K, Lee JH, Lim SC and Kim KK: KITENIN increases invasion and
migration of mouse squamous cancer cells and promotes pulmonary
metastasis in a mouse squamous tumor model. FEBS Lett. 583:711–717.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho SB, Park YL, Park SJ, Park SY, Lee WS,
Park CH, Choi SK, Heo YH, Koh YS, Cho CK, et al: KITENIN is
associated with activation of AP-1 target genes via MAPK cascades
signaling in human hepatocellular carcinoma progression. Oncol Res.
19:115–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryu HS, Park YL, Park SJ, Lee JH, Cho SB,
Lee WS, Chung IJ, Kim KK, Lee KH, Kweon SS, et al: KITENIN is
associated with tumor progression in human gastric cancer.
Anticancer Res. 30:3479–3486. 2010.PubMed/NCBI
|
|
21
|
Lee S, Song YA, Park YL, Cho SB, Lee WS,
Lee JH, Chung IJ, Kim KK, Rew JS and Joo YE: Expression of KITENIN
in human colorectal cancer and its relation to tumor behavior and
progression. Pathol Int. 61:210–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JK, Yoon TM, Seo DJ, Sun EG, Bae JA,
Lim SC, Choi YD, Lee JH, Joo YE and Kim KK: KAI1 COOH-terminal
interacting tetraspanin (KITENIN) expression in early and advanced
laryngeal cancer. Laryngoscope. 120:953–958. 2010.PubMed/NCBI
|
|
23
|
Yoon TM, Kim SA, Lee JK, Park YL, Kim GY,
Joo YE, Lee JH, Kim KK and Lim SC: Expression of KITENIN and its
association with tumor progression in oral squamous cell carcinoma.
Auris Nasus Larynx. 40:222–226. 2013. View Article : Google Scholar
|
|
24
|
Lee KH, Ahn EJ, Oh SJ, Kim O, Joo YE, Bae
JA, Yoon S, Ryu HH, Jung S, Kim KK, et al: KITENIN promotes glioma
invasiveness and progression, associated with the induction of EMT
and stemness markers. Oncotarget. 6:3240–3253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mittal K, Ebos J and Rini B: Angiogenesis
and the tumor micro-environment: Vascular endothelial growth factor
and beyond. Semin Oncol. 41:235–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y and Adjei AA: Targeting
angiogenesis in cancer therapy: Moving beyond vascular endothelial
growth gactor. Oncologist. 20:660–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gomes FG, Nedel F, Alves AM, Nör JE and
Tarquinio SB: Tumor angiogenesis and lymphangiogenesis:
Tumor/endothelial crosstalk and cellular/microenvironmental
signaling mechanisms. Life Sci. 92:101–107. 2013. View Article : Google Scholar :
|
|
28
|
Stacker SA, Williams SP, Karnezis T,
Shayan R, Fox SB and Achen MG: Lymphangiogenesis and lymphatic
vessel remodelling in cancer. Nat Rev Cancer. 14:159–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duong T, Koopman P and Francois M: Tumor
lymphangiogenesis as a potential therapeutic target. J Oncol.
2012:2049462012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weng W, Feng J, Qin H and Ma Y: Molecular
therapy of colorectal cancer: Progress and future directions. Int J
Cancer. 136:493–502. 2015.
|
|
31
|
Marques I, Araújo A and de Mello RA:
Anti-angiogenic therapies for metastatic colorectal cancer: Current
and future perspectives. World J Gastroenterol. 19:7955–7971. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Royston D and Jackson DG: Mechanisms of
lymphatic metastasis in human colorectal adenocarcinoma. J Pathol.
217:608–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun XF and Zhang H: Clinicopathological
significance of stromal variables: Angiogenesis, lymphangiogenesis,
inflammatory infiltration, MMP and PINCH in colorectal carcinomas.
Mol Cancer. 5:432006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
American Joint Committee on Cancer
Classification (AJCC): Cancer Staging Manual. 6 edition. revised.
Lippincott-Raven; Philadelphia: pp. 113–123. 2002
|
|
35
|
Weider N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar
|
|
36
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
37
|
Svagzdys S, Lesauskaite V, Pavalkis D,
Nedzelskiene I, Pranys D and Tamelis A: Microvessel density as new
prognostic marker after radiotherapy in rectal cancer. BMC Cancer.
9:952009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Des Guetz G, Uzzan B, Nicolas P, Cucherat
M, Morere JF, Benamouzig R, Breau JL and Perret GY: Microvessel
density and VEGF expression are prognostic factors in colorectal
cancer. Meta-analysis of the literature. Br J Cancer. 94:1823–1832.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Donizy P, Rudno-Rudzinska J, Halon A,
Dziegala M, Kabarowski J, Frejlich E, Dziegiel P, Kielan W and
Matkowski R: Intratumoral but not peritumoral lymphatic vessel
density measured by D2-40 expression predicts poor outcome in
gastric cancer - ROC curve analysis to find cut-off point.
Anticancer Res. 34:3113–3118. 2014.PubMed/NCBI
|
|
40
|
Pula B, Wojnar A, Witkiewicz W, Dziegiel P
and Podhorska-Okolow M: Podoplanin expression in cancer-associated
fibroblasts correlates with VEGF-C expression in cancer cells of
invasive ductal breast carcinoma. Neoplasma. 60:516–524. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao J, Knutsen A, Arbman G, Carstensen J,
Frånlund B and Sun XF: Clinical and biological significance of
angiogenesis and lymphangiogenesis in colorectal cancer. Dig Liver
Dis. 41:116–122. 2009. View Article : Google Scholar
|
|
42
|
Sundov Z, Tomic S, Alfirevic S, Sundov A,
Capkun V, Nincevic Z, Nincevic J, Kunac N, Kontic M, Poljak N, et
al: Prognostic value of MVD, LVD and vascular invasion in lymph
node-negative colon cancer. Hepatogastroenterology. 60:432–438.
2013.PubMed/NCBI
|
|
43
|
Yan G, Zhou XY, Cai SJ, Zhang GH, Peng JJ
and Du X: Lymph-angiogenic and angiogenic microvessel density in
human primary sporadic colorectal carcinoma. World J Gastroenterol.
14:101–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Holmqvist A, Gao J, Adell G, Carstensen J
and Sun XF: The location of lymphangiogenesis is an independent
prognostic factor in rectal cancers with or without preoperative
radiotherapy. Ann Oncol. 21:512–517. 2010. View Article : Google Scholar
|
|
45
|
Jakob C, Aust DE, Liebscher B, Baretton
GB, Datta K and Muders MH: Lymphangiogenesis in regional lymph
nodes is an independent prognostic marker in rectal cancer patients
after neoadjuvant treatment. PLoS One. 6:e274022011. View Article : Google Scholar : PubMed/NCBI
|