Introduction
Hepatocellular carcinoma (HCC) is one of the most
common cancers and the third leading cause of cancer-related deaths
in the world (1). Recently data
showed that 782,500 new cases and 745,500 deaths occurred worldwide
during 2012, with China alone accounting for approximately 50% of
the total number of cases and deaths (2). The majority of HCC occurs in the
setting of chronic liver disease from viral hepatitis, alcohol
abuse, heavy exposure to aflatoxin or algal hepatotoxins in
contaminated water, betel nut chewing and diabetes mellitus
(3). The process of HCC involves a
series of sequential and complex steps. Over the past decades a
large number of studies mostly focused on the cancer stem cells
(CSCs).
CSCs are a subset of tumor cells that are capable of
self-renewal, chemo/radio-therapeutic resistance, tumorigenicity
and differentiation, similar to normal stem cells (4,5), and
these characteristics could further result in aggressive phenotype
of cancer and poor prognosis. Therefore, CSCs may serve as an
effective therapeutic target in the treatment of HCC and may
improve the current poor prognosis of this disease.
NIMA-related kinase 2 (NEK2), a member of the Nek
family of serine/threonine kinases, is structurally related to the
essential mitotic regulator NIMA and is highly enriched at the
centrosome (6). Recent data
indicate that NEK2 has emerged as an important player in cancer
progression. Overexpression of NEK2 in myeloma (7), colorectal carcinomas (8,9),
breast carcinoma (10,11), and lung cancer (12) has been associated with aggressive
disease, poor differentiation, development of metastases and poor
clinical prognosis. Previous studies also revealed expression of
Nek2 and β-catenin were correlated with each other in clinical
specimens of colorectal cancer and breast carcinoma (8,10).
Furthermore, β-catenin was proved as a Nek2 substrate involved in
centrosome separation (13), and
Nek2 could bind to β-catenin to prevent its ubiquitination and
degradation (14). β-catenin was
well-known as a key components of canonical wnt/β-catenin signal
pathway which regulate the CSC features.
However, up to now, no data are available regarding
the role of NEK2 in HCC and CSCs. In the present study, we
evaluated that NEK2 is highly expressed in HCC, and associated with
tumor recurrence and poor prognosis. Furthermore, this study also
revealed the role of NEK2 in CSCs, including maintaining
self-renewal property by means of Wnt/β-catenin signaling, and
influencing chemotherapeutic resistance by preferential regulating
the expression of ABCG2 and ALDH1A1 in HCC cells.
Materials and methods
Patients and specimens
The tumor tissues with adjacent non-tumor tissues
were from 40 patients, and tumor specimens with clinicopathological
features and follow-up, were obtained in 104 patients. All patients
underwent curative hepatectomy for HCC at Sir Run Run Shaw
Hospital, College of Medicine, Zhejiang University, from January
2006 to December 2010. All the cases conformed to the following
criteria: diagnosed by postoperative histopathology, no
perioperative extrahepatic metastasis, no other malignant diseases,
did not die from perioperative complications and no preoperative
anticancer therapy. Curative resection was defined as removal of
all recognizable tumors with a clear microscopic margin. Follow-up
data were recorded from the patient's medical records and completed
by a telephone survey. All subjects selected were required to
provide written informed consent on the use of clinical specimens
for medical research. The study was approved by the Ethics
Committee of our hospital.
Cell lines and cell culture
Normal human liver cell line (Chang liver) and HCC
cell lines Hep-G2, Hep-3B, HuH-7, SMMC7721, HCCLM3, Snu387 and
Snu475 were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA) or the Cell Bank of Shanghai Institutes
for Biological Sciences, the Chinese Academy of Sciences (Shanghai,
China). Chang liver, HuH-7, SMMC7721 and HCCLM3 were routinely
maintained in Dulbecco's modified Eagle's medium (DMEM). Hep-G2 and
Hep-3B were cultured in Minimum Essential Medium (MEM). Snu387 and
Snu475 were in RPMI-1640 medium. All cell lines were supplemented
with 10% fetal bovine serum (FBS; Gibco-Invitrogen Carlsbad, CA,
USA), 100 units/ml penicillin, and 100 mg/ml streptomycin at 37°C
in a humidified incubator under 5% CO2.
Immunohistochemistry (IHC) and IHC
evaluation
Immunohistochemical stainings were performed
following standard procedure. Briefly, formalin-fixed and paraffin
embedded human samples were first cut into 5-µm-thick
sections. Then the antigen retrieval was accomplished by
deparaffinization, rehydration, and boiling in a microwave oven
with citrated buffer. Hydrogen peroxide (3%) in PBS was used to
block the endogenous peroxidase activity and BSA was used to block
non-specific staining. Sections were incubated with NEK2 antibody
(AP8074c; Abgent, San Diego, CA, USA) and β-catenin antibody
(#9582; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C
overnight. The EnVision kit (Dako, Carpinteria, CA, USA) was used
to detect primary antibody followed by staining with DAB reagent
and counterstaining with hematoxylin.
To evaluate IHC staining of NEK2/β-catenin in the
nuclear and cytoplasmic regions, the expression of NEK2/β-catenin
was scored as absent staining (−), weak staining (+), moderate
staining (++) and strong staining (+++). In the present study, we
characterize a low (−/+) score of NEK2/β-catenin as 'NEK2/β-catenin
negative' and a high (++/+++) score of NEK2/β-catenin as
'NEK2/β-catenin positive', respectively (15,16).
Assessments of the staining were scored in a double-blinded manner
by two experienced pathologists. When a discrepancy arose for any
case, the two pathologists discussed it and reached the final score
by consensus.
Protein extraction and western blot
analysis
Cell lysates were generated and total proteins were
separated by standard SDS-PAGE, followed by transfer to PVDF
membranes. The membranes were then washed and blocked before
incubation of primary antibody (NEK-2, AP8074c, Abgent; β-catenin,
#9582, Cell Signaling Technology; Bim1, #6964, Cell Signaling
Technology; Sox2, #3579, Cell Signaling Technology; Nanog,
AP21336c, Abgent; and β-actin, BS6007M, Bioworld Technology, St.
Louis Park, MN, USA), followed by incubation of horseradish
peroxidase (HRP)-conjugated secondary antibodies. The reactions
were detected by enhanced chemiluminescence assay. β-actin was used
as control.
RNA preparation and quantitative
real-time PCR
All procedures were performed according to the
manufacturer's instructions. Total RNA was extracted using the
Ultrapure RNA kit (CWbio, Co., Ltd., Beijing, China). RNA was
reverse transcribed into cDNA using iScript cDNA Synthesis kits
(Bio-Rad Laboratories, Hercules, CA, USA). Quantitative real-time
PCR (qRT-PCR) was performed using SYBR-Green PCR kit (Applied
Biosystems). GADPH was used as loading control. Specific primers
for the amplification of target genes and GAPDH were listed in
Table I.
 | Table ISequences of gene-specific primers
used for real-time RT-PCR. |
Table I
Sequences of gene-specific primers
used for real-time RT-PCR.
| Gene | Primer sequence
forward (5′-3′) | Primer sequence
reverse (5′-3′) |
|---|
| NEK2 |
CATTGGCACAGGCTCCTAC |
GAGCCATAGTCAAGTTCTTTCCA |
| ABCG2 |
CACCTTATTGGCCTCAGGAA |
CCTGCTTGGAAGGCTCTATG |
| ALDHIA1 |
TGGAATGTGGAGGAGGCCCGT |
CACCAAAGGGGCACTGGGCA |
| β-catenin |
GTCTTACCTGGACTCTGGAATCC |
GGTATCCACATCCTCTTCCTCAG |
| c-Myc |
GGCTCCTGGCAAAAGGTCA |
CTGCGTAGTTGTGCTGATGT |
| EpCAM |
TAATCGTCAATGCCAGTGTACTTC |
CTTCTCCCAAGTTTTGAGCCAT |
| CD133 |
TGGATGCAGAACTTGACAACGT |
ATACCTGCTACGACAGTCGTGGT |
| K19 |
TTTGAGACGGAACAGGCTCT |
TCAGTAACCTCGGACCTGCT |
| LIN28 |
TGTAAGTGGTTCAACGTGCG |
CCTCACCCTCCTTCAAGCTC |
| NOTCH1 |
GAGGCGTGGCAGACTATGC |
CTTGTACTCCGTCAGCGTGA |
| GAPDH |
GGTCTCCTCTGACTTCAACA |
GTGAGGGTCTCTCTCTTCCT |
Small interfering RNA and
transfection
NEK2 siRNA sequences were previously performed
(17). The NEK2 siRNA-NEK2 sequence
were 5′-UGCACUUGGACUUAGAUGUGAGCUG-3′ (sence) and
5′-CAGCACAUCUAAGUCCAAGUGCA-3′ (antisence). The NEK2 siRNA-control
sequences were 5′-UUCUCCGAACGUGUCACGUTT-3′ (sence) and
5′-ACGUGACACGUUCGGAGAATT-3′ (antisence).
Transfection of the siRNAs for HCC cells was
performed with Lipofectamine 3000 (Invitrogen) according to the
manufacturer's instructions. After 48 h of transfection, cells were
lysed for western blot analysis and quantitative real-time PCR. For
chemosensitivity, colony formation and spheroid formation assay,
cells were collected 24 h after transfection.
Spheroid formation assay, colony
formation assay and chemosensitivity assay
For spheroid formation assays, single cell
suspensions of 2×104 cells were seeded in 6-well
ultra-low attachment plates (Corning, Inc., Corning, New York, USA)
in complete mammoCult™ medium (Stem Cell Technologies, Vancouver,
BC, Canada). Cells were cultured in mammoCult media according to
the manufacturer's instructions. The number of spheres for each
well was evaluated after 7 days of culture.
For colony formation assays, cells were seeded in a
6-well plate (1×103 cells/well). After incubation at
37°C for 7 days, the cells were washed twice with PBS and stained
with 0.1% crystal violet solution. The dishes were photographed and
the colonies were counted.
Chemosensitivity assay was determined by Cell
Counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan).
Briefly, 6×103 cells in 100 µl medium was
dispensed into a 96-well plate. After overnight incubation, cells
were exposed to 5-fluorouracil (5-Fu) and cisplatin for 72 h,
respectively. Then CCK-8 was added to the wells and incubated for 1
h. Finally, the absorbance of the sample taken from each well was
detected at 450 nm.
Statistical analysis
The statistical analyses were performed using
GraphPad Prism software version 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA) and SPSS version 19.0 (SPSS, Inc., Chicago, IL,
USA). A statistical analysis for group differences was performed by
using the Chi-square test or the Student's t-test. The mean SD of
three independent experiments is reported. Recurrence free survival
(RFS) and overall survival (OS) after the operation were calculated
using the Kaplan-Meier method. Multivariate analysis of prognostic
factors for survival was performed by a Cox stepwise regression
model. A P-value of <0.05 was considered to be statistically
significant.
Results
NEK2 expression is upregulated in
HCC
To determine the significance of NEK2 in HCC, we
first analyzed multiple microarray data sets in the Oncomine
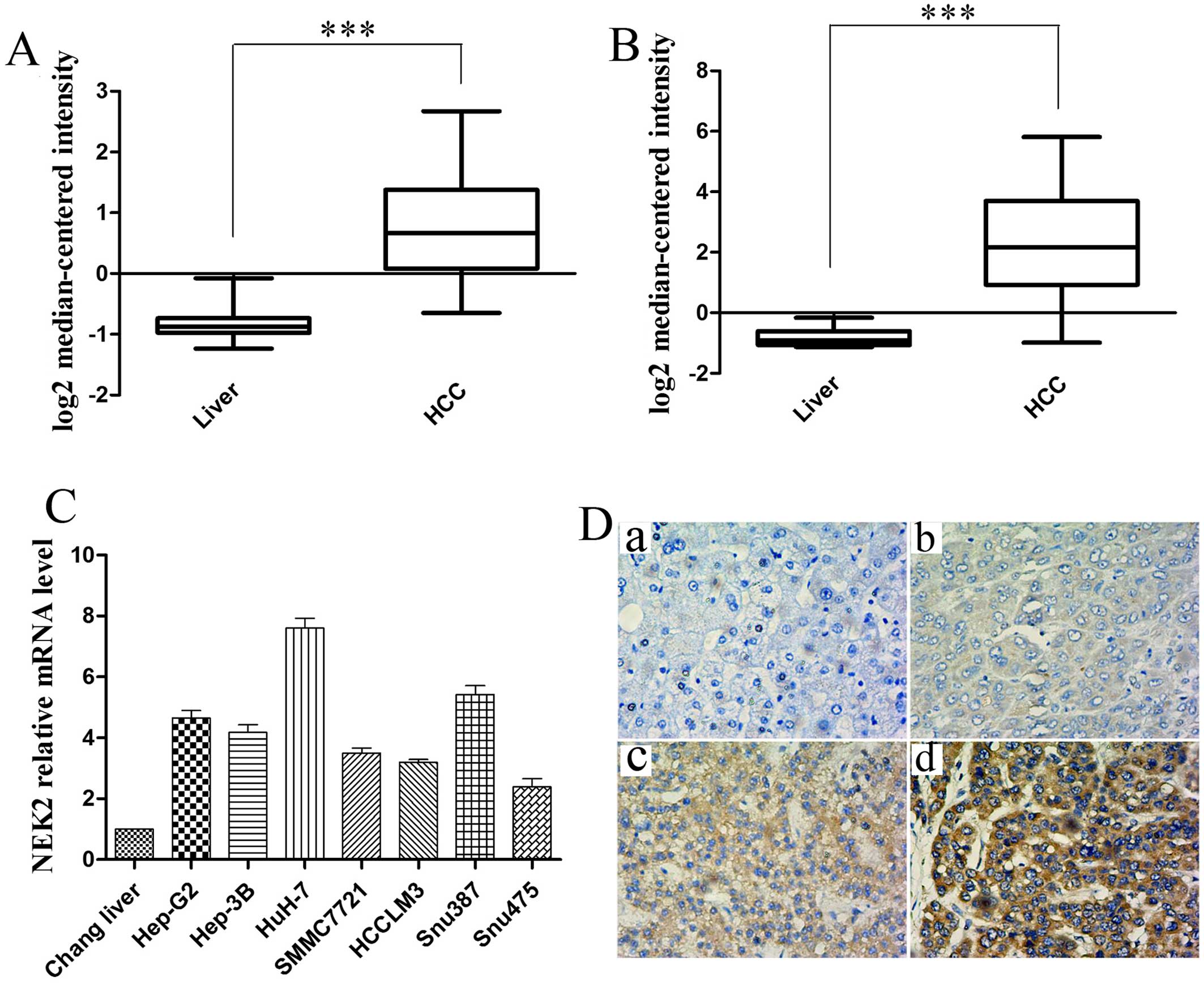
database (www.oncomine.com). As showed in Fig. 1A and B, we found NEK2 mRNA levels
were significantly increased in HCC samples as compared to normal
liver tissue from two published HCC gene expression studies
Wurmbach Liver Statistics (18) and
Roessler Liver Statistics (19)
(all P<0.001). We further assessed the mRNA expression of NEK2
in multiple HCC cell lines by real-time RT-PCR test. As shown in
Fig. 1C, the mRNA levels of NEK2
were higher in the seven HCC cell lines including Hep-G2, Hep-3B,
HuH-7, SMMC7721, HCCLM3, Snu387 and Snu475 than that in Chang
liver.
To verify the microarray analysis results, we
performed IHC experiments on 40 pairs human HCC specimens and their
matched normal tissues. Immunohistochemical analysis showed NEK2
positive staining was detected in the nuclear and cytoplasmic
regions. The expression of NEK2 was classified into negative (−) or
weak positive (+) and moderate positive (++) or strong positive
(+++) staining in Fig. 1D. Positive
staining of NEK2 could be observed in 23 of 40 (57.5%) cases of
HCCs, whereas NEK2 showed positive staining in only 3 of 40 (7.5%)
cases of adjacent non-tumor tissues.
Correlation between NEK2 expression and
clinical outcome
To better understand the clinical significance of
NEK2 expression in HCC, we investigated the clinicopathological
features of NEK2 in 104 HCC samples. As shown in Table II, a positive NEK2 protein level
was significantly associated with β-catenin expression
(P<0.001). In contrast, NEK2 expression was not correlated with
age, gender, HBsAg, differentiation, tumor number, tumor size, TNM
stage, BCLC stage and portal vein tumor thrombus (PVTT) (all
P>0.05).
 | Table IIRelationship between NEK2 expression
and clinicopathological features. |
Table II
Relationship between NEK2 expression
and clinicopathological features.
|
Characteristics | NEK2 expression n
(%)
| P-value |
|---|
| Negative (44) | Positive (60) |
|---|
| Age (years) | | | 0.539 |
| ≤50 | 23 | 35 | |
| >50 | 21 | 25 | |
| Gender | | | 0.123 |
| Male | 32 | 51 | |
| Female | 12 | 9 | |
|
Differentiation | | | 0.572 |
| Well/medium | 21 | 32 | |
|
Poorly/undifferentiated | 23 | 28 | |
| Cirrhosis | | | 0.656 |
| Absent | 21 | 26 | |
| Present | 23 | 34 | |
| Tumor number | | | 0.286 |
| Single | 41 | 52 | |
| Multiple | 3 | 8 | |
| Tumor size
(cm) | | | 0.539 |
| ≤5 | 29 | 36 | |
| >5 | 15 | 24 | |
| TNM stage | | | 0.262 |
| I | 37 | 45 | |
| II/III | 7 | 15 | |
| BCLC stage | | | 0.945 |
| A | 12 | 16 | |
| B | 32 | 44 | |
| PVTT | | | 0.764 |
| Absent | 39 | 52 | |
| Present | 5 | 8 | |
| β-catenin
expression | | | <0.001 |
| Positive | 23 | 11 | |
| Negative | 21 | 49 | |
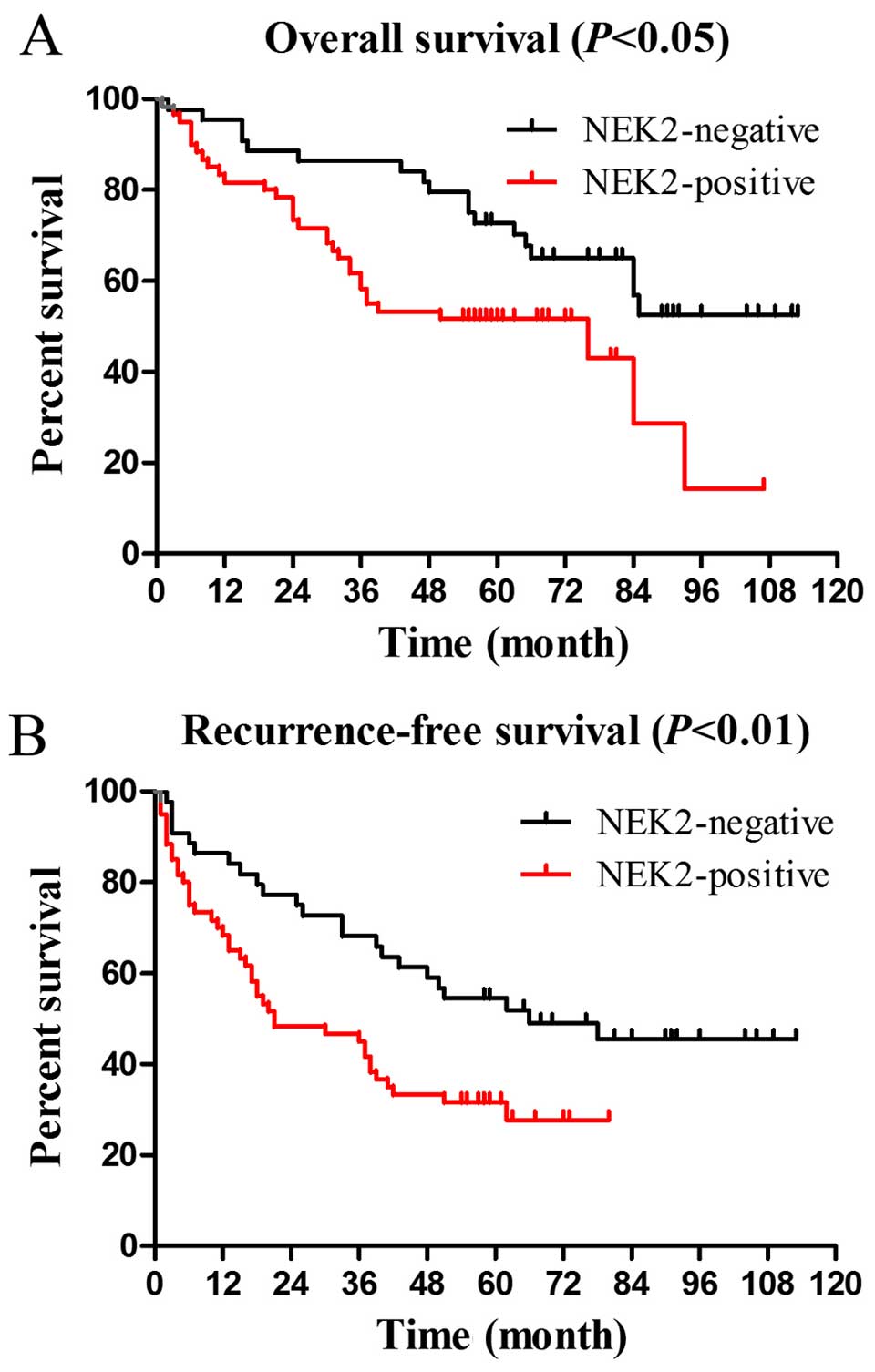
To assess the prognostic significance of NEK2
expression, Kaplan-Meier curves for OS and RFS were analyzed. The
OS and RFS rates at 5 years were 51.7 and 31.7% for NEK2 positive
patients (n=60) compared with 72.7 and 54.5% for NEK2 negative
patients (n=44), respectively (OS P<0.05, Fig. 2A; RFS P<0.01, Fig. 2B).
Univariate Cox regression analysis was conducted to
identify important prognostic factors of OS. NEK2 expression
(P=0.014), PVTT (P<0.001), TNM stage (P<0.001) and tumor size
(P=0.034) were identified as important risk factors for OS
(Table III). However, in
multivariate Cox analysis, NEK2 expression (P=0.033) and tumor size
(P=0.032) were found to be independent negative prognostic factors
for OS (Table III).
 | Table IIIUnivariate and multivariate Cox
regression analysis for OS (HR hazard ratio, CI confidence
interval). |
Table III
Univariate and multivariate Cox
regression analysis for OS (HR hazard ratio, CI confidence
interval).
| Variables | OS
|
|---|
Univariate analysis
| Multivariate
analysis
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) (≤50
vs. >50) | 0.981
(0.559–1.720) | 0.946 | 1.217
(0.657–2.254) | 0.533 |
| Gender (Male vs.
female) | 1.213
(0.619–2.376) | 0.573 | 1.511
(0.728–3.134) | 0.268 |
| Cirrhosis (Absent
vs. present) | 1.627
(0.911–2.908) | 0.100 | 1.872
(0.998–3.512) | 0.051 |
| Tumor number
(Single vs. multiple) | 1.384
(0.588–3.255) | 0.457 | 0.538
(0.129–2.246) | 0.395 |
| Tumor size (≤5 vs.
>5 cm) | 1.827
(1.046–3.191) | 0.034 | 2.140
(1.069–4.283) | 0.032 |
| TNM stage (I vs.
II/III) | 3.129
(1.707–5.735) | <0.001 | 2.311
(0.414–12.885) | 0.339 |
| BCLC stage (A vs.
B) | 1.566
(0.801–3.063) | 0.190 | 0.998
(0.432–2.303) | 0.996 |
|
Differentiation | | | | |
| (Well/medium vs.
poorly/undifferentiated) | 1.285
(0.738–2.240) | 0.376 | 0.673
(0.352–1.286) | 0.230 |
| PVTT (Absent vs.
present) | 5.765
(2.925–11.364) | <0.001 | 3.569
(0.734–17.365) | 0.115 |
| NEK2 expression
(Positive vs. negative) | 2.122
(1.162–3.875) | 0.014 | 1.984
(1.058–3.719) | 0.033 |
These results indicated that the positive expression
of NEK2 was associated with unfavorable outcomes in HCC
patients.
NEK2 knockdown decreases the self-renewal
property of CSCs
β-catenin is a key downstream molecule in the
Wnt/β-catenin signaling pathway, which is one of the well-known
pathways to play a critical role in CSC formation and maintenance
(20,21). The results from the clinical samples
indicated a correlation between NEK2 levels and β-catenin, so we
next investigated whether NEK2 knockdown influenced the stemness
characteristics of CSCs in HCC. Given that self-renewal is a
hallmark of CSCs, we performed sphere forming assay and colony
formation assay to investigate the role of NEK2 in maintaining
self-renewal property in HCC cells.
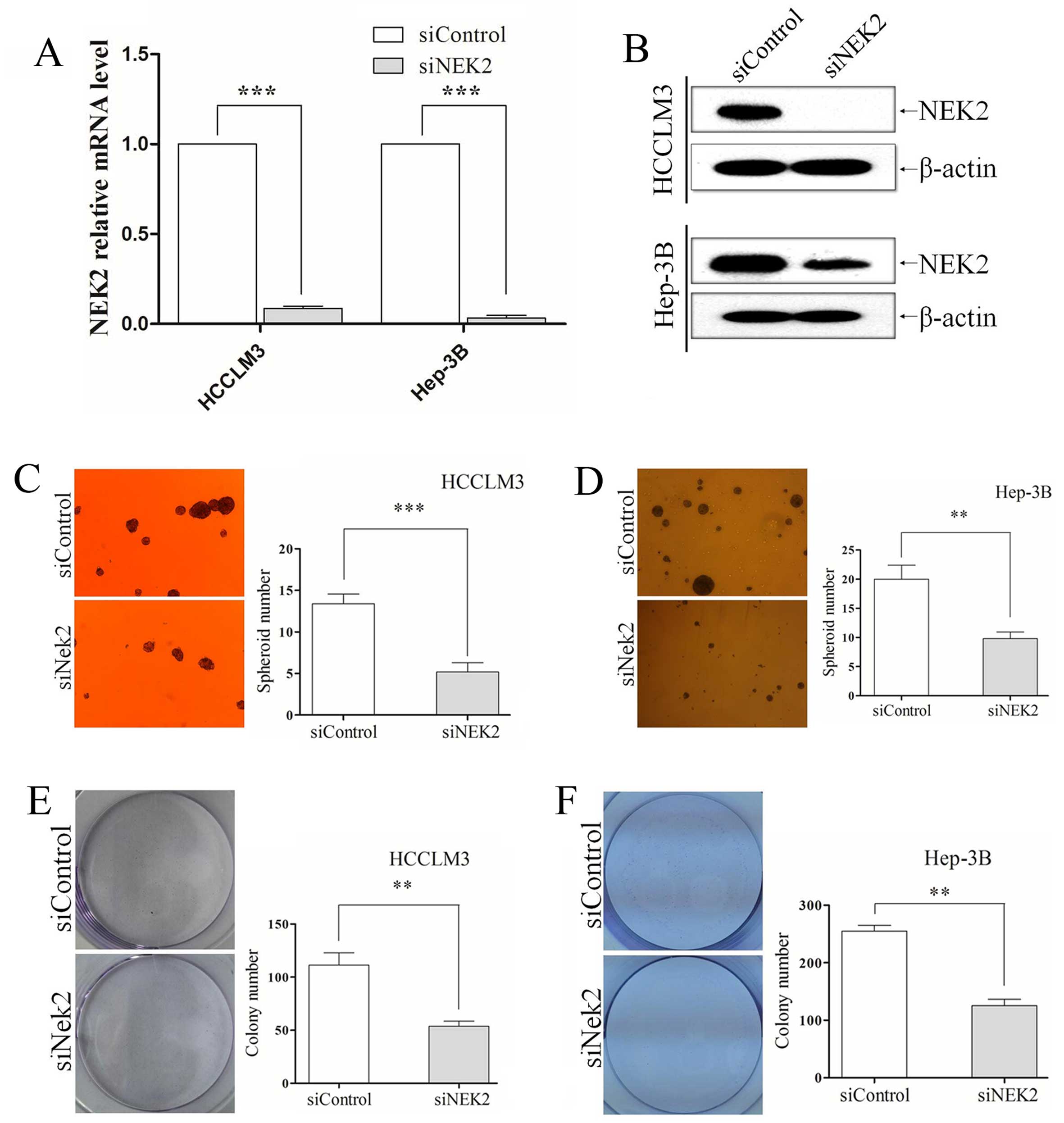
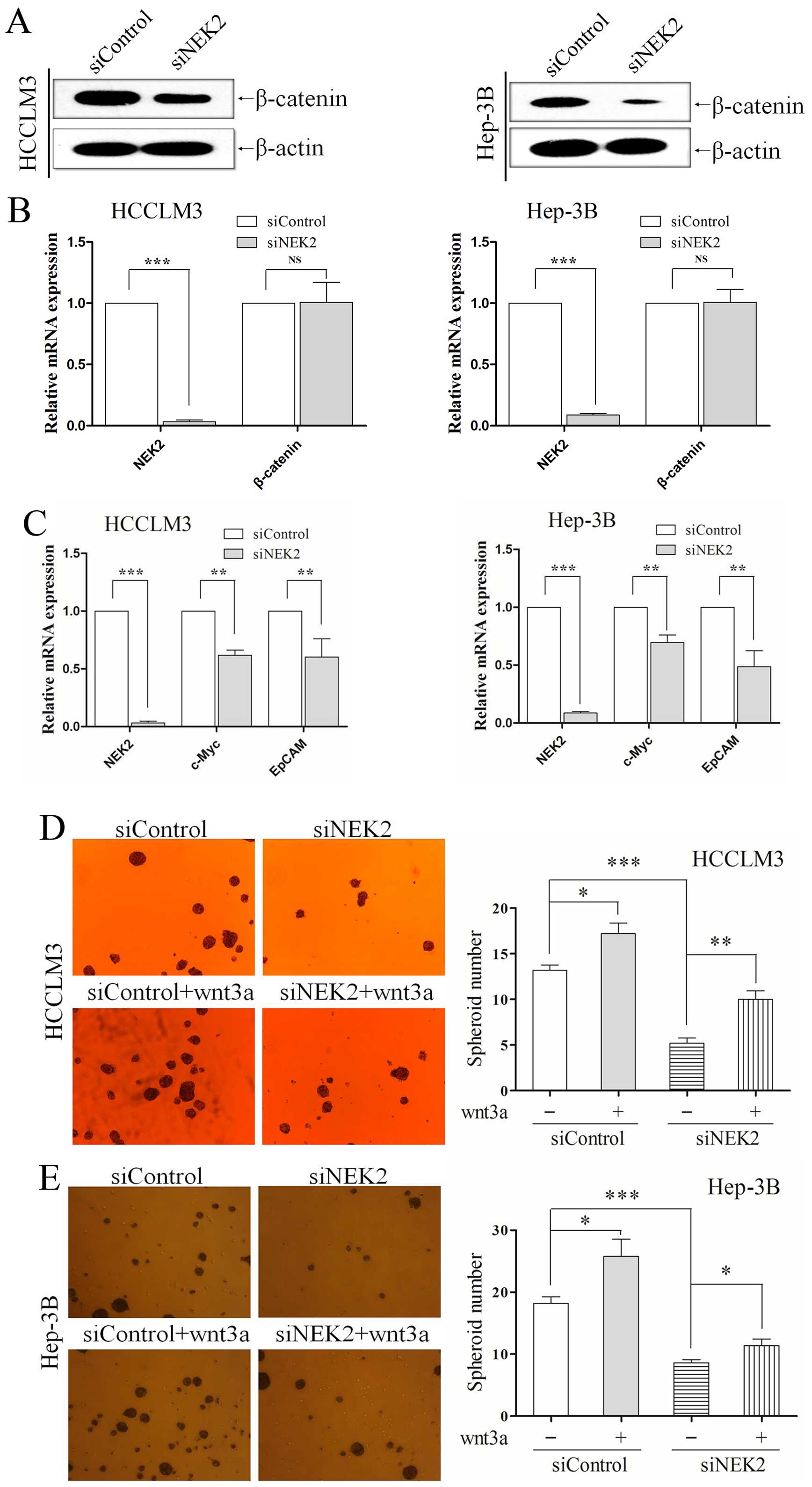
siRNA technology was used to knockdown NEK2, and its
levels were effectively downregulated in HCCLM3 and Hep-3B,
verified by qRT-PCR (all P<0.001; Fig. 3A) and western blotting (Fig. 3B). In the sphere formation assay, we
found that NEK2 knockdown group and control group grew in the form
of suspended individual cells on the first day. As 7 days passed,
HCCLM3 and Hep-3B cells in which NEK2 expression was knocked down
exhibited fewer and smaller spheres (all P<0.01; Fig. 3C and D). Colony formation assay
showed that NEK2 deletion formed less and smaller colonies than
their control groups. NEK2 knocked-down also resulted in generation
of a decreased ability to form colonies in HCCLM3 and Hep-3B cells
(all P<0.01; Fig. 3E and F).
Deletion of NEK2 reduces the expression
of stemness associated genes of CSCs
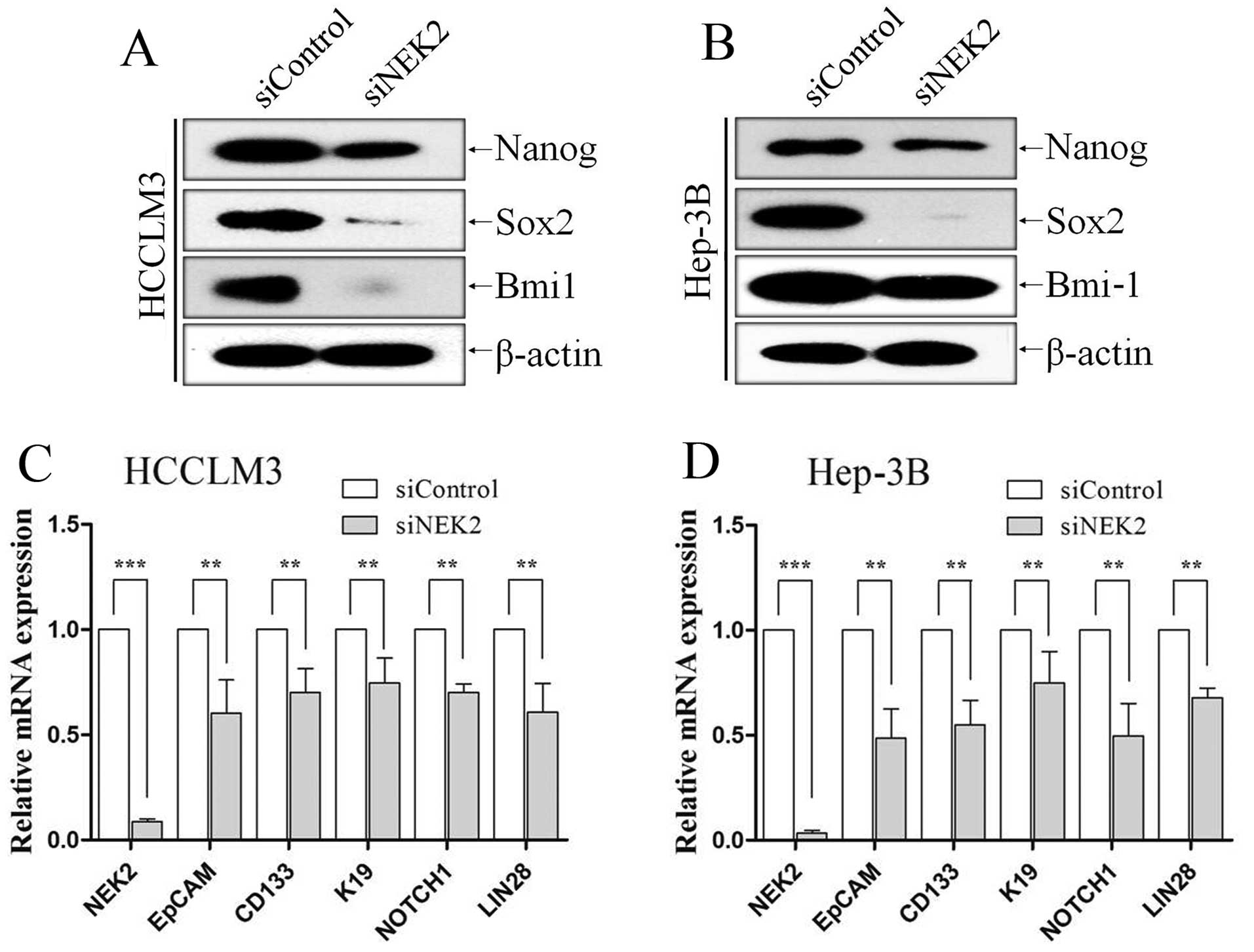
NEK2 had been proved to influence self-renewal of
HCC CSCs, then we investigate whether the knockdown of NEK2
suppresses stemness associated genes by means of western blot
analysis and qRT-PCR. We investigated whether NEK2 influences the
expression of transcriptional factors including Bmi-1, Sox2 and
Nanog, which are essential for maintaining stem cell phenotypes.
Compared with the control group, western blot analysis showed NEK2
knockdown decreased the levels of stem cell genes Bmi-1, Sox2 and
Nanog in HCCLM3 (Fig. 4A) and
Hep-3B (Fig. 4B) cells.
Additionally, the expression levels of the hepatic CSC markers
EpCAM, CD133, K19, LIN28 and NOTCH1 were analyzed by qRT-PCR. All
of these markers have been reported to be enriched in hepatic CSCs.
QRT-PCR analysis also revealed that NEK2 deletion in HCCLM3 and
Hep-3B cells expressed lower mRNA levels of EpCAM, CD133, K19,
LIN28 and NOTCH1 (all P<0.01; Fig.
4C and D).
NEK2 regulates the self-renewal property
of CSCs through the canonical wnt/β-catenin signal pathway
Next, we explored the molecular mechanisms by which
NEK2 affects the self-renewal traits of CSCs. Recent studies
indicate that Wnt/β-catenin pathway play a key role in hepatic CSCs
(22) and its deregulation has been
extensively reported in HCC (23,24).
We examined both expression of the NEK2 and β-catenin from previous
HCC specimens by IHC. Nek2 positive expression was found to
associate with β-catenin positive expression (r=0.358, P<0.001;
Table III). Western blot analysis
also showed NEK2 knockdown reduced β-catenin protein (Fig. 5A), but the mRNA level of β-catenin
was not altered (P<0.01; Fig.
5B) in HCC cells, which meant NEK2 regulated β-catenin through
post-translational modification. This result was in accordance with
that previously shown, i.e. NEK2 binds to β-catenin to
prevent its ubiquitination and degradation (14). Subsequently, we used qRT-PCR to
assessed the expression of downstream target genes of wnt/β-catenin
signal pathway including EpCAM and c-Myc. The expression of EpCAM
and c-Myc in NEK2 knockdown group was significantly decreased
compared with controls (all P<0.01; Fig. 5C).
Wnt3a, a canonical Wnt ligand, activates the
canonical Wnt signaling pathway (25,26).
Moreover, to confirm that the self-renewal effect of NEK2 was
caused by activation of the Wnt/β-catenin pathway, we enhanced
canonical Wnt signaling with exogenous recombinant human Wnt3A
(R&D Systems, Minneapolis, MN, USA; 20 ng/ml). HCC cells were
transfected with siRNA for 24 h, subsequently were cultured in the
presence of recombinant Wnt3a. As confirmed, NEK2 knockdown reduced
the ability to form tumorspheres in vitro, conversely, Wnt3a
addition increased the ability of tumor-sphere formation to
partially compensate NEK2 inhibition in HCCLM3 (all P<0.01;
Fig. 5D) and Hep-3B (all P<0.01;
Fig. 5E). All these results
demonstrate the important role of NEK2 in maintaining self-renewal
property by means of Wnt/β-catenin signaling.
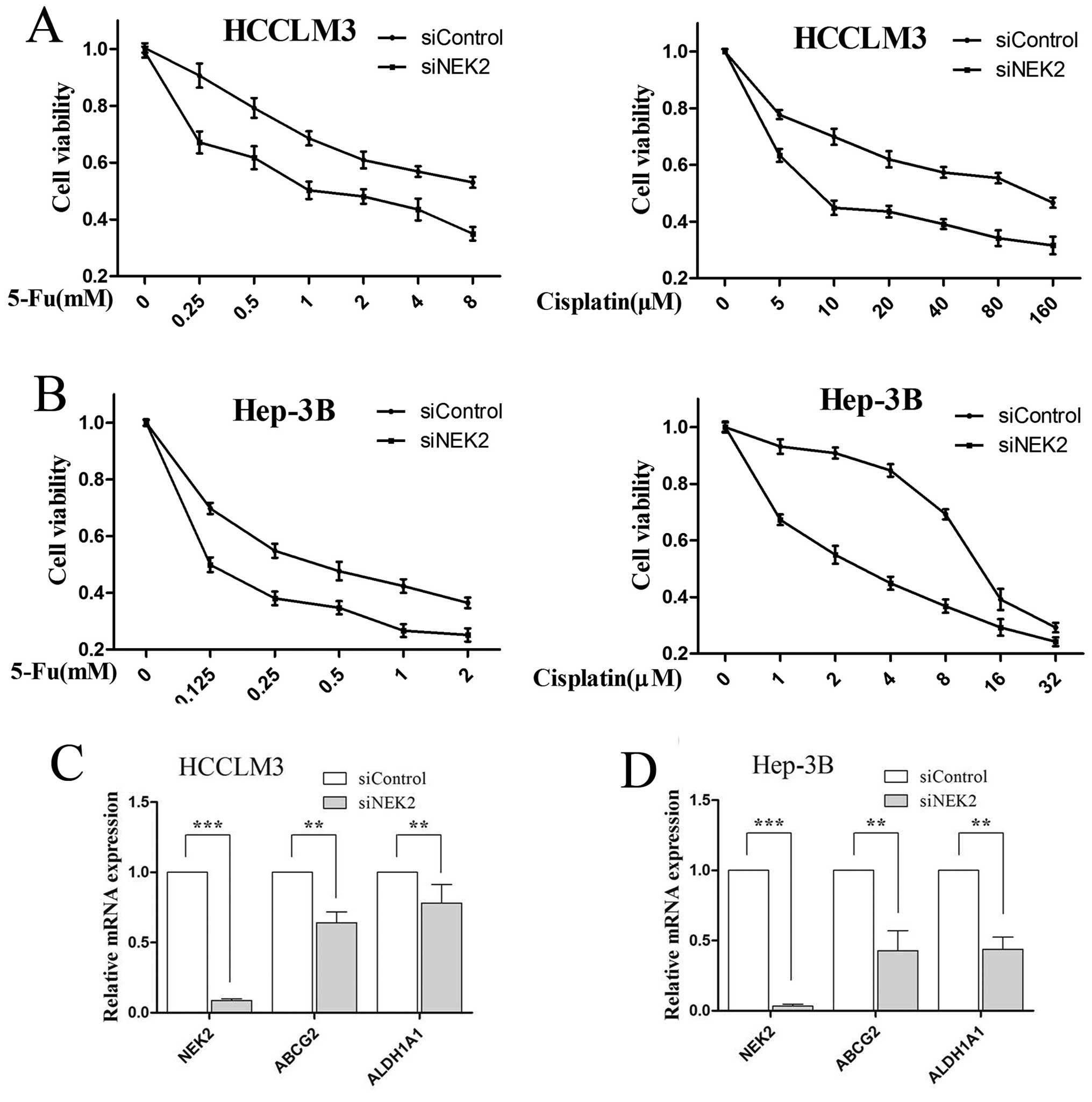
NEK2 deletion reduces chemotherapeutic
resistance of CSCs
Chemotherapeutic resistance is an important
characteristic of CSCs, which are known to show strong resistance
to chemotherapy, thus, we examined 5-Fu and cisplatin resistance of
HCC cells for NEK2 inhibition. Compared with control cells, NEK2
knockdown cells displayed significantly higher sensitivity to 5-Fu
and cisplatin chemotherapeutic agents in HCCLM3 (P<0.01;
Fig. 6A) and Hep-3B cells
(P<0.01; Fig. 6B). Finally, to
investigate the mechanism responsible for 5-Fu and cisplatin
resistance, we analyzed the mRNA expressions of multidrug resistant
genes (ABCG2) and aldehyde dehydrogenase 1 family, member A1
Aliases (ALDH1A1). QRT-PCR showed that ABCG2 and ALDH1A1 were
lowered in siNEK2 HCC cells (all P<0.01; Fig. 6C and D). All together, these data
indicate that NEK2 may influence chemotherapeutic resistance
through regulating the expression of ABCG2 and ALDH1A1 in HCC
cells.
Discussion
The association between aberrant NEK2 expression and
the prognosis of patients with HCC has not been previously
reported. However, a few studies have estimated the impact of NEK2
expression on the prognosis of several other types of cancers. In
the present study, we firstly demonstrated that increased
expression of NEK2 was significantly associated with poor prognosis
and was an independent prognostic factor in patients with HCC. The
effects of NEK2 expression on CSC-like properties have not been
examined in HCC or other cancers. In addition, our results from
in vitro experiments indicated that knockdown of NKE2
expression contributed to the inhibition of CSC-like properties in
HCC, including the self-renewal and chemotherapeutic resistance
properties.
CSC was first reported in acute myeloid leukemia
(AML) (27), and subsequently found
in some solid tumors, including HCC. NEK2 was associated with
cancer cells in proliferation, drug resistance, apoptosis,
tumorigenicity, invasion and migration (11,28,29).
To better elucidate the role of NEK2 in CSCs, we investigated the
effect of NEK2 depletion on the self-renewal properties. As
expected, the ability to form spheres and colonies was inhibited.
Nanog, Sox2 and Bmi-1 are three core transcription factors
regulating cellular pluripotency and are known to suppress
differentiation in ES cells (4,30).
CD133, EpCAM, K19, LIN28 and NOTCH1 which is frequently expressed
in HCC, has been predicted to be a CSC marker (31–34).
Silencing NEK2 expression in HCC cells also downregulated the
expression of stemness associated genes of CSCs, including Nanog,
Sox2, Bmi-1, EpCAM, CD133, K19, LIN28 and NOTCH1.
The canonical Wnt/β-catenin signaling pathway has
emerged as a critical regulator of stem cells. In many tissues,
activation of Wnt/β-catenin signaling has also been associated with
cancer. This has raised the possibility that the tightly regulated
self-renewal mediated by Wnt/β-catenin in stem and progenitor cells
is converteded in cancer cells to allow malignant proliferation
(35). Further investigation
revealed that NEK2 influenced the self-renewal properties in HCC
cells though the Wnt/β-catenin signaling pathway. EpCAM and c-Myc
has been shown to be a direct transcriptional target in the
Wnt/β-catenin signaling pathway (22,36),
which has been suggested to play an important role in governing the
self-renewal of cancer cells (37,38).
Our data indicated that the expression of β-catenin, EpCAM and
c-Myc reduced accordingly when NEK2 was silenced. Furthermore,
Wnt3a increased the ability of tumorsphere formation to partially
compensate NEK2 inhibition. Taken together, these results suggest
that the role of NEK2 in regulating self-renewal property is via
activation of the Wnt/β-catenin signaling pathway.
For HCC, traditional chemotherapeutic strategies do
not completely eliminate tumors, which results in tumor recurrence
and drug resistance. The CSC model might explain this situation,
and drug resistance is one of its properties (39,40).
We examined the influence of NEK2 to drug resistance of HCC. Our
results showed that NEK2 deletions were more sensitive to 5-Fu and
cisplatin in HCC cells. This results was consistent with previous
reports that NEK2 induced drug resistance in other cancers through
different mechanisms, such as activation of efflux drug pumps
(7) and regulation
ALDH1A1-dependent drug resistance (41). Further qRT-PCR analyses revealed
that knockdown of NEK2 decreased ABCG2 and ALDH1A1 level.
As an important multidrug resistance transporter,
ABCG2 has the capability to efflux various chemotherapy drugs and
contributes to drug resistance of cancer cells (42,43).
ABCG2 was also considered as a potential marker of CSCs in HCC and
relevant with tumor stages and poor prognosis (44). ALDH1A1, belonged to the aldehyde
dehydrogenases family of proteins, plays an important role in the
metabolization of reactive acetaldehyde, and is expressed at high
levels in stem cells and regulates stem cell function (45). Enforced expression of ALDH1A1 led to
increased activity of the drug efflux pump to induce drug
resistance (41). ALDH1A was also
reported as a marker of several stem cancer cells (46–48).
These findings further support the idea that NEK2 knockdown could
increase the susceptibility of chemotherapy drugs and decrease the
stemness marker for HCC by preferentially mediating ABCG2 and
ALDH1A1.
In conclusion, we firstly demonstrated that
upregulation of NEK2 expression contributed to poor prognosis in
patients with HCC. Furthermore, the study also revealed the role of
NEK2 in maintaining self-renewal property by means of Wnt/β-catenin
signaling, and influencing chemotherapeutic resistance by
preferential regulating the expression of ABCG2 and ALDH1A1 in HCC
cells. The present study provides a foundation for future studies
that hold great promise for the development of a novel clinical
biomarker for prediction of the malignant potential of HCC, and
therapeutic strategies for HCC patients.
Acknowledgments
The present study is financially supported by grants
from the Health and Family Planning Commission of Zhejiang Province
(grant nos. 2014KYB138 and 2015RCB014), and the National Natural
Science Foundation of China (grant no. C0707).
References
|
1
|
Giordano S and Columbano A: MicroRNAs: New
tools for diagnosis, prognosis, and therapy in hepatocellular
carcinoma? Hepatology. 57:840–847. 2013. View Article : Google Scholar
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poon D, Anderson BO, Chen LT, Tanaka K,
Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al Asian
Oncology Summit: Management of hepatocellular carcinoma in Asia:
Consensus statement from the Asian Oncology Summit 2009. Lancet
Oncol. 10:1111–1118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sainz B Jr and Heeschen C: Standing out
from the crowd: Cancer stem cells in hepatocellular carcinoma.
Cancer Cell. 23:431–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang B and Jacob ST: Role of cancer stem
cells in hepatocarcinogenesis. Genome Med. 3:112011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu W, Baxter JE, Wattam SL, Hayward DG,
Fardilha M, Knebel A, Ford EM, da Cruz e Silva EF and Fry AM:
Alternative splicing controls nuclear translocation of the cell
cycle-regulated Nek2 kinase. J Biol Chem. 282:26431–26440. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou W, Yang Y, Xia J, Wang H, Salama ME,
Xiong W, Xu H, Shetty S, Chen T, Zeng Z, et al: NEK2 induces drug
resistance mainly through activation of efflux drug pumps and is
associated with poor prognosis in myeloma and other cancers. Cancer
Cell. 23:48–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neal CP, Fry AM, Moreman C, McGregor A,
Garcea G, Berry DP and Manson MM: Overexpression of the Nek2 kinase
in colorectal cancer correlates with beta-catenin relocalization
and shortened cancer-specific survival. J Surg Oncol. 110:828–838.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi Y, Iwaya T, Sawada G, Kurashige
J, Matsumura T, Uchi R, Ueo H, Takano Y, Eguchi H, Sudo T, et al:
Up-regulation of NEK2 by microRNA-128 methylation is associated
with poor prognosis in colorectal cancer. Ann Surg Oncol.
21:205–212. 2014. View Article : Google Scholar
|
|
10
|
Wang S, Li W, Lv S, Wang Y, Liu Z, Zhang
J, Liu T and Niu Y: Abnormal expression of Nek2 and β-catenin in
breast carcinoma: Clinicopathological correlations. Histopathology.
59:631–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marina M and Saavedra HI: Nek2 and Plk4:
Prognostic markers, drivers of breast tumorigenesis and drug
resistance. Front Biosci (Landmark Ed). 19:352–365. 2014.
View Article : Google Scholar
|
|
12
|
Zhong X, Guan X, Dong Q, Yang S, Liu W and
Zhang L: Examining Nek2 as a better proliferation marker in
non-small cell lung cancer prognosis. Tumour Biol. 35:7155–7162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bahmanyar S, Kaplan DD, Deluca JG,
Giddings TH Jr, O'Toole ET, Winey M, Salmon ED, Casey PJ, Nelson WJ
and Barth AI: beta-Catenin is a Nek2 substrate involved in
centrosome separation. Genes Dev. 22:91–105. 2008. View Article : Google Scholar :
|
|
14
|
Mbom BC, Siemers KA, Ostrowski MA, Nelson
WJ and Barth AI: Nek2 phosphorylates and stabilizes β-catenin at
mitotic centrosomes downstream of Plk1. Mol Biol Cell. 25:977–991.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen L, Yuan YF, Li Y, Chan TH, Zheng BJ,
Huang J and Guan XY: Clinical significance of CHD1L in
hepatocellular carcinoma and therapeutic potentials of
virus-mediated CHD1L depletion. Gut. 60:534–543. 2011. View Article : Google Scholar
|
|
16
|
Chen D, Xing W, Hong J, Wang M, Huang Y,
Zhu C, Yuan Y and Zeng W: The beta2-adrenergic receptor is a
potential prognostic biomarker for human hepatocellular carcinoma
after curative resection. Ann Surg Oncol. 19:3556–3565. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Li W, Liu N, Zhang F, Liu H, Liu
F, Liu J, Zhang T and Niu Y: Nek2A contributes to tumorigenic
growth and possibly functions as potential therapeutic target for
human breast cancer. J Cell Biochem. 113:1904–1914. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wurmbach E, Chen YB, Khitrov G, Zhang W,
Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J, et
al: Genome-wide molecular profiles of HCV-induced dysplasia and
hepatocellular carcinoma. Hepatology. 45:938–947. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roessler S, Jia HL, Budhu A, Forgues M, Ye
QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX, et al: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dravid G, Ye Z, Hammond H, Chen G, Pyle A,
Donovan P, Yu X and Cheng L: Defining the role of Wnt/beta-catenin
signaling in the survival, proliferation, and self-renewal of human
embryonic stem cells. Stem Cells. 23:1489–1501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato N, Meijer L, Skaltsounis L, Greengard
P and Brivanlou AH: Maintenance of pluripotency in human and mouse
embryonic stem cells through activation of Wnt signaling by a
pharmacological GSK-3-specific inhibitor. Nat Med. 10:55–63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamashita T, Budhu A, Forgues M and Wang
XW: Activation of hepatic stem cell marker EpCAM by
Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res.
67:10831–10839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu
LX, Zhang SH, Huang DD, Tang L, Kong XN, et al: Wnt/beta-catenin
signaling contributes to activation of normal and tumorigenic liver
progenitor cells. Cancer Res. 68:4287–4295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monga SP: β-catenin signaling and roles in
liver homeostasis, injury, and tumorigenesis. Gastroenterology.
148:1294–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan W, Choi SC, Wang H, Qin Y,
Volpicelli-Daley L, Swan L, Lucast L, Khoo C, Zhang X, Li L, et al:
Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate
regulates LRP6 phosphorylation. Science. 321:1350–1353. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang KL, Han L, Chen LY, Shi ZD, Yang M,
Ren Y, Chen LC, Zhang JX, Pu PY and Kang CS: Blockage of a
miR-21/EGFR regulatory feedback loop augments anti-EGFR therapy in
glioblastomas. Cancer Lett. 342:139–149. 2014. View Article : Google Scholar
|
|
27
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cappello P, Blaser H, Gorrini C, Lin DC,
Elia AJ, Wakeham A, Haider S, Boutros PC, Mason JM, Miller NA, et
al: Role of Nek2 on centrosome duplication and aneuploidy in breast
cancer cells. Oncogene. 33:2375–2384. 2014. View Article : Google Scholar
|
|
29
|
Naro C, Barbagallo F, Chieffi P, Bourgeois
CF, Paronetto MP and Sette C: The centrosomal kinase NEK2 is a
novel splicing factor kinase involved in cell survival. Nucleic
Acids Res. 42:3218–3227. 2014. View Article : Google Scholar :
|
|
30
|
Pei D: Regulation of pluripotency and
reprogramming by transcription factors. J Biol Chem. 284:3365–3369.
2009. View Article : Google Scholar
|
|
31
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamashita T, Honda M, Nakamoto Y, Baba M,
Nio K, Hara Y, Zeng SS, Hayashi T, Kondo M, Takatori H, et al:
Discrete nature of EpCAM+ and CD90+ cancer
stem cells in human hepatocellular carcinoma. Hepatology.
57:1484–1497. 2013. View Article : Google Scholar
|
|
33
|
Kawai T, Yasuchika K, Ishii T, Katayama H,
Yoshitoshi EY, Ogiso S, Kita S, Yasuda K, Fukumitsu K, Mizumoto M,
et al: Keratin 19, a cancer stem cell marker in human
hepatocellular carcinoma. Clin Cancer Res. 21:3081–3091. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng YW, Nie YZ and Taniguchi H: Cellular
reprogramming and hepatocellular carcinoma development. World J
Gastroenterol. 19:8850–8860. 2013. View Article : Google Scholar :
|
|
35
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kanwar SS, Yu Y, Nautiyal J, Patel BB and
Majumdar AP: The Wnt/beta-catenin pathway regulates growth and
maintenance of colonospheres. Mol Cancer. 9:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Terris B, Cavard C and Perret C: EpCAM, a
new marker for cancer stem cells in hepatocellular carcinoma. J
Hepatol. 52:280–281. 2010. View Article : Google Scholar
|
|
38
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al:
EpCAM-positive hepatocellular carcinoma cells are tumor-initiating
cells with stem/progenitor cell features. Gastroenterology.
136:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu X, Ghisolfi L, Keates AC, Zhang J,
Xiang S, Lee DK and Li CJ: Induction of cancer cell stemness by
chemotherapy. Cell Cycle. 11:2691–2698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Zhou W, Xia J, Gu Z, Wendlandt E,
Zhan X, Janz S, Tricot G and Zhan F: NEK2 mediates
ALDH1A1-dependent drug resistance in multiple myeloma. Oncotarget.
5:11986–11997. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Robey RW, To KK, Polgar O, Dohse M, Fetsch
P, Dean M and Bates SE: ABCG2: A perspective. Adv Drug Deliv Rev.
61:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang G, Wang Z, Luo W, Jiao H, Wu J and
Jiang C: Expression of potential cancer stem cell marker ABCG2 is
associated with malignant behaviors of hepatocellular carcinoma.
Gastroenterol Res Pract. 2013:7825812013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koppaka V, Thompson DC, Chen Y, Ellermann
M, Nicolaou KC, Juvonen RO, Petersen D, Deitrich RA, Hurley TD and
Vasiliou V: Aldehyde dehydrogenase inhibitors: A comprehensive
review of the pharmacology, mechanism of action, substrate
specificity, and clinical application. Pharmacol Rev. 64:520–539.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luo Y, Dallaglio K, Chen Y, Robinson WA,
Robinson SE, McCarter MD, Wang J, Gonzalez R, Thompson DC, Norris
DA, et al: ALDH1A isozymes are markers of human melanoma stem cells
and potential therapeutic targets. Stem Cells. 30:2100–2113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Balicki D: Moving forward in human mammary
stem cell biology and breast cancer prognostication using ALDH1.
Cell Stem Cell. 1:485–487. 2007. View Article : Google Scholar
|
|
48
|
Choi SA, Lee JY, Phi JH, Wang KC, Park CK,
Park SH and Kim SK: Identification of brain tumour initiating cells
using the stem cell marker aldehyde dehydrogenase. Eur J Cancer.
50:137–149. 2014. View Article : Google Scholar
|