Introduction
Osteosarcoma is the most common tumor among
malignant tumors in children, and mainly occurs during childhood
and adolescence (1,2). Currently, the combination of surgery
and auxiliary chemotherapy significantly benefits to improve the
survival of patients, but is not ideal for patients suffering
recurrent, metastatic or drug-resistant osteosarcoma (3,4). As a
new promising approach for treating solid tumors (5–7),
photodynamic therapy (PDT) enables the absorption of a
photosensitizer to produce reactive oxygen species (ROS) which
kills tumor cells and facilitates local photoradiation therapy
(8). These studies suggest that PDT
plays an important role in treating tumors; however, the related
mechanism is not clear.
Aloe-emodin (AE) is a novel anthraquinone compound
extracted from traditional Chinese medicine (TCM) plants and has
been verified to have antitumor effects (9,10).
However, the genotoxicity (e.g. gene lesion and gene mutation)
limits its application in large dosage (11). Furthermore, AE has been found to
have a certain fluorescence and can be used as a photosensitizer
(12,13). The combination of light emitting
diode (LED) and AE-mediated photodynamic therapy (AE-PDT) can
reduce the AE concentration to induce the apoptosis of tumor cells
and therefore reduce its toxicity (14,15).
The present study aimed to investigate the effect of AE-PDT on
human osteosarcoma cell line MG-63 and to explore the related
mechanisms.
Materials and methods
Cell culture
The human osteosarcoma cell line MG-63 [purchased
from the American Type Culture Collection (ATCC); Manassas, VA,
USA] was incubated in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (both from Gibco,
Carlsbad, CA, USA), 100 IU/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere of 5%
CO2. When the confluency reached 70%, the cells were
divided into a control group (no treatment), an AE group (treatment
with only AE), a LED alone group (treatment with only
photoradiation) and an AE-PDT group (treatment with AE and
photoradiation). For some experiments, the cells were pretreated
with ROS inhibitor N-acetyl-L-cysteine (NAC) (5 mM;
Beyotime, China), or JNK inhibitor SP600125 (10 µM; Sigma,
St. Louis, MO, USA). AE at different concentrations (0, 5, 10, 20
and 50 µM; Sigma) was added to the culture medium as
indicated for 6 h and thereafter rinsed 3 times with normal medium.
The PDT was applied with a LED light source at 430 nm of
wavelength, continuous input model and density of 40
mW/cm2 at different radiation periods of 0, 60, 120 and
160 sec aiming to produce energy density of 0, 2.4, 4.8 and 6.4
J/cm2, respectively.
Measurement of the cell viability by
CCK-8
The cells were inoculated in 96-well plates at the
density of 5×103 cells/well for 24 h, and 3 repetitive
wells were prepared for each group. After the corresponding
treatments, the cells were cultured for another 12 h and then
treated with 10 µl Cell Counting Kit-8 (CCK-8) (Beyotime) in
an incubator for 1 h. The absorption values (A) of CCK-8 [optical
density (OD)] at 450 nm were measured by a microplate reader to
calculate the cell growth inhibition rate (%) according to the
formulation: Inhibition rate (%) = (ODblank −
ODexperiment)/ODblank × 100%. Based on the
inhibition rate, an AE concentration of 10 µM and energy
density of PDT at 4.8 J/cm2 were selected for subsequent
experiments.
Measurement of intracellular ROS
The cells were inoculated in 24-well plates at the
density of 5×104 cells/well. Following corresponding
treatments, the cells were incubated with 200 µl DCFH-DA
(Sigma) at a concentration of 10 µM at 37°C for 20 min.
After rinsing with phosphate-buffered saline (PBS) for 3 times, the
cells were digested using 0.25% trypsin and re-suspended in PBS,
and the intensity of fluorescence was measured by flow cytometry
according to the manual.
Monodansylcadaverine (MDC) staining
After the corresponding treatment, the cells were
rinsed with PBS for 3 times and incubated in 200 µl of 0.05
mM MDC (Sigma) at 37°C for 30 min. Then, the cells were rinsed with
PBS for 2 times and the aggregation of autophagic vacuoles was
observed under a fluorescence microscope (DMI4000 B; Leica
Microsystems, Wetzlar, Germany) with an excitation wavelength of
460–500 nm and an emission wavelength of 512–542 nm.
Hoechst 33342 staining
Following the corresponding treatment, cells
incubated in 24-well plates were stained with Hoechst (10
µM; Sigma) staining at 37°C in the dark for 5 min, and
subsequently observed under a fluorescence microscope (DMI4000 B)
with an excitation wavelength of 355–366 nm and an emission
wavelength of 465–480 nm.
Transmission electron microscope
(TEM)
After the different treatments, cells collected in
EP tubes were centrifuged, fixed with 2.5% glutaraldehyde and 1%
osmic acid, dehydrated with gradient ethanol and acetone, embedded
and sliced, and stained with 3% uranyl acetate-lead citrate.
Finally, the cells were observed with TEM.
Measurement of apoptosis by flow
ctyometry
After treatment, the MG-63 cells incubated at
1×106 cells/well into a 6-well plate were then stained
with Annexin V/propidium iodide (PI) (KeyGen Biotech, China) for
flow cytometry, according to the manual.
Western blotting
The collected cells were rinsed with PBS and lysed.
An equal volume of protein lysates was added for sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins
were transferred to nitrocellulose membranes, blocked with
Tris-buffered saline and Tween-20 (TBST) containing 5% skimmed milk
at room temperature for 1 h, rinsed and incubated with the
corresponding primary antibodies β-actin (1:1,000), Bcl-2
(1:1,000), total-JNK (1:1,000), p-JNK (1:1,000), cleaved caspase-3
(1:1,000) (all from Cell Signaling Technology, Danvers, MA, USA),
Beclin-1 (1:1,000) and LC-3 (1:1,000) (both from Sigma) at 4°C
overnight. After rinsing with TBST, the proteins were incubated
with secondary antibodies at room temperature for 1 h. The film was
developed with an enhanced chemiluminescence (ECL) detection
system.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). Data were compared with one- or two-way ANOVA for
intergroup data and the SNK-q test for intragroup. The differences
were considered significant if p<0.05.
Results
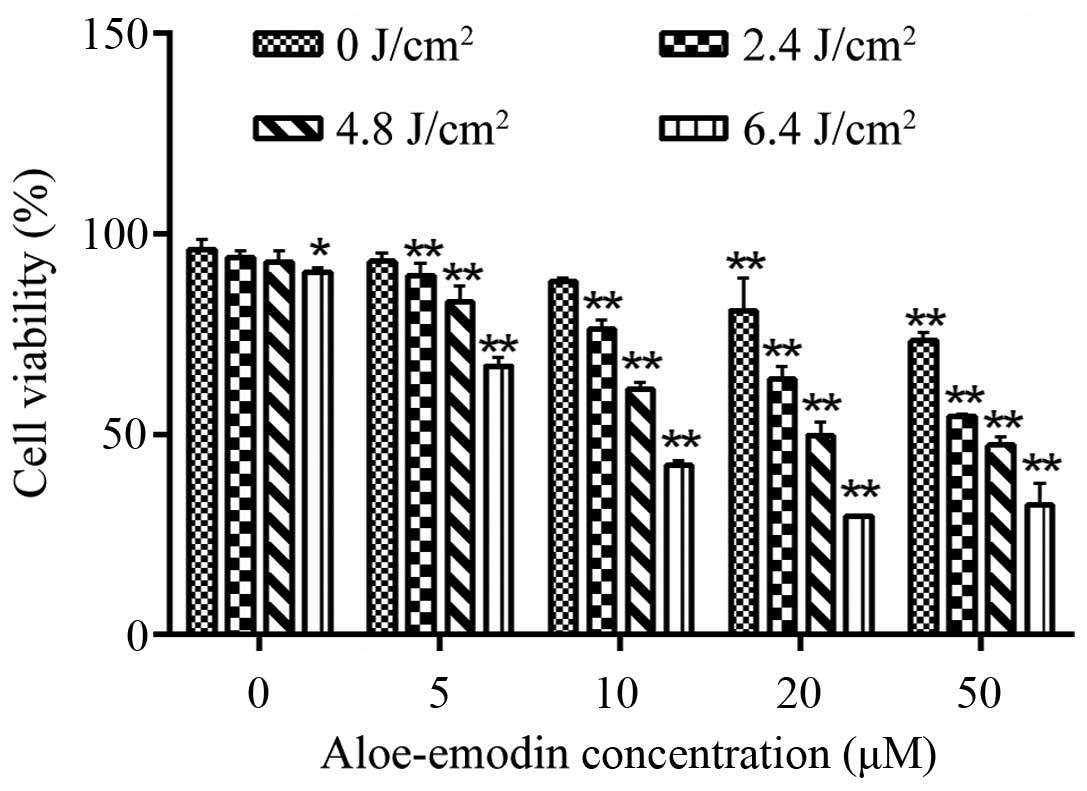
Cell viability of the human osteosarcoma
cell line MG-63
The measurement of cell viability by CCK-8 indicated
that AE alone at the concentration of 0–10 µM had no
significant inhibition on the viability of MG-63 cells (p>0.05).
In contrast, cell viability was significantly reduced following
treatment of AE at a concentration of >10 µM (p<0.05;
Fig. 1). Similarly, LED alone with
an energy density of 0–4.8 J/cm2 revealed no obvious
effect on the viability of the MG-63 cells (p>0.05). When the
LED energy density was >4.8 J/cm2, the viability of
the MG-63 cells was substantially inhibited (p<0.05; Fig. 1). Furthermore, AE-PDT caused
significant inhibition of the viability of MG-63 cells in a
dose-dependent manner (p<0.01; Fig.
1). Therefore, we chose AE at 10 µM and PDT with an
energy density of 4.8 J/cm2 for the subsequent
experiments.
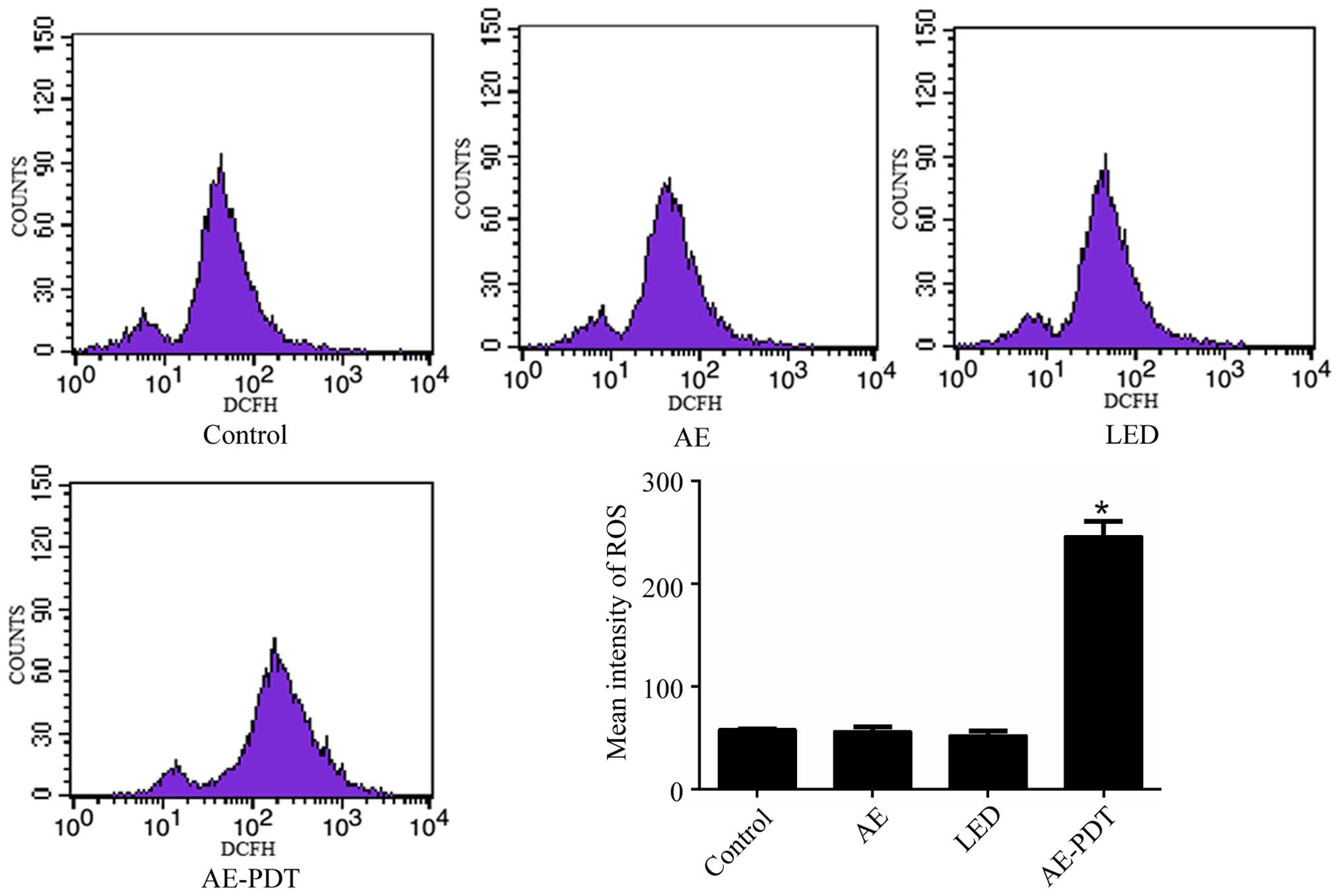
Intracellular ROS
Flow cytometry demonstrated that there was no
significant difference in the intracellular ROS level between the
LED alone group, AE alone group and control group (p>0.05).
However, the ROS level in the AE-PDT group was substantially
increased when compared to the LED alone group, AE alone and
control group, respectively (p<0.05; Fig. 2).
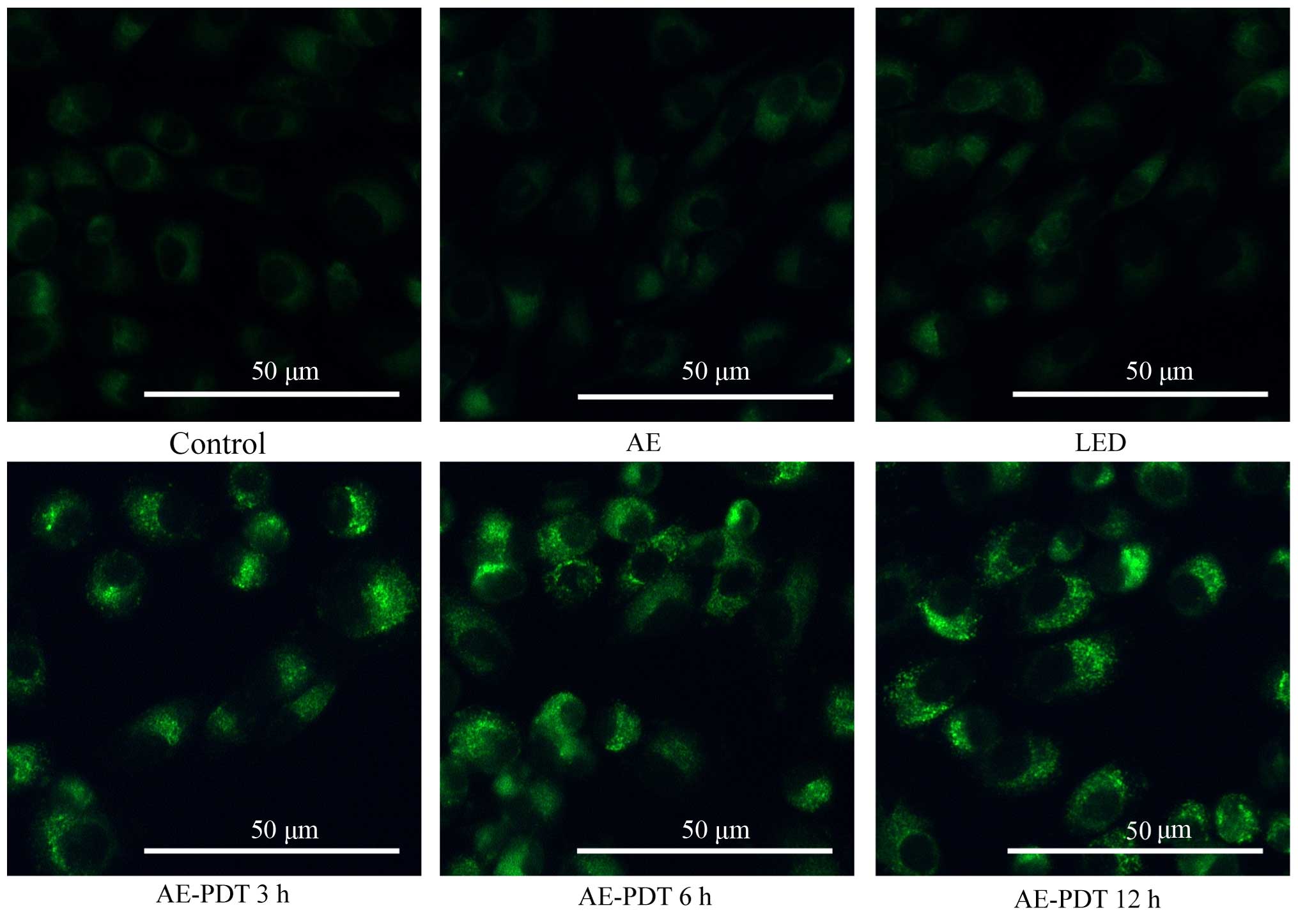
Cell morphologic changes
After staining with MDC under a fluorescence
microscope, the autophagic vacuoles displayed green spots mainly
distributed in the perineuclei. There were no significant changes
between the control, AE alone and LED alone group. However in the
AE-PDT group, the MG-63 cellular nuclei were surrounded by the
aggregation of massive autophagic vacuoles, and a significant
difference was found compared with the control, AE alone and LED
alone group (Fig. 3).
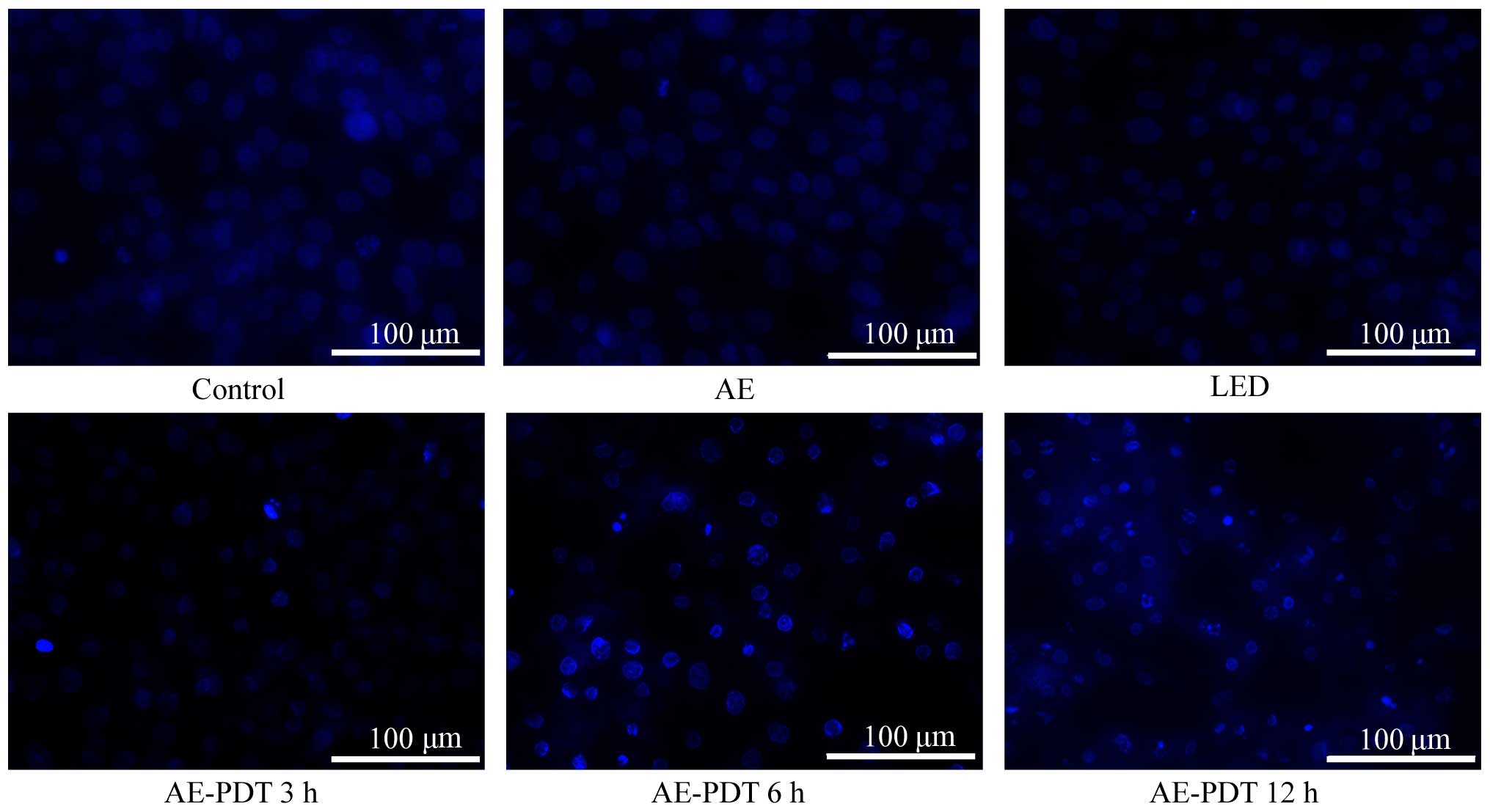
For Hochest 33342 staining, the MG-63 cells
demonstrated a higher density of nuclear chromatin showing
highlight blue, obvious pycnosis, aggregation and rapture resulting
in typical apoptotic bodies after treatment with AE-PDT (Fig. 4), and these cell changes were
increased with an increase in the treatment periods. However, no
significant changes occurred in the control, AE alone and LED alone
group.
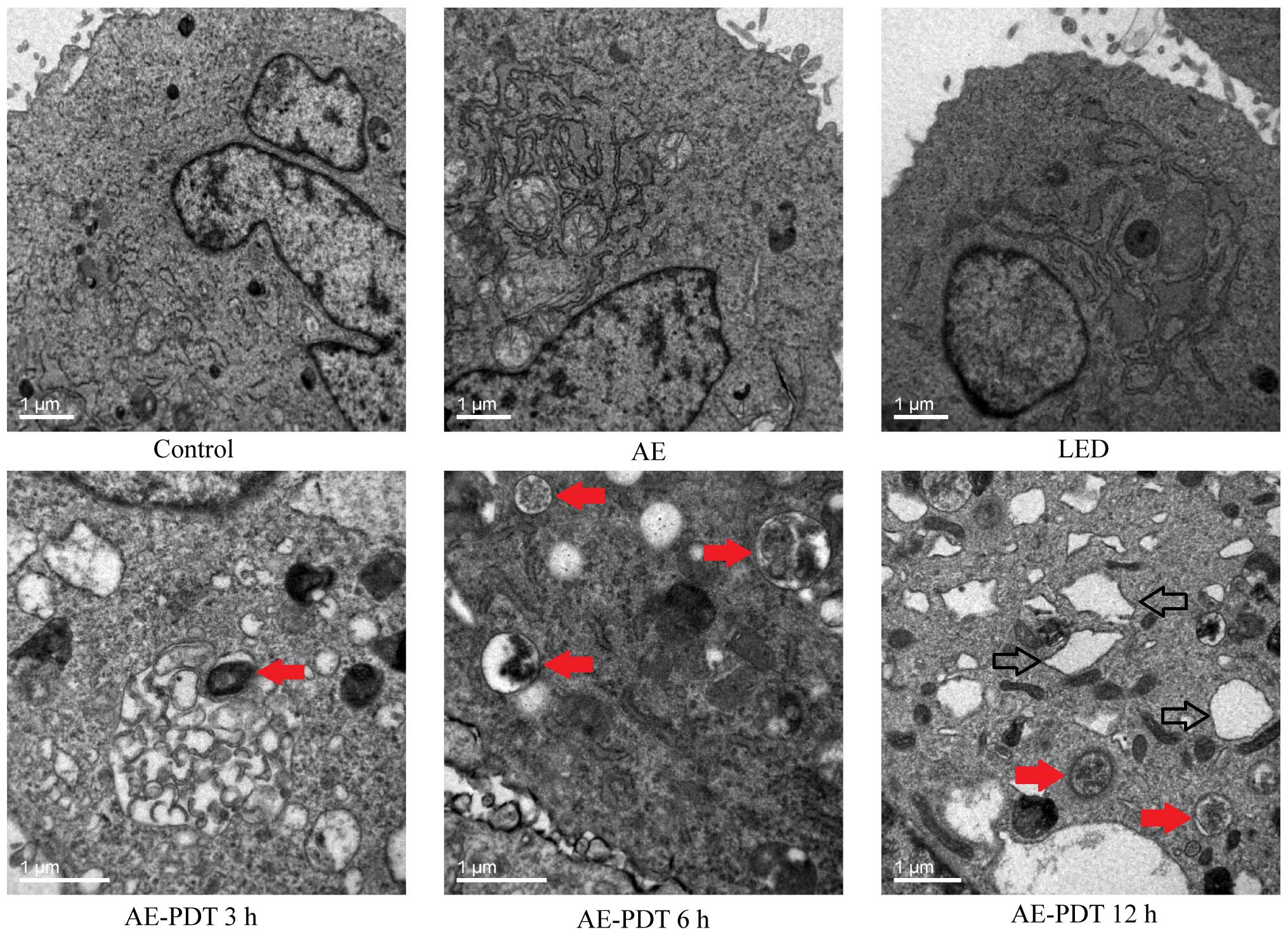
Under TEM, the treatment of MG-63 cells using AE-PDT
contributed to produce massive vesicular structure wrapping
cellular organelles or protein which was fused with lysosomes to
form typical autophagic lysosomes. The autophagic lysosomes were
obviously different from necrotic and apoptotic cells and were
significantly increased in the AE-PDT group when compared to the
control, AE alone group, and LED alone group (red arrow, Fig. 5). Noticeably, there were numerous
expanded endoplastic reticulum in the AE-PDT group (hollow arrow,
Fig. 5).
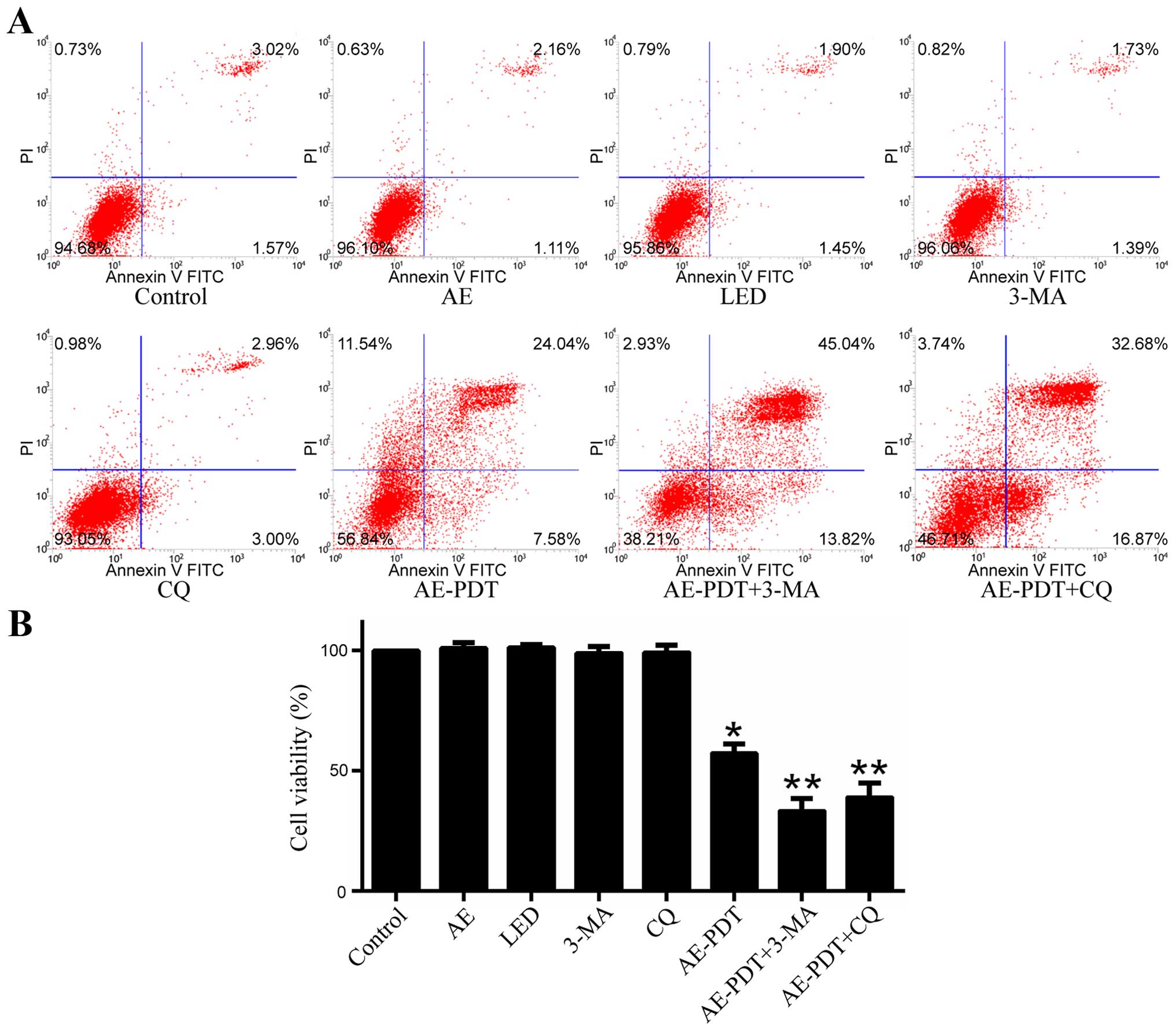
Apoptosis and cell viability
When compared to the control group, the apoptosis
rate in the AE alone and LED alone group was not significantly
different (p>0.05; Fig. 6A).
However, the apoptosis rate in the AE-PDT group was substantially
increased and higher relative to that in the control group, AE
alone and LED alone group (p<0.05; Fig. 6A). Pretreatment with 5 mM 3-MA
(AE-PDT+3-MA group) and 15 µM chloroquine (CQ) (AE-PDT+CQ
group) significantly increased the apoptosis rate (p<0.05;
Fig. 6A) and promoted cell death
when compared to the AE-PDT group (p<0.05; Fig. 6B).
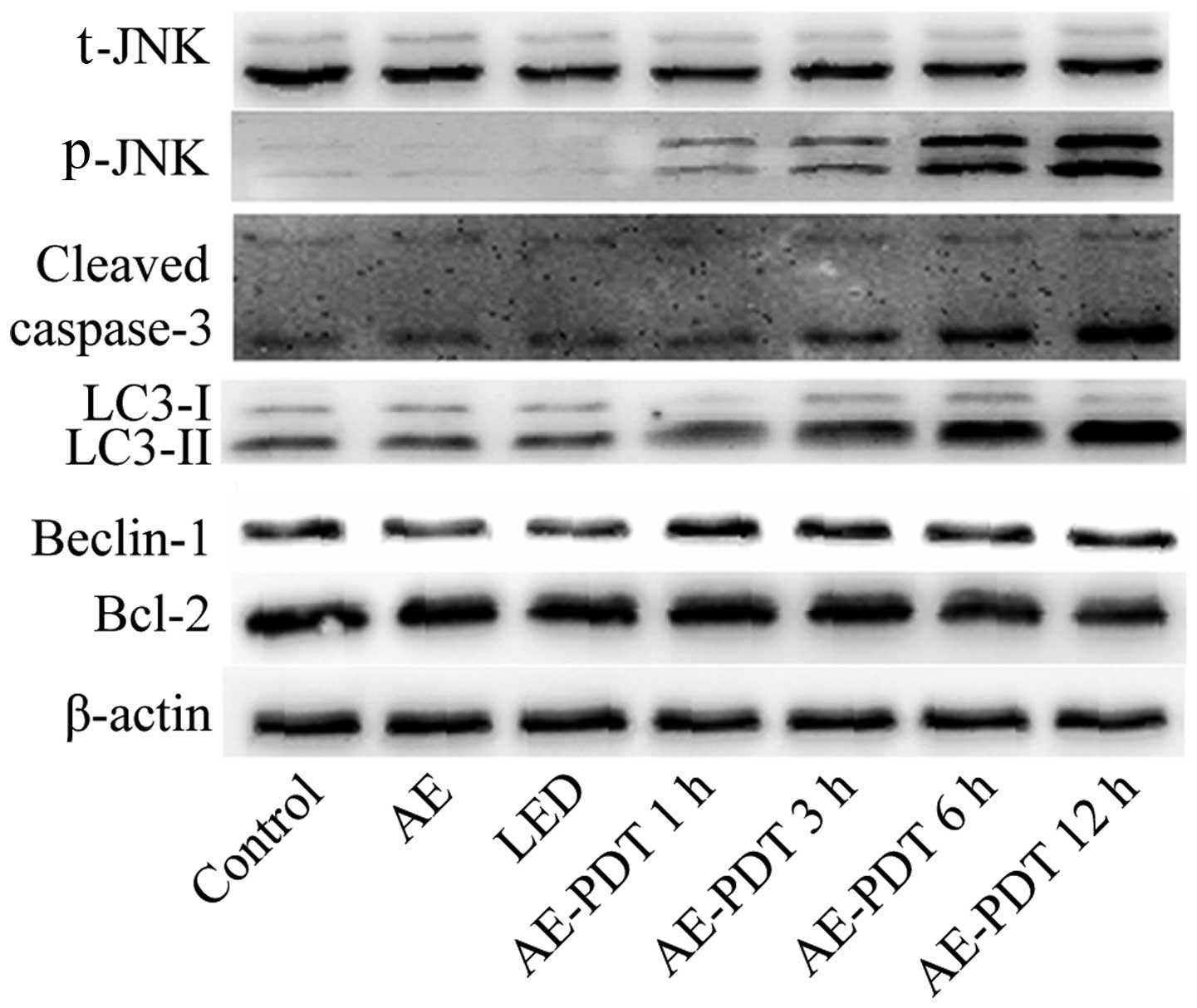
Expression of apoptosis and
autophagy-related protein
The results indicated that the AE alone and LED
alone treatments had no significant influence on the expression of
apoptotic and autophagic proteins including LC-3II, cleaved
caspase-3, Beclin-1, Bcl-2 and p-JNK (p>0.05; Fig. 7). However, AE-PDT was able to
significantly increased the expression of LC-3II, cleaved
caspase-3, Beclin-1 and p-JNK in a time-dependent manner
(p<0.05; Fig. 7).
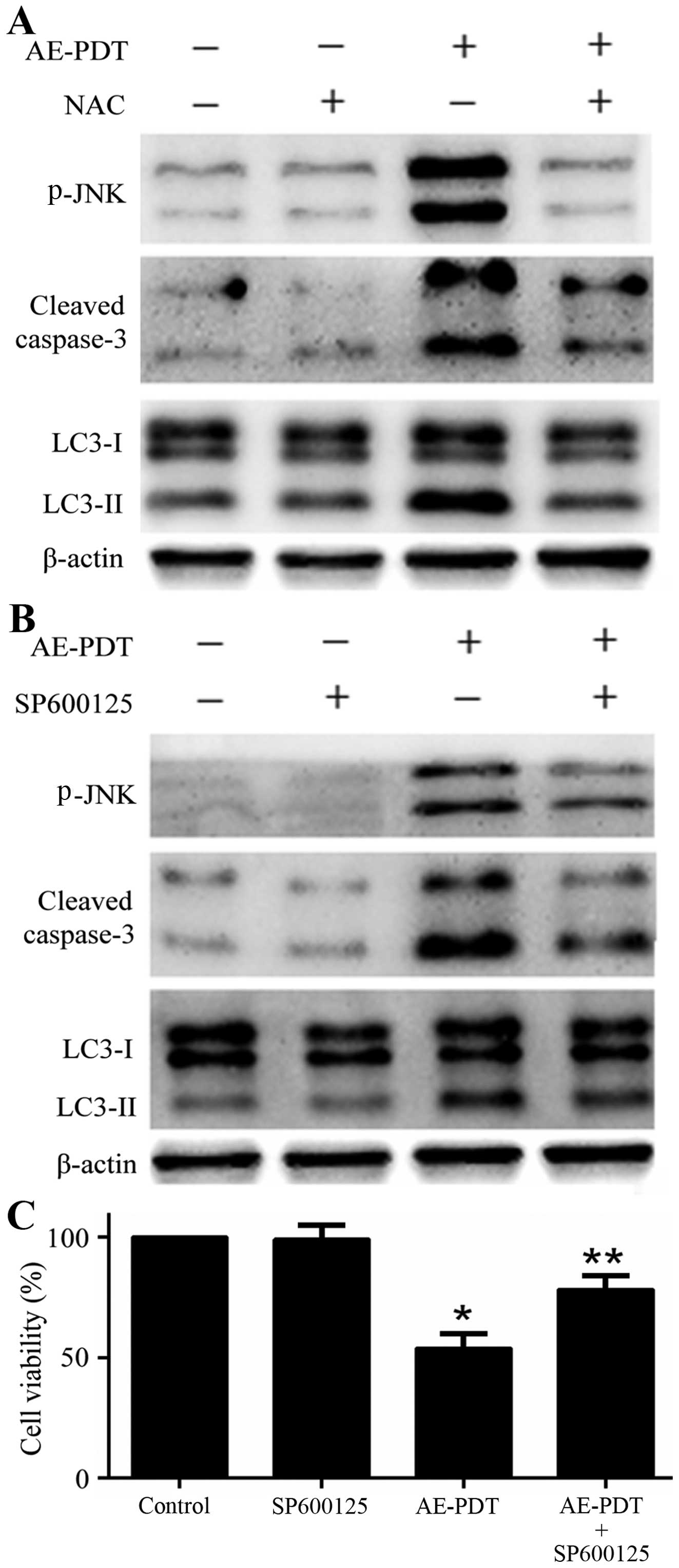
Effects of NAC and SP600125 on autophagy,
apoptosis and cell viability
The pretreatment of cells with ROS inhibitor NAC (5
mM) was found to inhibit the expression of apoptotic and autophagic
proteins p-JNK, cleaved caspase-3 and LC3-II followed by the
treatment of AE-PDT (p<0.05; Fig.
8A). Similarly, the pretreatment of cells with JNK inhibitor
SP600125 (10 µM) significantly reduced the expression of
apoptotic and autophagic proteins p-JNK, cleaved caspase-3 and
LC3-II followed by the treatment of AE-PDT (p<0.05; Fig. 8B). Furthermore, the pretreatment
with SP600125 (10 µM) significantly increased the viability
of the MG-63 cells when compared to that noted in the AE-PDT group
(p<0.05; Fig. 8C).
Discussion
PDT is a newly developed invasive therapeutic
approach for the treatment of tumors by utilizing visible light at
a certain wavelength to excite a photosensitizer aggregated around
tumors. After excitation, the photosensitizer can induce a series
of photochemical and photobiological reactions to produce ROS,
further damaging subcellular organelles, inducing cell death and
causing antitumor effects (8).
Although PDT is a promising therapeutic strategy for solid tumors,
the molecular mechanism of PDT is still not completely clear.
AE is known as an active extract of traditional
Chinese herbs, and has fluorescence and an important antitumor
effect with dose-dependent cytotoxicity (11–15).
In the present study, only AE at a concentration higher than 10
µM showed potential in inducing the apoptosis of human
osteosarcoma MG-63 cells, suggesting that a high concentration of
AE is required for antitumor therapy. However, its dosage for
clinical application must be reduced due to genotoxicity leading to
gene lesions and gene mutations (11). A previous study indicated that AE
alone at the concentration of 50 µM has the ability to
promote the apoptosis of pulmonary carcinoma, while AE-PDT (AE at a
concentration of 20 µM and PDT at an energy density of 1.6
J/cm2) showed a comparable antitumor effect on lung
cancer cell line H460 (15). In the
present study, the results indicated that AE-PDT (AE at a
concentration of 10 µM and PDT at an energy density of 4.8
J/cm2) significantly reduced the viability of human
osteosarcoma MG-63 cells, and triggered autophagy and apoptosis.
Thus, these studies suggested that AE-PDT has a significant
antitumor effect at a low dosage by utilizing the photosensitizing
features of AE, and at the same time reducing the related
side-effects triggered by a higher concentration of drugs.
The ROS-JNK pathway is involved in AE-PDT-induced
autophagy and apoptosis. ROS signaling plays an important role in
PDT and a low dosage of ROS contributes to improve cellular
function and cell survival. In contrast, excessive ROS cause the
capability to promote programmed cell death (16). The present study indicated that the
ROS level in the human osteosarcoma MG-63 cells treated with AE-PDT
was significantly increased and cleaved caspase-3 was also
gradually increased. ROS inhibitor NAC was found to prevent these
changes, suggesting that ROS were involved in the apoptosis induced
by AE-PDT. Following stress stimulus, ROS can induce the formation
of autophagosome, and previous studies indicate that ROS play a
critical role in promoting autophagy by activating the JNK pathway
(17,18). The present study revealed that
AE-PDT significantly activated the JNK pathway in MG-63 cells while
inhibition of ROS prevented the activation of JNK, suggesting that
ROS were the proximal event of JNK. Furthermore, the JNK inhibitor
was found to partially block the expression of autophagic and
apoptotic proteins, and reverse the AE-PDT-induced inhibition of
cell viability. Based on these data, we conclude that JNK was
involved in the AE-PDT-induced autophagy and apoptosis in MG-63
cells and acted as a downstream of ROS, and thus the mechanism of
the AE-PDT-mediated antitumor effect was associated with ROS-JNK
interaction. This finding is consistent with some recent studies
suggesting that ROS-induced JNK activation plays an important role
in inducing tumor cell apoptosis and autophagy (16,19).
Autophagy can protect tumor cells through the
cleavage of ROS and inhibition of apoptosis while in some cases it
can render the autophagic death of tumor cells and contribute to
tumor suppression (20,21). This suggests that autophagy plays a
dual roles in antitumor effects. Synthesized pheophorbide
a-mediated PDT was found to hold some promise in killing oral
squamous carcinoma cells through inducing apoptosis and autophagy,
and inhibition of autophagy enhanced the apoptosis (22). These results suggest that autophagy
plays a role in PDT-mediated antitumor therapy. This hypothesis was
supported by the finding that pretreatment of MG-63 cells with
3-MA, a specific inhibitor of the early stage autophagic process
(23), and CQ, a late stage
autophagy inhibitor (23),
significantly increased the apoptosis rate, and further reduced the
cell viability when compared to the AE-PDT group. Therefore, we
propose that autophagy may have a protective effect during the
early stage of AE-PDT.
In summary, the present study indicated that AE-PDT
effectively decreased the viability of human osteocarcinoma MG-63
cells in an AE concentration- and PDT energy density-dependent
manner, and induced autophagy and apoptosis through activation of
the ROS-JNK signaling pathway. Furthermore, autophagy may play a
protective role during the early stage of AE-PDT. Further research
is needed to enhance the antitumor effect of AE-PDT by modulating
autophagy and apoptosis.
Abbreviations:
|
PDT
|
photodynamic therapy
|
|
ROS
|
reactive oxygen species
|
|
AE
|
aloe-emodin
|
|
AE-PDT
|
aloe-emodin-mediated photodynamic
therapy
|
|
TCM
|
traditional Chinese medicine
|
|
LED
|
light emitting diode
|
|
TEM
|
transmission electron microscope
|
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (81572634), the Graduate
Scientific Innovation Project of Chongqing Education Committee
(CYS14122 and CYS15141) and the Technologic and Scientific
Development Program of Chongqing Science and Technology Committee
(CSTC2012gg-yyjs10018). The authors wish to thank Mr. Yong Zhu, Mr.
Bin He and Mr. Zenghui Zhao, of the Department of Orthopedics, The
First Affiliated Hospital of Chongqing Medical University,
Chongqing, China, for their advice and supervision with regards to
the statistical analysis and modification of the manuscript.
References
|
1
|
Whelan J, McTiernan A, Cooper N, Wong YK,
Francis M, Vernon S and Strauss SJ: Incidence and survival of
malignant bone sarcomas in England 1979–2007. Int J Cancer1.
31:E508–E517. 2012. View Article : Google Scholar
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014.PubMed/NCBI
|
|
4
|
Mittal N, Kent PM and Ording J: Metastatic
and recurrent bone primary bone cancers. Curr Probl Cancer.
37:215–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shirasu N, Nam SO and Kuroki M:
Tumor-targeted photodynamic therapy. Anticancer Res. 33:2823–2831.
2013.PubMed/NCBI
|
|
6
|
Calabrò G, Patalano A, Lo Conte V and
Chianese C: Photodynamic chemotherapy in the treatment of
superficial mycoses: An evidence-based evaluation. G Ital Dermatol
Venereol. 148:639–648. 2013.
|
|
7
|
Master A, Livingston M and Sen Gupta A:
Photodynamic nanomedicine in the treatment of solid tumors:
Perspectives and challenges. J Control Release. 168:88–102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mfouo-Tynga I and Abrahamse H: Cell death
pathways and phthalocyanine as an efficient agent for photodynamic
cancer therapy. Int J Mol Sci. 16:10228–10241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen R, Zhang J, Hu Y, Wang S, Chen M and
Wang Y: Potential antineoplastic effects of Aloe-emodin: A
comprehensive review. Am J Chin Med. 42:275–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tabolacci C, Lentini A, Mattioli P,
Provenzano B, Oliverio S, Carlomosti F and Beninati S: Antitumor
properties of aloe-emodin and induction of transglutaminase 2
activity in B16-F10 melanoma cells. Life Sci. 87:316–324. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sevcovicova A, Bodnarova K, Loderer D,
Imreova P, Galova E and Miadokova E: Dual activities of emodin -
DNA protectivity vs mutagenicity. Neuro Endocrinol Lett. 35(Suppl
2): S149–S154. 2014.
|
|
12
|
Zaffaroni M, Mucignat C, Pecere T, Zagotto
G, Frapolli R, D'Incalci M and Zucchetti M: High-performance liquid
chromatographic assay for the determination of Aloe Emodin in mouse
plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 796:113–119.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vargas F, Rivas C and Medrano M:
Interaction of emodin, aloe-emodin, and rhein with human serum
albumin: A fluorescence spectroscopic study. Toxicol Mech Methods.
14:227–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HZ: Effects and mechanisms of emodin
on cell death in human lung squamous cell carcinoma. Br J
Pharmacol. 134:11–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HZ, Yang WH, Hour MJ, Wu CY, Peng WH,
Bao BY, Han PH and Bau DT: Photodynamic activity of aloe-emodin
induces resensitization of lung cancer cells to anoikis. Eur J
Pharmacol. 648:50–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trachootham D, Lu W, Ogasawara MA, Nilsa
RD and Huang P: Redox regulation of cell survival. Antioxid Redox
Signal. 10:1343–1374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodríguez-Blanco J, Martín V,
García-Santos G, Herrera F, Casado-Zapico S, Antolín I and
Rodriguez C: Cooperative action of JNK and AKT/mTOR in
1-methyl-4-phenylpyri-dinium-induced autophagy of neuronal PC12
cells. J Neurosci Res. 90:1850–1860. 2012. View Article : Google Scholar
|
|
18
|
Erikstein BS, Hagland HR, Nikolaisen J,
Kulawiec M, Singh KK, Gjertsen BT and Tronstad KJ: Cellular stress
induced by resazurin leads to autophagy and cell death via
production of reactive oxygen species and mitochondrial impairment.
J Cell Biochem. 111:574–584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou P, Xia Y, Chen T, Zhang J, Wang Z,
Chen W, Chen M, Kanchana K, Yang S and Liang G: Selective killing
of gastric cancer cells by a small molecule targeting ROS-mediated
ER stress activation. Mol Carcinog. Jun 18–2015.Epub ahead of
print. View
Article : Google Scholar
|
|
20
|
Gewirtz DA: The four faces of autophagy:
Implications for cancer therapy. Cancer Res. 74:647–651. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bincoletto C, Bechara A, Pereira GJ,
Santos CP, Antunes F, Peixoto da-Silva J, Muler M, Gigli RD,
Monteforte PT, Hirata H, et al: Interplay between apoptosis and
autophagy, a challenging puzzle: New perspectives on antitumor
chemotherapies. Chem Biol Interact. 206:279–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahn MY, Yoon HE, Kwon SM, Lee J, Min SK,
Kim YC, Ahn SG and Yoon JH: Synthesized Pheophorbide a-mediated
photodynamic therapy induced apoptosis and autophagy in human oral
squamous carcinoma cells. J Oral Pathol Med. 42:17–25. 2013.
View Article : Google Scholar
|
|
23
|
Abe A and Kokuba H: Harmol induces
autophagy and subsequent apoptosis in U251MG human glioma cells
through the downregulation of survivin. Oncol Rep. 29:1333–1342.
2013.PubMed/NCBI
|