Introduction
Endometrial cancer (EC) is one of the most common
cancers of the female reproductive system with an estimated 54,870
newly diagnosed cases and 10,170 deaths in the USA alone in 2015
(1). In China, the incidence of EC
has increased markedly with a higher prevalence in younger women
due in part to factors such as obesity and lifestyle changes
(2,3). EC is usually diagnosed in early stages
(90%) and is often successfully treated with surgical intervention
(4). However, EC may metastasize to
the pelvic, para-aortic lymph nodes or to distant sites via
different routes, and most deaths from EC are caused by metastases
that are resistant to conventional therapies. Therefore, it is
significant to elucidate the molecular mechanism underlying EC
metastasis so as to gain insight into better diagnostic and
prognostic biomarkers, as well as novel therapies.
Tumor metastasis consists of several steps, all of
which are required for the spread of tumor cells (5). Notably, during tumor progression,
epithelial-mesenchymal transition (EMT) is activated in certain
cancer cells and enables them to acquire cellular characteristics
associated with high-grade malignancy, including the capacity to
complete various steps in the metastatic cascade (6). In addition, several studies have
elucidated a link between EMT and stem cell characteristics and
drug resistance, reinforcing the opinion that EMT is closely
related to tumor progression (7,8).
During EMT, epithelial cells undergo extensive alterations in gene
expression patterns, resulting in the loss of apico-basal polarity,
fracture of intercellular adhesive junctions, and degradation of
basement-membrane components (9).
In this way, epithelial cells adopt mesenchymal traits by altering
their morphology, cellular architecture, adhesion, and migratory
capacity (10). However, the
mechanisms and pathways that initiate EMT are not comprehensively
clear.
EMT is a dynamic procedure and triggered by
interactions between extracellular components from the
microenvironment and secreted factors, such as the wingless-type
MMTV integration site family members (Wnts), transforming growth
factor-β (TGF-β), fibroblast growth factors, and epidermal growth
factors (9). These factors
participate in multiple signaling pathways and initiate the
expression of downstream transcription factors, such as Snail and
Twist, as well as cytokines, such as MMP2 and MMP9. Among the
involved signaling pathways, the Wnt signaling pathway plays a
critical role in inducing EMT (11–13).
Wnt family proteins bind to and activate one or more of the 10
seven-transmembrane Fzd family receptors, playing roles in
proliferation, migration, and invasion (14). Previous studies have shown that
during EMT, Wnt5a/b ligand and/or its cognate receptor Fzd2 are
generally overexpressed in cell lines derived from late-stage
mesenchymal-type cancers, such as melanoma and cancers of the
breast, lung, colon, liver, and the gastric tract (15–18).
However, whether Wnt5a/b-Fzd2 induces EMT in EC and a mechanistic
understanding of signaling pathway regulation have been left
unanswered by previous investigations.
This study explored the association between the
expression of Wnt receptor Fzd2 and EMT markers in EC tissues and
investigated the role of Fzd2 in the regulation of EMT in EC cell
lines. The findings shed light on the correlation between Fzd2 and
the promotion of an EMT phenotype and cell migration in EC, and
could potentially guide the development of new therapies for EC
metastasis.
Materials and methods
Patients and tissues
Thirteen cases of fresh EC and para-tumor normal
endometrial tissues were obtained from Chinese female patients who
underwent surgical treatment during 2014 and 2015 at the Shanghai
First Maternity and Infant Hospital (Shanghai, China). No patient
had undergone endocrine therapy, radiotherapy, or chemotherapy
before surgery. This study was approved by the Human Investigation
Ethics Committee of the Shanghai First Maternity and Infant
Hospital. The samples were collected after written informed consent
was obtained from the patients.
Cell culture
The human EC cell lines HEC-1B and Ishikawa were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM)/F12 (Gibco, Auckland, New Zealand), supplemented with
10% fetal bovine serum (FBS) (Gibco Life Technologies, Carlsbad,
CA, USA) and 100 U/ml penicillin/streptomycin, and maintained in a
5% CO2 humidified incubator at 37°C.
Transient and stable transfection
For stable overexpression of human Fzd2 in EC cells,
Fzd2 coding sequences were cloned into lentiviral vectors with
Ubi-MCS-3FLAG-SV40-puromycin using Gateway technology (Invitrogen
Life Technologies, Carlsbad CA, USA) by GeneChem Biotech Co., Ltd.
(Shanghai, China). HEC-1B and Ishikawa cells were infected with
nontarget or Fzd2-specific lentiviral particles in 6-well plates in
the presence of Polybrene (5 mg/ml). The cells were treated with
puromycin (1 µg/ml) to generate stable Fzd2-overexpressing
clones. The siRNA targeting Frizzled2 (si-Fzd2) and the negative
control (si-Ctl) were purchased from Hanyin Biotech (Shanghai,
China). The cells were transfected with the siRNA in Opti-MEM using
Lipofectamine 2000 (11668-019; both from Invitrogen Life
Technologies) according to the manufacturer's instructions.
RNA extraction and qRT-PCR
Total RNA was extracted from the cultured cells
using Trizol reagent (Invitrogen Life Technologies) and converted
into cDNA with the One Step PrimeScript RT reagent kit (Takara,
Dalian, China). The gene expression was detected by real-time
polymerase chain reaction (PCR) using SYBR Green Master Mix
(Takara) on an ABI Prism 7000 thermal cycler (Applied Biosystems,
Foster City, CA, USA). The gene expression was calculated using the
2−ΔΔCt formula and normalized against glyceraldehyde
3-phosphate dehydrogenase (GAPDH). The oligonucleotide primers used
for quantitative reverse transcription (qRT)-PCR are listed in
Table I. The data were obtained in
triplicate from three independent experiments.
 | Table IPrimer sequences for real-time PCR
analysis. |
Table I
Primer sequences for real-time PCR
analysis.
| Gene | Primer sequence |
|---|
| GAPDH | F:
5′-AGGGCTGCTTTTAACTCTGGT-3′ |
| R:
5′-CCCCACTTGATTTTGGAGGGA-3′ |
| N-cadherin | F:
5′-TGCGGTACAGTGTAACTGGG-3′ |
| R:
5′-GAAACCGGGCTATCTGCTCG-3′ |
| Vimentin | F:
5′-GACGCCATCAACACCGAGTT-3′ |
| R:
5′-CTTTGTCGTTGGTTAGCTGGT-3′ |
| SPP1 | F:
5′-GAAGTTTCGCAGACCTGACAT-3′ |
| R:
5′-GTATGCACCATTCAACTCCTCG-3′ |
| Cytokeratin7 | F:
5′-TCCGCGAGGTCACCATTAAC-3′ |
| R:
5′-GCTCTGTCAACTCCGTCTCAT-3′ |
| Cytokeratin19 | F:
5′-ACCAAGTTTGAGACGGAACAG-3′ |
| R:
5′-CCCTCAGCGTACTGATTTCCT-3′ |
Protein extraction and western blot
analysis
Total protein was extracted with lysis buffer
(Beyotime Biotech, Jiangsu, China) containing a 1% dilution of the
protease inhibitor phenylmethanesulfonyl fluoride (Beyotime
Biotech). The protein concentrations were determined using a
bicinchoninic acid protein assay kit (Beyotime Biotech). Equal
amounts of protein were loaded onto each lane of a SDS-PAGE gel for
protein separation and transferred to polyvinylidene fluoride
membranes (Millipore, Billerica, MA, USA). The membranes were
blocked with 5% bovine serum albumin for 2 h and incubated with
antibodies against Fzd2 (1 µg/ml; R&D Systems, Inc.,
Minneapolis, MN, USA), N-cadherin (1:1,000), vimentin (1:1,000),
E-cadherin (1:1,000) (all from Cell Signaling Technology, Danvers,
MA, USA), and GAPDH (1:5,000; Abcam, Cambridge, MA, USA) at 4°C
overnight. Peroxidase-linked secondary anti-rabbit (1:2,000) or
anti-mouse antibodies (1:2,000; both from Cell signaling
Technology) were used to detect the bound primary antibodies, and
the blotted proteins were visualized using an enhanced
chemiluminescence kit (Pierce Biotechnology, Inc., Rockford, IL,
USA). The intensity of protein bands was quantified using ImageJ
software (National Institutes of Health, Bethesda, MD, USA). The
relative expression of target proteins was described as a ratio
relative to the expression of GAPDH, and statistical data from at
least three experiments were graphed.
Cell proliferation assay
HEC-1B and Ishikawa cells were seeded into a 96-well
plate (3,000 cells/well). Then, 20 µl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well
and subsequently incubated at 37°C for 1 h. The absorbance was
measured at 490 nm on a plate reader (Bio-Rad, USA). Wells
containing known cell numbers (0, 1×103,
2×103, 5×103, 10×103,
20×103, or 40×103 cells/well; 6 wells/cell
density) were treated in a similar fashion to establish standard
curves. Each individual experiment was repeated three times in
triplicate.
Migration assay
The cells were seeded in 6-well plates and allowed
to adhere for 24 h. Confluent monolayer cells were scratched using
a 200-µl pipette tip and then washed three times with 1X
phosphate-buffered saline to clear cell debris and suspension
cells. Fresh serum-free medium was added, and images were captured
at 0 and 24 h at the same position of the wound. For the Transwell
assay, a total of 4×104 cells were resuspended in 200
µl of the serum-free medium and seeded on the top chamber of
the Transwell cell culture chambers (8 µm pore size; Corning
Costar, no. 3422). The complete medium (800 µl) was added to
the bottom chamber as a chemoattractant. After 16 h, the cells that
had migrated to the basal side of the membranes were stained with
calcein-AM (0.2 µg/ml; Invitrogen Life Technologies, no.
C3100MP) for 30 min and counted at a ×200 magnification.
Recombinant human Wnt5a and Wnt5b (250 ng/ml; R&D Systems,
Inc.) were added to both top and bottom chambers. All experiments
were repeated at least three times. The number of cells that had
migrated was estimated using MetaMorph image analysis software
(Molecular Devices, LLC, Sunnyvale, CA, USA), and the data are
expressed as mean average ± standard deviation (SD) (n=3).
Construction of reporter plasmids and
luciferase assays
T-cell factor/lymphoid enhancer factor (TCF/LEF)
reporter M50 Super 8× TOPFlash and M51 Super 8× FOPFlash (TOP Flash
mutant) (plasmids #12456 and #12457 respectively; Addgene
Cambridge, MA, USA) driving the expression of green fluorescent
protein (GFP) (TOP/FOP-GFP) were gifts from Randall Moon
(Cambridge, MA, USA). HEC-1B and Ishikawa cells (2×104)
were plated in 24-well plates 24 h before transfection. The cells
were co-transfected with 500 ng FOP/TOP reporter plasmid and
Renilla luciferase plasmid. The luciferase activity was
assayed 24 h after transfection and measured using Dual-Glo
Luciferase reagents (E1531; Promega Corp., Madison, WI, USA). The
results were normalized against Renilla activity. All
experiments were performed in triplicate.
Statistical analysis
Statistical analyses were performed using the
Statistical Package for the Social Sciences software version 17.0
(SPSS, Inc., Chicago, IL, USA). All data are represented as the
mean ± SD. Measurement data were analyzed using unpaired Student's
t test or one-way analysis of variance for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
result.
Results
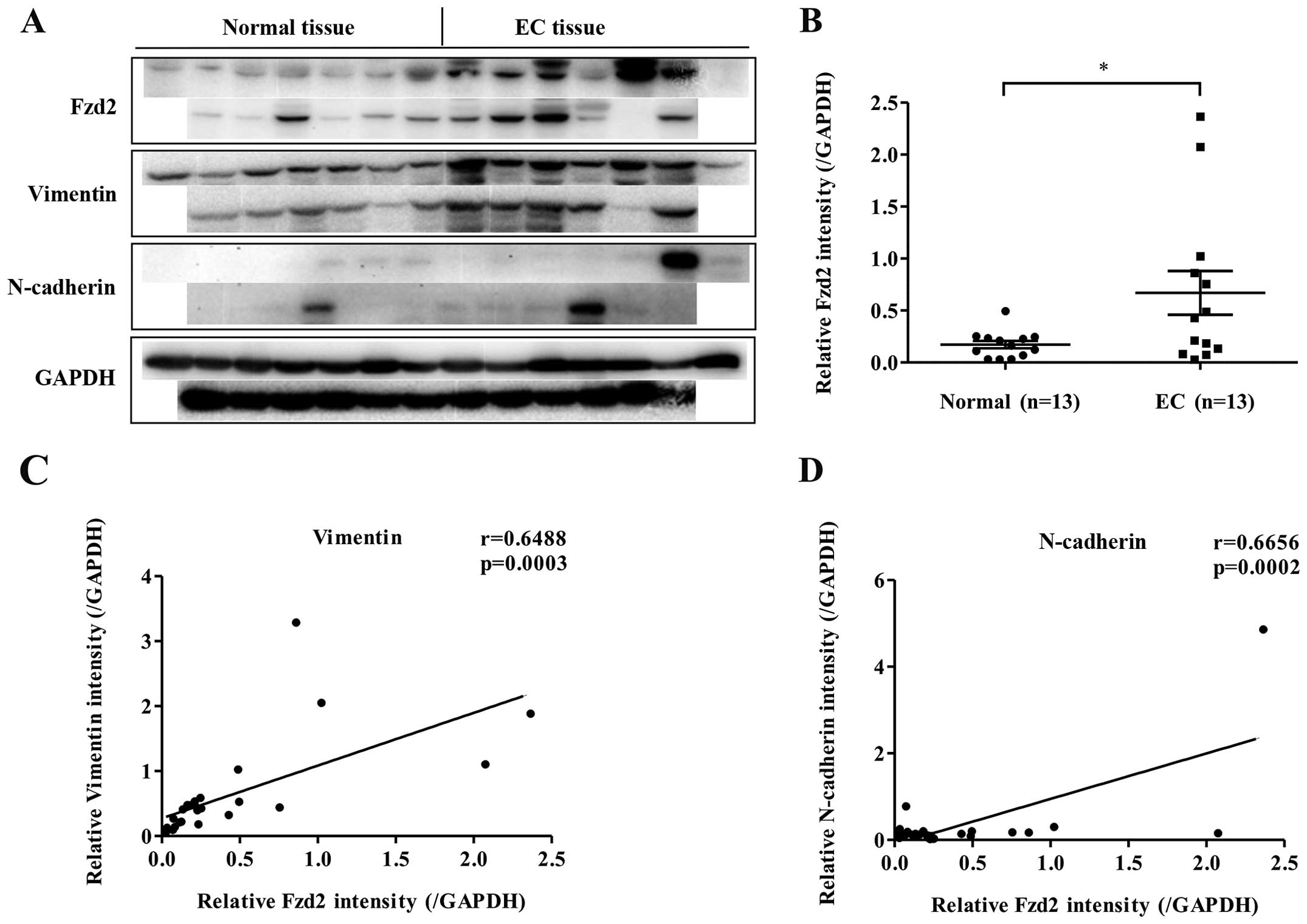
Fzd2 expression in EC tissues
A previous study showed Fzd2 overexpression to be
associated with many types of human cancers; the present study
assessed whether this was also true for EC. Western blot analysis
was used to evaluate the expression of Fzd2 in EC tissues and
paired adjacent normal tissues. In a panel of 13 patient tissues,
Fzd2 was overexpressed in EC tissues relative to the level in
normal tissues (Fig. 1A and B).
Moreover, the expression of Fzd2 was positively correlated with
markers of mesenchymal cells, such as vimentin (VIM) and N-cadherin
(CDH2) (Fig. 1A, C and D).
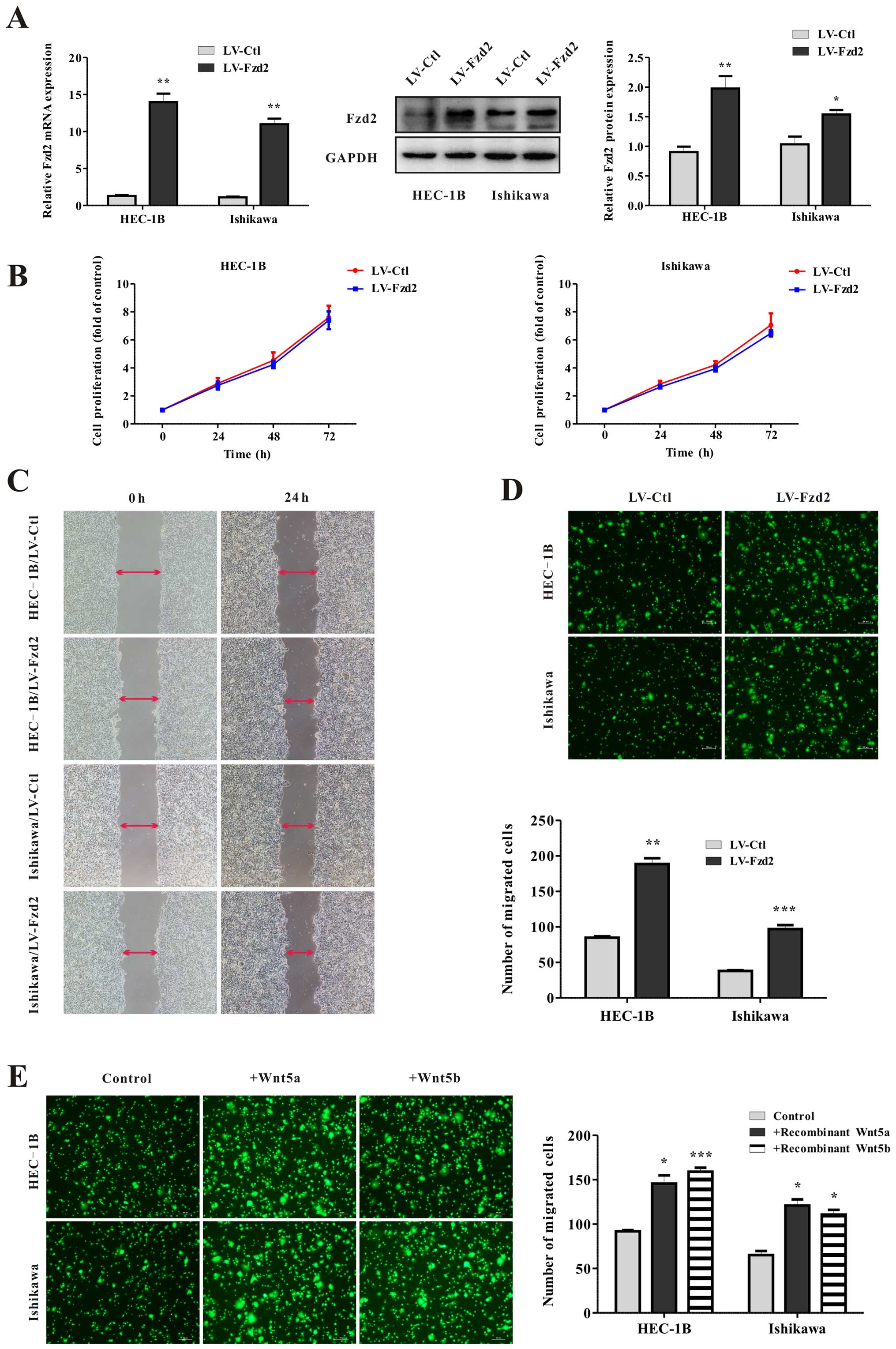
Wnt5-Fzd2 regulates cell migration
To determine whether Fzd2 is required for cell
growth or migration, EC cell lines HEC-1B and Ishikawa were stably
transfected with lentiviral vectors encoding human Fzd2
(HEC-1B/LV-Fzd2, Ishikawa/LV-Fzd2) and an empty vector as a control
(HEC-1B/LV-Ctl, Ishikawa/LV-Ctl). To examine the efficiency of Fzd2
overexpression, the levels of mRNA and protein expression were
detected before cellular assays (Fig.
2A). No differences were observed in cell viability between the
control and Fzd2-overexpressing cells (Fig. 2B). However, wound-healing and
Transwell migration assays both demonstrated that the migration
ability of the HEC-1B and Ishikawa cells was markedly increased by
2- to 3-fold after Fzd2 overexpression (Fig. 2C and D). Additionally, exposure of
HEC-1B and Ishikawa cells to human recombinant protein Wnt5a or
Wnt5b also appropriately increased cell migration potential
(Fig. 2E). Furthermore, when Fzd2
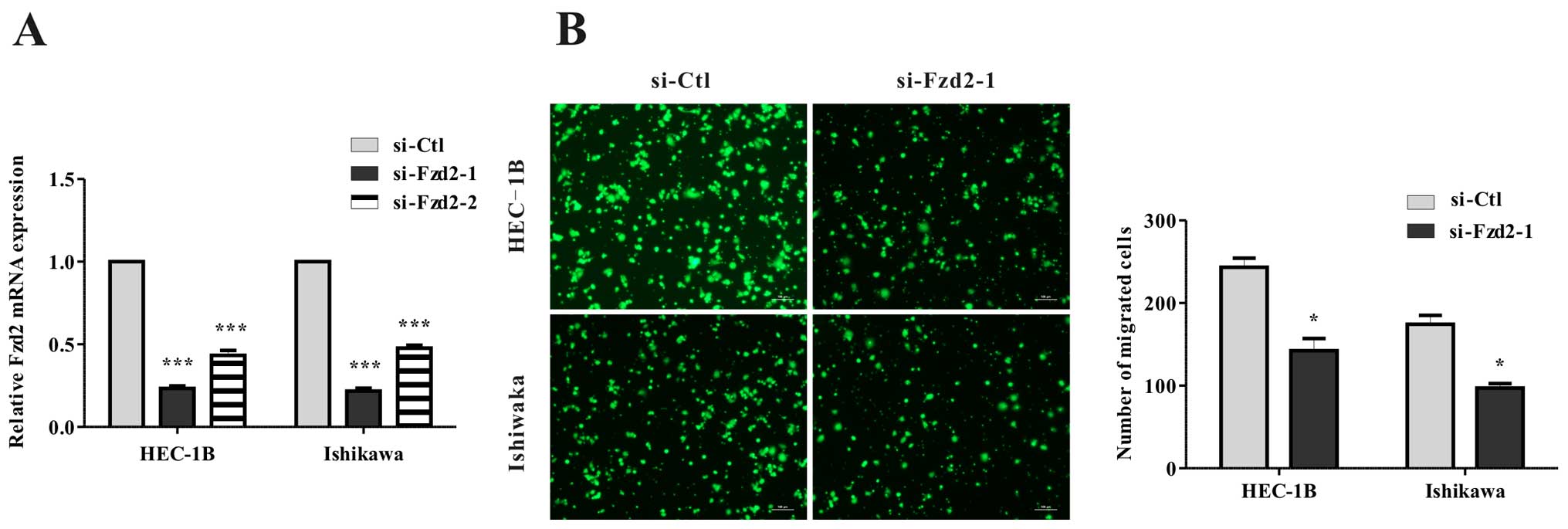
was depleted in HEC-1B and Ishikawa cells using siRNA (Fig. 3A), a significant reduction in
migration (Fig. 3B) was found.
Overall, these data showed that Fzd2 plays a causal role in EC cell
motility.
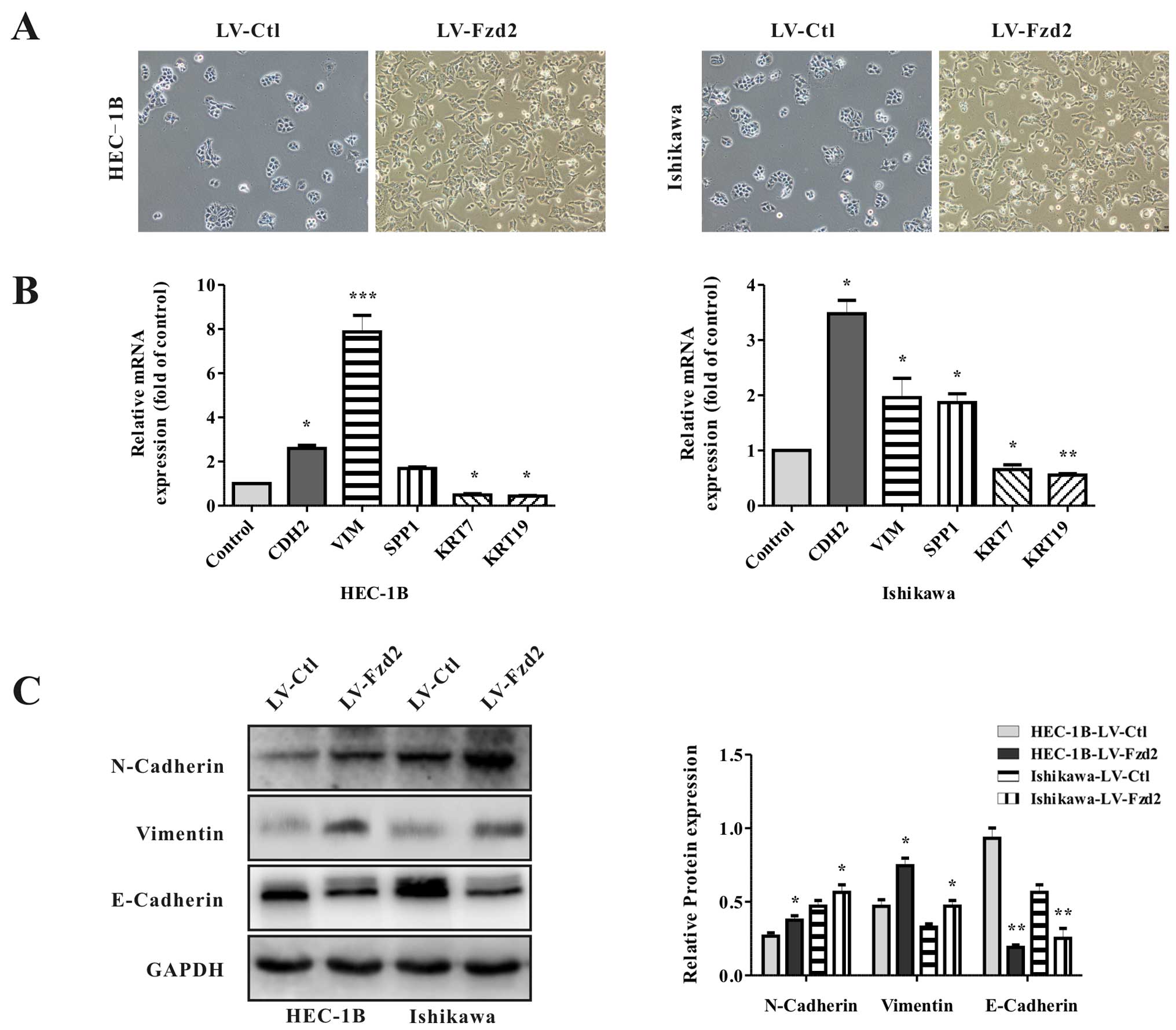
Fzd2 overexpression promotes the EMT
phenotype in EC cells
Because Fzd2 levels are correlated with mesenchymal
markers in EC tissues, and the processes involved in EMT are
closely correlated with cell motility and cancer metastasis, it was
hypothesized that Fzd2 overexpression may drive EMT. To test this,
the cellular morphology of the Fzd2-overexpressing EC cells was
microscopically examined. HEC-1B/LV-Fzd2 and Ishikawa/LV-Fzd2 cells
gained a spindle-shaped morphology and lost cell-cell contacts
compared with their control cells, suggesting an EMT phenotype
(Fig. 4A). To identify whether this
transformation represented EMT, the levels of EMT-associated genes
were detected by qRT-PCR and western blot analysis (Fig. 4B and C). Relative to the controls,
the levels of epithelial marker E-cadherin in the HEC-1B/LV-Fzd2
and Ishikawa/LV-Fzd2 cells were decreased, whereas the levels of
mesenchymal markers CDH2 and VIM were increased. Thus, it was
concluded that Fzd2 was involved in the EMT of EC cells.
Fzd2-mediated cell migration is dependent
on the canonical Wnt pathway
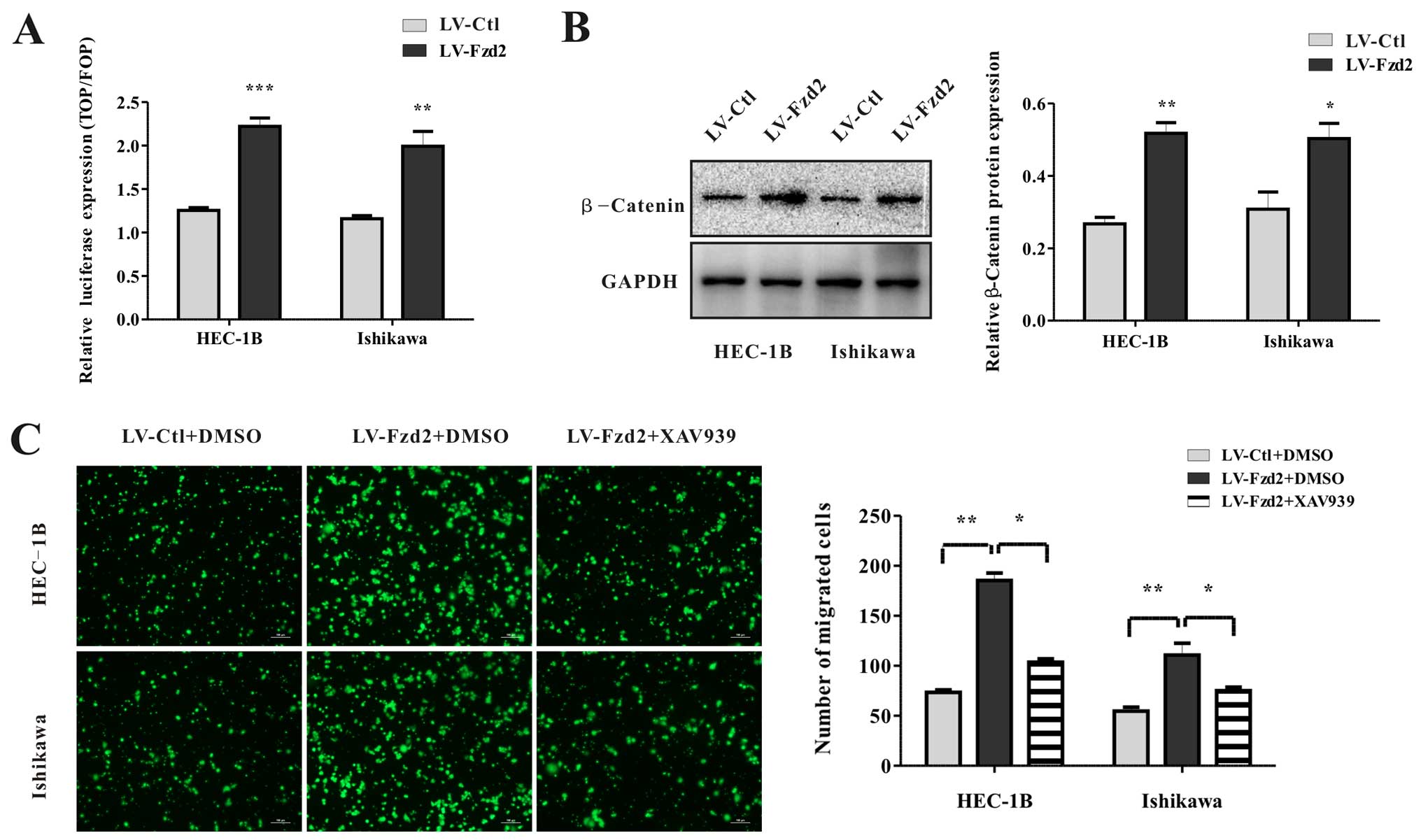
The activation of the Wnt pathway plays a vital role
in EMT during cancer progression. Previous data showed that Fzd2
could activate β-catenin-dependent (canonical) signaling by
activating the transcription factor TCF, whose activity can be
monitored using well-characterized TOP/FOP-GFP reporter plasmids.
Overexpression of Fzd2 in the HEC-1B and Ishikawa cells induced a
2-fold increase in luciferase activity compared with that noted in
the vector-only cells (Fig. 5A).
Consistently, the levels of β-catenin protein expression in these
cells were also elevated (Fig. 5B).
To investigate whether Fzd2-mediated migration depended on the
β-catenin-TCF pathway, HEC-1B/LV-Fzd2 and Ishikawa/LV-Fzd2 cells
were treated with an inhibitor of β-catenin stabilization (XAV939).
XAV939 abolished cell migration compared with dimethyl sulfoxide
(DMSO) (Fig. 5C). All in all, all
these data suggested that Fzd2-mediated cell migration depended on
the β-catenin-TCF pathway in EC cell lines HEC-1B and Ishikawa.
Discussion
Tumor metastasis and dissemination are the leading
causes of death in EC and as much as 90% of cancer-associated
mortality in general (19).
Regrettably, the progress in developing efficient strategies
specifically targeting tumor metastasis or cells with metastatic
potential has been limited (20).
Enhancing the understanding of the molecular mechanisms of the
metastatic process might improve the clinical management and
outcomes of patients with the disease.
Malignant epithelial tumor cells can disseminate
from the primary tumor and invade distant organs through a variety
of mechanisms. EMT is considered to be an important means of tumor
metastasis in many common cancers, including EC (21). Previous studies have indicated that
the activation of Fzd2-mediated signaling might be important in
metastatic and late-stage cancers, and a high expression of Fzd2
and its ligand Wnt5a could be a potential marker of poor outcome of
patients with hepatocellular carcinoma and prostate cancer,
respectively (17,18) The present results are consistent
with previous findings and prove for the first time that Fzd2 plays
an important role in tumorigenesis and acquisition of the
metastatic phenotype in EC. This study found that overexpression of
Fzd2 in EC cell lines HEC-1B and Ishikawa could promote cell
migration potential and an EMT phenotype with an increase in
E-cadherin and concomitant reduction in CDH2 and VIM. The loss of
E-cadherin protein appears to be a crucial step, reducing
cell-to-cell adhesion and destabilizes the epithelial architecture
(22). Additionally, even with
limited patient numbers, Fzd2 was overexpressed and correlated with
EMT markers in EC tissues relative to paired normal tumor-adjacent
tissues. Although these studies are not sufficient to confirm Fzd2
as a prognostic factor for EC, the expression data combined with
functional data indicate that Fzd2 may serve as a target for
anticancer therapy.
However, how Fzd2 regulates EMT transition in EC
development remains unknown and must be further investigated. The
Wnt signaling pathway is highly conserved and regulates the
specification of cell fate, stem cell maintenance, and initiation
of EMT (14,23). The present results showed that Fzd2
overexpression activated the canonical Wnt signaling pathway and
increased the β-catenin protein level. As a result, stable,
nonphosphorylated β-catenin accumulated and translocated into the
nucleus, binding to the N terminus of TCF/LEF transcription factors
(24). The TCF/LEF reporter that
indicates the expression of fluorescent protein (TOP-GFP) provides
direct evidence for the activation of the canonical Wnt signaling.
In the present study, Fzd2 overexpression significantly enhanced
the luciferase expression of the TOP/FOP reporter plasmid,
indicating the dependence on the canonical Wnt pathway. Exposing EC
cells to β-catenin stabilization antagonist XAV939 did affect
Fzd2-mediated cell migration, further suggesting the dependence on
the canonical Wnt pathway. However, previous studies have shown
that Wnt5-Fzd2 activates both β-catenin-dependent (canonical) and
β-catenin-independent (noncanonical) signaling (25–27).
Moreover, at some stage of tumor progression, a switch appears to
arise between β-catenin-dependent and β-catenin-independent
signaling, consistent with the finding that XAV939 could not fully
reverse the Fzd2-mediated cell migration. Overall, further
investigations are needed to prove that alternative signaling
pathways are involved in Fzd2-mediated cell migration and EMT.
Once the cells pass through EMT, an ongoing
autocrine Wnt signaling could be established to maintain their
residence in the resulting mesenchymal state. However, the
concomitant secreted inhibitors of the Wnt signaling pathway, such
as DKK1 and SFRP1, would destabilize the autocrine loop. Previous
data showed that EMT could be induced by reducing the levels of
secreted inhibitors (28). Thus, it
remains to be demonstrated whether such a mechanism operates in EC
and is critical to EMT induction. Furthermore, a series of
additional factors have been found capable of inducing EMT in
various types of epithelial cells, including TGF-β, Notch, Sonic
hedgehog, and multiple growth factors secreted into the
microenvironment of tumor cells. All things considered, it is also
possible to reveal an alternative mechanism in the tumor
microenvironment for inducing and maintaining the mesenchymal state
in EC.
A delicate balance between estrogen and progesterone
signaling underlies the normal functioning of the female
reproductive tract and menstrual cycle. The development of EC,
especially Type I, is correlated with estrogen excess (29). Previous studies have shown that
estrogen enhances Wnt/β-catenin signaling in the proliferative
phase during the menstrual cycle, while progesterone inhibits
Wnt/β-catenin signaling, restraining proliferative actions of
estrogens in the secretory phase (30,31).
Thus, when exposed to enhanced or unopposed estrogen signaling, the
constitutive activation of Wnt/β-catenin signaling in endometrium
would trigger endometrial hyperplasia, which may develop further
into EC.
Although surgery is the standard treatment for
early-stage EC patients, patients with lymph node or distant-organ
metastases also require chemoradiotherapy and often have poor
clinical outcomes. Therefore, identifying biomarkers correlated
with cell metastatic potential might help to optimize treatment
strategies. This study found that Fzd2 levels in EC tissues are
positively correlated with the markers of EMT. Furthermore, our
findings concerning EC cells showed that Fzd2 overexpression
promoted the EMT phenotype, and these effects involved the
activation of the Wnt/β-catenin pathway. Thus, Fzd2 might be a
potential marker for EC metastasis and a target for future
therapies for this disease.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81172476, 81272885 and 81472427),
the Science and Technology Commission of Shanghai Municipality (no.
13JC1404501), the Doctoral Fund of Ministry of Education of China
(no. 20120073110090), the Program for Young Excellent Talents in
Tongji University (no. 1400813).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu WH, Xiang YB, Zhang X, Ruan Z, Cai H,
Zheng W and Shu XO: Association of dietary glycemic index and
glycemic load with endometrial cancer risk among Chinese women.
Nutr Cancer. 67:89–97. 2015. View Article : Google Scholar
|
|
3
|
Gao J, Yang G, Wen W, Cai QY, Zheng W, Shu
XO and Xiang YB: Impact of known risk factors on endometrial cancer
burden in Chinese women. Eur J Cancer Prev. 25:329–334. PubMed/NCBI
|
|
4
|
Colombo N, Preti E, Landoni F, Carinelli
S, Colombo A, Marini C and Sessa C; ESMO Guidelines Working Group:
Endometrial cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24(Suppl 6):
vi33–vi38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: Migrating cancer stem cells-an integrated
concept of malignant tumour progression. Nat Rev Cancer. 5:744–749.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and β-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG and
Weiss SJ: Canonical Wnt signaling regulates Slug activity and links
epithelial-mesenchymal transition with epigenetic breast cancer 1,
early onset (BRCA1) repression. Proc Natl Acad Sci USA.
109:16654–16659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang YW, Su YJ, Hsiao M, Wei KC, Lin WH,
Liang CL, Chen SC and Lee JL: Diverse targets of β-catenin during
the epithelial-mesenchymal transition define cancer stem cells and
predict disease relapse. Cancer Res. 75:3398–3410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Willert K, Brown JD, Danenberg E, Duncan
AW, Weissman IL, Reya T, Yates JR III and Nusse R: Wnt proteins are
lipid-modified and can act as stem cell growth factors. Nature.
423:448–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Du J, Zheng J, Liu J, Xu R, Shen
T, Zhu Y, Chang J, Wang H, Zhang Z, et al: EGF-reduced Wnt5a
transcription induces epithelial-mesenchymal transition via
Arf6-ERK signaling in gastric cancer cells. Oncotarget.
6:7244–7261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dissanayake SK, Wade M, Johnson CE,
O'Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal
DT, Indig FE, et al: The Wnt5A/protein kinase C pathway mediates
motility in melanoma cells via the inhibition of metastasis
suppressors and initiation of an epithelial to mesenchymal
transition. J Biol Chem. 282:17259–17271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto H, Oue N, Sato A, Hasegawa Y,
Yamamoto H, Matsubara A, Yasui W and Kikuchi A: Wnt5a signaling is
involved in the aggressiveness of prostate cancer and expression of
metalloproteinase. Oncogene. 29:2036–2046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gujral TS, Chan M, Peshkin L, Sorger PK,
Kirschner MW and MacBeath G: A noncanonical Frizzled2 pathway
regulates epithelial-mesenchymal transition and metastasis. Cell.
159:844–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weigelt B, Peterse JL and van 't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sleeman J and Steeg PS: Cancer metastasis
as a therapeutic target. Eur J Cancer. 46:1177–1180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wheelock MJ and Johnson KR: Cadherins as
modulators of cellular phenotype. Annu Rev Cell Dev Biol.
19:207–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clevers H: Wnt/β-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Tu C, Zhang D, Zheng Y, Peng Z,
Feng Y, Xiao S and Li Z: Wnt/β-catenin and Wnt5a/Ca2+
pathways regulate proliferation and apoptosis of keratinocytes in
psoriasis lesions. Cell Physiol Biochem. 36:1890–1902. 2015.
View Article : Google Scholar
|
|
26
|
Grumolato L, Liu G, Mong P, Mudbhary R,
Biswas R, Arroyave R, Vijayakumar S, Economides AN and Aaronson SA:
Canonical and noncanonical Wnts use a common mechanism to activate
completely unrelated coreceptors. Genes Dev. 24:2517–2530. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang E, Li Z, Xu Z, Duan W, Sun C and Lu
L: Frizzled2 mediates the migration and invasion of human oral
squamous cell carcinoma cells through the regulation of the signal
transducer and activator of transcription-3 signaling pathway.
Oncol Rep. 34:3061–3067. 2015.PubMed/NCBI
|
|
28
|
Scheel C, Eaton EN, Li SH, Chaffer CL,
Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, et al:
Paracrine and autocrine signals induce and maintain mesenchymal and
stem cell states in the breast. Cell. 145:926–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kandoth C, Schultz N, Cherniack AD, Akbani
R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al
Cancer Genome Atlas Research Network: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, van der Zee M, Fodde R and Blok
LJ: Wnt/B-catenin and sex hormone signaling in endometrial
homeostasis and cancer. Oncotarget. 1:674–684. 2010. View Article : Google Scholar
|
|
31
|
Wang Y, Hanifi-Moghaddam P, Hanekamp EE,
Kloosterboer HJ, Franken P, Veldscholte J, van Doorn HC, Ewing PC,
Kim JJ, Grootegoed JA, et al: Progesterone inhibition of
Wnt/β-catenin signaling in normal endometrium and endometrial
cancer. Clin Cancer Res. 15:5784–5793. 2009. View Article : Google Scholar : PubMed/NCBI
|