Introduction
The mortality rate of prostate cancer has greatly
increased globally in recent years, due to the fact that this type
of tumor is not easily detected. Owing to its lack of noticeable
symptoms, prostate cancer is commonly ignored. Once it is
diagnosed, the tumor has usually invaded other tissues or organs
and has undergone metastasis. It is essential to find a
satisfactory strategy by which to treat prostate cancer, because to
date traditional therapies applied including surgery, radiotherapy,
chemotherapy and comprehensive therapy have not been effective in
treating this disease. The reason is that these methods do not
inhibit invasion and metastasis, thus the development of a new drug
to suppress invasion and metastasis is the key for clinical
treatment.
Epidemiological studies have shown that cruciferous
vegetables reduce the risk of a variety of cancers (1). Sulforaphane (SFN), as an
isothiocyanate, effectively inhibits the growth of various tumor
cells (2). SFN metabolizes and
generates metabolites such as sulforaphane-glutathione (SFN-GSH),
sulforaphane-cysteine-glycine (SFN-CG), sulforaphane-cysteine
(SFN-Cys), and sulforaphane-N-acetyl-cysteine (SFN-NAC). SFN
metabolites may be the main compounds in tissues, rather than SFN
(3). The metabolites, especially
SFN-Cys, were found to inhibit histone deacetylase (HDAC) activity
and have a higher plasma concentration and longer half-life
(4,5), which may contribute to cancer
inhibition. Our previous studies showed that SFN inhibited invasion
through phosphorylation of extracellular signal-regulated kinase
1/2 (ERK1/2) and CD44v6 downregulation in human prostate cancer
DU145 cells (6). However, the key
mechanisms of SFN-Cys in the inhibition of prostate cancer
migration and invasion are not yet clear.
ERK1/2 are members of the mitogen-activated protein
kinase (MAPK) family, which can be activated by various
extracellular stimuli. ERK1/2 phosphorylation regulates the
activation of downstream substrate molecules (7) and mediates signal transduction
processes in many cancer cells. Sustained ERK1/2 phosphorylation
was found to inhibit growth and invasion, and induce cell cycle
arrest and apoptosis (8–11). Transient (<15 min) ERK1/2
phosphorylation was found to contribute to cancer proliferation and
invasion (12,13). Further studies to find out the
downstream signaling molecules are necessary for an overview of the
whole signaling cascade.
Galectins are a β-galactoside-binding protein
family, consisting of 15 members. It was reported that the
expression level of galectin-1 is increased in various tumor cells
including hepatocellular carcinoma (14), pancreatic ductal adenocarcinoma
(15), oral squamous cell carcinoma
(16), vulvar neoplasia (17) and colorectal cancer (18). Knockdown of galectin-1 through small
interfering RNA in highly invasive cancer cells reduced invasion.
Moreover, the invasion levels in poorly invasive cancer cells were
significantly increased after overexpression of galectin-1
(19). Galectin-1 is involved in
cell-to-cell, cell-to-extracellular matrix (ECM) adhesion and
aggregation (20). Furthermore,
transient activation of ERK1/2 contributed to galectin-1 increase,
and the high expression of galectin-1 was reversed by the ERK1/2
inhibitor U0126 in T lymphocytes (21). Therefore, sustained ERK1/2
phosphorylation may lead to galectin-1 downregulation in human
prostate cancer cells. The possible mechanisms of galectin-1 that
contribute to cancer invasion need to be further investigated and
discussed.
In the present study, we investigated the effects of
SFN-Cys on prostate cancer cell proliferation, invasion and the
underlying mechanisms, which will help us identify more targets and
provide a basis for the clinical application of SFN-Cys in the
treatment of prostate cancer.
Materials and methods
Reagents
D,L-sulforaphane-L-cysteine (SFN-Cys) and the
anti-galectin-1 antibody were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Dimethyl sulfoxide (DMSO) was
acquired from AppliChem GmbH (Darmstadt, Germany). RPMI-1640
culture medium was purchased from HyClone (Logan, UT, USA). Fetal
bovine serum (FBS) and penicillin-streptomycin were obtained from
Invitrogen (Carlsbad, CA, USA). β-actin antibody was purchased from
ProteinTech Group, Inc. (Chicago, IL, USA). The phosphorylated
ERK1/2 (pERK1/2), ERK1/2 and ERK1/2 inhibitor (PD98059) were
obtained from Cell Signaling Technology, Inc. (Shanghai, China).
The MTS assay kit was purchased from Promega (Madison, WI, USA).
Transwell plates and Matrigel basement membrane matrix for invasion
assay were obtained from BD Biosciences (Bedford, MA, USA). The
DAPI staining solution was purchased from Beyotime Institute of
Biotechnology (Nantong, China).
Cell culture
Human prostate cancer cell lines DU145 and PC3 were
purchased from the Cell Resource Center, Peking Union Medical
College (CRC/PUMC). Cells were cultured in RPMI-1640 medium with
10% FBS, 100 U/ml penicillin and streptomycin. The cells were
maintained at 37°C in a humidified incubator containing 5%
CO2.
Cell morphology
DU145 and PC3 cells at 80% confluency were exposed
to SFN-Cys at different concentrations (0, 5, 10, and 15 µM)
for 24 h in 6-well plates. Cell morphology was observed with phase
contrast microscope at x100 magnification (Leica, Germany). Digital
cameras recorded the morphological change of the prostate cancer
cells.
MTS assay
The cell viability was determined using the MTS
assay kit (Promega). The cells (4–6×103) were seeded in
96-well plates and treated with various doses of SFN-Cys for 24 h.
Then 20 µl of MTS reagent was added to each well and
incubated at 37°C for 1 h. The absorbance was measured at 490 nm on
a BioTek Synergy HT Multi-Detection Microplate Reader (BioTek,
Winooski, VT, USA).
Scratch assay
The cells were cultured in 6-well plates for 10 h.
Then, a 200-µl pipette tip was used to make two parallel
wounds and one vertical wound per well. After being washed with
PBS, the cells were incubated in serum-free medium at different
doses of SFN-Cys for 24 h. The image of the wound area was captured
by a phase-contrast microscope (Leica) at 0 and 24 h, and measured
by the ImageJ processing program.
Invasion assay
The 24-well invasion chamber with 8-µm pores
coated with Matrigel matrix was used for the cell invasion assay.
Matrigel matrix was diluted with FBS-free medium to 2 mg/ml. The
Transwell chambers were rehydrated with FBS-free medium at 37°C for
30 min, and then the cells (1×105) were seeded in the
upper chamber with 10% FBS culture medium. The 500 µl of
culture medium was added to the lower chambers. After incubation at
different doses of SFN-Cys for 24 h, the cells in the upper chamber
were wiped off with cotton swab. The invaded cells in the lower
chamber were fixed with 100% methanol for 20 min, and subsequently
stained with 0.5% crystal violet solution for 20 min. Then, the
cells were rinsed with distilled water and observed in five
randomly selected fields per well under microscope. The ImageJ
processing program was used for data analysis.
Immunoblotting
The cells were harvested and lysed with lysis buffer
(Thermo Fisher Scientific, Waltham, MA, USA) for 30 min. Then, the
cell lysate was centrifuged at 12,000 × g for 10 min. The BCA
protein assay kit (Invitrogen) was used to detect protein
concentrations. Equal amounts of protein were separated using
SDS-PAGE gels and transferred to nitrocellulose membranes. The
membranes were blocked with 1.5% BSA for 1 h. After incubation with
primary antibodies overnight at 4°C, the fluorescence-labeled
secondary antibody (LI-COR Biosciences, Lincoln, NE, USA) was
incubated with the membranes. After being washed, the protein bands
were detected using the Odyssey Infrared Imaging System (LI-COR
Biosciences). β-actin was used as an internal control.
Immunofluorescence assays
The cells (4×104) were seeded in a
24-well with glass coverslips and incubated for 10 h at 37°C.
Following treatment with 15 µM SFN-Cys for 24 h, the cells
were fixed with 4% paraformaldehyde for 15 min and permeabilized
with 0.5% Triton X-100 for 20 min at room temperature. After
blocking through 5% BSA for 30 min, the cells were incubated with
primary antibodies for 2 h and incubated with the
fluorescence-labeled secondary antibody for 1 h. The glass
coverslips were stained with DAPI and examined on confocal laser
scanning microscope (Olympus FV1000; Olympus Corp., Tokyo,
Japan).
Statistical analysis
The results are expressed as the mean ± SD, and
analyzed using SPSS 18.0 software package by one-way ANOVA. The
differences were considered statistically significant at
p<0.05.
Results
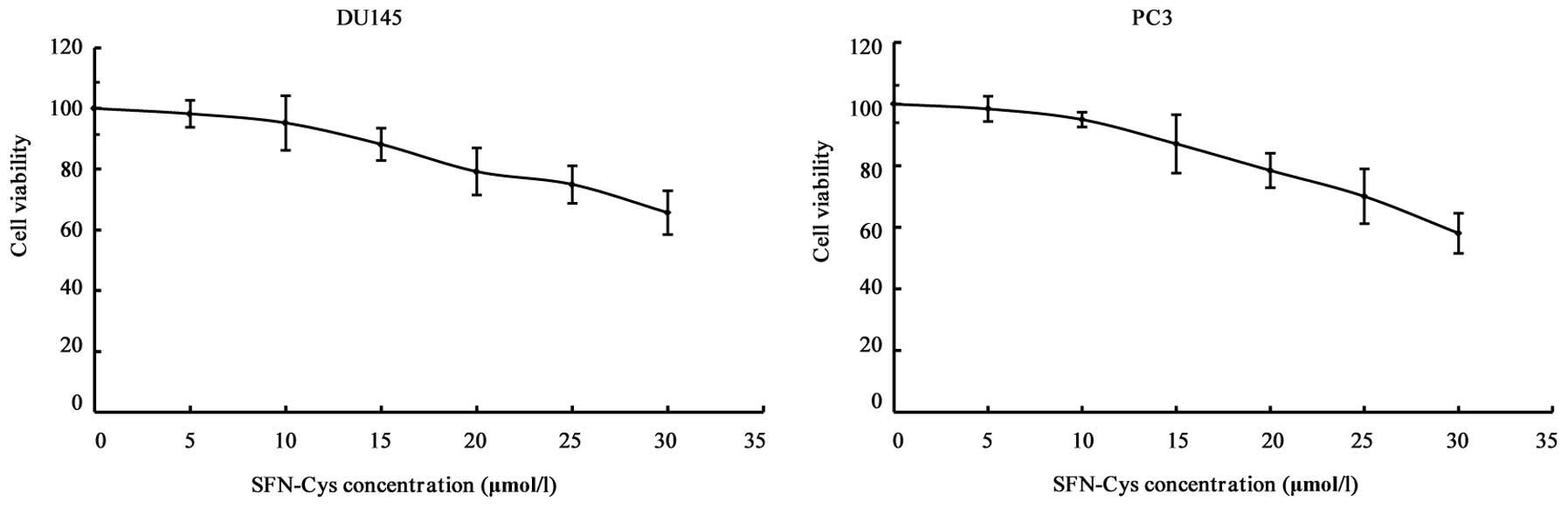
SFN-Cys inhibits cell proliferation
MTS assay was used to assess the effect of SFN-Cys
on cell viability. The cells were treated with 0, 5, 10, 15, 20, 25
and 30 µM SFN-Cys for 24 h. The results showed that cell
viability was inhibited by SFN-Cys in a concentration-dependent
manner (Fig. 1). Our study showed
that 20 µM of SFN-Cys inhibited cell growth, but 15
µM of SFN-Cys did not markedly decrease cell viability.
Thus, we chose 15 µM SFN-Cys as an optimal concentration for
the invasion studies.
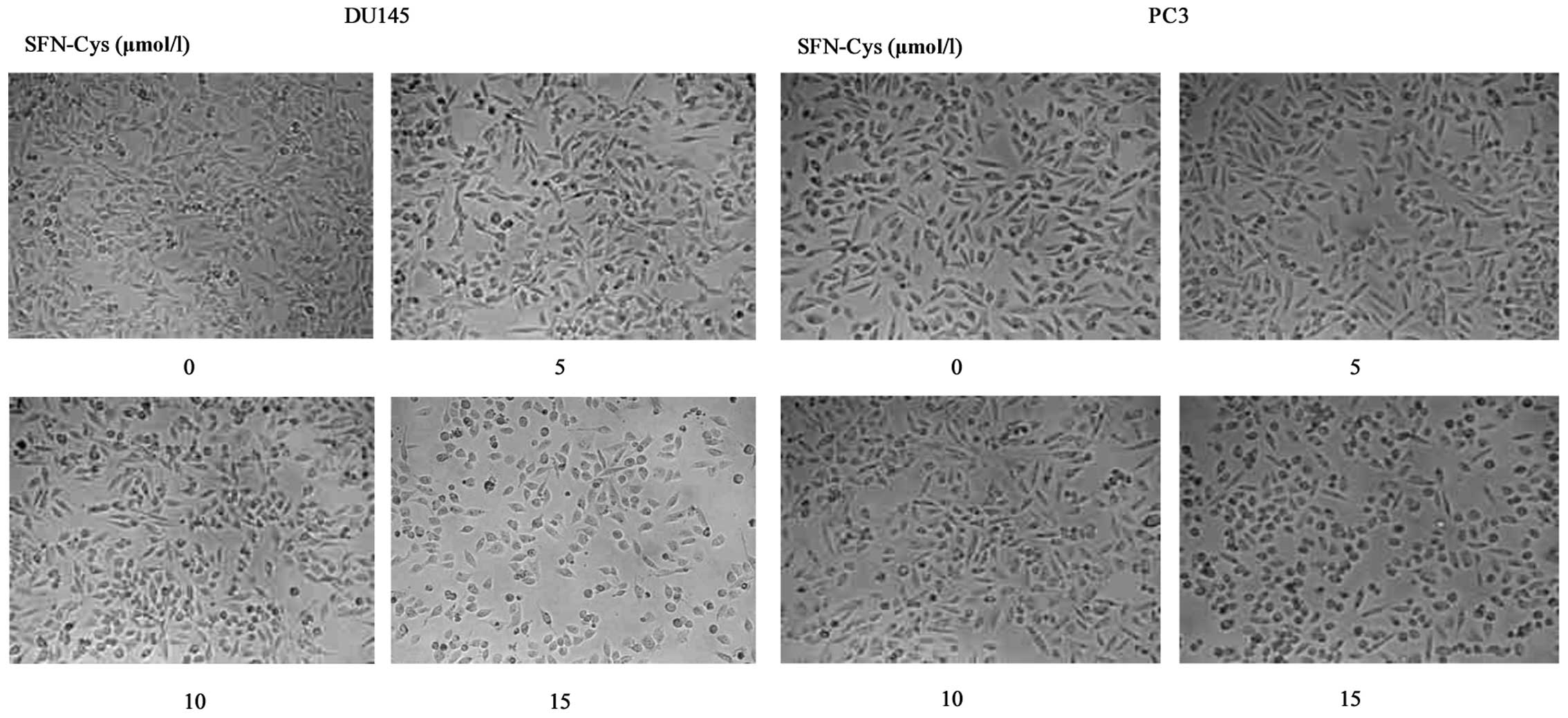
SFN-Cys induces morphological
changes
Following a 24 h treatment with 15 µM
SFN-Cys, we observed obvious morphological changes in the DU145 and
PC3 cells, such as cell contraction and pseudopodia shortening
(Fig. 2). Because the cellular
pseudopodia are closely related to tumor invasion, we speculated
that SFN-Cys inhibited cell invasion in the DU145 and PC3 cells.
Therefore, 15 µM was the optimal concentration for the
invasion studies.
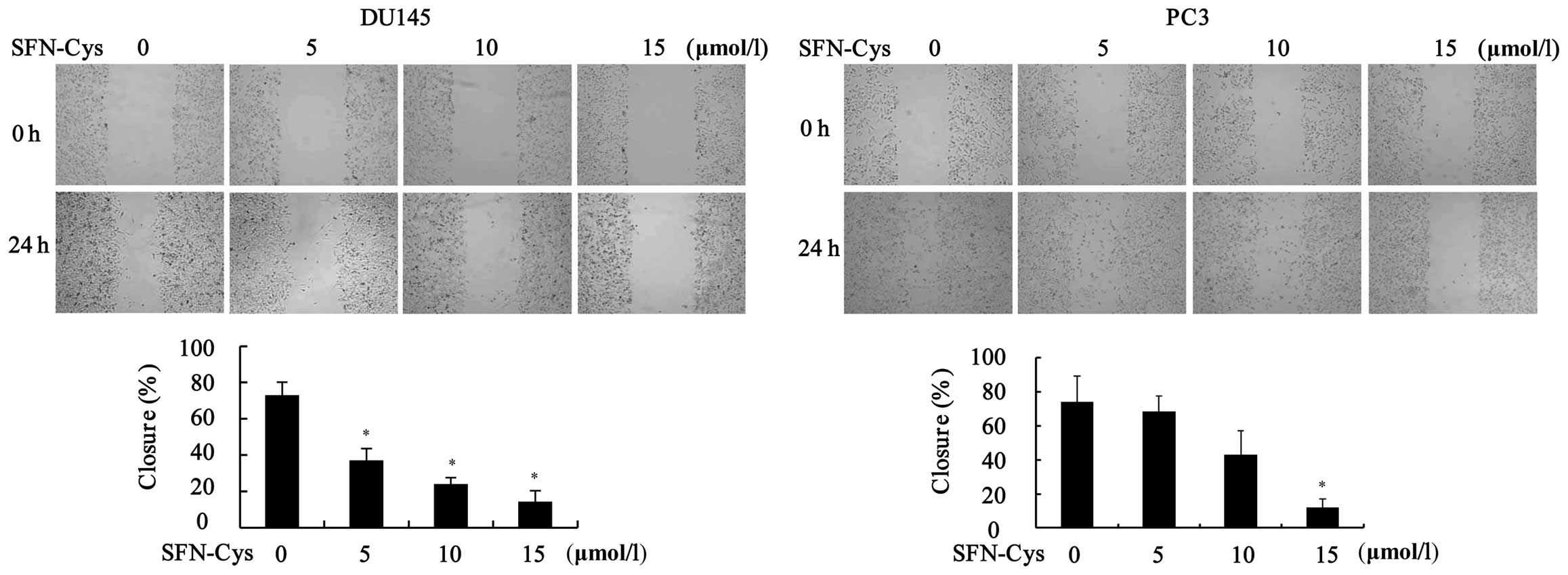
SFN-Cys inhibits migration in a
dose-dependent manner
We evaluated the effects of SFN-Cys on cell
migration by scratch assay. After being treated with different
doses of SFN-Cys, the area of the wound was observed under a
microscope at 0 and 24 h (Fig. 3).
The results showed that SFN-Cys significantly decreased cell
migration when compared to the control (0 µM) in the DU145
and PC3 cells.
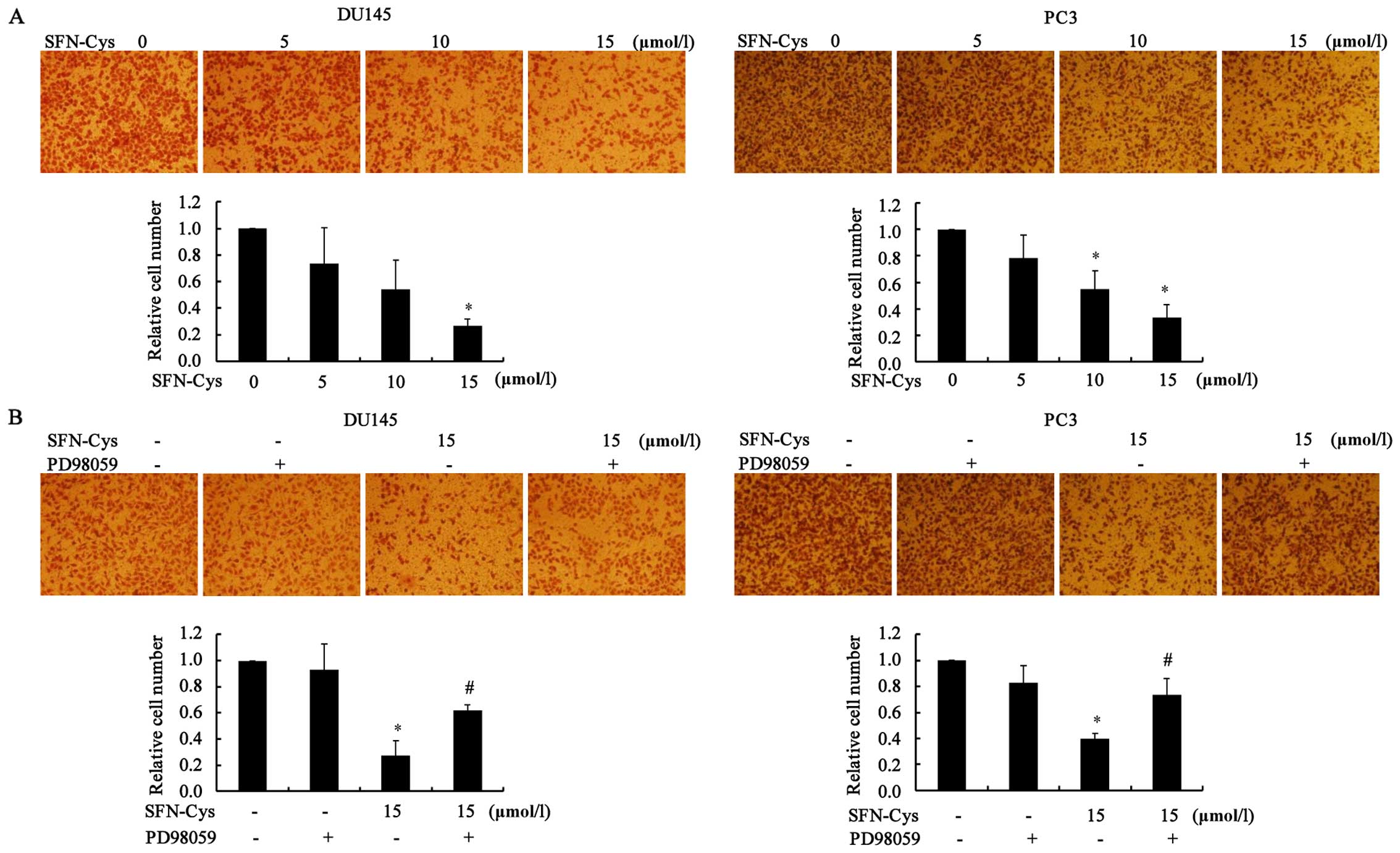
SFN-Cys inhibits cell invasion in the
DU145 and PC3 cells
Transwell invasion assays were used to assess the
effects of SFN-Cys on cell invasion. The cells were treated with
different concentrations of SFN-Cys (0, 5, 10 and 15 µM).
Then, the invaded cells were counted as described in Materials and
methods. The results showed that the cell invasiveness was
significantly reduced when compared to the control group in a
dose-dependent manner (Fig. 4A).
Meanwhile, we aimed to ascertain whether SFN-Cys inhibits invasion
via ERK1/2 activation. The ERK1/2 inhibitor PD98059 (25 µM)
was added to the medium for 30 min, and then the cells were treated
with 15 µM of SFN-Cys for 24 h. The results showed that the
invaded cells were significantly increased when compared to the
SFN-Cys-only group in the DU145 and PC3 cells, respectively
(Fig. 4B). These results suggest
that SFN-Cys inhibited invasion via activation of ERK1/2 signaling
in the human prostate cancer cells.
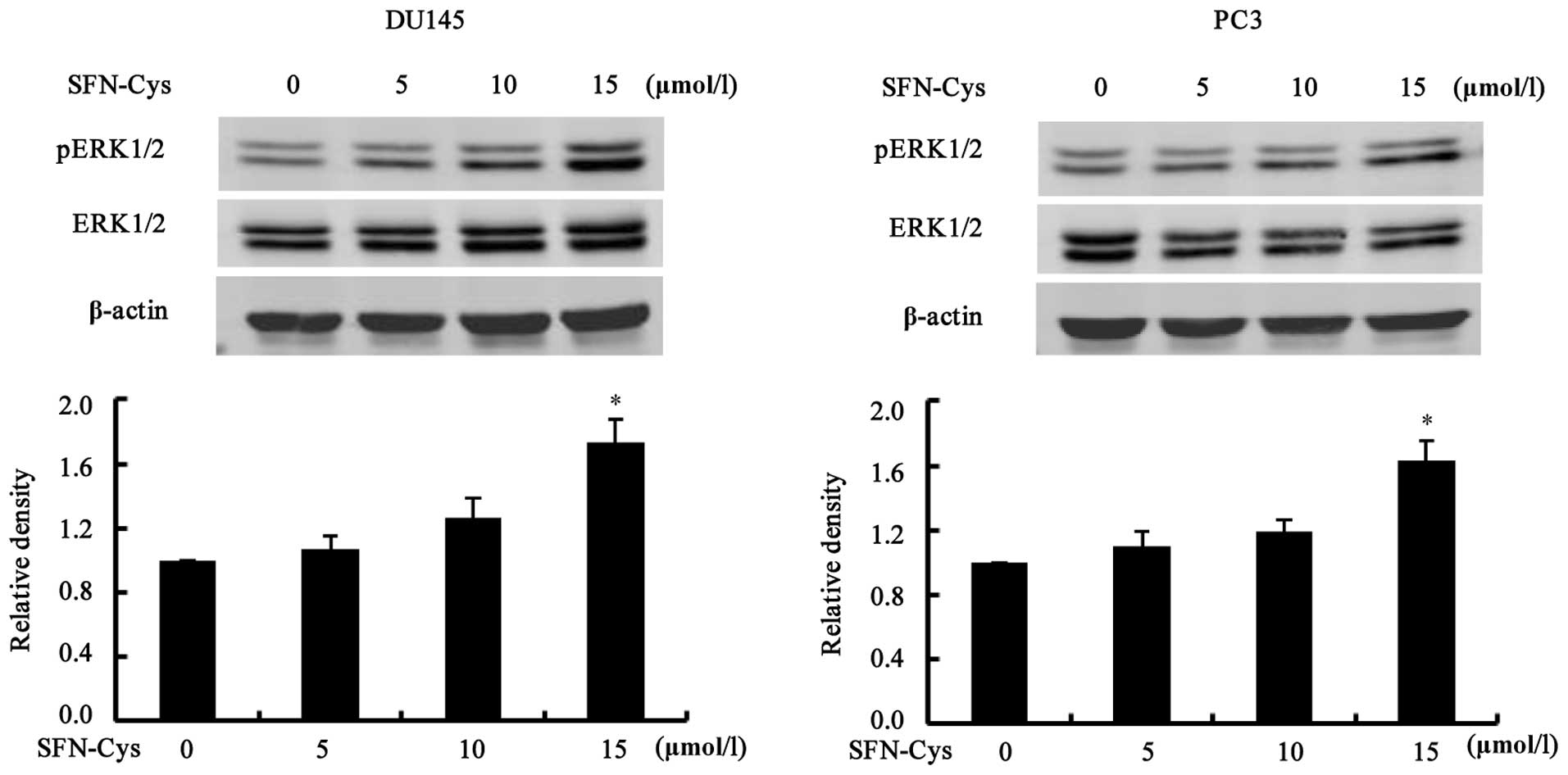
SFN-Cys inhibits cell invasion of DU145
and PC3 cells via sustained ERK1/2 phosphorylation
We further explored the molecular mechanisms
involved in SFN-Cys-triggered invasion. Our previous studies showed
that phosphorylation of ERK1/2 reached the highest degree at 24 h.
Therefore, we chose 24 h as the optimal time for subsequent study.
The cells were treated with increasing doses of SFN-Cys (0, 5, 10
and 15 µM) for 24 h. Western blot analysis showed that
phosphorylation of ERK1/2 was significantly increased at 15
µM of SFN-Cys (Fig. 5). The
results indicated that SFN-Cys inhibited invasion via activation of
ERK1/2 in both the DU145 and PC3 cells.
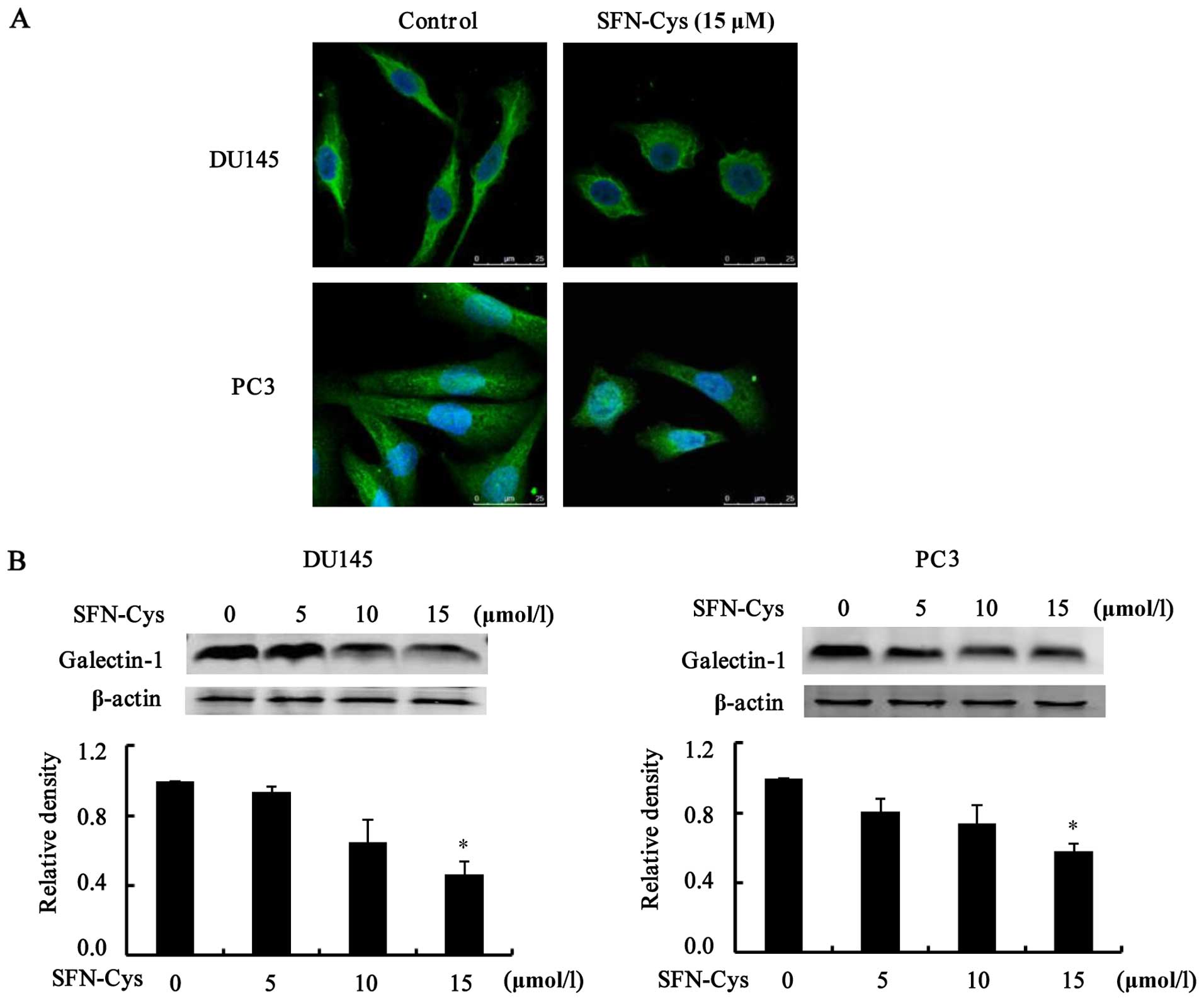
SFN-Cys inhibits galectin-1-related
invasion
Overexpression of galectin-1 promotes tumor cell
invasion. To elucidate the mechanisms of SFN-Cys-induced invasion
inhibition, we detected the expression of galectin-1 in the DU145
and PC3 cells. Immunofluorescence showed that galectin-1 was
located in both the cytoplasm and the cell membrane of the prostate
cancer cells. SFN-Cys (15 µM) induced cellular pseudopodia
shortening (Fig. 6A). Next, we used
western blot analysis to examine the expression of galectin-1
protein. The results showed that the expression level of galectin-1
was markedly reduced with the increasing SFN-Cys concentrations
(Fig. 6B). These results suggested
that SFN-Cys inhibited invasion via downregulation of galectin-1 in
the DU145 and PC3 cells.
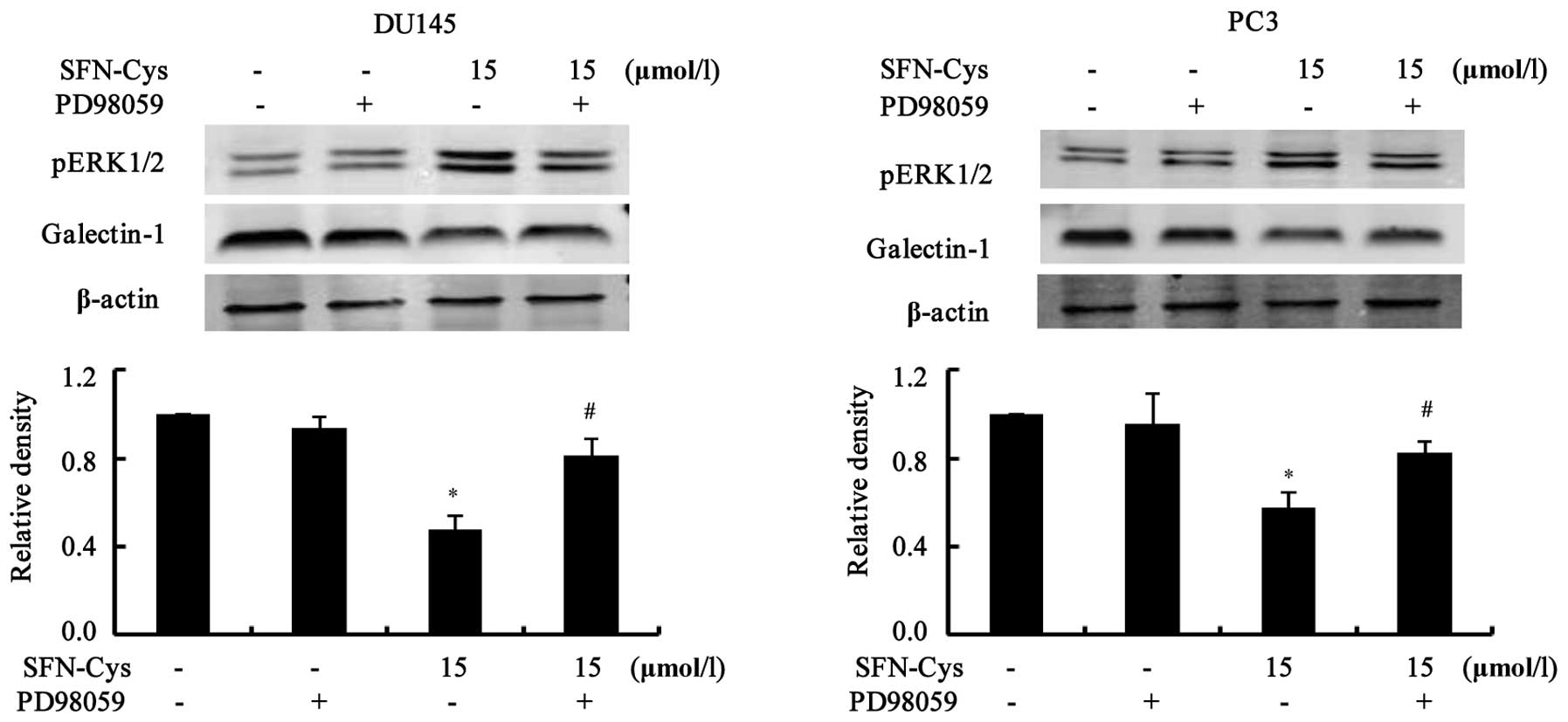
SFN-Cys downregulates galectin-1 via
activation of ERK1/2
We examined the link between ERK1/2 phosphorylation
and galectin-1 expression. First, cells were treated with ERK1/2
inhibitor PD98059 (25 µM) for 30 min, then 15 µM of
SFN-Cys was added to the medium for 24 h. Western blot analysis
showed that phosphorylation of ERK1/2 was markedly reduced, but
downregulation of galectin-1 was reversed by PD98059 (Fig. 7), implying that galectin-1 is the
downstream effector of ERK1/2 in the DU145 and PC3 cells. All the
data indicated that SFN-Cys suppressed invasion via ERK1/2-mediated
downregulation of galectin-1 in the human prostate cancer
cells.
Discussion
SFN suppresses invasion in a variety of tumor cells
(22–24). Due to a short half-life, SFN has not
been used in clinical treatment. SFN-Cys, as a major metabolite of
SFN, was found to have extensive tissue distribution in treated
mice and a longer half-life (3,5).
Therefore, it was more valuable to investigate the mechanisms
involved in the inhibition of invasion in prostate cancer cells by
SFN-Cys. In the present study, we found that SFN-Cys inhibited cell
proliferation by MTS assay in the DU145 and PC3 cells. This
provided an optimum concentration and treatment time with which to
investigate invasion inhibition. Meanwhile, these data confirmed
that SFN-Cys also inhibited tumor growth, which may be related to
inhibition of cell proliferating signaling, such as transient
activation of ERK1/2 and downstream oncoproteins. In our model,
SFN-Cys triggered sustained activation of ERK1/2. These data
triggered different results which include cell cycle arrest and
apoptosis. More importantly, the phosphorylation of ERK1/2 also
caused inhibition of invasion. We demonstrated that SFN-Cys
significantly suppressed invasion in the cells following treatment
with 15 µM SFN-Cys by scratch and invasion assays,
indicating that broccoli-derived SFN-Cys has anti-invasion
potential in human prostate cancer cells.
In addition, we further explored the molecular
mechanism of SFN-Cys-mediated inhibition of invasion. The ERK1/2
signaling pathway is associated with intracellular protein-protein
interactions and the regulation of multiple cellular processes,
such as proliferation, differentiation, invasion and apoptosis. It
was reported that high expression of pERK1/2 is found in benign
prostate lesions, suggesting a good prognosis (25). Moreover, activation of ERK1/2
inhibited invasion in various tumor cells. Our previous studies
demonstrated that SFN inhibited invasion via persistent ERK1/2
phosphorylation in human glioblastoma cells (10) and prostate cancer cells (6). In this study, SFN-Cys significantly
increased ERK1/2 phosphorylation in a dose-dependent manner, and
effectively inhibited invasion in the DU145 and PC3 cells, which
could be blocked by PD98059. These results indicated that SFN-Cys
inhibited tumor invasion through sustained ERK1/2 activation in
human prostate cancer cells.
Tumor invasion is a complex process, including
adhesion and degradation of ECM, angiogenesis, and proliferation.
Galectin-1 contributes to cell-to-ECM adhesion and migration
(26). Studies have shown that
galectin-1 promoted tumor invasion in oral cancer and lung
adenocarcinoma (19). It was
reported that galectin-1 expression is significantly correlated
with tumor stage (27) and clinical
prognosis (28). Our results showed
that SFN-Cys markedly downregulated galectin-1 levels in the DU145
and PC3 cells. When the cells were treated with PD98059 and
SFN-Cys, the downregulation of galectin-1 was reversed by PD98059.
The immunofluorescence assays showed that galectin-1 was mainly
located in the cytoplasm and the cell membrane of prostate cancer
cells. These results demonstrated that SFN-Cys downregulated
galectin-1 via sustained ERK1/2 phosphorylation in human prostate
cancer cells. The question is how does ERK1/2 phosphorylation lead
to galectin-1 downregulation? Studies have shown that some
transcription factors modulate galectin-1 expression such as
hypoxia inducible factor-1 (29)
and activator protein-1 (30);
several transcription factors function by ERK 1/2 phosphorylation
(31,32). Therefore, we aimed to ascertain that
SFN-Cys may downregulate galectin-1 via ERK1/2-relevant
transcription factors, such as AP-1 and Egr-1. Galectin-1 was
confirmed to promote cell migration and invasiveness, which were
found to be major hallmarks in tumor progression. Cell migration
occurs through multiple adhesion and spreading events, especially
the degradation of ECM proteins by serine proteases, cathepsins,
and matrix metalloproteinases (MMPs) such as MMP-2, MMP-9 and
MMP-14. As a result, the proteasome pathway may be a major player
in the regulation of galectin-1 and tumor invasion; however further
studies are needed.
Galectin-1, as a glycoprotein, plays roles in the
cell membrane and the ECM. The carbohydrate chains of galectin-1
could interact with adhesion molecules, such as integrin and
E-cadherin, on cell surfaces and in the ECM, regulating subsequent
motility and adhesion (33).
Studies have shown that galectin-1 stimulated collagen, fibronectin
synthesis and laminin expression (16,34)
that promoted cell-matrix adhesion. Furthermore, galectin-1 induced
epithelial-to-mesenchymal transition (EMT) and upregulated
integrins that mediated cell-ECM interactions (35). Upregulated galectin-1 was found to
stimulate platelets to release angiogenesis-related factors
(36). Furthermore, galectin-1
overexpression was found to cause chemoresistance and promoted
carcinogenesis and invasion (37).
In the present study, SFN-Cys significantly decreased galectin-1
expression, indicating that the use of SFN-Cys possesses a better
chemotherapeutic effect. Meanwhile, SFN-Cys is absorbed and
maintains appropriate blood and tissue concentrations (5), which suggests that SFN-Cys shows
promise as an anticancer agent for clinical trial.
In summary, our results revealed that SFN-Cys
inhibited invasion in human prostate cancer cells via persistent
ERK1/2 phosphorylation which triggers galectin-1 downregulation.
This study demonstrated that SFN-Cys has potential as an anticancer
agent for prostate cancer therapy.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272843).
References
|
1
|
Abdull Razis AF and Noor NM: Cruciferous
vegetables: Dietary phytochemicals for cancer prevention. Asian Pac
J Cancer Prev. 14:1565–1570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lenzi M, Fimognari C and Hrelia P:
Sulforaphane as a promising molecule for fighting cancer. Cancer
Treat Res. 159:207–223. 2014. View Article : Google Scholar
|
|
3
|
Clarke JD, Hsu A, Williams DE, Dashwood
RH, Stevens JF, Yamamoto M and Ho E: Metabolism and tissue
distribution of sulforaphane in Nrf2 knockout and wild-type mice.
Pharm Res. 28:3171–3179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Myzak MC, Karplus PA, Chung FL and
Dashwood RH: A novel mechanism of chemoprotection by sulforaphane:
Inhibition of histone deacetylase. Cancer Res. 64:5767–5774. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gasper AV, Al-Janobi A, Smith JA, Bacon
JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA and
Mithen RF: Glutathione S-transferase M1 polymorphism and metabolism
of sulforaphane from standard and high-glucosinolate broccoli. Am J
Clin Nutr. 82:1283–1291. 2005.PubMed/NCBI
|
|
6
|
Peng X, Zhou Y, Tian H, Yang G, Li C, Geng
Y, Wu S and Wu W: Sulforaphane inhibits invasion by phosphorylating
ERK1/2 to regulate E-cadherin and CD44v6 in human prostate cancer
DU145 cells. Oncol Rep. 34:1565–1572. 2015.PubMed/NCBI
|
|
7
|
Futran AS, Link AJ, Seger R and Shvartsman
SY: ERK as a model for systems biology of enzyme kinetics in cells.
Curr Biol. 23:R972–R979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goulet AC, Chigbrow M, Frisk P and Nelson
MA: Selenomethionine induces sustained ERK phosphorylation leading
to cell-cycle arrest in human colon cancer cells. Carcinogenesis.
26:109–117. 2005. View Article : Google Scholar
|
|
9
|
Krishna-Subramanian S, Hanski ML,
Loddenkemper C, Choudhary B, Pagès G, Zeitz M and Hanski C: UDCA
slows down intestinal cell proliferation by inducing high and
sustained ERK phosphorylation. Int J Cancer. 130:2771–2782. 2012.
View Article : Google Scholar
|
|
10
|
Li C, Zhou Y, Peng X, Du L, Tian H, Yang
G, Niu J and Wu W: Sulforaphane inhibits invasion via activating
ERK1/2 signaling in human glioblastoma U87MG and U373MG cells. PLoS
One. 9:e905202014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang TY, Chang GC, Chen KC, Hung HW, Hsu
KH, Sheu GT and Hsu SL: Sustained activation of ERK and
Cdk2/cyclin-A signaling pathway by pemetrexed leading to S-phase
arrest and apoptosis in human non-small cell lung cancer A549
cells. Eur J Pharmacol. 663:17–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas W, Coen N, Faherty S, Flatharta CO
and Harvey BJ: Estrogen induces phospholipase A2 activation through
ERK1/2 to mobilize intracellular calcium in MCF-7 cells. Steroids.
71:256–265. 2006. View Article : Google Scholar
|
|
13
|
Liu Z, Yu X and Shaikh ZA: Rapid
activation of ERK1/2 and AKT in human breast cancer cells by
cadmium. Toxicol Appl Pharmacol. 228:286–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bacigalupo ML, Manzi M, Rabinovich GA and
Troncoso MF: Hierarchical and selective roles of galectins in
hepatocarcinogenesis, liver fibrosis and inflammation of
hepatocellular carcinoma. World J Gastroenterol. 19:8831–8849.
2013. View Article : Google Scholar :
|
|
15
|
Tang D, Zhang J, Yuan Z, Gao J, Wang S, Ye
N, Li P, Gao S, Miao Y, Wang D, et al: Pancreatic satellite cells
derived galectin-1 increase the progression and less survival of
pancreatic ductal adenocarcinoma. PLoS One. 9:e904762014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu MH, Hong HC, Hong TM, Chiang WF, Jin YT
and Chen YL: Targeting galectin-1 in carcinoma-associated
fibroblasts inhibits oral squamous cell carcinoma metastasis by
downregulating MCP-1/CCL2 expression. Clin Cancer Res.
17:1306–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kohrenhagen N, Voelker HU, Kapp M, Dietl J
and Kämmerer U: The expression of galectin-1 in vulvar neoplasia.
Anticancer Res. 30:1547–1552. 2010.PubMed/NCBI
|
|
18
|
Barrow H, Rhodes JM and Yu LG: The role of
galectins in colorectal cancer progression. Int J Cancer. 129:1–8.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu MH, Hong TM, Cheng HW, Pan SH, Liang
YR, Hong HC, Chiang WF, Wong TY, Shieh DB, Shiau AL, et al:
galectin-1-mediated tumor invasion and metastasis, upregulated
matrix metalloproteinase expression, and reorganized actin
cytoskeletons. Mol Cancer Res. 7:311–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elola MT, Wolfenstein-Todel C, Troncoso
MF, Vasta GR and Rabinovich GA: Galectins: Matricellular
glycan-binding proteins linking cell adhesion, migration, and
survival. Cell Mol Life Sci. 64:1679–1700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuertes MB, Molinero LL, Toscano MA,
Ilarregui JM, Rubinstein N, Fainboim L, Zwirner NW and Rabinovich
GA: Regulated expression of galectin-1 during T-cell activation
involves Lck and Fyn kinases and signaling through MEK1/ERK, p38
MAP kinase and p70S6 kinase. Mol Cell Biochem. 267:177–185. 2004.
View Article : Google Scholar
|
|
22
|
Wang L, Tian Z, Yang Q, Li H, Guan H, Shi
B, Hou P and Ji M: Sulforaphane inhibits thyroid cancer cell growth
and invasiveness through the reactive oxygen species-dependent
pathway. Oncotarget. 6:25917–25931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mokhtari RB, Kumar S, Islam SS,
Yazdanpanah M, Adeli K, Cutz E and Yeger H: Combination of carbonic
anhydrase inhibitor, acetazolamide, and sulforaphane, reduces the
viability and growth of bronchial carcinoid cell lines. BMC Cancer.
13:3782013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pastorek M, Simko V, Takacova M, Barathova
M, Bartosova M, Hunakova L, Sedlakova O, Hudecova S, Krizanova O,
Dequiedt F, et al: Sulforaphane reduces molecular response to
hypoxia in ovarian tumor cells independently of their resistance to
chemotherapy. Int J Oncol. 47:51–60. 2015.PubMed/NCBI
|
|
25
|
Deschênes-Simard X, Gaumont-Leclerc MF,
Bourdeau V, Lessard F, Moiseeva O, Forest V, Igelmann S, Mallette
FA, Saba-El-Leil MK, Meloche S, et al: Tumor suppressor activity of
the ERK/MAPK pathway by promoting selective protein degradation.
Genes Dev. 27:900–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fulcher JA, Hashimi ST, Levroney EL, Pang
M, Gurney KB, Baum LG and Lee B: galectin-1-matured human
mono-cyte-derived dendritic cells have enhanced migration through
extracellular matrix. J Immunol. 177:216–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HJ, Do IG, Jeon HK, Cho YJ, Park YA,
Choi JJ, Sung CO, Lee YY, Choi CH, Kim TJ, et al: Galectin 1
expression is associated with tumor invasion and metastasis in
stage IB to IIA cervical cancer. Hum Pathol. 44:62–68. 2013.
View Article : Google Scholar
|
|
28
|
Chen J, Zhou SJ, Zhang Y, Zhang GQ, Zha
TZ, Feng YZ and Zhang K: Clinicopathological and prognostic
significance of galectin-1 and vascular endothelial growth factor
expression in gastric cancer. World J Gastroenterol. 19:2073–2079.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao XY, Chen TT, Xia L, Guo M, Xu Y, Yue
F, Jiang Y, Chen GQ and Zhao KW: Hypoxia inducible factor-1
mediates expression of galectin-1: The potential role in
migration/invasion of colorectal cancer cells. Carcinogenesis.
31:1367–1375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Juszczynski P, Ouyang J, Monti S, Rodig
SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA and
Shipp MA: The AP1-dependent secretion of galectin-1 by Reed
Sternberg cells fosters immune privilege in classical Hodgkin
lymphoma. Proc Natl Acad Sci USA. 104:13134–13139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon S and Seger R: The extracellular
signal-regulated kinase: Multiple substrates regulate diverse
cellular functions. Growth Factors. 24:21–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC
and Lu KH: Silibinin suppresses human osteosarcoma MG-63 cell
invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of
MMP-2. Carcinogenesis. 28:977–987. 2007. View Article : Google Scholar
|
|
33
|
Barondes SH, Cooper DN, Gitt MA and
Leffler H: galectins. Structure and function of a large family of
animal lectins. J Biol Chem. 269:20807–20810. 1994.PubMed/NCBI
|
|
34
|
Yun SP, Lee SJ, Jung YH and Han HJ:
galectin-1 stimulates motility of human umbilical cord
blood-derived mesenchymal stem cells by downregulation of
smad2/3-dependent collagen 3/5 and upregulation of NF-κB-dependent
fibronectin/laminin 5 expression. Cell Death Dis. 5:e10492014.
View Article : Google Scholar
|
|
35
|
Rizqiawan A, Tobiume K, Okui G, Yamamoto
K, Shigeishi H, Ono S, Shimasue H, Takechi M, Higashikawa K and
Kamata N: Autocrine galectin-1 promotes collective cell migration
of squamous cell carcinoma cells through up-regulation of distinct
integrins. Biochem Biophys Res Commun. 441:904–910. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Etulain J, Negrotto S, Tribulatti MV,
Croci DO, Carabelli J, Campetella O, Rabinovich GA and Schattner M:
Control of angiogenesis by galectins involves the release of
platelet-derived proangiogenic factors. PLoS One. 9:e964022014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Le Mercier M, Lefranc F, Mijatovic T,
Debeir O, Haibe-Kains B, Bontempi G, Decaestecker C, Kiss R and
Mathieu V: Evidence of galectin-1 involvement in glioma
chemoresistance. Toxicol Appl Pharmacol. 229:172–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|