Introduction
Breast cancer is the most common type of cancer and
remains the leading cause of cancer death among women in the world
(1). Metastasis is the main cause
of breast cancer death (2). Breast
cancer metastasis is a complex pathophysiological process involving
epithelial-mesenchymal transition (EMT), migration and motility,
invasion, and metastatic growth (3). The molecular mechanism underlying
breast cancer metastasis is not fully understood.
Mounting evidence shows that cancer cells are
different from normal cells in their metabolic properties. Normal
cells mostly depend on mitochondrial oxidative phosphorylation to
produce energy in the form of ATP. In contrast, cancer cells prefer
breakdown of glucose for energy rather than oxidative
phosphorylation, even in the presence of available oxygen. This
phenomenon is known as aerobic glycolysis, or Warburg effect, which
is a hallmark of cancer cells (4–9).
However, there is no report that directly links increased
glycolysis to breast cancer cell migration.
Fatty acid synthase (FASN) is a key enzyme involved
in neoplastic lipogenesis and the production of long-chain fatty
acids from acetyl-coenzyme A (CoA) and malonyl-CoA. In breast
cancer cells, there is a positive correlation between
over-expression of FASN and amplification and/or overexpression of
ErbB2 (10,11). ErbB2 (Her2/neu) is an
oncogene that is over-expressed in 25–30% of human primary breast
cancer and is associated with a poor prognosis (12). It has been demonstrated that both
ErbB2 and FASN enhance the transformation and/or metastatic
potentials of breast cancer cells (13–18).
Our previous study showed that ErbB2 promotes breast cancer cell
glycolysis via upregulation of lactate dehydrogenase A (LDHA), a
key enzyme that catalyzes the production of lactate (19). Therefore, we hypothesize that both
FASN and ErbB2 promote cell migration via glycolysis in breast
cancer.
Heregulin-β1 (HRG-β1, also known as neuregulin-β1),
a member of the epidermal growth factor (EGF) family (20), is an ErbB3 and ErbB4 ligand that
leads to the formation of ErbB2-ErbB3/ErbB4 heterodimers, resulting
in activation of the ErbB2 signaling pathway (21). HRG-β1 contributes to breast cancer
cell migration, invasion, and metastasis (22–24).
Whether HRG-β1 can induce glycolysis, and whether HRG-β1-induced
glycolysis contributes to cell migration, is unknown. To explore
HRG-β1's ability to induce glycolysis, MCF7 cells were used because
they express ErbB2, ErbB3, and ErbB4 receptors (25).
In this study, we investigated the importance of
FASN, ErbB2-mediated glycolysis in breast cancer cell migration. We
report that FASN, ErbB2-high-expressing SK-BR-3 cells displayed
higher levels of glycolysis and migration than FASN,
ErbB2-low-expressing MCF7 cells. In addition, inhibition of FASN by
cerulenin impaired glycolysis and migration in SK-BR-3 cells.
Finally, we show that inhibition of glycolysis by 2-DG or OX
reverses HRG-β1-induced migration. These results indicate that
breast cancer cells are dependent on FASN, ErbB2-mediated
glycolysis for migration, suggesting that glycolysis is a novel
factor influencing migration and that it plays an important role in
breast cancer progression.
Materials and methods
Cell lines and cell cultures
Human breast cancer cell line MCF7 was obtained from
the Cell Bank of Chinese Academy of Sciences, Shanghai, China.
SK-BR-3 was a gift from Professor Shengyong Yang of Sichuan
University. MCF7 cells were cultured in DMEM/F12 (Hyclone) with 10%
fetal bovine serum (FBS, Biological Industries) and
penicillin/streptomycin (PS, Hyclone). SK-BR-3 cells were
maintained in DMEM (Hyclone) supplemented with 10% FBS and PS.
Cells were cultured at 37°C in a 5% CO2 incubator.
Cell treatments with reagents
SK-BR-3 cells (1×106 cells/well) were
plated in 6-well plates for 24 h and DMSO or cerulenin (10
µg/ml, Cayman) was added into the medium. Twenty-four hours
after treatment, media was collected for glucose uptake and lactate
production assays and cells were trypsinized for Transwell and
western blot assays. MCF7 cells (8×105 cells/well) were
plated in 6-well plates for 24 h. The medium was changed to 0.5%
FBS medium and cells were starved for 24 h. Cells were treated with
vehicle, heregulin-β1 (HRG-β1, 100 ng/ml, PeproTech), HRG-β1 plus
cerulenin (10 µg/ml), 2-deoxyglucose (2-DG, 0.5 mM, Sigma)
or oxamate (OX, 50 mM, Sigma). 24 h after treatment, media was
collected for glucose uptake and lactate production assays and
cells were trypsinized for Transwell and western blot assays.
Glucose uptake and lactate production
assays
Glucose uptake was measured using Glucose Oxidase
Assay kit (Applygen, China) and results were normalized to the
total cellular protein amounts. Lactate production in the medium
was performed by using Lactate Assay kit (Nanjing Jiancheng
Bioengineering Institute, China) according to the manufacturer's
protocol. Results were normalized to the total cellular protein
amounts.
Western blot assay
Cells were harvested and lysed in a buffer
containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1%
Triton, 1 mM PMSF and Protease Inhibitor Cocktail (Sigma) for 20
min on ice. Lysates were cleared by centrifugation at 15,000 g at
4°C for 20 min. Supernatants were collected and protein
concentrations were determined by Bradford assay (Bio-Rad).
Proteins were subjected to electrophoresis on SDS-polyacrylamide
gels and were then transferred to PVDF membranes (Bio-Rad).
Membranes were blocked in Tris buffered saline (TBS) (pH 7.4) with
5% non-fat dry milk and probed with primary antibodies as indicated
in the figure legends. The following antibodies were used: FASN
(sc-55580, Santa Cruz), ErbB2 (2156, Cell Signaling Technology),
LDHA (3582, Cell Signaling Technology), β-actin (A2228, Sigma),
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (AG019, Beyotime,
China). Immunoreactive bands were visualized by horseradish
peroxidase (HRP)-conjugated secondary antibodies (Bio-Rad) using
ECL Western Blotting Substrate (Pierce). The fold expression of
protein was quantified using Image Lab software. Protein expression
in control group was set as 1.0.
Cell transfection
pcDNA3.0 was purchased from Hangzhou Baosai
Biotechnology, China and pcDNA3.0/ErbB2 was purchased from Addgene.
Transient transfection was performed using the Lipofectamine 2000
transfection reagent (Invitrogen) according to the manufacturer's
protocol. Forty-eight hours after transfection, cells were
trypsinized for Transwell and western blot assays, and culture
media was collected for glucose uptake and lactate production
assays.
Wound healing assay
MCF7 and SK-BR-3 cells were plated in 6-well plates
and allowed to grow to confluence. Wounds were made using a 10
µl pipette tip and indicated reagents were added to the
cells. Wound closure was monitored over a 24 or 48 h period and
photographs were taken at 0, 24 or 48 h after wounding. Wound width
at 0, 24 or 48 h was measured and the results were expressed as
wound closure (%) = (width at 0 h − width at × h)/width at 0 h ×
100% (x=24 or 48).
Transwell migration assay
Transwell migration assay was performed using Falcon
cell culture inserts (Corning). Cells were trypsinized and
suspended in medium with 0.5% FBS. MCF7 cells (3×105) or
SK-BR-3 cells (1×105) were seeded in the top of the
inserts and placed in the wells containing medium with 10% FBS.
After 48 h incubation at 37°C, the inserts were washed with PBS
twice and cells on the upper surface of the inserts were gently
removed with a cotton swab. The inserts were fixed with 10%
methanol, rinsed with ultra-pure water, and stained with 0.1%
crystal violet for 20 min. Cells that migrated onto the lower
surface were counted under a microscope in five random fields.
Statistical analysis
All experiments were repeated three times.
Statistical analysis was performed using GraphPad Prism version
5.0. The data were expressed as means ± SE. The difference between
groups was detected using Student's t-test. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
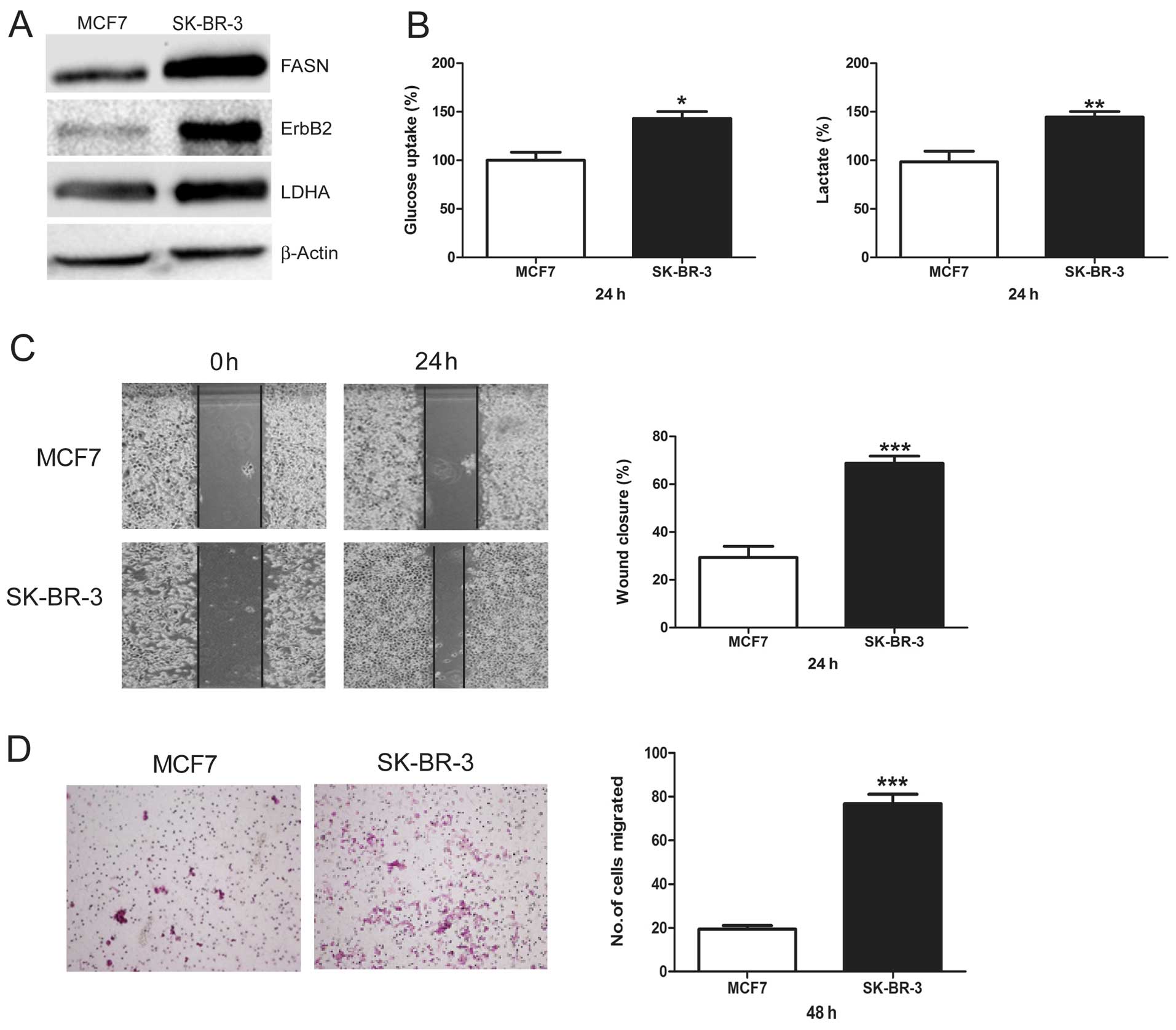
FASN, ErbB2-high-expressing SK-BR-3 cells
show increased glycolysis and migration versus FASN,
ErbB2-low-expressing MCF7 cells
First, we demonstrated that MCF7 cells displayed low
levels of FASN and ErbB2 and SK-BR-3 cells had high levels of FASN
and ErbB2 (Fig. 1A). Then, we
compared LDHA protein levels between these two cell lines. We found
that SK-BR-3 cells showed much higher LDHA protein levels than MCF7
cells (Fig. 1A). Next, glucose
uptake and lactate production, which are two important markers of
glycolysis, were measured and compared between MCF7 and SK-BR-3
cells. SK-BR-3 cells showed a significantly higher glucose uptake
and lactate production than MCF7 cells (Fig. 1B). Finally, we compared the
migratory potential between MCF7 and SK-BR-3 cells using wound
healing assay and Transwell assay. Compared to MCF7 cells, SK-BR-3
cells showed a higher percentage of wound closure (Fig. 1C) and migrated cell numbers
(Fig. 1D), indicating that SK-BR-3
cells had a greater basal migration capacity than MCF7 cells. These
results suggest a consistent relationship between FASN, ErbB2,
glycolysis, and cell migration, indicating that increased
glycolysis predicts higher migratory potential in breast cancer
cells.
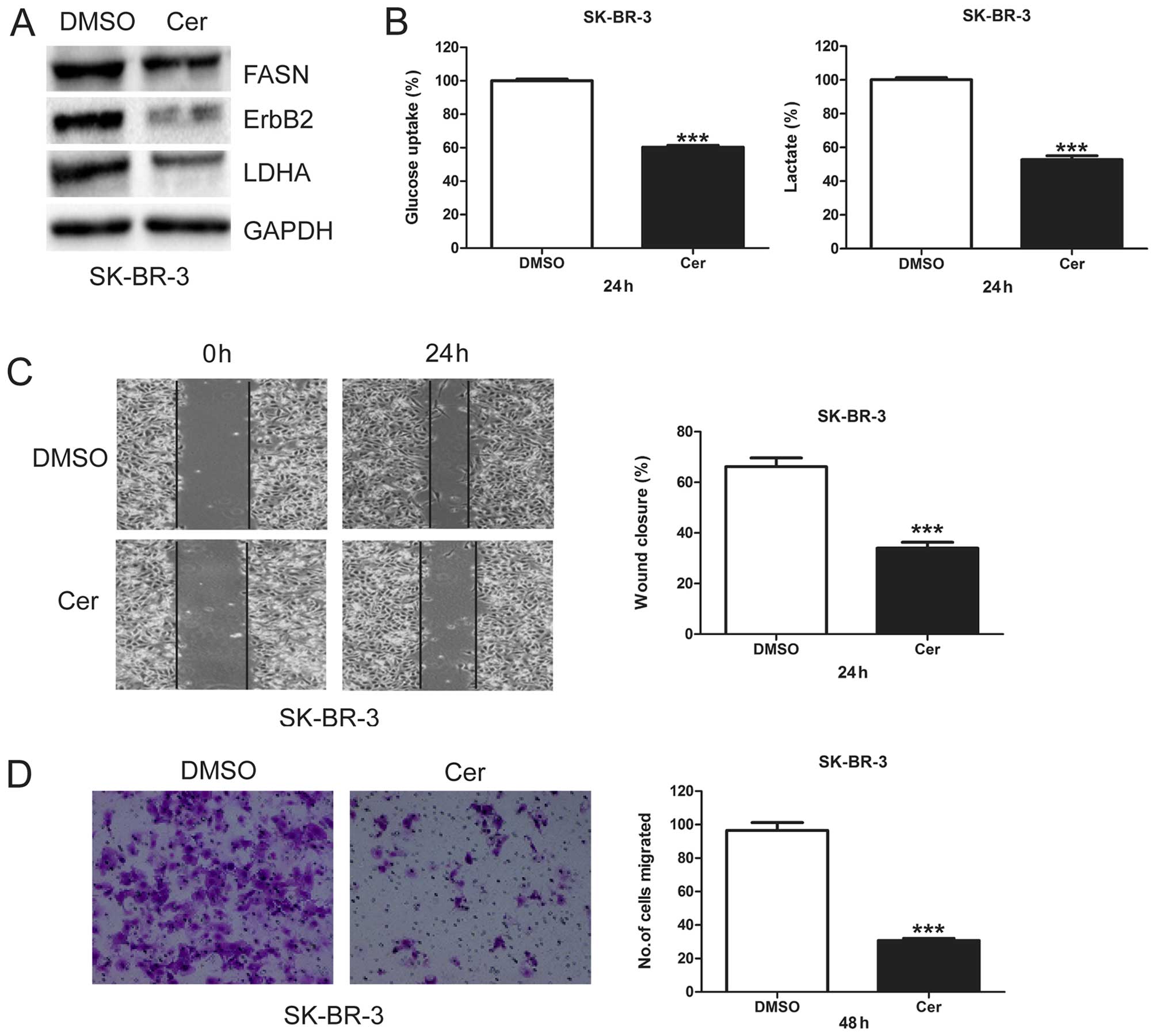
Cerulenin decreases glycolysis and
migration in SK-BR-3 cells
To investigate the role of FASN in glycolysis and
cell migration, we treated SK-BR-3 cells with cerulenin, a specific
inhibitor of FASN, and measured its effects on ErbB2 and LDHA
protein levels, glycolysis, and cell migration. When FASN protein
levels were inhibited by cerulenin, both ErbB2 and LDHA proteins
levels were downregulated (Fig.
2A). Cerulenin treatment in SK-BR-3 cells also significantly
decreased glucose uptake and lactate production (Fig. 2B), indicating that inhibition of
FASN by cerulenin inhibits glycol-ysis. We further found that
cerulenin significantly decreased migratory capacity of SK-BR-3
cells (Fig. 2C and D). These
results suggest FASN may play an important role in glycolysis and
migration of breast cancer cells.
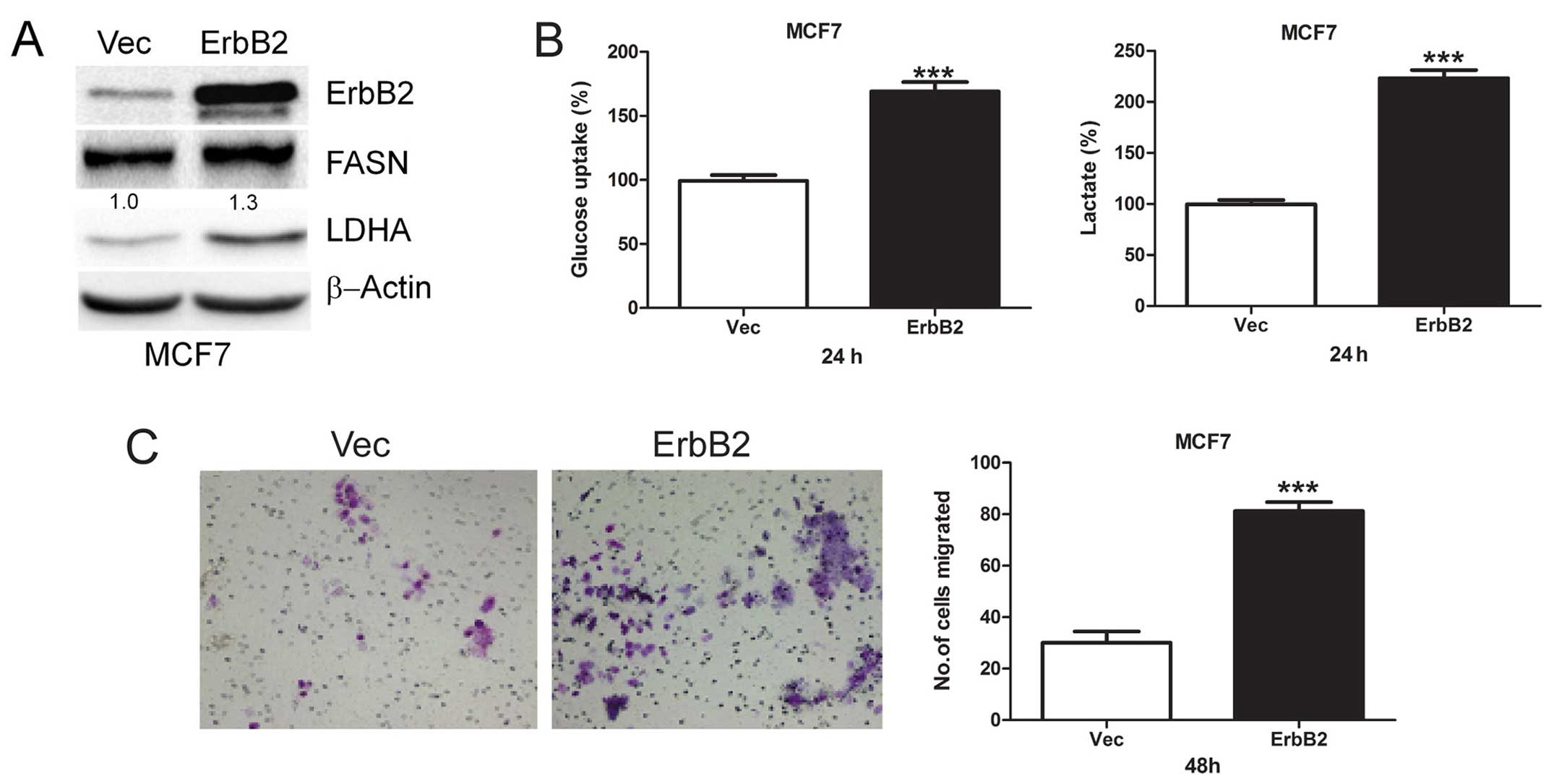
Overexpression of ErbB2 promotes
glycolysis and cell migration
To investigate the role of ErbB2-mediated glycolysis
in cell migration, we transiently transfected ErbB2 plasmid into
MCF7 cells and then detected FASN and LDHA protein levels. We found
that overexpression of ErbB2 upregulates FASN and LDHA protein
levels (Fig. 3A). Moreover, we
found that acute overexpression of ErbB2 increased both glucose
uptake and lactate production (Fig.
3B), indicating increased glycolysis. Then, the role of
ErbB2-mediated glycolysis in cell migration was investigated, and
we found that overexpression of ErbB2 greatly enhanced cell
migration (Fig. 3C). These results
suggest ErbB2 plays an important role in glycolysis and migration
of breast cancer cells.
Cerulenin reverses HRG-β1-induced
glycolysis and migration in MCF7 cells
We treated MCF7 cells with HRG-β1 and detected FASN,
ErbB2, and LDHA protein levels. We found that HRG-β1 upregulated
FASN, ErbB2 and LDHA protein levels whereas cerulenin reversed such
upregulation (Fig. 4A). We further
found that HRG-β1 induced both glucose uptake and lactate
production with cerulenin reversing such an induction in MCF7 cells
(Fig. 4B). Moreover, HRG-β1
treatment in MCF7 cells greatly increased wound closure and number
of migrated cells, while cerulenin overcame such increases
(Fig. 4C and D). This result
suggests that FASN plays an important role in HRG-β1-indcued
glycolysis and migration.
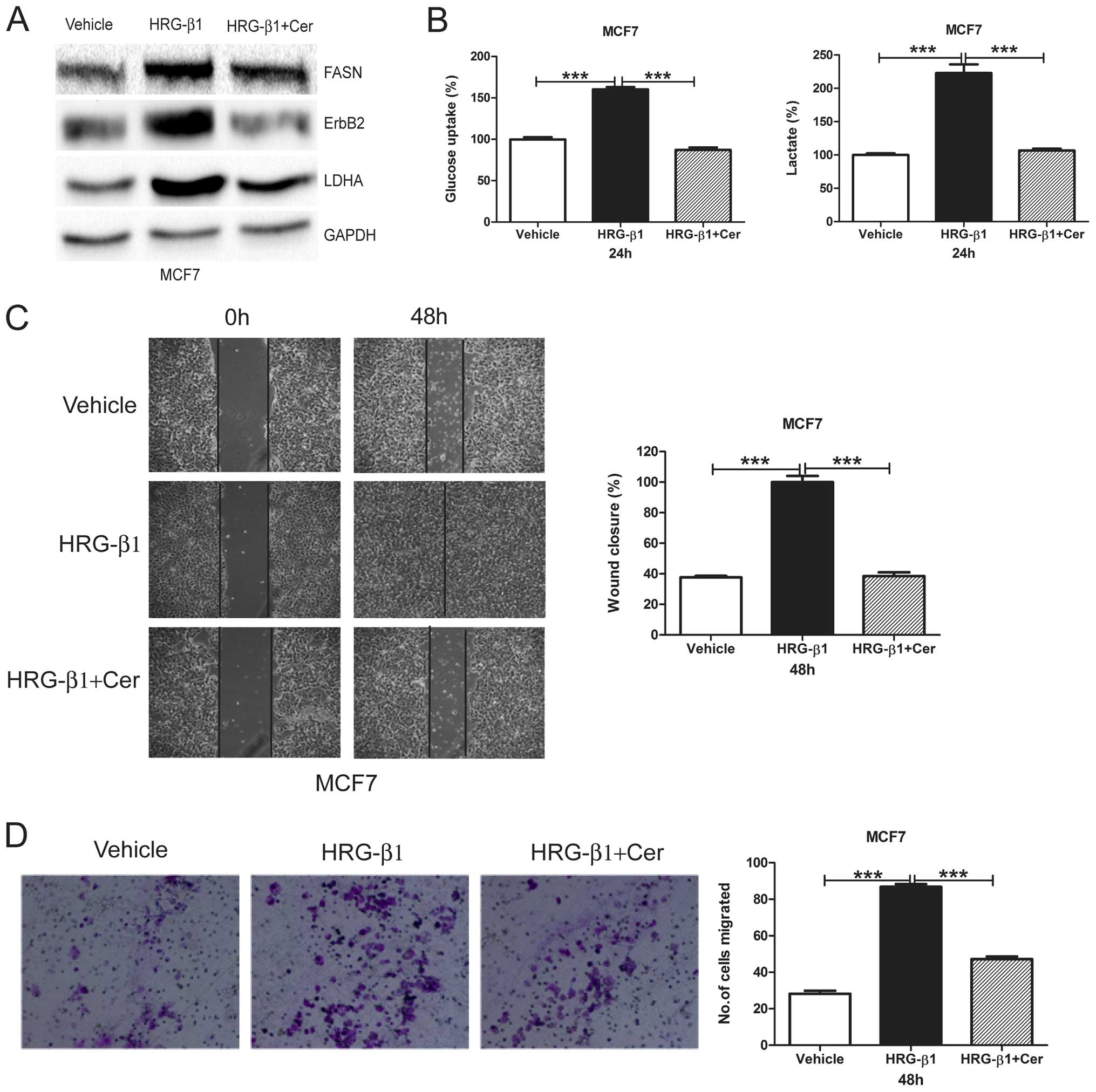 | Figure 4Cerulenin reverses HRG-β1-induced
glycolysis and cell migration. (A) MCF7 cells were treated with
Vehicle, HRG-β1 (100 ng/ml), HRG-β1 (100 ng/ml) plus Cer (10
µg/ml) for 24 h. Cells were collected and western blot
analysis of ErbB2, FASN and LDHA protein levels was performed.
GAPDH served as a loading control. (B) MCF7 cells were treated with
Vehicle, HRG-β1 (100 ng/ml), HRG-β1 (100 ng/ml) plus Cer (10
µg/ml) for 24 h. Cell media were collected for glucose
uptake and lactate production assay. Data are shown in percentage
relative to Vehicle-treated MCF7 cells. (C) Confluent monolayers of
MCF7 cells were wounded using a pipette tip and were treated with
Vehicle, HRG-β1 (100 ng/ml), HRG-β1 (100 ng/ml) plus Cer (10
µg/ml) for 48 h. Wound closure was monitored by microscopy
and representative photomicrographs (×40 magnification) are shown
(left). Wound width was measured at 0 and 48 h and plotted as
percentage of wound closure (right). (D) MCF7 cells were treated
with Vehicle, HRG-β1 (100 ng/ml), HRG-β1 (100 ng/ml) plus Cer (10
µg/ml) for 24 h. Then, cells were plated into inserts for
Transwell migration assay. After 48 h of incubation, migrated cell
numbers were counted under a microscope in five random fields per
insert. Columns, mean of three independent experiments; bars, SE;
***P<0.001. |
2-DG or OX reverses HRG-β1-induced
glycolysis and migration in MCF7 cells
In order to further demonstrate that glycolysis is
required for cell migration, we treated MCF7 cells with HRG-β1 and
detected LDHA protein levels. We found that HRG-β1 greatly
upregulated LDHA protein levels while both 2-DG and OX reversed the
upregulation (Fig. 5A). Moreover,
both 2-DG and OX reversed HRG-β1-induced glycolysis (Fig. 5B) and cell migration (Fig. 5C and D). These results suggest that
HRG-β1-induced glycolysis promotes cell migration whereas
glycolysis inhibition by 2-DG or OX reverses such effects.
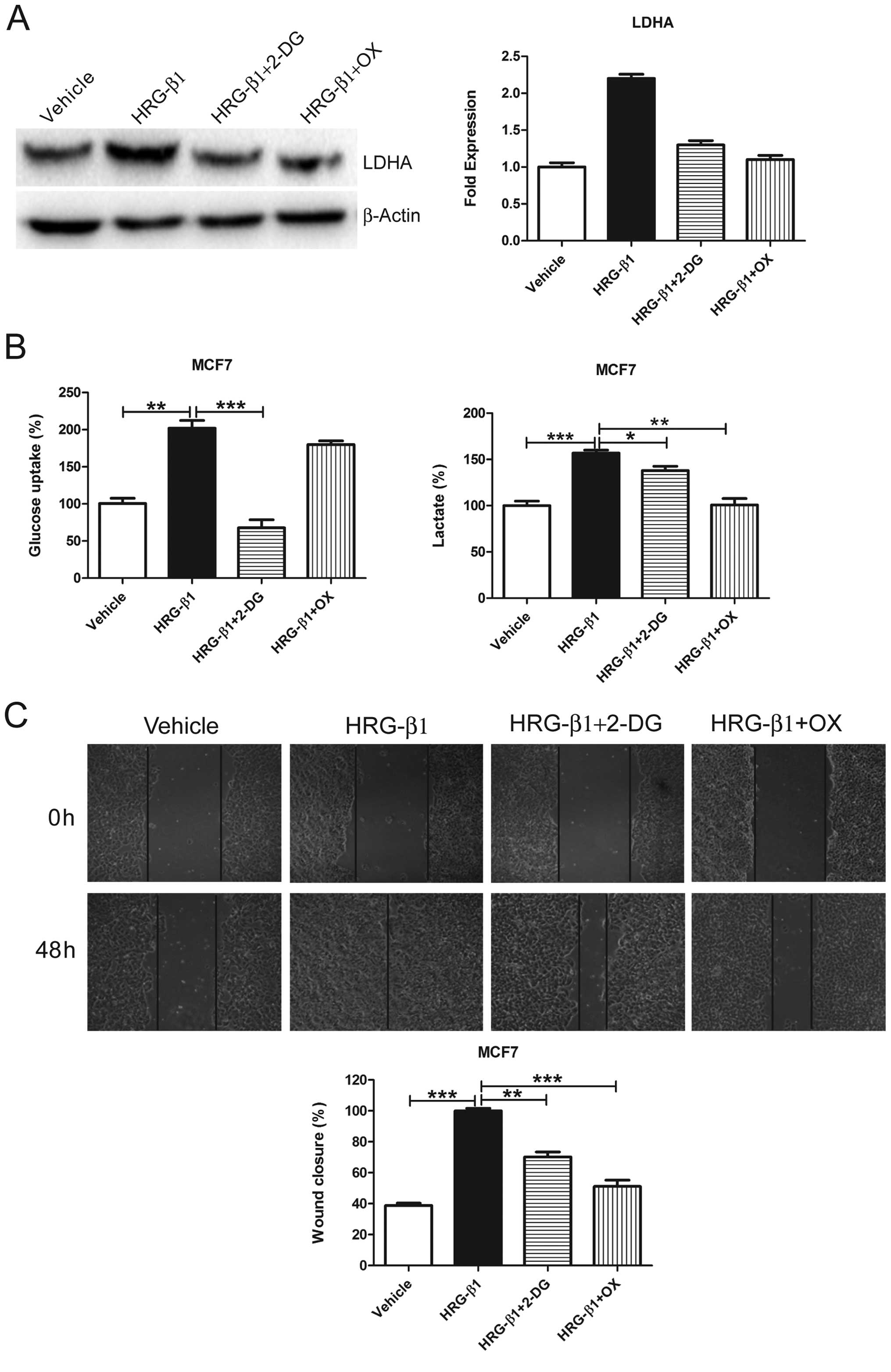 | Figure 52-DG and OX reverses HRG-β1-induced
glycolysis and cell migration. (A) MCF7 cells were treated with
Vehicle, HRG-β1 (100 ng/ml), HRG-β1 (100 ng/ml) plus 2-DG (0.5 mM)
or OX (50 mM) for 24 h. Cells were collected and western blot
analysis of LDHA protein levels was performed. β-actin served as a
loading control (left). The fold expression of LDHA was quantified
using the protein level treated with vehicle as 1.0 (right). (B)
MCF7 cells were treated with Vehicle, HRG-β1 (100 ng/ml), HRG-β1
(100 ng/ml) plus 2-DG (0.5 mM) or OX (50 mM) for 24 h. Cell media
were collected for glucose uptake and lactate production assay.
Data are shown in percentage relative to Vehicle-treated MCF7
cells. (C) Confluent monolayers of MCF7 cells were wounded using a
pipette tip and were treated with Vehicle, HRG-β1 (100 ng/ml),
HRG-β1 (100 ng/ml) plus 2-DG (0.5 mM) or OX (50 mM) for 48 h. Wound
closure was monitored by microscopy and representative
photomicrographs (×40 magnification) are shown. Wound width was
measured at 0 and 48 h and plotted as percentage of wound closure.
Columns, mean of three independent experiments; bars, SE.
*P<0.05, **P<0.01,
***P<0.001. |
Discussion
Thus far, there has been no evidence reported to
directly link glycolysis to cell migration. Here, for the first
time, we have shown that FASN, ErbB2-mediated glycolysis is
required for breast cancer cell migration. We found that FASN,
ErbB2-overexpressing SK-BR-3 cells displayed higher LDHA protein
levels, a higher level of glycolysis, and a greater migratory
capacity when compared to FASN, ErbB2-low-expressing MCF7 cells.
Inhibition of FASN by cerulenin decreased ErbB2 and LDHA protein
levels, as well as glycolysis and migration in SK-BR-3 cells.
Activation of ErbB2 by overexpression, or by HRG-β1, promotes
glycolysis and cell migration while inhibition of glycolysis by
2-DG or OX reverses such induction. These results demonstrated that
glycolysis is directly linked to breast cancer cell migration.
Lactate is the end product of glycolysis and a
number of studies have shown that it plays important roles in the
malignant development of tumor, such as cell migration, invasion
and metastasis (26-29). However, all in vitro studies
have been performed by treating cancer cells with exogenous
lactate. To our knowledge, this is the first report showing that
endogenous lactate directly induced by glycolysis promotes breast
cancer cell migration.
ErbB2 and FASN are reciprocally regulated in breast
cancer cells. On the one hand, overexpression of ErbB2 upregulates
FASN expression, both at the transcriptional level and at the
translational level (10,30). Activation of ErbB2 by HRG-β1 induces
tyrosine phosphorylation of FASN and upregulation of FASN activity,
leading to increased cell invasion (31). On the other hand, overexpression of
FASN activates ErbB2 and induces an invasive breast cancer-like
phenotype (18) and FASN regulates
ErbB2 expression at the transcriptional level via PEA3 (32). Our results showed that both ErbB2
and FASN protein levels are directly consistent with LDHA protein
level, glycolysis, and migration, while inhibition of FASN also
decreased ErbB2 and LDHA protein levels, glycolysis, and migration.
Our previous study (19) showed
that ErbB2 upregulates LDHA at the transcriptional level. However,
whether FASN directly regulates LDHA, or indirectly regulates it
via ErbB2, deserves further investigation.
There are several possible mechanisms by which
HRG-β1 drives breast cancer cell migration and invasion: these
include upregulation of tumor necrosis factor receptor (TNFR)
super-family member Fn14 expression (25), induction of EMT (22,23),
and upregulation of GPR30 expression (24,33).
We have found a novel mechanism whereby HRG-β1 can induce
glycolysis and drive migration in MCF7 cells. Phosphatidylinositol
3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) are two
important pathways downstream of ErbB2 signaling induced by HRG-β1
(34). PI3K pathway plays a
dominant role in cellular transformation in ErbB2/ErbB3
coreceptor-mediated heregulin signaling (34) and AKT stimulates aerobic glycolysis
in cancer cells (35). Therefore,
we speculate that activation of PI3K/AKT pathway by HRG-β1 promotes
glycolysis resulting in enhanced cell migration.
In summary, to the best of our knowledge, for the
first time, we report that FASN, ErbB2-mediated glycolysis is
required for breast cancer cell migration. Inhibition of FASN by
cerulenin, or inhibition of glycolysis by 2-DG or OX, reverses
HRG-β1- induced glycolysis and migration. This study provides
direct evidence in support of a causal relationship between
glycolysis and breast cancer cell migration. These novel findings
prompt us to further investigate the role of glycolysis in invasion
and metastasis of breast cancer, which is important for elucidating
the molecular mechanism of breast cancer metastasis.
Abbreviations:
|
2-DG
|
2-deoxyglucose
|
|
Cer
|
cerulenin
|
|
EGF
|
epidermal growth factor
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FASN
|
fatty acid synthase
|
|
FBS
|
fetal bovine serum
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
HRG-β1
|
heregulin-β1
|
|
HRP
|
horseradish peroxidase
|
|
LDHA
|
lactate dehydrogenase A
|
|
MAPK
|
mitogen-activated protein kinase
|
|
OX
|
oxamate
|
|
PI3K
|
phosphatidylinositol 3 kinase
|
|
PS
|
penicillin and streptomycin
|
|
TBS
|
Tris buffered saline
|
|
TNFR
|
tumor necrosis factor receptor
|
Acknowledgments
The authors acknowledge financial support for the
projects supported by National Natural Sciences Foundation of China
(81272907, J1103604) and the Project 2012-1707-7-7 sponsored by the
Scientific Research Foundation for the Returned Overseas Chinese
Scholars, State Education Ministry.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scully OJ, Bay BH, Yip G and Yu Y: Breast
cancer metastasis. Cancer Genomics Proteomics. 9:311–320.
2012.PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JW and Dang CV: Cancer's molecular
sweet tooth and the Warburg effect. Cancer Res. 66:8927–8930. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.PubMed/NCBI
|
|
10
|
Kumar-Sinha C, Ignatoski KW, Lippman ME,
Ethier SP and Chinnaiyan AM: Transcriptome analysis of HER2 reveals
a molecular connection to fatty acid synthesis. Cancer Res.
63:132–139. 2003.PubMed/NCBI
|
|
11
|
Menendez JA, Mehmi I, Atlas E, Colomer R
and Lupu R: Novel signaling molecules implicated in
tumor-associated fatty acid synthase-dependent breast cancer cell
proliferation and survival: Role of exogenous dietary fatty acids,
p53-p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-kappaB. Int J
Oncol. 24:591–608. 2004.PubMed/NCBI
|
|
12
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guy CT, Webster MA, Schaller M, Parsons
TJ, Cardiff RD and Muller WJ: Expression of the neu protooncogene
in the mammary epithelium of transgenic mice induces metastatic
disease. Proc Natl Acad Sci USA. 89:10578–10582. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson E, Seachrist DD, DeLeon-Rodriguez
CM, Lozada KL, Miedler J, Abdul-Karim FW and Keri RA:
HER2/ErbB2-induced breast cancer cell migration and invasion
require p120 catenin activation of Rac1 and Cdc42. J Biol Chem.
285:29491–29501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Dong L, Wei D, Wang X, Zhang S and
Li H: Fatty acid synthase mediates the epithelial-mesenchymal
transition of breast cancer cells. Int J Biol Sci. 10:171–180.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan M, Li P, Klos KS, Lu J, Lan KH, Nagata
Y, Fang D, Jing T and Yu D: ErbB2 promotes Src synthesis and
stability: Novel mechanisms of Src activation that confer breast
cancer metastasis. Cancer Res. 65:1858–1867. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan M, Yao J and Yu D: Overexpression of
the c-erbB-2 gene enhanced intrinsic metastasis potential in human
breast cancer cells without increasing their transformation
abilities. Cancer Res. 57:1199–1205. 1997.PubMed/NCBI
|
|
18
|
Vazquez-Martin A, Colomer R, Brunet J,
Lupu R and Menendez JA: Overexpression of fatty acid synthase gene
activates HER1/HER2 tyrosine kinase receptors in human breast
epithelial cells. Cell Prolif. 41:59–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao YH, Zhou M, Liu H, Ding Y, Khong HT,
Yu D, Fodstad O and Tan M: Upregulation of lactate dehydrogenase A
by ErbB2 through heat shock factor 1 promotes breast cancer cell
glycolysis and growth. Oncogene. 28:3689–3701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montero JC, Rodríguez-Barrueco R, Ocaña A,
Díaz-Rodríguez E, Esparís-Ogando A and Pandiella A: Neuregulins and
cancer. Clin Cancer Res. 14:3237–3241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agus DB, Akita RW, Fox WD, Lewis GD,
Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K,
et al: Targeting ligand-activated ErbB2 signaling inhibits breast
and prostate tumor growth. Cancer Cell. 2:127–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng L, Zha Z, Lang B, Liu J and Yao X:
Heregulin-beta1 promotes metastasis of breast cancer cell line
SKBR3 through upregulation of Snail and induction of
epithelial-mesenchymal transition. Cancer Lett. 280:50–60. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J, Jeong H, Lee Y, Kim C, Kim H and
Kim A: HRG-β1-driven ErbB3 signaling induces epithelial-mesenchymal
transition in breast cancer cells. BMC Cancer. 13:3832013.
View Article : Google Scholar
|
|
24
|
Ruan SQ, Wang ZH, Wang SW, Fu ZX, Xu KL,
Li DB and Zhang SZ: Heregulin-β1-induced GPR30 upregulation
promotes the migration and invasion potential of SkBr3 breast
cancer cells via ErbB2/ErbB3-MAPK/ERK pathway. Biochem Biophys Res
Commun. 420:385–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asrani K, Keri RA, Galisteo R, Brown SA,
Morgan SJ, Ghosh A, Tran NL and Winkles JA: The HER2- and heregulin
β1 (HRG)-inducible TNFR superfamily member Fn14 promotes HRG-driven
breast cancer cell migration, invasion, and MMP9 expression. Mol
Cancer Res. 11:393–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonuccelli G, Tsirigos A, Whitaker-Menezes
D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N,
Howell A, Martinez-Outschoorn UE, et al: Ketones and lactate 'fuel'
tumor growth and metastasis: Evidence that epithelial cancer cells
use oxidative mitochondrial metabolism. Cell Cycle. 9:3506–3514.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goetze K, Walenta S, Ksiazkiewicz M,
Kunz-Schughart LA and Mueller-Klieser W: Lactate enhances motility
of tumor cells and inhibits monocyte migration and cytokine
release. Int J Oncol. 39:453–463. 2011.PubMed/NCBI
|
|
28
|
Hirschhaeuser F, Sattler UG and
Mueller-Klieser W: Lactate: A metabolic key player in cancer.
Cancer Res. 71:6921–6925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stern R, Shuster S, Neudecker BA and
Formby B: Lactate stimulates fibroblast expression of hyaluronan
and CD44: The Warburg effect revisited. Exp Cell Res. 276:24–31.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoon S, Lee MY, Park SW, Moon JS, Koh YK,
Ahn YH, Park BW and Kim KS: Up-regulation of acetyl-CoA carboxylase
alpha and fatty acid synthase by human epidermal growth factor
receptor 2 at the translational level in breast cancer cells. J
Biol Chem. 282:26122–26131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin Q, Yuan LX, Boulbes D, Baek JM, Wang
YN, Gomez-Cabello D, Hawke DH, Yeung SC, Lee MH, Hortobagyi GN, et
al: Fatty acid synthase phosphorylation: A novel therapeutic target
in HER2-overexpressing breast cancer cells. Breast Cancer Res.
12:R962010. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Menendez JA, Vellon L, Mehmi I, Oza BP,
Ropero S, Colomer R and Lupu R: Inhibition of fatty acid synthase
(FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in
cancer cells. Proc Natl Acad Sci USA. 101:10715–10720. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruan SQ, Wang SW, Wang ZH and Zhang SZ:
Regulation of HRG-β1-induced proliferation, migration and invasion
of MCF-7 cells by upregulation of GPR30 expression. Mol Med Rep.
6:131–138. 2012.PubMed/NCBI
|
|
34
|
Vijapurkar U, Kim MS and Koland JG: Roles
of mitogen-activated protein kinase and phosphoinositide 3′-kinase
in ErbB2/ErbB3 coreceptor-mediated heregulin signaling. Exp Cell
Res. 284:291–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elstrom RL, Bauer DE, Buzzai M, Karnauskas
R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, et
al: Akt stimulates aerobic glycolysis in cancer cells. Cancer Res.
64:3892–3899. 2004. View Article : Google Scholar : PubMed/NCBI
|