Introduction
Carcinoma of the ampulla of Vater is the most common
cancer of the small intestine and is commonly adenocarcinoma
(1). The ampulla of Vater is
located in the second portion of the duodenum, at the confluence of
the common bile and pancreatic duct. Long-term exposure to bile
acids is a possible reason for malignant transformation. Bile acids
are potential carcinogens in gastrointestinal cancer, including
gastric, esophageal and colon cancer, and cholangiocarcinoma
(2). Incidences of intestinal
metaplasia and gastric cardia cancer are increased in patients with
gastroesophageal reflux disease (3). Treatment of squamous cell carcinoma
cell lines of the esophagus with bile acids induces cell cycle
progression and production of G1-regulating molecules (4). The secondary bile acids, deoxycholate
and lithocholate, are the most well-known carcinogens in human
colon cancer and are associated with the generation of reactive
oxygen/nitrogen species (ROS/RNS) (5). The accumulation of ROS/RNS was found
to cause oxidative DNA damage and further mutation in colon cancer
(6). The conjugated bile acid
glycochenodeoxycholate (GCDA) induced expression of cyclooxygenase
2 (COX-2) and genes that are related to cell proliferation in
cholangiocytes (7). Another type of
conjugated bile acid, taurochenodeoxycholate (TCDA), induced
phosphorylation of epidermal growth factor receptor (EGFR) and its
downstream signaling in a human cholangiocarcinoma cell line
(8).
Bile acids interact with several types of nuclear
receptors, including farnesoid X receptor (FXR), vitamin D
receptor, pregnane X receptor, and constitutive androstane receptor
(9). Nuclear receptors are
ligand-modulated transcription factors and regulate uptake,
detoxification and secretion of bile acids. FXR is the main form of
nuclear receptor of bile acids expressed in the gastrointestinal
tract where it mediates homeostasis of bile acids, lipids and
glucose. Expression of FXR is reduced in human colon cancer or
Barrett's esophageal cancer. FXR functions as a tumor suppressor in
colon, liver and esophageal cancer (10–12).
The oncosuppressive roles of FXR in these cancers include
suppression of proliferation and induction of apoptosis after
exposure to bile acids. Bile acids also act as systemic hormones
when interacting with membrane receptors, such as G protein-coupled
bile acid receptor 1 (gene GPBAR1; also known as TGR5, M-BAR
and BG37). Bile acid-dependent TGR5 activation is involved in the
immunomodulatory properties of bile acids, synthesis of endothelial
nitric oxide, and mitochondrial energy homeostasis (2,9). In
the normal physiologic condition, the functions of TGR5 include
modulation of gallbladder filling, improvement of insulin
sensitivity, maintenance of glucose homeostasis, and increased
energy expenditure to attenuate diet-induced obesity (13,14).
The function of TGR5 is variable in different types of cancer
(15). Expression of TGR5 is
increased in the intestinal subtype of gastric adenocarcinoma and
intestinal metaplasia, but not in normal gastric epithelium. Mild
to strong TGR5 staining is associated with poor patient survival,
and TCDA increased proliferation of a gastric adenocarcinoma cell
line through the TGR5-dependent pathway (16). TDCA-induced ROS production and cell
proliferation are mediated through TGR5 in Barrett's esophageal and
esophageal adenocarcinoma cell lines (17). Stimulation with bile acids prompted
the proliferation of an endometrial cell line by activating TGR5
and inducing cyclin D1 expression (18). In contrast to patients with gastric,
esophageal and endometrial cancer, the binding of bile acids to
TGR5 induced c-Jun-N terminal kinase (JNK) activation and enhanced
apoptosis in hepatocytes (19).
TGR5-deficient mice are much more susceptible to chemically induced
acute liver injury with increased incidence of liver cancer
(20). TGR5 may promote or suppress
carcinogenesis after stimulation by bile acids (2). Since the role of bile acids in
ampullary cancer is largely unknown, in the present study, we
investigated the role of TGR5 in ampullary adenocarcinoma.
Materials and methods
Bioinformatic analysis
First, we conducted a search of the Kaplan-Meier
plotter database (http://kmplot.com/analysis/) to systematically assess
the expression level of the GPBAR1 gene in gastric, breast,
lung and ovarian cancer patients (21–23).
Kaplan-Meier Plot survival curves were drawn. Second, a PrognoScan
database (http://www.abren.net/PrognoScan/) analysis was
conducted. The expression level of the GPBAR1 gene was
correlated with the survival of cancer patients. Third, data of
GPBAR1 gene expression from genomics studies of 30 types of
human cancer in the cBioPortal database (http://www.cbioportal.org/index.do) were examined
(24,25). Mutation of the GPBAR1 gene
was documented by oncogenomic analysis.
Patients
A total of 99 patients who were diagnosed as having
ampullary adenocarcinoma and who underwent radical resection at
National Cheng Kung University Hospital from January 1990 to
January 2010 were enrolled. Patients who received conservative
treatment or exhibited other cell types of ampullary cancer were
excluded. Demographics, histopathological findings and clinical
outcomes were collected by conducting a retrospective chart review.
A formal written informed consent was obtained from each patient.
Their medical charts were reviewed until January 2016. The
disease-specific survival rate was defined as the period from
surgery until cancer-related death. The present study was approved
by the Institutional Review Board of the National Cheng Kung
University Hospital (NCKUH IRB no. A-ER-101-390 and
B-ER-103-408).
Western blotting
Total protein lysates from tumor specimens and
corresponding specimens of normal duodenum were obtained from the
same patient and the protein concentration of the supernatants was
measured using the amido black method. Equivalent amounts of
protein (30 µg) were separated on 10–15% polyacrylamide gels
by SDS-gel electrophoresis, transferred to polyvinylidene
difluoride membranes, and probed with the antibody against TGR5
(Abcam Biotechnology, Cambridge, UK), FXR (R&D, Abingdon, UK),
and GAPDH (Cell Signaling Technology, Danvers, MA, USA) proteins.
Protein expression was visualized by ECL chemiluminescence
(Promega, Madison, WI, USA) and quantitated by comparison with
GAPDH.
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
The fresh cancer tissues and normal duodenum from
the same patient, were obtained for RT-PCR. The total RNA was
extracted from fresh tissues, and single-stranded cDNA was
synthesized using oligo(dT) as the random primer. The cDNA was
amplified using the primers for β-actin, GPBAR1 and
FXR genes, which were: β-actin sense,
5′-AGCGGGAAATCGTGCGTG-3′; and β-actin antisense,
5′-CAGGGTACATGGTGGTGGTGCC-3′; GPBAR1 sense,
5′-CCCAGGCTATCTTCCCAGC-3′ and GPBAR1 antisense,
5′-GCCAGGACTGAGAGGAGCA-3′; FXR sense,
5′-GACTTTGGACCATGAAGACCAG-3′ and FXR antisense,
5′-GCCCAGACGGAAGTTTCTTATT-3′. The RT-PCR products were analyzed
using agarose gel electrophoresis, and the GPBAR1 or FXR bands were
semi-quantified using densitometric analysis and subsequently
normalized relative to the β-actin bands.
Immunohistochemical (IHC) staining
Samples of ampullary adenocarcinoma and the
surrounding duodenum were fixed in 4% formalin and embedded in
paraffin. IHC staining was performed using a monoclonal mouse
anti-human TGR5 antibody (Abcam Biotechnology). The sections were
incubated using an avidin-biotin complex reagent (Dako,
Carpinteria, CA, USA), incubated with 3-amino-9-ethyl carbazole
(Zymed Laboratories, South San Francisco, CA, USA) to develop the
final color, and counterstained with hematoxylin. The
immunoreactivity of the TGR5 protein was assessed using a
semi-quantitative method and according to the Remmele and Stegner
immunoreactive scoring (IRS) system (26). The IRS scores ranged from 0 to 12
and immunoreactivity was characterized as negative, weak, mild and
strong. One researcher assessed the lesions (H.P. Hsu).
Statistical analysis
All statistical analyses were conducted using SPSS
version 12.0 (SPSS, Inc., New York, NY, USA). A univariate analysis
of the categorical variables was performed using the Chi-square
test. The continuous variables were compared using the
non-parametric Kruskal-Wallis H test. Any association between
specific markers and the recurrence-free survival of patients was
assessed using the Kaplan-Meier method, and the level of
significance was tested using the log-rank test. A P-value of
<0.05 was considered to indicate a statistically significant
result.
Results
Analysis of microarray GAPBR1 gene
expression data
Several gene expression databases of human cancer
genetics are available at websites, including the Kaplan-Meier
plotter, PrognoScan and cBioPortal system. There is no public
database of ampullary adenocarcinoma genetics. Other human cancer
types were used to study the function of TGR5 (gene name:
GPBAR1). These three databases were used to assess
GPBAR1 gene expression and correlate it with clinical
outcome. Analysis of the relationship of the GPBAR1 gene
expression level (based on Kaplan-Meier plotter data) to survival
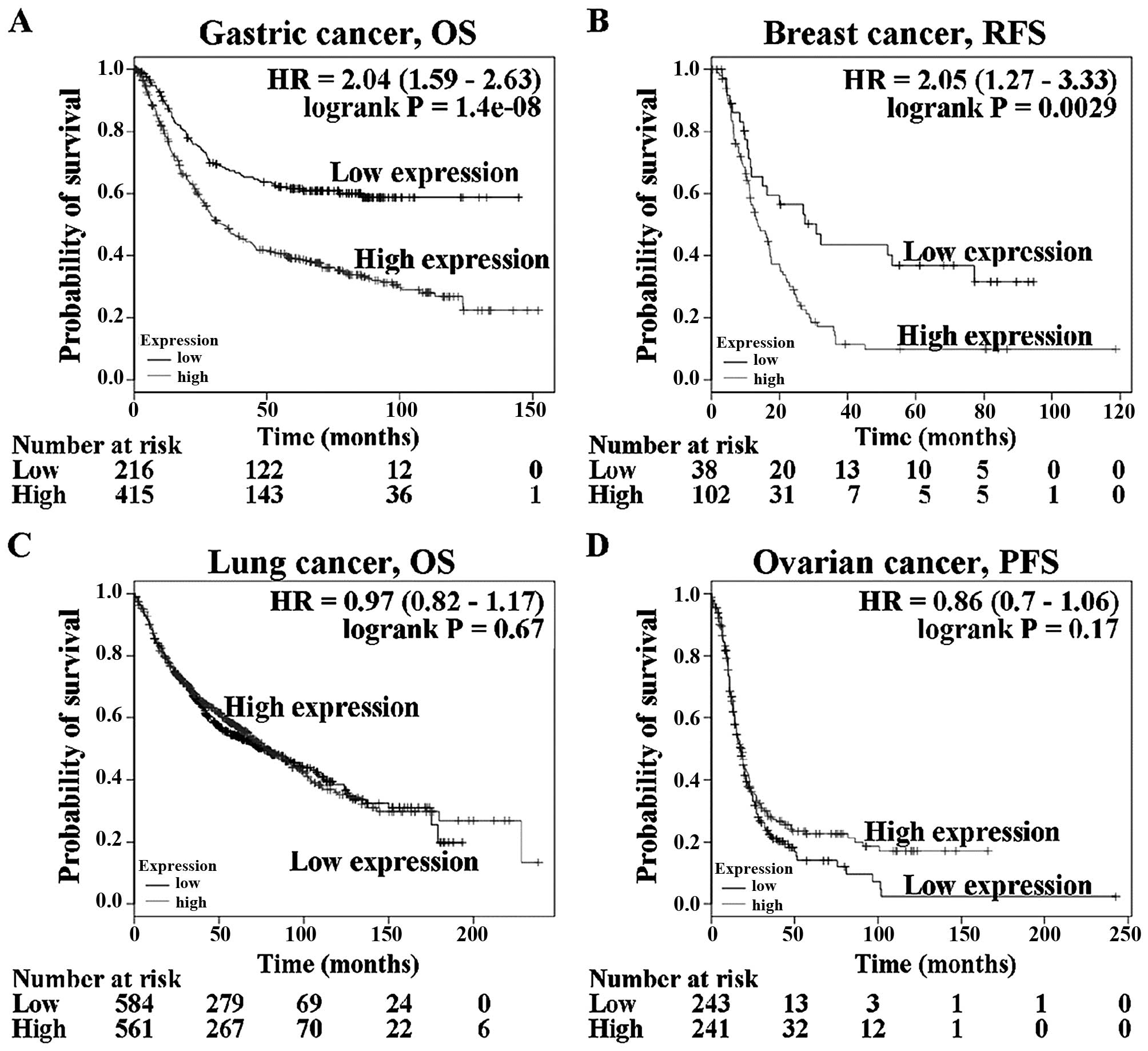
in patients with gastric, breast, lung and ovarian cancer (Fig. 1) revealed that prognosis was poorer
in gastric cancer and breast cancer patients with high
GPBAR1 gene expression than in those with low expression
(Fig. 1A and B). GPBAR1 gene
expression was not correlated with overall survival of patients
with lung cancer (Fig. 1C). Ovarian
cancer patients with high GPBAR1 gene expression tended to
have a better prognosis than those with low expression (Fig. 1D). The function of GPBAR1
gene in tumor development differed between these four types of
cancer.
Prognoscan is a collection of human
cancer microarray data-sets
High GPBAR1 gene expression predicted a trend
toward poor prognosis in 12 datasets (Table I) and good prognosis in 15 datasets
(Table II). In most datasets, the
GPBAR1 gene expression was not significantly correlated with
survival and only one dataset in each group displayed predictive
power (GSE13507 and GSE8894). The level of GPBAR1 expression
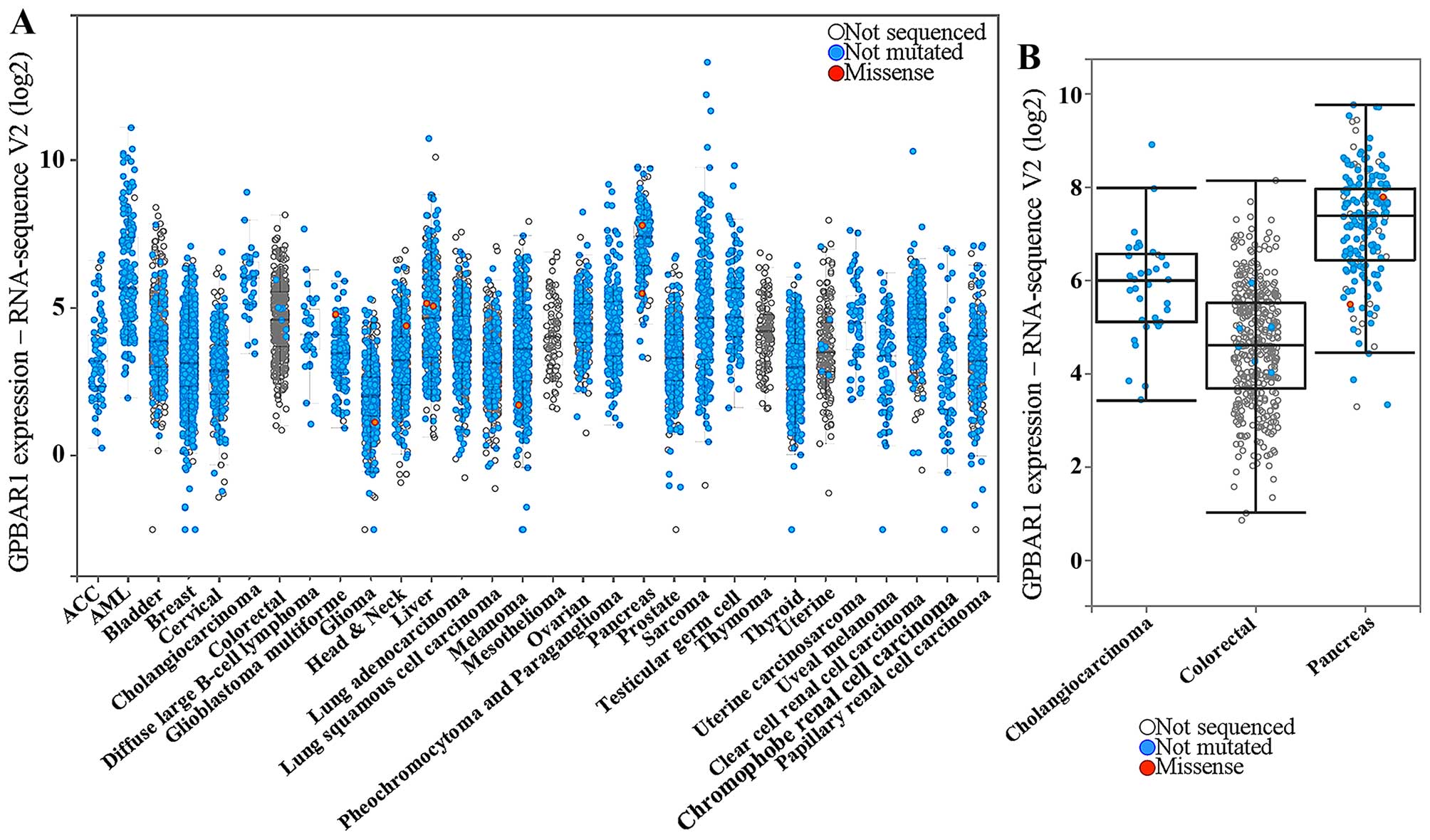
in human cancer was investigated using the cBioPortal system and
analyzed in 30 types of cancer (Fig.
2A). Three specific types of cancer (cholangiocarcinoma,
pancreatic and colorectal cancer) were compared (Fig. 2B). Cholangiocarcinoma and pancreatic
cancer are continuously exposed to bile and toxic bile acids are
well-established carcinogens for cholangiocarcinoma and colorectal
cancer. Cholangiocarcinoma and pancreatic cancer, compared to other
cancers, had a higher level of GPBAR1 expression (Fig. 2B). Thus the function of
GPBAR1 is distinct in different cancers, and its expression
may be correlated with bile exposure.
 | Table IPrognoScana microarray analysis of the prognostic
value of GPBAR1 in human cancer. (High GPBAR1 gene
expression predicted poor prognosis in these datasets). |
Table I
PrognoScana microarray analysis of the prognostic
value of GPBAR1 in human cancer. (High GPBAR1 gene
expression predicted poor prognosis in these datasets).
| Dataset | Cancer type | End point | Cohort | Contributor | Array type | Probe ID | No. | Cut point | Minimum
P-value | Corrected
P-value | ln
(HR-high/HR-low) | COX P-value | ln (HR) | HR (95% CI) |
|---|
| GSE13507 | Bladder | OS | CNUH | Kim | Human-6 v2 | ILMN_1727709 | 165 | 0.61 | 0.000 | 0.014b | 0.83 | 0.80 | −0.06 | 0.94
(0.57–1.54) |
| GSE2658 | Blood | DSS | Arkansas Z | han | HG-U133_Plus_2 | 1552501_a_at | 559 | 0.53 | 0.030 | 0.358 | 0.44 | 0.62 | 0.08 | 1.08
(0.79–1.48) |
| GSE7696 | Brain | OS | Lausanne | Murat | HG-U133_Plus_2 | 1552501_a_at | 70 | 0.89 | 0.127 | – | 0.61 | 1.00 | 0.00 | 1.00
(0.18–5.57) |
| GSE19615 | Breast | DMFS | DF/HCC | Li | HG-U133_Plus_2 | 1552501_a_at | 115 | 0.30 | 0.194 | – | 0.95 | 0.77 | 0.18 | 1.20
(0.36–3.99) |
| GSE12276 | Breast | RFS | EMC | Bos | HG-U133_Plus_2 | 1552501_a_at | 204 | 0.43 | 0.051 | – | 0.28 | 0.58 | 0.05 | 1.05
(0.89–1.24) |
| GSE6532-GPL570 | Breast | RFS | GUYT | Loi | HG-U133_Plus_2 | 1552501_a_at | 87 | 0.83 | 0.232 | – | 0.55 | 0.67 | −0.33 | 0.72
(0.16–3.31) |
| | DMFS | | Loi | | | 87 | 0.83 | 0.232 | – | 0.55 | 0.67 | −0.33 | 0.72
(0.16–3.31) |
| GSE9195 | Breast | RFS | GUYT2 | Loi | HG-U133_Plus_2 | 1552501_a_at | 77 | 0.16 | 0.102 | – | 15.30 | 0.29 | 1.50 | 4.47
(0.29–69.89) |
| GSE17537 | Colorectal | DFS | VMC | Smith | HG-U133_Plus_2 | 1552501_a_at | 55 | 0.49 | 0.047 | 0.472 | 1.03 | 0.48 | 1.21 | 3.36
(0.12–96.71) |
| | OS | | | | | 55 | 0.67 | 0.288 | – | 0.48 | 0.90 | −0.18 | 0.83
(0.05–14.36) |
| GSE3141 | Lung | OS | Duke | Bild | HG-U133_Plus_2 | 1552501_a_at | 111 | 0.22 | 0.024 | 0.304 | 0.88 | 0.08 | 0.28 | 1.33
(0.96–1.83) |
| GSE17710 | Lung | RFS | UNC | Wilkerson |
Agilent-UNC-custom-4X44K | 25074 | 56 | 0.68 | 0.159 | – | 0.50 | 0.56 | 0.32 | 1.38
(0.46–4.11) |
 | Table IIPrognoScana microarray analysis of the prognostic
value of GPBAR1 in human cancer. (High GPBAR1 gene
expression predicted good prognosis in these datasets.) |
Table II
PrognoScana microarray analysis of the prognostic
value of GPBAR1 in human cancer. (High GPBAR1 gene
expression predicted good prognosis in these datasets.)
| Dataset | Cancer type | End point | Cohort | Contributor | Array type | Probe ID | No. | Cut point | Minimum
P-value | Corrected
P-value | ln
(HR-high/HR-low) | COX P-value | ln (HR) | HR (95% CI) |
|---|
|
GSE12417-GPL570 | Blood | OS | AMLCG (2004) | Metzeler | HG-U133_Plus_2 | 1552501_a_at | 79 | 0.27 | 0.114 | – | −0.50 | 0.41 | −0.28 | 0.76
(0.39–1.47) |
| GSE16581 | Brain | OS | UCLA | Lee | HG-U133_Plus_2 | 1552501_a_at | 67 | 0.25 | 0.021 | 0.275 | −1.44 | 0.63 | −0.81 | 0.44
(0.02–12.73) |
| GSE9195 | Breast | DMFS | GUYT2 | Loi | HG-U133_Plus_2 | 1552501_a_at | 77 | 0.74 | 0.058 | – | −15.50 | 1.00 | −0.01 | 0.99
(0.06–16.54) |
| GSE17536 | Colorectal | DFS | MCC | Smith | HG-U133_Plus_2 | 1552501_a_at | 145 | 0.70 | 0.005 | 0.087 | −1.39 | 0.12 | −1.87 | 0.15
(0.01–1.62) |
| | OS | | | | | 177 | 0.85 | 0.035 | 0.389 | −0.94 | 0.25 | −0.95 | 0.39
(0.08–1.96) |
| | DSS | | | | | 177 | 0.84 | 0.023 | 0.295 | −1.27 | 0.58 | −0.52 | 0.60
(0.10–3.68) |
| GSE14333 | Colorectal | DFS | Melbourne | Jorissen | HG-U133_Plus_2 | 1552501_a_at | 226 | 0.85 | 0.076 | – | −1.01 | 0.25 | −0.17 | 0.85
(0.64–1.13) |
| GSE22138 | Eye | DMFS | BRCIC | Laurent | HG-U133_Plus_2 | 1552501_a_at | 63 | 0.81 | 0.015 | 0.216 | −1.60 | 0.35 | −6.72 | 0.00
(0–1599.31) |
| GSE2837 | Head and neck | RFS | VUMC, VAMC, UTMDACC
(1992–2005) | Chung | U133_X3P | Hs2.160954.1.
S1_3p_s_at | 28 | 0.25 | 0.117 | – | −0.90 | 0.23 | −3.90 | 0.02
(0.00–12.57) |
| GSE13213 | Lung | OS | Nagoya (1995–1999,
2002–2004) | Tomida | G4112F | A_23_P400378 | 117 | 0.31 | 0.107 | – | −0.47 | 0.97 | −0.01 | 0.99
(0.62–1.59) |
| GSE31210 | Lung | OS | NCCRI | Okayama | HG-U133_Plus_2 | 1552501_a_at | 204 | 0.87 | 0.121 | – | −1.45 | 0.81 | 0.05 | 1.05
(0.70–1.58) |
| | RFS | | | | | 204 | 0.88 | 0.052 | – | −1.30 | 0.48 | −0.11 | 0.90
(0.67–1.21) |
| GSE17537 | Colorectal | DSS | VMC | Smith | HG-U133_Plus_2 | 1552501_a_at | 49 | 0.90 | 0.151 | – | −15.26 | 0.87 | 0.30 | 1.35
(0.04–44.67) |
| GSE8894 | Lung | RFS | Seoul
(1995–2005) | Lee | HG-U133_Plus_2 | 1552501_a_at | 138 | 0.26 | 0.002 | 0.048b | −0.75 | 0.08 | −10.60 | 0.00
(0.00–3.68) |
| GSE17710 | Lung | OS U | NC | Wilkerson |
Agilent-UNC-custom-4X44K | 25074 | 56 | 0.21 | 0.267 | – | −0.44 | 0.83 | −0.11 | 0.89
(0.32–2.53) |
| GSE9891 | Ovarian | OS | AOCS, RBH, WH,
NKI-AVL (1992–2006) | Tothill | HG-U133_Plus_2 | 1552501_a_at | 278 | 0.74 | 0.024 | 0.304 | −0.50 | 0.37 | −0.35 | 0.71
(0.33–1.52) |
| GSE17260 | Ovarian | PFS | Niigata
(1997–2008) | Yoshihara | G4112A | A_23_P400378 | 110 | 0.70 | 0.077 | – | −0.46 | 0.99 | 0.00 | 1.00
(0.58–1.73) |
| | OS | | | | | 110 | 0.69 | 0.106 | – | −0.57 | 0.56 | −0.20 | 0.82
(0.41–1.63) |
| GSE19234 | Skin | OS | NYU | Bogunovic | HG-U133_Plus_2 | 1552501_a_at | 38 | 0.79 | 0.093 | – | −1.20 | 0.31 | −0.66 | 0.52
(0.14–1.86) |
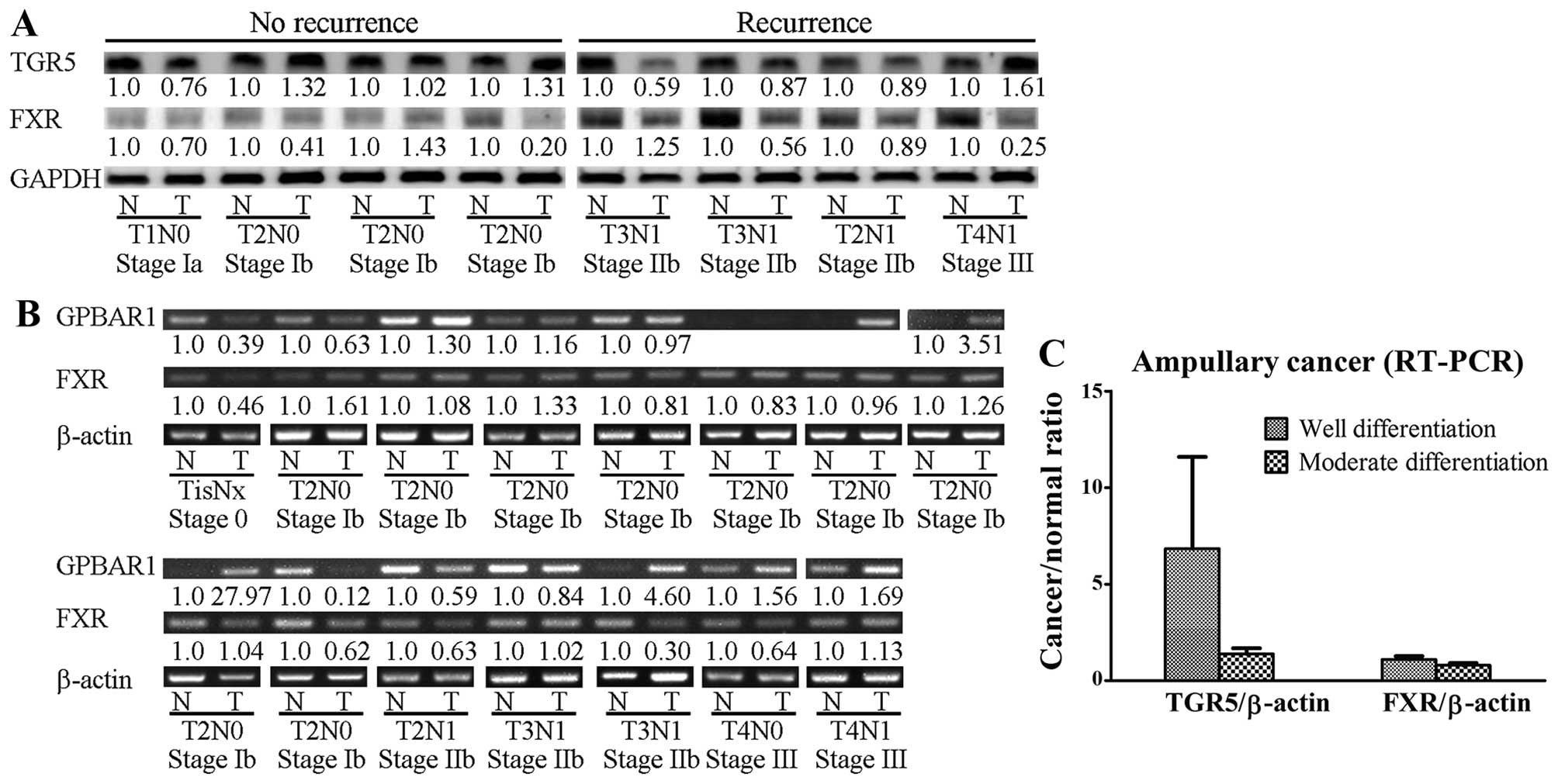
Expression of TGR5 protein in ampullary
cancer
The ampulla of Vater is located in the second part
of the duodenum and is exposed to bile acids under normal
physiological conditions. TGR5 protein (product of the
GPBAR1 gene) and GPBAR1 mRNA were detected in
clinical samples of ampullary cancer and the surrounding normal
duodenum (Fig. 3). In the patients
with cancer recurrence, the TGR5 protein level was lower in the
tumor than that noted in the normal duodenum. In the patients
without cancer recurrence, the tumor TGR5 level was similar to the
normal tissue level. In contrast, no such pattern was found for FXR
protein (Fig. 3A). Increased
GPBAR1 mRNA was detected in 7 of 15 specimens of ampullary
cancer, particularly in well-differentiated ampullary
adenocarcinoma (Fig. 3B and C).
Expression of FXR mRNA was not correlated with histological
differentiation (Fig. 3C).
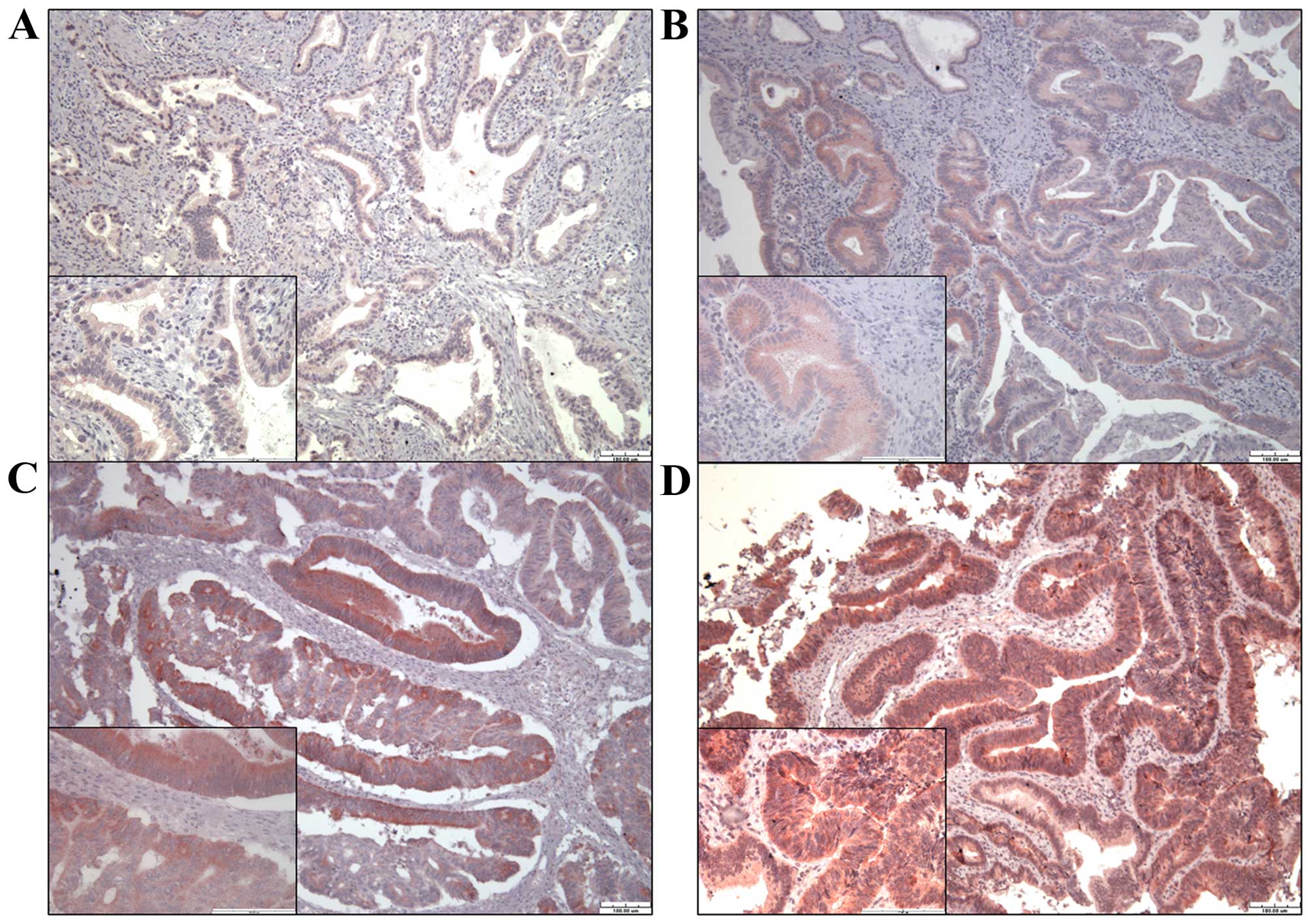
Immunohistochemical staining of TGR5 in
ampullary cancer
To study the relationship of TGR5 protein expression
with clinical outcome, 99 specimens of ampullary adenocarcinoma
were immunostained for TGR5. TGR5 was detected in the cytoplasm and
nucleus of each cancer cell (Fig.
4). We divided the result as negative, weak, mild and strong
expression of TGR5. Expression of TGR5 was negative in 14 patients,
weak in 33, mild in 29, and strong in 23 (Table I). Negative, weak or mild expression
of TGR5 was correlated with younger age (P=0.043) and higher level
of direct bilirubin (P=0.023, separately) and tended to be
correlated with higher level of total bilirubin (P=0.059), higher
plasma level of cancer antigen-125 (CA-125) (P=0.099), advanced
tumor stage and AJCC TNM stage (P=0.063 and 0.062, separately)
(Table III).
 | Table IIICorrelation of TGR5 expression with
demographics and histopathological findings in patients with
ampullary adenocarcinoma who underwent radical resection. |
Table III
Correlation of TGR5 expression with
demographics and histopathological findings in patients with
ampullary adenocarcinoma who underwent radical resection.
| Expression of TGR5
| P-value |
|---|
| Negative, weak,
mild | Strong |
|---|
| Patients, n
(%) | 76 (77) | 23 (23) | |
| Gender, n (%) | | | 0.481 |
| Female | 30 (73) | 11 (27) | |
| Male | 46 (79) | 12 (21) | |
| Age at surgery
(years)a | 65 (32–90) | 68 (35–83) | 0.043 |
| Total bilirubin
(mg/dl)a | 3.4 (0.2–19.6) | 1.3 (0.4–16.3) | 0.059 |
| Direct bilirubin
(mg/dl)a | 2.6 (0–18.0) | 0.6 (0–7.3) | 0.023 |
| Preoperative bile
decompression, n (%) | 41 (77) | 12 (23%) | 1.000 |
| CEA (ng/ml)a | 1.9
(0.1–296.3) | 2.5 (0–13.0) | 0.306 |
| CA-125
(U/ml)a | 15.4
(5.2–164.1) | 11.5
(0.5–66.7) | 0.099 |
| CA-199
(U/ml)a | 55.4
(0.3–7512.9) | 44.2
(1.4–1860) | 0.892 |
| Subtype, n
(%)b | | | 0.206 |
| Intestinal
type | 37 (74) | 13 (26) | |
|
Pancreaticoduodenal type | 17 (90) | 2 (10) | |
| Tumor type, n
(%) | | | 0.315 |
| Polypoid | 40 (74) | 14 (26) | |
| Ulcerative | 21 (87) | 3 (13) | |
| Mixed | 15 (71) | 6 (29) | |
| Resection margin, n
(%) | | | 1.000 |
| Free | 66 (76) | 21 (24) | |
| Microscopically
positive | 8 (80) | 2 (20) | |
| Lymph node
metastasis, n (%)b | | | 0.176 |
| Negative | 42 (75) | 14 (25) | |
| Positive | 30 (88) | 4 (12) | |
| Lymphovascular
invasion, n (%)b | | | 0.192 |
| Negative | 27 (69) | 12 (31) | |
| Positive | 34 (83) | 7 (17) | |
| Perineural
invasion, n (%)b | | | 0.373 |
| Negative | 28 (68) | 13 (32) | |
| Positive | 18 (82) | 4 (18) | |
| Histological
differentiation, n (%)b | | | 0.847 |
| Well | 31 (74) | 11 (26) | |
| Moderate | 37 (77) | 11 (23) | |
| Poor | 5 (83) | 1 (17) | |
| Pancreatic
invasion, n (%)b | | | 0.149 |
| Negative | 34 (71) | 14 (29) | |
| Positive | 42 (84) | 8 (16) | |
| Tumor size
(cm)a | 2.4 (0.7–8.0) | 2.5 (1.0–6.0) | 0.783 |
| Tumor stage, n
(%) | | | 0.063 |
| T1 | 5 (45) | 6 (55) | |
| T2 | 27 (75) | 9 (25) | |
| T3 | 29 (83) | 6 (17) | |
| T4 | 15 (88) | 2 (12) | |
| AJCC TNM stage, n
(%) | | | 0.062 |
| I | 26 (63) | 15 (37) | |
| II | 34 (85) | 6 (15) | |
| III | 15 (88) | 2 (12) | |
| IV | 1 (100) | 0 (0) | |
Correlation of TGR5 expression with
clinical outcomes of ampullary cancer patients
In 95 patients with regular follow-up (range, 3–249
months), 55 patients developed recurrence. The recurrences in
patients with negative, weak or mild TGR5 expression tended to be
earlier (within postoperative 12 months) (P=0.089), although the
level of TGR5 expression was not associated with recurrence
patterns (Table IV). The
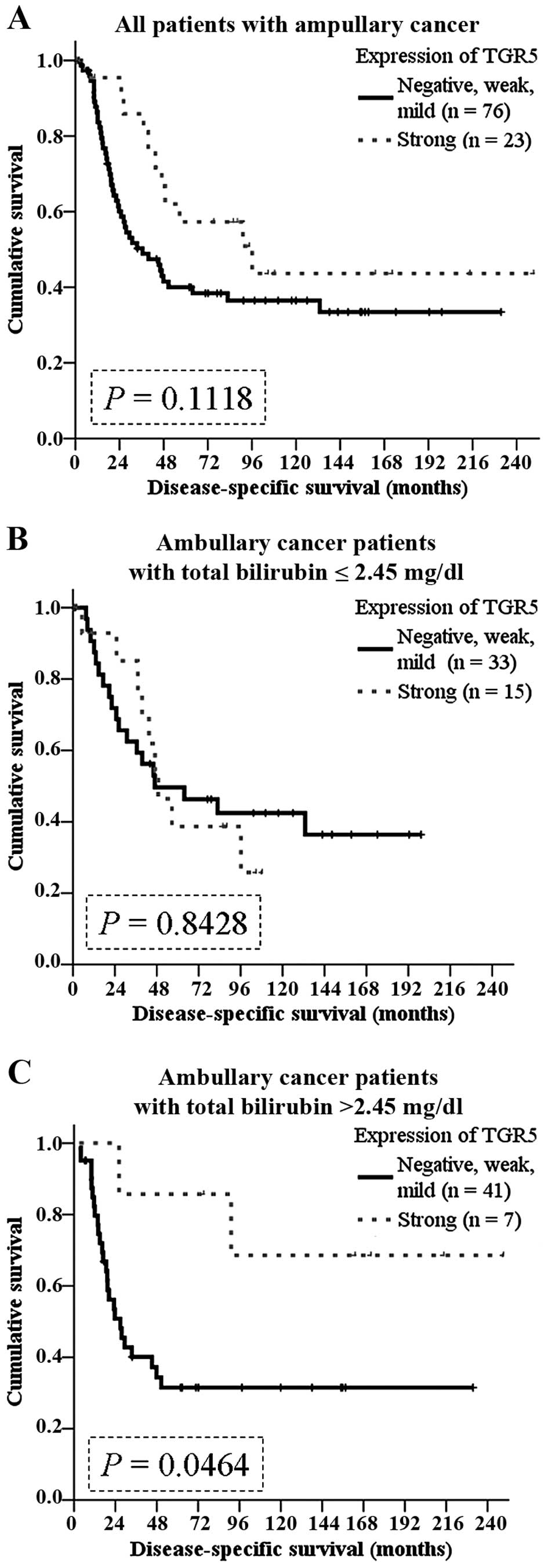
disease-specific survival rate tended to be better in patients with
strong TGR5 expression (P=0.1118; Fig.
5A).
 | Table IVCorrelation between disease
recurrence and TGR5 expression in patients with ampullary
adenocarcinoma who underwent radical resection. |
Table IV
Correlation between disease
recurrence and TGR5 expression in patients with ampullary
adenocarcinoma who underwent radical resection.
| Expression of TGR5
| P-value |
|---|
| Negative, weak,
mild | Strong |
|---|
| Patients, n
(%) | 76 (77) | 23 (23) | |
| No recurrence, n
(%)a | 28 (70) | 12 (30) | |
| Recurrence, n
(%)a | 44 (80) | 11 (20) | 0.089 |
| Delayed
recurrence, n (%)(after postoperative 12 months) | 16 (70) | 7
(30) | |
| Early recurrence,
n (%)(within postoperative 12 months) | 28 (90) | 3
(10) | |
| Patterns of
recurrence, n (%)a,b |
| Liver metastasis,
n (%) | 19 (86) | 3
(14) | 0.260 |
| Local recurrence,
n (%) | 28 (85) | 5
(15) | 0.205 |
| Peritoneal
carcinomatosis, n (%) | 12 (86) | 2
(14) | 0.506 |
| Bone metastasis, n
(%) | 6
(67) | 3
(33) | 0.437 |
| Other metastasis,
n (%)c | 14 (82) | 3
(18) | 0.754 |
In the literature, the function of TGR5 depends on
dysregulation of bile acid homeostasis (2). We hypothesized that high levels of
preoperative bilirubin interacts with TGR5 in ampullary cancer. We
grouped the patients according to the median level of total
bilirubin (2.45 mg/dl). Although not correlated with survival in
the patients with total bilirubin ≤2.45 mg/dl (Fig. 5B), TGR5 expression predicted a
better prognosis in patients with higher than 2.45 mg/dl (P=0.0464;
Fig. 5C).
Discussion
The ampullary of Vater is normally exposed to bile.
This is the first study to investigate expression of the membrane
bile acid receptor, TGR5, in ampullary adenocarcinoma. The results
of analysis of GPBAR1 gene expression data (gene of TGR5) in
microarray databases was correlated with clinical outcomes and
varied between types of cancers. The pathological function of TGR5
in cancer is regulated by a complex mechanism. In ampullary
adenocarcinoma, the present study detected TGR5 protein and
GPBAR1 mRNA in the tumor and surrounding normal duodenum.
Negative, weak or mild TGR5 expression was correlated with
elevation of plasma bilirubin. In the patients with plasma total
bilirubin higher than the median, strong TGR5 expression predicted
a better prognosis.
There are two types of ampullary adenocarcinoma:
intestinal and pancreaticobiliary types. These differ in clinical
behavior (27) and the differences
may be intrinsic. Nuclear accumulation of β-catenin promotes WNT
activation and cancer progression; however, loss of the β-catenin
protein in ampullary cancer is correlated with poor prognosis
(28). Nestin, a stemness protein,
performs a dual role in ampullary adenocarcinoma, as a predictor of
good prognosis in early cancer and as a promoter of metastasis in
advanced cancer (29). The
epithelial cell marker, EpCAM, is one of the signatures of cancer
stem cells with oncogenic potential mediated via upregulation of
c-myc and cyclins. However, loss of EpCAM is linked to a more
aggressive phenotype of ampullary cancer, suggesting that EpCAM may
play a different role in ampullary cancer than in other cancers
(30). Ampullary adenocarcinoma is
a unique cancer with a 5-year survival rate <50% after curative
resection (27,31,32).
Further study of ampullary adenocarcinoma is required to develop
new treatment modalities and improve clinical outcomes.
The ampullary of Vater is located at the confluence
of the common bile and pancreatic ducts, and second portion of the
duodenum. Long-term exposure to bile acids increases oxidative
stress, generates ROS/RNS, and induces cell damage and mutation
rates in gastrointestinal cancer (2,5,6).
Alteration of the bile contents of gastroesophageal reflux is
correlated with the increased incidence of cancer in cell culture,
animal models and epidemiology studies (3,4,33).
Toxic bile acids induce expression of COX-2 or activation of EGFR
and promote carcinogenesis in cholangiocarcinoma (7,8). In
our previous study, preoperative plasma concentration of total
bilirubin was elevated in non-survivors of ampullary cancer
(32). However, no relationship was
found between hyperbilirubinemia and ampullary cancer
recurrence.
Bile acids interact not only with nuclear receptors,
but also with membrane receptors. TGR5 is a G protein-coupled bile
acid receptor that mediates bile acid-regulated energy and glucose
homeostasis (2,9,34).
Bile acids induce cell proliferation and cell cycle progression
through the TGR5-dependent pathway and TGR5 acts such as an
oncoprotein (16–18). In hepatocytes, suppression of TGR5
enhances chemical-induced carcinogenesis and activation of TGR5
promotes cell apoptosis (19,20).
Whether TGR5 promotes or suppresses carcinogenesis depends on the
composition of the bile acids (2).
We analyzed multiple microarray datasets and found that high
GPBAR1 gene expression predicted poor prognosis in some
datasets, but good prognosis in others (Tables I and II; Fig.
1). Bile acid-exposed cancers (such as cholangiocarcinoma and
pancreatic cancer) had a higher ratio of GPBAR1 expression
(Fig. 2B). Since the ampulla of
Vater is also exposed to bile acids under normal physiological
conditions, the study of bile acid receptors, such as TGR5 and FXR,
is indicated in ampullary cancer. In the present study, the mRNAs
or proteins of TGR5 or FXR were detected in specimens of ampullary
adenocarcinoma (Fig. 3). Increased
expression of GPBAR1 mRNA but not FXR mRNA was correlated
with histological differentiation and well-differentiated
adenocarcinoma (Fig. 3C). Taken
together, our results indicate that the membrane receptor of bile
acids, TGR5, may be activated in ampullary cancer.
Activation of TGR5 plays a role in cyclic adenosine
monophosphate (cAMP), EGFR, mitogen-activated protein kinase (MAPK,
such as JNK, ERK-1/2), cyclooxygenase-2 (COX-2) or signal
transducer and activator of transcription 3 (STAT3) signaling
(35). TGR5 functions in a cell
type-dependent and context-dependent manner in cancer. In gastric
and esophageal cancer, the TGR5-dependent pathway mediates bile
acid-induced ROS production and cell proliferation as well as
deoxycholate-induced EGFR phosphorylation and ERK1/2 activation.
Moreover, TGR5 expression is associated with the poor prognosis of
patients (36–38), suppresses STAT3 signaling and
inhibits cell cycle progression, angiogenesis, metastasis and
evasion of the immune system in gastric cancer (36). Interaction of TGR5 and EGFR depends
on lipid rafts. Deoxycholate induces EGFR phosphorylation and
ERK1/2 activation through the TGR5-dependent pathway (38). However, TGR5 performs as a
tumor-suppressor in liver cancer. TGR5-deficient mice have an
increased incidence of liver cancer (19). Bile acids conjugate TGR5 to induce
JNK activation and enhance apoptosis in hepatocytes (20). In the present study, strong TGR5
expression was correlated with lower plasma concentration of total
and direct bilirubin. The patients with strong TGR5 expression
tended to have a lower plasma level of CA-125, earlier tumor stage,
and earlier AJCC TNM stage and also a better disease-specific
survival rate, particularly those patients with total bilirubin
concentration higher than 2.45 mg/dl. We conclude that TGR5
performs as a tumor suppressor in hyperbilirubinemic patients with
ampullary adenocarcinoma.
In summary, high TGR5 expression was correlated with
lower plasma concentration of total/direct bilirubin, lower plasma
level of CA-125, early tumor stage and AJCC TNM stage. High TGR5
expression also predicted a good survival in patients with total
bilirubin levelss higher than 2.45 mg/dl. TGR5 performs as a tumor
suppressor in hyperbilirubinemia condition of ampullary
adenocarcinoma patients.
Abbreviations:
|
AJCC TNM stage
|
American Joint Committee on Cancer
tumor-node-metastases staging system
|
|
CA-125
|
cancer antigen-125
|
|
CA-199
|
cancer antigen-199
|
|
cAMP
|
cyclic adenosine monophosphate
|
|
CEA
|
carcinoembryonic antigen
|
|
COX-2
|
cyclooxygenase-2
|
|
EGFR
|
epidermal growth factor receptor
|
|
FXR
|
farnesoid X receptor
|
|
GCDC
|
glycochenodeoxycholate
|
|
GPBAR1
|
G protein-coupled bile acid receptor
1
|
|
IHC
|
immunohistochemistry
|
|
IRS
|
immunoreactive score of Remmele and
Stegner
|
|
JNK
|
c-Jun-N terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PDGFR
|
platelet-derived growth factor
receptor
|
|
RNS
|
reactive nitrogen species
|
|
ROS
|
reactive oxygen species
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
TCDA
|
taurochenodeoxycholate
|
Acknowledgments
The present study was supported by grants from the
National Science Council (grant NSC-102-B-2314-B-006-043), the
Ministry of Science and Technology (grant
MOST-104-B-2314-B-006-049), the National Cheng Kung University
Hospital (to H.-P.H.), and the Chi Mei Medical Center (to M.-C.C.).
The authors are grateful for the support from the Human Biobank,
Research Center of Clinical Medicine, National Cheng Kung
University Hospital. We were blessed with support from the late
superintendent, Professor Pin-Wen Lin. Furthermore, we thank Mr.
Chih-Yang Wang and Ms. Yu-Hsuan Hung for their support.
References
|
1
|
Albores-Saavedra J, Schwartz AM, Batich K
and Henson DE: Cancers of the ampulla of vater: Demographics,
morphology, and survival based on 5,625 cases from the SEER
program. J Surg Oncol. 100:598–605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuei J, Chau T, Mills D and Wan YJ: Bile
acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp
Biol Med. 239:1489–1504. 2014. View Article : Google Scholar
|
|
3
|
Ye W, Chow WH, Lagergren J, Yin L and
Nyrén O: Risk of adenocarcinomas of the esophagus and gastric
cardia in patients with gastroesophageal reflux diseases and after
antireflux surgery. Gastroenterology. 121:1286–1293. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishioka K, Doki Y, Miyata H, Tamura S,
Yasuda T, Kimura Y, Kishi K, Yoshida K, Fujiwara Y, Yano M, et al:
Bile acid promotes the proliferation of squamous cell carcinoma of
the esophagus, independent of its inducing COX-2 expression. J Surg
Res. 132:130–135. 2006. View Article : Google Scholar
|
|
5
|
Bernstein C, Payne CM and Bernstein H:
Bile acids: Promoters or carcinogens in colon cancer? J Carcinogene
Mutagene. 2:101e View Article : Google Scholar : 2011.
|
|
6
|
Payne CM, Bernstein C, Dvorak K and
Bernstein H: Hydrophobic bile acids, genomic instability, Darwinian
selection, and colon carcinogenesis. Clin Exp Gastroenterol.
1:19–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Komichi D, Tazuma S, Nishioka T, Hyogo H
and Chayama K: Glycochenodeoxycholate plays a carcinogenic role in
immortalized mouse cholangiocytes via oxidative DNA damage. Free
Radic Biol Med. 39:1418–1427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoon JH, Higuchi H, Werneburg NW, Kaufmann
SH and Gores GJ: Bile acids induce cyclooxygenase-2 expression via
the epidermal growth factor receptor in a human cholangiocarcinoma
cell line. Gastroenterology. 122:985–993. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomas C, Pellicciari R, Pruzanski M,
Auwerx J and Schoonjans K: Targeting bile-acid signalling for
metabolic diseases. Nat Rev Drug Discov. 7:678–693. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lax S, Schauer G, Prein K, Kapitan M,
Silbert D, Berghold A, Berger A and Trauner M: Expression of the
nuclear bile acid receptor/farnesoid X receptor is reduced in human
colon carcinoma compared to nonneoplastic mucosa independent from
site and may be associated with adverse prognosis. Int J Cancer.
130:2232–2239. 2012. View Article : Google Scholar
|
|
11
|
van de Winkel A, van Zoest KP, van Dekken
H, Moons LM, Kuipers EJ and van der Laan LJ: Differential
expression of the nuclear receptors farnesoid X receptor (FXR) and
pregnane X receptor (PXR) for grading dysplasia in patients with
Barrett's oesophagus. Histopathology. 58:246–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim I, Morimura K, Shah Y, Yang Q, Ward JM
and Gonzalez FJ: Spontaneous hepatocarcinogenesis in farnesoid X
receptor-null mice. Carcinogenesis. 28:940–946. 2007. View Article : Google Scholar :
|
|
13
|
Li T, Holmstrom SR, Kir S, Umetani M,
Schmidt DR, Kliewer SA and Mangelsdorf DJ: The G protein-coupled
bile acid receptor, TGR5, stimulates gallbladder filling. Mol
Endocrinol. 25:1066–1071. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomas C, Gioiello A, Noriega L, Strehle
A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski
M, et al: TGR5-mediated bile acid sensing controls glucose
homeostasis. Cell Metab. 10:167–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duboc H, Taché Y and Hofmann AF: The bile
acid TGR5 membrane receptor: From basic research to clinical
application. Dig Liver Dis. 46:302–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao W, Tian W, Hong J, Li D, Tavares R,
Noble L, Moss SF and Resnick MB: Expression of bile acid receptor
TGR5 in gastric adenocarcinoma. Am J Physiol Gastrointest Liver
Physiol. 304:G322–G327. 2013. View Article : Google Scholar :
|
|
17
|
Hong J, Behar J, Wands J, Resnick M, Wang
LJ, DeLellis RA, Lambeth D, Souza RF, Spechler SJ and Cao W: Role
of a novel bile acid receptor TGR5 in the development of
oesophageal adenocarcinoma. Gut. 59:170–180. 2010. View Article : Google Scholar
|
|
18
|
Casaburi I, Avena P, Lanzino M, Sisci D,
Giordano F, Maris P, Catalano S, Morelli C and Andò S:
Chenodeoxycholic acid through a TGR5-dependent CREB signaling
activation enhances cyclin D1 expression and promotes human
endometrial cancer cell proliferation. Cell Cycle. 11:2699–2710.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang JI, Yoon JH, Myung SJ, Gwak GY, Kim
W, Chung GE, Lee SH, Lee SM, Kim CY and Lee HS: Bile acid-induced
TGR5-dependent c-Jun-N terminal kinase activation leads to enhanced
caspase 8 activation in hepatocytes. Biochem Biophys Res Commun.
361:156–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen WD, Yu D, Forman BM, Huang W and Wang
YD: Deficiency of G-protein-coupled bile acid receptor Gpbar1
(TGR5) enhances chemically induced liver carcinogenesis.
Hepatology. 57:656–666. 2013. View Article : Google Scholar :
|
|
21
|
Györffy B, Lánczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar
|
|
22
|
Győrffy B, Surowiak P, Budczies J and
Lanczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e82241.1–e82241.8. 2013.
View Article : Google Scholar
|
|
23
|
Győrffy B, Lánczky A and Szállási Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar
|
|
24
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBio-Portal. Sci Signal. 6:pl12013. View Article : Google Scholar
|
|
25
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Remmele W and Schicketanz KH:
Immunohistochemical determination of estrogen and progesterone
receptor content in human breast cancer. Computer-assisted image
analysis (QIC score) vs. subjective grading (IRS). Pathol Res
Pract. 189:862–866. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schiergens TS, Reu S, Neumann J, Renz BW,
Niess H, Boeck S, Heinemann V, Bruns CJ, Jauch KW and Kleespies A:
Histomorphologic and molecular phenotypes predict gemcitabine
response and overall survival in adenocarcinoma of the ampulla of
Vater. Surgery. 158:151–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu HP, Shan YS, Jin YT, Lai MD and Lin
PW: Loss of E-cadherin and beta-catenin is correlated with poor
prognosis of ampullary neoplasms. J Surg Oncol. 101:356–362.
2010.PubMed/NCBI
|
|
29
|
Shan YS, Chen YL, Lai MD and Hsu HP:
Nestin predicts a favorable prognosis in early ampullary
adenocarcinoma and functions as a promoter of metastasis in
advanced cancer. Oncol Rep. 33:40–48. 2015.
|
|
30
|
Piscuoglio S, Lehmann FS, Zlobec I,
Tornillo L, Dietmaier W, Hartmann A, Wünsch PH, Sessa F, Rümmele P,
Baumhoer D, et al: Effect of EpCAM, CD44, CD133 and CD166
expression on patient survival in tumours of the ampulla of Vater.
J Clin Pathol. 65:140–145. 2012. View Article : Google Scholar
|
|
31
|
Rostain F, Hamza S, Drouillard A, Faivre
J, Bouvier AM and Lepage C: Trends in incidence and management of
cancer of the ampulla of Vater. World J Gastroenterol.
20:10144–10150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu HP, Yang TM, Hsieh YH, Shan YS and Lin
PW: Predictors for patterns of failure after
pancreaticoduodenectomy in ampullary cancer. Ann Surg Oncol.
14:50–60. 2007. View Article : Google Scholar
|
|
33
|
Goldstein SR, Yang GY, Curtis SK, Reuhl
KR, Liu BC, Mirvish SS, Newmark HL and Yang CS: Development of
esophageal metaplasia and adenocarcinoma in a rat surgical model
without the use of a carcinogen. Carcinogenesis. 18:2265–2270.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pols TW, Noriega LG, Nomura M, Auwerx J
and Schoonjans K: The bile acid membrane receptor TGR5: A valuable
metabolic target. Dig Dis. 29:37–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stepanov V, Stankov K and Mikov M: The
bile acid membrane receptor TGR5: A novel pharmacological target in
metabolic, inflammatory and neoplastic disorders. J Recept Signal
Transduct Res. 33:213–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo C, Su J, Li Z, Xiao R, Wen J, Li Y,
Zhang M, Zhang X, Yu D, Huang W, et al: The G-protein-coupled bile
acid receptor Gpbar1 (TGR5) suppresses gastric cancer cell
proliferation and migration through antagonizing STAT3 signaling
pathway. Oncotarget. 6:34402–34413. 2015.PubMed/NCBI
|
|
37
|
Yasuda H, Hirata S, Inoue K, Mashima H,
Ohnishi H and Yoshiba M: Involvement of membrane-type bile acid
receptor M-BAR/TGR5 in bile acid-induced activation of epidermal
growth factor receptor and mitogen-activated protein kinases in
gastric carcinoma cells. Biochem Biophys Res Commun. 354:154–159.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jensen DD, Godfrey CB, Niklas C, Canals M,
Kocan M, Poole DP, Murphy JE, Alemi F, Cottrell GS, Korbmacher C,
et al: The bile acid receptor TGR5 does not interact with
β-arrestins or traffic to endosomes but transmits sustained signals
from plasma membrane rafts. J Biol Chem. 288:22942–22960. 2013.
View Article : Google Scholar : PubMed/NCBI
|