Introduction
After decades of development, immunotherapy has
arrived as a potent cancer therapeutic option (1). This is best demonstrated by the
clinical efficacy of immune checkpoint inhibitors targeting
CTLA-4/CD80/CD86 and PD-1/PD-L1 in cutaneous melanoma and other
malignancies such as lung cancer (reviewed in refs. 2–7).
Cancer immunotherapy is based on the ability of immune system to
specifically distinguish and target non-self from self (8). The robustness and specificity of
antitumor immune responses are like two wheels of a cart in the
success of cancer immunotherapy. The checkpoint inhibitors can
induce robust antitumor immune responses through activation of
systemic immunity and enhancement of T cell activity by blocking
negative signals, enhancing positive signals or altering the
cytokine milieu (9). However, a
significant population of patients experienced immune-related
adverse events (irAE) in the treatment with checkpoint inhibitors
although the majority of them were low-grade. Most commonly
reported irAEs were gastrointestinal and dermatological, and less
commonly endocrine, hepatic and neurological toxicities (10). In addition, it is likely that any
successful immunotherapy strategy will rely to some extent on
adaptive immunity, since any sustained antitumor immune responses
will depend on the development of immunological memory (8). Therefore, immunotherapies targeting
tumor-associated-antigens (TAAs) that induce tumor-specific immune
responses are all the more needed in the new era of immune
checkpoint inhibitors.
Protein synthesis is a fundamental metabolic process
of cells and its deregulation critically affects cellular
functions. Protein synthesis (mRNA translation) consists of three
steps: initiation, elongation and termination. One of important
regulatory mechanisms of protein synthesis is eukaryotic elongation
factor 2 kinase (eEF2K), eukaryotic elongation factor 2 (eEF2)
pathway. eEF2 is a gene which catalyzes the translocation of the
elongated peptidyl-tRNA from A to P sites of the ribosome and plays
an essential role in elongation step of protein synthesis (11,12).
eEF2K inhibits eEF2 activity through phosphorylation of eEF2 and
slows down the rate of protein synthesis (13–15).
Growing evidence has shown the importance of eEF2K-eEF2 pathway in
physiological and pathological settings (16). In the nervous system, eEF2K-eEF2
pathway is involved in processes such as learning and memory
(17–19). In malignancies, eEF2K mRNA levels
were increased in glioblastoma and medulloblastoma and the
increased eEF2K mRNA expression was associated with poor prognosis,
which could be explained by eEF2K-induced resistance to nutrient
deprivation (20). In addition,
mTORC1 pathway and the oncogenic Ras/Raf/MEK/ERK pathway cooperate
to inhibit eEF2K activity through regulation of its phosphorylation
(21).
In previous studies, we showed that eEF2 was
overexpressed in various types of solid tumors such as intestinal,
lung, pancreatic and breast cancers, glioblastoma multiforme and
non-Hodkin's lymphoma (22,23). Knockdown of eEF2 by eEF2-specific
shRNA inhibited cell growth of these tumor cells and eEF2 promoted
progression of G2/M of the cell cycle in association with
activation of Akt and a G2/M regulator, cdc2 proteins, resulting in
promotion of in vivo cancer cell growth (22). Moreover, we identified
HLA-A*02:01- or HLA-A*24:02-restricted 9-mer
eEF2 peptides and demonstrated that these eEF2 peptides could
induce eEF2-specific cytotoxic T lymphocytes (CTLs) from peripheral
blood mononuclear cells of healthy volunteers (23). These results indicated that
eEF2-targeting, peptide-based cancer immunotherapy should be worth
exploring.
In the present study, we identified two mouse MHC
class I-restricted eEF2-derived 9-mer peptides, EF17 (17–25 aa) and
EF180 (180–188 aa) and examined the safety and efficacy of
eEF2-targeting, peptide-based cancer immunotherapy using a mouse
model.
Materials and methods
Mice
Male C57BL/6 (H-2Db) mice were obtained
from Clea Japan, Inc. (Tokyo, Japan), maintained in a specific
pathogen-free (SPF) containment facility in accordance with the
guidelines of the Regulations on Animal Experimentation at Osaka
University, and were used for experiments at 6–8 weeks of age.
Animal experiments were approved by Osaka University Gene
Modification Experiments Safety Committee and Animal
Experimentation Committee.
Peptide synthesis and adjuvant
The primary amino acid sequence of mouse eEF2 was
analyzed for consensus motifs for 9-mer peptides capable of binding
to mice MHC class I molecule (H-2Db) using peptide
binding prediction programs; NetMHC 3.0 (http://www.cbs.dtu.dk/services/NetMHC/), SYFPEITHI
(http://www.syfpeithi.de), HLA peptide motif
search (http://www-bimas.cit.nih.gov/molbio/hla_bind/) and
RANKPEP (http://imed.med.ucm.es/Tools/rankpep.html). Then, the
top 2 candidate peptides for H-2Db, EF17 (17–25
a.a.ANIRNMSVI) and EF180 (a.a.180–188 RIVENVNVI) (Table I) were synthesized in immunological
purity (Sigma-Genosys, Hokkaido, Japan). Synthesized peptide was
dissolved in distilled water and stored at −20°C until use. An
incomplete Freund's adjuvant (IFA) Montanide ISA 51 was obtained
from Seppic S.A. (Orsay, France).
 | Table IBinding of mouse eEF2 peptide to
H-2Db molecules. |
Table I
Binding of mouse eEF2 peptide to
H-2Db molecules.
| Peptide | Position | | Amino acid
sequence |
|---|
| EF17 | 17–25 aa | | ANIRNMSVI |
| EF180 | 180–188 aa | | RIVENVNVI |
|
| Program | Rank | Peptide | Affinity (nM) |
|
| NetMHC3.0 | 1 | EF17 | 130 |
| 2 | EF180 | 414 |
|
| Program | Rank | Peptide | Binding score |
|
| SYFPEITHI | 1 | EF17 | 26 |
| 2 | EF180 | 22 |
| HLA peptide | 1 | EF180 | 720 |
| Motif search | 2 | EF17 | 660 |
| RANKPEP | 1 | EF17 | 21.6 |
| 4 | EF180 | 16.2 |
Cells
RMAS, a transporter associated with antigen
processing (TAP)-deficient subline of Rauscher leukemia
virus-induced lymphoma cell line of C57BL/6 origin (RMA) was kindly
provided by Dr K. Kärre (Karolinska Institute, Sweden) through Dr
H.-G. Rammensee (University of Tubingen, Germany) (24). C1498, a WT1-non-expressing murine
leukemia cell line of C57BL/6 origin, was purchased from American
Type Culture Collection (ATCC) (Rockville, MD, USA). WT1-expressing
murine WT1-C1498 (mWT1-C1498) was generated by transduction of
C1498 cells with murine WT1 17AA(+)KTS(+) isoform cDNA that was
inserted into pcDNA3.1(+) mammalian expression vector (Invitrogen,
Tokyo, Japan) (25).
Vaccination schedule and in vivo tumor
challenge
For experiments to evaluate the elicitation of
eEF2-specific CTL responses and the damage in normal organs by
vaccination with mouse eEF2 peptide, either EF17 or EF180 peptide
(100 µg) emulsified with Montanide ISA51 was intradermally
administered eight times into flank region of mice at one week
interval.
To evaluate in vivo antitumor effects of
mouse eEF2 peptide vaccine, 3×105 eEF2 expressing
mWT1-C1498 cells in 100 µl of phosphate-buffered saline
(PBS) were intradermally implantated to abdominal region of mice on
day 0, and then one each of EF17 and EF180 (100 µg)
emulsified with Montanide ISA51 or PBS was intradermally
administered into flank region of mice on days 1, 8, 15 and 22.
Tumor growth was assessed by measuring the longest diameter of the
palpable mass.
51Cr release cytotoxicity and
flow cytometric cytokine assays
Seven or 8 days after the last vaccination,
splenocytes were collected from the vaccinated or PBS-treated mice.
The splenocytes were then stimulated with their respective peptides
and cultured in complete medium containing 10% heat-inactivated
FCS, 45% RPMI-1640 medium, 45% AIM-V medium, 1 × non-essential
amino acid (Gibco), 50 µM 2-mercaptoethanol, 50 IU/ml
penicillin and 50 µg/ml streptomycin. Two and four days
later, recombinant interleukin-2 (rIL-2; Shionogi Biomedical
Laboratories, Osaka, Japan) was added to the culture medium at the
concentration of 20 IU/ml. After six days of culture, a
51Cr release cytotoxicity assay was performed against
eEF2 peptide-pulsed or -unpulsed RMAS cells for eEF2-specific CTL
activity, as previously described (25).
For cytokine assay, after 10 days of culture,
splenocytes (2×105 cells) were stimulated with 20
µg/ml of their respective vaccinated peptide and brefeldin A
(BFA; Sigma) was added to the concentration of 10 µg/ml for
5 h in 96-well U-bottom plates. After 5 h of culture, the cells
were stained with anti-mouse CD3 (17A2) and anti-mouse CD8 (53–6.7)
(BD Biosciences, San Jose, CA, USA). Then, the cells were fixed and
permeabilized by fixation and permeabilization solution, and
stained with anti-mouse IFN-γ (XMG1.2) and anti-mouse TNF-α
(MP6-XT22) (both from BD Biosciences). Stained cells were gated for
CD3+ CD8+ cells and analyzed by FACSAria (BD
Biosciences).
Colony assay
For colony assay of colony-forming-unit
granulocyte-macrophage (CFU-GM), bone marrow cells were collected
from mouse limbs seven days after the last vaccination, plated at
1×104 cells/plate in methylcellulose medium containing
10 ng/ml IL-3, 10 ng/ml IL-6, 50 ng/ml SCF and 3 U/ml
erythropoietin (EPO) (MethoCult M3434; Stem Cell Technologies,
Vancouver, BC, Canada), and cultured at 37°C in a humidified
incubator under 5% CO2. Colonies were counted on day
8.
Histological analysis
Formalin-fixed tissue sections of brain, heart,
lung, liver, pancreas, kidney, urogenital organs, stomach,
intestines, bone marrow and spleen of the EF2 peptide-vaccinated or
PBS-treated mice were cut from each paraffin-block. After dewaxing
and rehydration, the sections were stained with hematoxylin and
eosin. The tissues from EF2-peptide vaccinated mice were
pathologically evaluated in comparison with those from PBS-treated
mice by a pathologist.
Statistical analysis
The statistical significance in a difference of
organ weights and colony numbers among test groups was assessed by
Kruskal-Wallis and Student's t-tests, respectively. Significant
differences in disease-free survival among test groups were
evaluated with the log-rank test.
Results
In vivo induction of eEF2-specific CTL
responses
Primary sequence of mouse eEF2 protein was analyzed
for 9-mer peptides with consensus motifs that were essential for
binding to the mouse MHC class I molecule (H-2Db) using
peptide binding prediction programs and two candidate peptides,
EF17 (17–25 aa ANIRNMSVI) and EF180 (180–188 aa RIVENVNVI)
(Table I) were selected.
To investigate whether vaccination with these two
eEF2 protein-derived 9-mer peptides induced in vivo eEF2
peptide-specific CTL responses, mice were eight times vaccinated
with one each of Montanide ISA51-adjuvanted EF17 and EF180 at
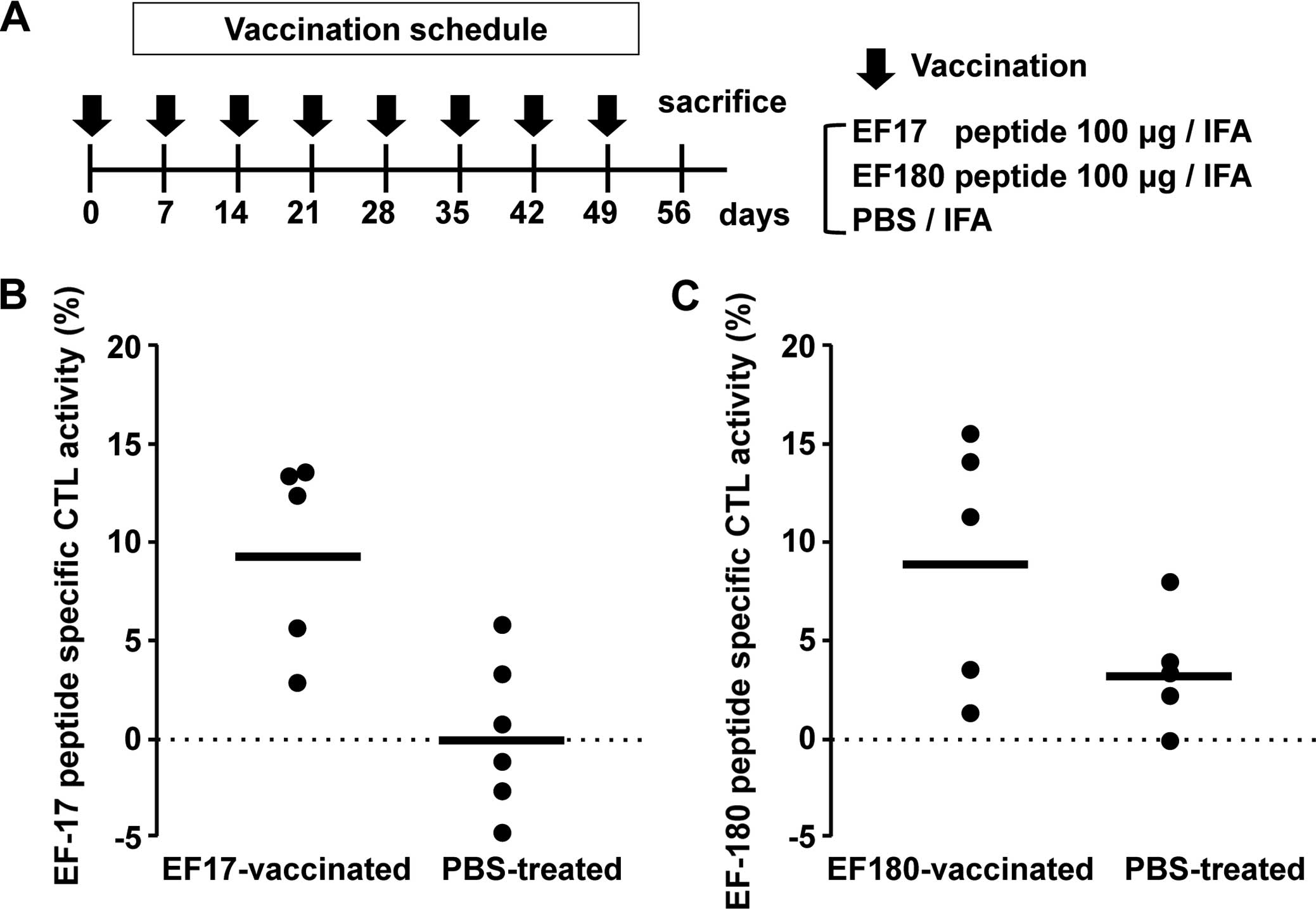
weekly intervals (Fig. 1A).
Splenocytes were collected from these mice 7 days after the last
vaccination, stimulated in vitro with respective eEF2
peptide, and assayed for eEF2 peptide-specific cytotoxic activity
against eEF2 peptide-pulsed RMAS cells. The splenocytes from
EF17-vaccinated mice showed higher EF17 peptide-specific cytotoxic
activity than those from Montanide ISA51-adjuvanted PBS-treated
mice (Fig. 1B). Similarly, the
splenocytes from EF180-vaccinated mice showed higher EF180
peptide-specific cytotoxic activity than those from Montanide
ISA51-adjuvanted PBS-treated mice (Fig.
1C).
These results indicated that vaccination with
EF2-derived 9-mer peptides, EF17 and EF180, induced in vivo
eEF2-specific CTL responses.
No damage of normal tissues by eEF2
peptide vaccination
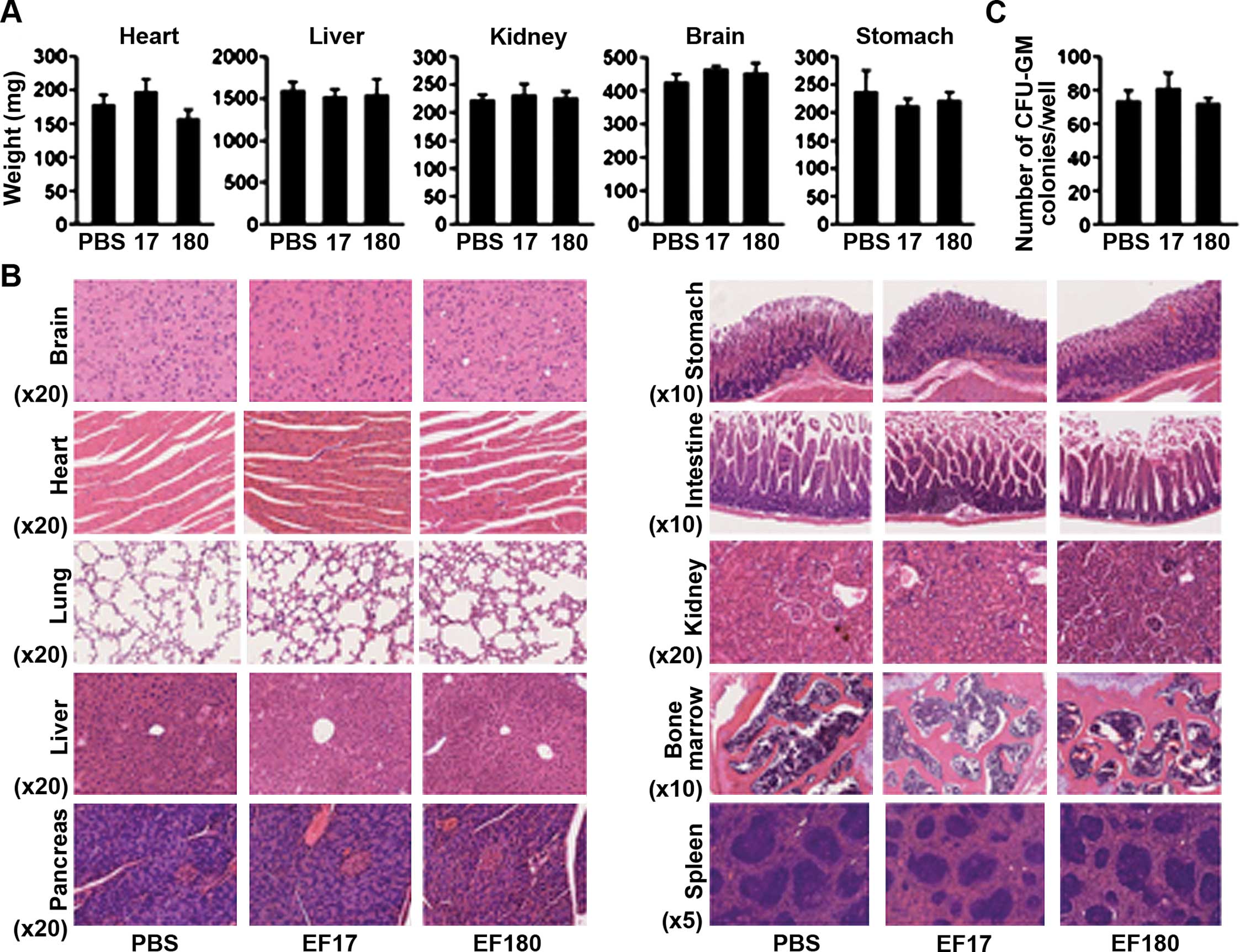
To evaluate the safety of EF2 peptide vaccination,
adverse effects of EF2 peptide vaccination on normal organs were
examined (Fig. 2). First, the
weights of organs such as heart, liver, kidney, brain and stomach
were examined. There were no significant differences in the weights
of such organs among PBS-treated, EF17- and EF180-vaccinated mice
(Fig. 2A). Next, specimen from
formalin-fixed paraffin-embedded blocks of brain, heart, lung,
liver, pancreas, stomach, intestine, kidney, bone marrow and spleen
were pathologically examined. Representative results are shown in a
Fig. 2B. All organs showed normal
structure and cellularity in all mice examined, and no pathological
changes that would be caused by immune response such as mononuclear
cells (lymphocytes) infiltration and tissue destruction were
observed. Furthermore, the colony-forming ability of bone marrow
cells was examined. No differences in numbers of CFU-GM colonies
were found among the PBS-treated, EF17- and EF180-vaccinated mice
(Fig. 2C).
These results showed that eEF2 peptide vaccination
did not damage normal tissues.
Induction of in vivo antitumor effects
against eEF2-expressing tumor by eEF2 peptide vaccination
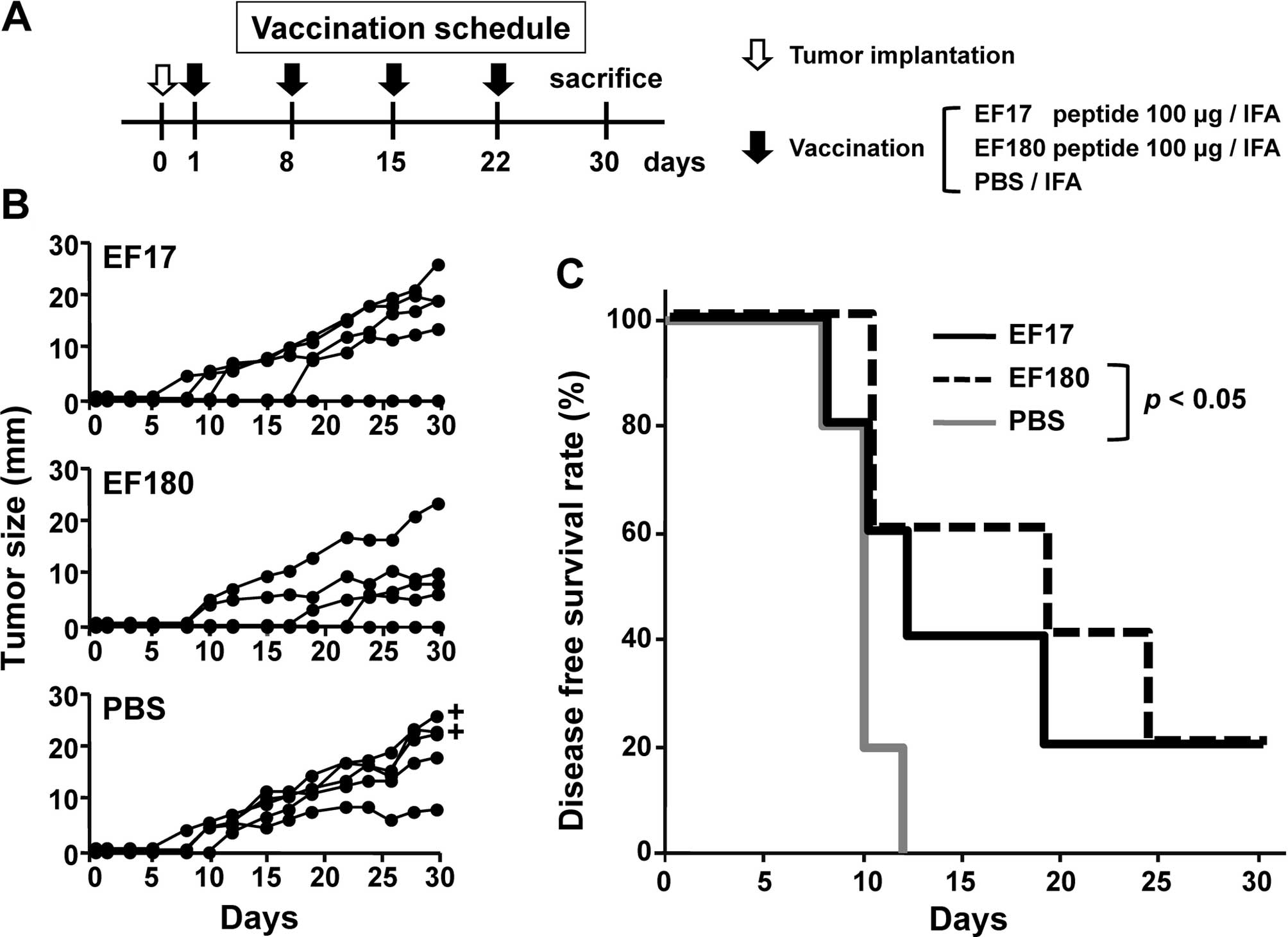
Whether or not vaccination with eEF2-derived 9-mer
peptides EF17 or EF180 induced in vivo antitumor effects
against eEF2-expressing tumor was investigated in a mouse
therapeutic model (Fig. 3A). Mice
were implanted with eEF2-expressing mWT1-C1498 cells on day 0 and
then vaccinated four times with either Montanide ISA51-adjuvanted
EF17 or EF180, or treated with Montanide ISA51-adjuvanted PBS at
weekly intervals and tumor size (Fig.
3B) and survival rates (Fig.
3C) were assessed. All of the five PBS-treated mice developed
tumors until day 15 and two of the five died on day 30. In
EF17-vaccinated mice, two did not develop tumors until day 15 and
one of the two rejected a tumor. All EF17-vaccinated mice were
alive on day 30. In EF180-vaccinated mice, three did not develop
tumors until day 15 and one of the three rejected a tumor. All
EF180-vaccinated mice were alive on day 30 (Fig. 3C). Disease-free survival rates of
PBS-treated, EF17- and EF180-vaccinated mice were 0, 20 and 20%,
respectively, on day 30. The disease-free survival rates of
EF180-vaccinated mice were significantly higher than those of
PBS-treated mice (p<0.05) (Fig. 3C).
These results indicated that vaccination with eEF2
peptides, EF17 and EF180 induced in vivo antitumor effects
against eEF2-expressing tumor.
Correlation between eEF2-specific CTL
responses and antitumor effects
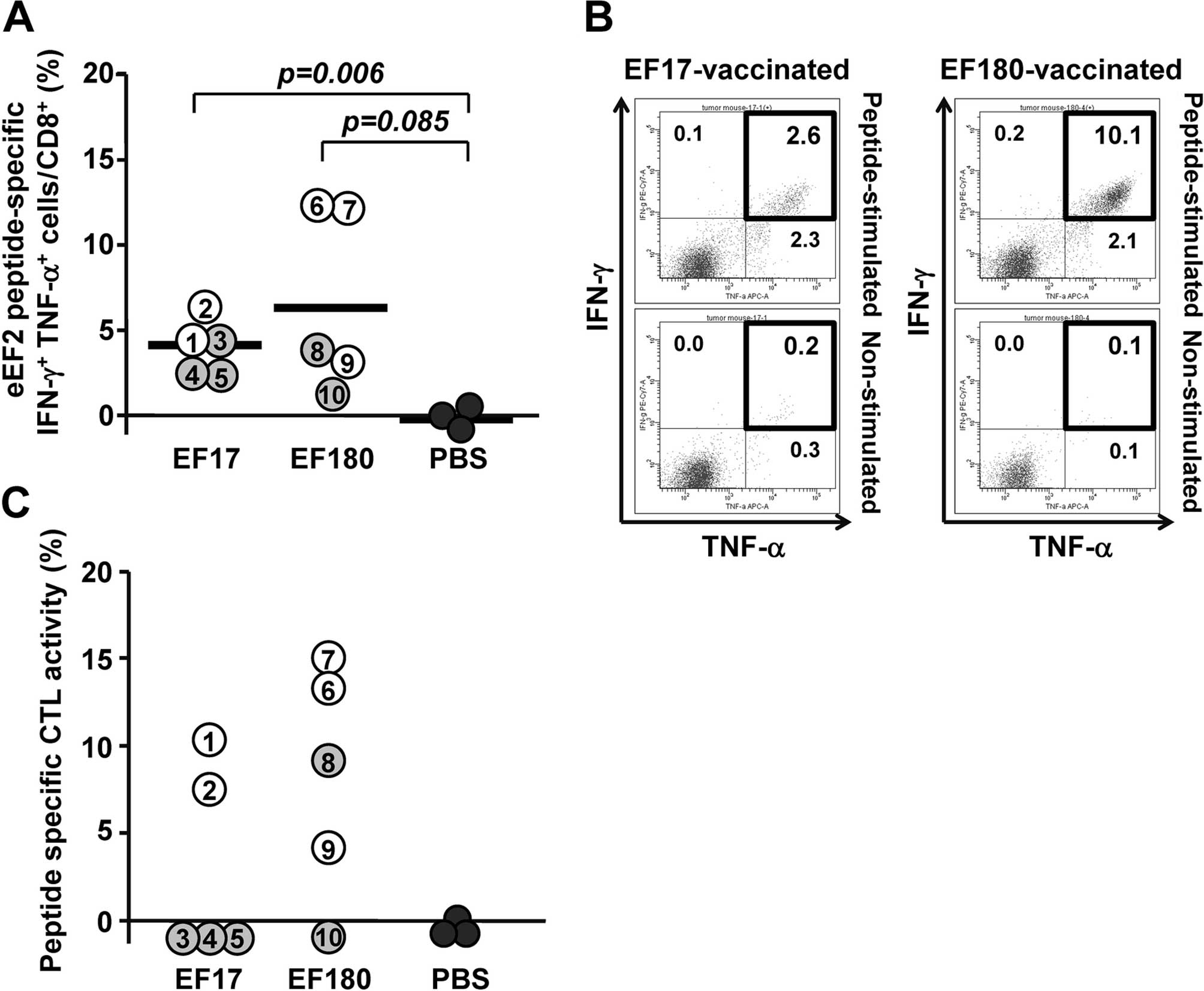
To analyze eEF2-specific CTL responses in the
therapeutic model, splenocytes were collected from eEF2
peptide-vaccinated mice on day 30 and examined for frequency of
CD8+ T cells producing Th1-type cytokines such as IFN-γ
and TNF-α, and for eEF2 peptide-specific cytotoxic activity. Flow
cytometric analysis showed that EF17-specific IFN-γ- and
TNF-α-producing (IFN-γ+ TNF-α+)
CD8+ T cells were detected in splenocytes from
EF17-vaccinated mice with frequency ranging from 2.5 to 6.5%, which
was significantly higher than that in splenocytes from Montanide
ISA51-adjuvanted PBS-treated mice (Fig.
4A). Similarly, EF180-specific IFN-γ+
TNF-α+ CD8+ T cells were detected in
splenocytes from EF180-vaccinated mice with frequency ranging from
1.0 to 12.1%, which was higher than that in splenocytes from
Montanide ISA51-adjuvanted PBS-treated mice although the difference
was not statistically significant (Fig.
4A). When responders and non-responders were defined to be mice
that did not and did develop tumors until day 15, respectively, the
frequency of eEF2 peptide-specific IFN-γ+
TNF-α+ CD8+ T cells was higher in responders
than non-responders in both EF17 and EF180 peptide-vaccinated mice
(Fig. 4A). Representative results
of flow cytometric analysis of IFN-γ+ TNF-α+
CD8+ T cells in splenocyes from mice that rejected the
implanted tumors are shown in Fig.
4B.
To examine a correlation between eEF2-specific CTL
responses and antitumor effects by eEF2 peptide vaccination, eEF2
peptide-specific cytotoxic activity was compared between responders
and non-responders (Fig. 4C). In
EF17-vaccinated mice, the two responders induced EF17-specific CTLs
whereas all the non-responders could not induce EF17-specific CTLs.
In EF180-vaccinated mice, all the three responders induced
EF180-specific CTLs whereas one of the two non-responder could not
induce EF180-specific CTLs.
These results indicated that vaccination with EF17
or EF180 elicited respective eEF2 peptide-specific CTL responses
and that antitumor effect correlated with the robustness of the
eEF2 peptide-specific CTL responses.
Discussion
In a previous study, we showed that eEF2 was
overexpressed in various types of cancers, that eEF2 gene product
elicited spontaneous humoral IgG immune responses in patients with
eEF2-expressing tumors, and that HLA-A*02:01- or
HLA-A*24:02-restriced 9-mer eEF2 peptides induced
eEF2-specific cytotoxic T lymphocytes (CTLs) from peripheral blood
mononuclear cells (PBMCs) of healthy volunteers (23). Importantly, induction of
eEF2-specific CTLs from PBMCs of healthy volunteers indicated that
precursors of eEF2-specific CTLs naturally existed in healthy
individuals without cancer. These results strongly indicated that
eEF2-expressing tumor-bearing patients should have eEF2-specific
CTLs and/or the CTL precursors and thus the eEF2 peptide-based
immunotherapy should be effective for such patients. In the present
study, two mouse eEF2-derived 9-mer peptides, EF17 (17–25 aa) and
EF180 (180–188 aa) were identified as eEF2-specific CTL epitopes,
and a mouse model of eEF2 peptide-based immunotherapy was
established.
eEF2 is an essential translational elongation factor
and is ubiquitously expressed in normal cells. However,
eEF2-specific CTLs that were induced by repeated eEF2 peptide
vaccination did not give rise to damage in normal cells. This can
be explained by the low expression of the target molecule eEF2 in
normal cells. Our previous immunohistochemical study showed that
eEF2 protein was undetectable in normal cells, whereas, it was
overexpressed in tumor cells in the majority of the cases examined.
Reportedly, the expression levels of target antigen proteins in
tumor cells were associated with recognition of tumor cells by CTLs
(26). A threshold level in antigen
expression in tumor cells could be determined for their lysis by
CTLs (27). Therefore, normal cells
with low or undetectable eEF2 expression should escape from the
attack by eEF2-specific CTLs. This is supported by the findings
that breast cancer MCF7 cells with undetectable eEF2 protein
expression escaped from the attack by eEF2-specific CTLs in an
in vitro killing assay (23).
eEF2 was overexpressed in 94.0% of non-Hodgkin's
lymphoma, 80.4% of lung cancer, 75.0% of glioblastoma multiforme,
75.0% of prostate cancer, 73.3% of esophageal squamous cell
carcinoma, 60.7% of pancreatic cancer, 52.4% of head and neck
squamous cell carcinoma and 50.0% of breast cancer. Particularly,
eEF2 was overexpressed in 100% (10/10) of follicular type of
non-Hodgkin's lymphoma (23).
Therefore, eEF2 peptide immunotherapy should be applicable to
various types of cancers, especially to non-Hodgkin's lymphoma.
The loss of target molecules can be a mechanism of
tumor escape from immune surveillance (28). We previously demonstrated that
knockdown of eEF2 molecule by eEF2-specific shRNA significantly
inhibited tumor cell growth. Furthermore, we showed that eEF2
promoted progression of G2/M in the cell cycle and enhanced in
vitro and in vivo tumor cell growth (22). These results indicated that it was
unlikely that loss of eEF2 antigen-mediated tumor cell escape from
eEF2-specific cellular immune responses since its loss should be
disadvantageous for growth and survival of the tumor cells.
It is well known that CD4+ T as well as
CD8+ T cells are critical components of antitumor
cellular immune responses (29–32).
CD4+ T cells can be involved in generation and
activation of CD8+ T cells (33–35)
and maintenance of CD8+ T cell memory functions
(36,37). Moreover, recent studies have shown
that CD4+ T cells could develop cytotoxic activity
against tumor cells (38,39). We have previously reported that
eEF2-specific IgG autoantibody was detectable in eEF2-expressing
tumor-bearing patients (23),
indicating that eEF2-specific CD4+ helper T cells had
been spontaneously elicited in such patients. Therefore, eEF2
peptide vaccination in clinical settings will induce eEF2-specific
CD8+ CTLs and the CTLs could eradicate eEF2-expressing
tumor cells in cooperation with spontaneously induced
CD4+ helper T cells. Therefore, eEF2-targeting
immunotherapy could be a promising treatment strategy for
cancer.
Acknowledgments
The present study was supported in part by a
Grant-in-Aid from the Ministry of Education, Culture, Sports,
Science and Technology, Japan, and the Ministry of Health, Labour
and Welfare, Japan.
Abbreviations:
|
BFA
|
brefeldin A
|
|
CFU-GM
|
colony-forming-unit
granulocyte-macrophage
|
|
CTLs
|
cytotoxic T lymphocytes
|
|
eEF2
|
eukaryotic elongation factor 2
|
|
eEF2K
|
eukaryotic elongation factor 2
kinase
|
|
EPO
|
erythropoietin
|
|
IFA
|
incomplete Freund's adjuvant
|
|
irAE
|
immune related adverse events
|
|
SPF
|
specific pathogen-free
|
|
TAAs
|
tumor-associated-antigens
|
|
TAP
|
a transporter associated with antigen
processing
|
|
rIL-2
|
recombinant interleukin-2
|
References
|
1
|
Madorsky Rowdo FP, Baron A, Urrutia M and
Mordoh J: Immunotherapy in cancer: A combat between tumors and the
immune system; you win some, you lose some. Front Immunol.
6:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ott PA, Hodi FS and Robert C: CTLA-4 and
PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable
clinical benefit in melanoma patients. Clin Cancer Res.
19:5300–5309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cranmer LD and Hersh E: The role of the
CTLA4 blockade in the treatment of malignant melanoma. Cancer
Invest. 25:613–631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarnaik AA and Weber JS: Recent advances
using anti-CTLA-4 for the treatment of melanoma. Cancer J.
15:169–173. 2009.PubMed/NCBI
|
|
5
|
Tsai KK, Zarzoso I and Daud AI: PD-1 and
PD-L1 antibodies for melanoma. Hum Vaccin Immunother. 10:3111–3116.
2014. View Article : Google Scholar
|
|
6
|
Anagnostou VK and Brahmer JR: Cancer
immunotherapy: A future paradigm shift in the treatment of
non-small cell lung cancer. Clin Cancer Res. 21:976–984. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guibert N, Delaunay M and Mazières J:
Targeting the immune system to treat lung cancer: Rationale and
clinical experience. Ther Adv Respir Dis. 9:105–120. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Monjazeb AM, Zamora AE, Grossenbacher SK,
Mirsoian A, Sckisel GD and Murphy WJ: Immunoediting and antigen
loss: Overcoming the achilles heel of immunotherapy with antigen
non-specific therapies. Front Oncol. 3:1972013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JE and Lim M: The role of checkpoints
in the treatment of GBM. J Neurooncol. 123:413–423. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Howell M, Lee R, Bowyer S, Fusi A and
Lorigan P: Optimal management of immune-related toxicities
associated with check-point inhibitors in lung cancer. Lung Cancer.
88:117–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spahn CM, Gomez-Lorenzo MG, Grassucci RA,
Jørgensen R, Andersen GR, Beckmann R, Penczek PA, Ballesta JP and
Frank J: Domain movements of elongation factor eEF2 and the
eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J.
23:1008–1019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taylor DJ, Nilsson J, Merrill AR, Andersen
GR, Nissen P and Frank J: Structures of modified eEF2 80S ribosome
complexes reveal the role of GTP hydrolysis in translocation. EMBO
J. 26:2421–2431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Redpath NT, Price NT, Severinov KV and
Proud CG: Regulation of elongation factor-2 by multisite
phosphorylation. Eur J Biochem. 213:689–699. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carlberg U, Nilsson A and Nygård O:
Functional properties of phosphorylated elongation factor 2. Eur J
Biochem. 191:639–645. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gismondi A, Caldarola S, Lisi G, Juli G,
Chellini L, Iadevaia V, Proud CG and Loreni F: Ribosomal stress
activates eEF2K-eEF2 pathway causing translation elongation
inhibition and recruitment of terminal oligopyrimidine (TOP) mRNAs
on polysomes. Nucleic Acids Res. 42:12668–12680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kenney JW, Moore CE, Wang X and Proud CG:
Eukaryotic elongation factor 2 kinase, an unusual enzyme with
multiple roles. Adv Biol Regul. 55:15–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verpelli C, Piccoli G, Zibetti C, Zanchi
A, Gardoni F, Huang K, Brambilla D, Di Luca M, Battaglioli E and
Sala C: Synaptic activity controls dendritic spine morphology by
modulating eEF2-dependent BDNF synthesis. J Neurosci. 30:5830–5842.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belelovsky K, Elkobi A, Kaphzan H, Nairn
AC and Rosenblum K: A molecular switch for translational control in
taste memory consolidation. Eur J Neurosci. 22:2560–2568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Im HI, Nakajima A, Gong B, Xiong X, Mamiya
T, Gershon ES, Zhuo M and Tang YP: Post-training dephosphorylation
of eEF-2 promotes protein synthesis for memory consolidation. PLoS
One. 4:e74242009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leprivier G, Remke M, Rotblat B, Dubuc A,
Mateo AR, Kool M, Agnihotri S, El-Naggar A, Yu B, Somasekharan SP,
et al: The eEF2 kinase confers resistance to nutrient deprivation
by blocking translation elongation. Cell. 153:1064–1079. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Regufe da Mota S, Liu R, Moore CE,
Xie J, Lanucara F, Agarwala U, Pyr Dit Ruys S, Vertommen D, Rider
MH, et al: Eukaryotic elongation factor 2 kinase activity is
controlled by multiple inputs from oncogenic signaling. Mol Cell
Biol. 34:4088–4103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura J, Aoyagi S, Nanchi I, Nakatsuka
S, Hirata E, Shibata S, Fukuda M, Yamamoto Y, Fukuda I, Tatsumi N,
et al: Overexpression of eukaryotic elongation factor eEF2 in
gastrointestinal cancers and its involvement in G2/M progression in
the cell cycle. Int J Oncol. 34:1181–1189. 2009.PubMed/NCBI
|
|
23
|
Oji Y, Tatsumi N, Fukuda M, Nakatsuka S,
Aoyagi S, Hirata E, Nanchi I, Fujiki F, Nakajima H, Yamamoto Y, et
al: The translation elongation factor eEF2 is a novel
tumor-associated antigen overexpressed in various types of cancers.
Int J Oncol. 44:1461–1469. 2014.PubMed/NCBI
|
|
24
|
Oka Y, Udaka K, Tsuboi A, Elisseeva OA,
Ogawa H, Aozasa K, Kishimoto T and Sugiyama H: Cancer immunotherapy
targeting Wilms' tumor gene WT1 product. J Immunol. 164:1873–1880.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakajima H, Oka Y, Tsuboi A, Tatsumi N,
Yamamoto Y, Fujiki F, Li Z, Murao A, Morimoto S, Hosen N, et al:
Enhanced tumor immunity of WT1 peptide vaccination by interferon-β
administration. Vaccine. 30:722–729. 2012. View Article : Google Scholar
|
|
26
|
Liu G, Ying H, Zeng G, Wheeler CJ, Black
KL and Yu JS: HER-2, gp100, and MAGE-1 are expressed in human
glioblastoma and recognized by cytotoxic T cells. Cancer Res.
64:4980–4986. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riker AI, Kammula US, Panelli MC, Wang E,
Ohnmacht GA, Steinberg SM, Rosenberg SA and Marincola FM: Threshold
levels of gene expression of the melanoma antigen gp100 correlate
with tumor cell recognition by cytotoxic T lymphocytes. Int J
Cancer. 86:818–826. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Budhu S, Wolchok J and Merghoub T: The
importance of animal models in tumor immunity and immunotherapy.
Curr Opin Genet Dev. 24:46–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hunder NN, Wallen H, Cao J, Hendricks DW,
Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA and Yee
C: Treatment of metastatic melanoma with autologous CD4+
T cells against NY-ESO-1. N Engl J Med. 358:2698–2703. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujiki F, Oka Y, Kawakatsu M, Tsuboi A,
Tanaka-Harada Y, Hosen N, Nishida S, Shirakata T, Nakajima H,
Tatsumi N, et al: A clear correlation between WT1-specific Th
response and clinical response in WT1 CTL epitope vaccination.
Anticancer Res. 30:2247–2254. 2010.PubMed/NCBI
|
|
31
|
Topalian SL: MHC class II restricted tumor
antigens and the role of CD4+ T cells in cancer
immunotherapy. Curr Opin Immunol. 6:741–745. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bourgeois C and Tanchot C: Mini-review CD4
T cells are required for CD8 T cell memory generation. Eur J
Immunol. 33:3225–3231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ridge JP, Di Rosa F and Matzinger P: A
conditioned dendritic cell can be a temporal bridge between a
CD4+ T-helper and a T-killer cell. Nature. 393:474–478.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujiki F, Oka Y, Tsuboi A, Kawakami M,
Kawakatsu M, Nakajima H, Elisseeva OA, Harada Y, Ito K, Li Z, et
al: Identification and characterization of a WT1 (Wilms Tumor Gene)
protein-derived HLA-DRB1*0405-restricted 16-mer helper
peptide that promotes the induction and activation of WT1-specific
cytotoxic T lymphocytes. J Immunother. 30:282–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujiki F, Oka Y, Kawakatsu M, Tsuboi A,
Nakajima H, Elisseeva OA, Harada Y, Li Z, Tatsumi N, Kamino E, et
al: A WT1 protein-derived, naturally processed 16-mer peptide, WT1,
is a promiscuous helper peptide for induction of WT1-specific 332
Th1-type CD4+ T cells. Microbiol Immunol. 52:591–600.
2008. View Article : Google Scholar
|
|
36
|
Shedlock DJ and Shen H: Requirement for
CD4 T cell help in generating functional CD8 T cell memory.
Science. 300:337–339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Janssen EM, Lemmens EE, Wolfe T, Christen
U, von Herrath MG and Schoenberger SP: CD4+ T cells are
required for secondary expansion and memory in CD8+ T
lymphocytes. Nature. 421:852–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Quezada SA, Simpson TR, Peggs KS, Merghoub
T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et
al: Tumor-reactive CD4+ T cells develop cytotoxic
activity and eradicate large established melanoma after transfer
into lymphopenic hosts. J Exp Med. 207:637–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin Y, Fujiki F, Katsuhara A, Oka Y,
Tsuboi A, Aoyama N, Tanii S, Nakajima H, Tatsumi N, Morimoto S, et
al: HLA-DPB1*05: 01-restricted WT1332-specific
TCR-transduced CD4+ T lymphocytes display a helper
activity for WT1-specific CTL induction and a cytotoxicity against
leukemia cells. J Immunother. 36:159–170. 2013. View Article : Google Scholar : PubMed/NCBI
|